Malignant lymphomas are characterized by specific gene rearrangements that result in particular fusion transcripts and proteins. For example, follicular lymphoma harbors the translocation t(14;18) while mantle cell lymphoma bears the translocation t(11;14). Both translocations result in specific gene fusions (Bcl-2 activation and cyclin D1 overexpression, respectively) that render the respective lymphoma cells resistant to apoptotic stimuli. The translocation t(11;18)(q21;q21) has been implicated recently in low-grade marginal-zone B-cell lymphomas of the mucosa associated lymphoid tissue (MALT).1,2 This translocation has been detected in MALT lymphomas of low grade only. The genes involved in the translocation have been cloned,3 showing that the 5′ partner gene is a member of the survivin family API2 (nhibitor ofpoptosis 2) and the 3′ partner is a novel gene of unknown function, designated MLT (ALTymphoma-associatedranslocation). This genetic aberration has been detected in approximately one third of low-grade MALT lymphomas.
The most frequent site in which MALT lymphomas are found is within the gastric mucosal wall. MALT lymphomas arising in the stomach have been linked to a chronic infection caused by Helicobacter pylori.4 Early gastric MALT lymphomas have been reported to respond to cure of the H pylori infection, with such remissions lasting for up to several years.5,6 We wanted to investigate whether the response of early gastric low-grade marginal-zone B-cell lymphomas of the MALT type to H pylorieradication therapy corresponded to the presence of t(11;18). Biopsies from 20 cases of low-grade gastric MALT lymphomas, recruited into a nationwide trial, were analyzed.7 All samples were reviewed by a central pathologist (M.S.). In 18 biopsy samples, MALT lymphoma response to H pylori eradication therapy was observed, whereas in 1 patient no response to H pylori eradication therapy occurred. In 3 patients the follow-up period has been too short to evaluate the response as yet.
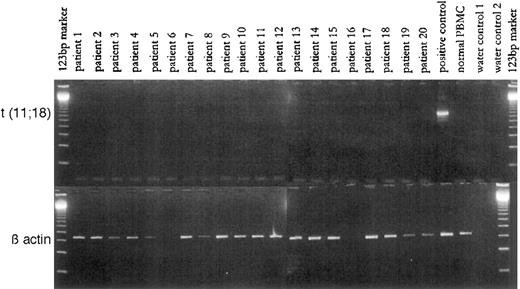
Reverse transcriptase polymerase chain reaction (RT-PCR) was used to detect the specific fusion transcript API2-MLT in RNA obtained from the biopsy samples. The fusion transcript was expressed in none of the 18 samples from which RNA could be successfully extracted (see Figure).
PCR-amplification for the t(11;18) fusion and for β actin (as reference).
PCR-amplification for the t(11;18) fusion and for β actin (as reference).
In contrast, the reference gene, β-actin, was expressed at a constant level in all samples (see Figure). Thus in these 18 cases of low-grade gastric marginal-zone B-cell lymphoma of MALT type, t(11;18) does not seem to be as frequent as has been reported for extranodal marginal-zone B-cell lymphomas. We feel that this discrepancy is unlikely to result from technical problems, because analysis of a further case with known t(11;18) clearly bore a specific fusion transcript detectable by our RT-PCR assay (see Figure). We suggest that, instead, t(11;18) plays no role in the response of gastric MALT lymphomas to cure of H pylori infection. Low-grade lymphomas harboring t(11;18) may be more advanced in their progression than those low-grade cases analyzed in this study. In agreement with this is the report that t(11;18) occurs predominantly in more progressed low-grade MALT lymphomas.3 Whether the presence of t(11;18) might predict nonresponse in MALT lymphomas to eradication of H pylori infection cannot be stated on the basis of our data, but this may be likely.
In summary, although t(11;18) is found in more progressed low-grade gastric MALT lymphomas, it is rare in those early gastric MALT lymphomas responding to H pylori eradication treatment. Thus t(11;18) may be a clonal marker of lymphoma progression in extranodal marginal-zone B-cell lymphomas.
References
Author notes
Supported in part by grants from the Deutsche Krebshilfe, Bonn, Germany (to A.N. and J.D.).