We have demonstrated that high molecular weight kininogen (HK) binds specifically on endothelial cells to domain 2/3 of the urokinase receptor (uPAR). Inhibition by vitronectin suggests that kallikrein-cleaved HK (HKa) is antiadhesive. Plasma kallikrein bound to HK cleaves prourokinase to urokinase, initiating cell-associated fibrinolysis. We postulated that HK cell binding domains would inhibit angiogenesis. We found that recombinant domain 5 (D5) inhibited endothelial cell migration toward vitronectin 85% at 0.27 μM with an IC50 (concentration to yield 50% inhibition) = 0.12 μM. A D5 peptide, G486-K502, showed an IC50 = 0.2 μM, but a 25-mer peptide from a D3 cell binding domain only inhibited migration 10% at 139 μM (IC50 > 50 μM). D6 exhibited weaker inhibitory activity (IC50 = 0.50 μM). D5 also potently inhibited endothelial cell proliferation with an IC50 = 30 nM, while D3 and D6 were inactive. Using deletion mutants of D5, we localized the smallest region for full activity to H441-D474. To further map the active region, we created a molecular homology model of D5 and designed a series of peptides displaying surface loops. Peptide 440-455 was the most potent (IC50 = 100 nM) in inhibiting proliferation but did not inhibit migration. D5 inhibited angiogenesis stimulated by fibroblast growth factor FGF2 (97%) in a chicken chorioallantoic membrane assay at 270 nM, and peptide 400-455 was also inhibitory (79%). HK D5 (for which we suggest the designation, “kininostatin”) is a potent inhibitor of endothelial cell migration and proliferation in vitro and of angiogenesis in vivo.
Angiogenesis is the formation of new capillaries from preexisting blood vessels. Many solid tumors are critically dependent on this new blood vessel formation to provide nutrients and oxygen and to support growth; thus, antiangiogenic therapy is an important goal for cancer therapy. Angiogenesis consists of the following steps: (1) endothelial cell detachment from adhesive proteins; (2) enzymatic degradation of the basement membrane by plasmin or plasmin-activated matrix metalloproteinases; (3) endothelial cell migration into perivascular spaces; (4) proliferation; and (5) tube formation. These steps result in new vessels. Stimuli include basic fibroblast growth factor FGF2, vascular endothelial cell growth factors (VEGF), other growth factors, and cytokines. Endogenous inhibitors of angiogenesis include plasma proteins such as thrombospondin and fragments of plasma proteins such as angiostatin, which is a proteolytic degradation product of plasminogen. Inhibition of angiogenesis can be mediated at 1 or more of the critical steps in the process.
The urokinase receptor (uPAR) plays a critical part in initiating cellular migration, regulating adhesion, and enhancing proteolysis, which are of central importance in the angiogenesis required for tumor growth and metastasis. This receptor binds prourokinase (proUK) with high affinity,1 thereby concentrating the expression of plasmin activity on the cell surface.2 Furthermore, uPAR is expressed on migrating cells, both in vivo and in vitro, indicating that uPAR enhances cell translocation.3 The urokinase receptor participates in the control of cell adhesion because it binds vitronectin with high affinity at a site within domains 2 and 3 of uPAR.4 In contrast, uPA is bound to uPAR within domain 1.5
Plasma HK (high molecular weight kininogen) is coded for by a single gene.6 The first 9 exons code for the heavy chain, and exon 10 codes for bradykinin (BK) domain 4 and the long light chain. The heavy chain of HK consists of domains 1, 2, and 3, and the light chain consists of domains 5 and 6. Mediation of the biologic effects of HK requires prior cell binding. Domain 3 (D3) inhibits thrombin action on platelets7 and cell binding to platelets8 and endothelial cells.9 Using recombinant fusion proteins and constrained peptides designed by homology modeling, we have mapped the region responsible for inhibiting thrombin binding to platelets to a heptapeptide within D38 coded for by exon 7 and found that it also blocks the binding of HK to neutrophils. Herwald et al9 have mapped the endothelial cell binding site on D3 and demonstrated that a peptide from D3 coded for by exon 9 inhibited thrombin-mediated tissue-type plasminogen activator release and prostacyclin synthesis. Following the cleavage of BK from HK, the resulting active cofactor, HKa, acquires the ability to bind to anionic surfaces.10 We have shown that within D5 of the light chain, critical amino acids (residues 441-457) in a histidine-glycine–rich region are responsible for binding to an artificial negatively charged surface.11 Using deletion mutagenesis, we have further defined a second subdomain—a histidine-glycine-lysine–rich region (residues 475-502) that also supports binding to an anionic surface.12 Our studies with recombinant polypeptides and monoclonal antibodies13 indicate that on neutrophils, D5 contains a binding site for HK, and we have mapped the site to residues 440-478,14 a site overlapping with the binding site for anionic surfaces. Hasan et al15 have mapped the endothelial cell binding domain on D5 to the histidine-glycine-lysine–rich region, specifically residues 471-496, which could represent a second region for cell interaction. The binding of D5 to cells appears to be responsible for the “antiadhesive” action of HKa. We showed that HKa can displace 125I-fibrinogen from both neutrophils and platelets.16 Asakura et al17 have confirmed and extended these results by showing that HKa inhibits the adhesion and spreading of human osteosarcoma cells on vitronectin-coated polystyrene plates and the spread of bovine aortic endothelial cells on vitronectin.17 The C-terminus of the light chain of HK in D6, which contains 31 amino acid residues 556-595, is required for the binding of prekallikrein (PK)18 and is exposed in HK. Because HK binds to cells via D3 or D5, D6 remains free to bind PK to endothelial cells.
We have recently observed that HKa binds to uPAR on endothelial cells.19 Domains 2 + 3 of uPAR are involved because both an antibody to these domains and vitronectin can inhibit its binding. HKa binds to purified soluble suPAR, while HK under the same conditions does not. In addition, we found that endothelial cell–bound HKa can bind PK, which, after activation to kallikrein by a cellular cysteine protease, can activate pro-uPA to uPA on uPAR.20 Hence, the interaction of kallikrein-cleaved HK (HKa) with uPAR may be important in both the ability of uPAR to catalyze the formation of cell surface plasmin as well as in the regulation of endothelial cell adhesion to extracellular matrix. Both of these activities are centrally involved in angiogenesis.21 22 We therefore tested HKa, D5, and 5 selected peptides from D5 for their ability to inhibit 2 other critical components of angiogenesis, endothelial proliferation and migration to vitronectin. We evaluated selected components in in vivo angiogenesis in a chicken chorioallantoic membrane (CAM) model.
Materials and methods
Materials
HK and HKa were purchased from Enzyme Research Laboratories, South Bend, IN. HK was more than 95% of a single band of 110 kd on both nonreduced and reduced SDS electrophoresis. HK had been digested with plasma kallikrein (molar ratio of 100:1 of HK to plasma kallikrein) for 20 minutes at 37°C. The resulting HKa was composed of 2 bands of 62 kd and 46 kd when analyzed by reduced SDS electrophoresis. Vitronectin (0.25 mg per vial) was purchased from Collaborative Biochemical Products, Bedford, MA.
Recombinant proteins
Deletion mutants of the light chain of HK were prepared as previously described.12 These mutants have been constructed in pGE × 2T vectors using a complementary DNA coding for the light chain of human HK as study material. Each has glutathione-S-transferase (GST) at the N-terminal of the polypeptide fused to the HK peptide. The amino acid sequence linked to GST is indicated. The mutants were purified on a glutathione column to more than 90% purity as previously described.12
Synthetic peptides
Peptide synthesis and high-performance-liquid-chromatography purification to more than 95% purity were performed by Dr John Lambris of the University of Pennsylvania, Philadelphia, PA. The cysteine-containing peptides were folded by dissolving each in a buffer containing 50 mM ammonium bicarbonate, pH 8.5, at a final concentration of 100 μg/mL and air oxidized for 3 days at 4°C with continuous agitation, then frozen and lyophilized. Additionally, 5,5-dithiobis (2-nitrobenzoic acid) was used to ensure that there were no detectable free-sulfhydryl groups in the peptide, and size exclusion on FPLC was used to ensure that the major population of peptide has intramolecular disulfide bonds and runs as a monomer.
Endothelial cell migration assay
Endothelial cells from human umbilical cords (HUVEC) were cultured as previously described.23 Endothelial cell adhesion to vitronectin was measured as described previously.24 We used a unique assay24 to measure the migration of endothelial cells to vitronectin or fibronectin. These assays were performed using a Neuroprobe 96-well disposable chemotaxis chamber with an 8–μm pore size. This chamber allows for quantitation of cellular migration toward a gradient of either vitronectin or fibronectin. Cultured cells were removed following a standardized method using ethylenediaminetetraacetic aicd (EDTA)/trypsin (0.01%/0.025%). Following removal, the cells were washed twice and resuspended (2 × 106/mL) in EBM (endothelial cell basal media; Clonetics, Inc, Walkersville, MD). The cell suspension (45 μL) containing 25 000 endothelial cells was added to a polypropylene plate containing 5 mL of test agent at different concentrations and incubated for 10 minutes at 22°C. Either vitronectin or fibronectin (28 μL) at 0.0125 to 100 μg/mL was added to the lower wells of a disposable chemotaxis chamber; the chamber was then assembled using the preframed filter. We added 25 μL of cell/test agent suspension to the upper filter wells and then incubated overnight (22 hours at 37°C, 5% CO2) in a humidified cell culture incubator. After the overnight incubation, nonmigrated cells and excess media were gently removed using a 12-channel pipette and a cell scraper. The filters were then washed twice in phosphate-buffered saline (PBS) (no Ca++ or Mg2+) and fixed with 1% formaldehyde in PBS (0.05 mol/L NaPO4 buffer containing 0.15 mol/L NaCl, pH 7.4). Membranes of migrated cells were permeated with Triton X-100 (0.2%) and then washed 2 to 3 times with PBS. The actin filaments of migrated cells were stained with rhodamine phalloidin (12.8 IU/mL) for 30 minutes (22°C). Rhodamine phalloidin was made fresh weekly and reused for up to 3 days when stored protected from light at 4°C. Chemotaxis was quantitatively determined by fluorescence detection using a Cytofluor II microfilter fluorimeter (530 excitation/590 emission). All cell treatments and subsequent washings were carried out using a uniquely designed treatment/wash station. This station consists of 6 individual reagent units, each with a 30-mL volume capacity. Individual units were filled with 1 of the following reagents: PBS, formaldehyde, Triton X-100, or rhodamine phalloidin. Using this technique, filters were gently dipped into the appropriate solution, thus minimizing migrated cell loss. This technique allows for maximum quantitation of cell migration and provides reproducible results with minimal inter- and intra-assay variability. The results presented are the mean of 3 separate experiments.
Endothelial cell proliferation assay
Endothelial cell proliferation was measured by using CyQUANT cell proliferation assay kit from Molecular Probes (Eugene, OR). The basis for the CyQUANT assay is the use of a proprietary green fluorescent dye (CyQUANT GR dye) that exhibits strong fluorescence enhancement when bound to cellular nucleic acids. HUVEC cells (50 000/well) in a 96-well microtiter plate were stimulated with 10 ng/mL FGF2 (Life Technologies, Gaithersburg, MD) in serum-free, M199 growth medium with or without PK or HK peptides for 48 hours at 37°C in a CO2 incubator. Cells were washed with serum-free medium and frozen at −40°C. Frozen cells were thawed and lysed with a buffer containing the CyQUANT GR dye. Fluorescence was measured using Cytofluor II fluorescence multi-well plate reader with excitation 485 nm and emission 530 nm.25The results presented are the mean of 3 separate experiments. In a 48-hour assay, there could be some contribution of an effect of these peptides in inhibiting apoptosis, which might enhance the stimulation or proliferation. The results are presented as a mean of 6 experiments ±SEM.
Neovascularization on the chicken chorioallantoic membrane
Ten-day-old embryos were purchased from Spafas, Inc (Preston, CT) and were incubated at 37°C with 55% humidity. A small hole was punctured in the shell concealing the air sac with a hypodermic needle. A second hole was punctured in the shell on the broad side of the egg directly over an avascular portion of the embryonic membrane, as observed during candling. A false air sac was created beneath the second hole by the application of negative pressure to the first hole, which caused the chicken CAM to separate from the shell. A window, approximately 1.0 cm2, was cut in the shell over the dropped CAM with the use of a small crafts grinding wheel (Dremel, Division of Emerson Electric Co, Racine, WI), which allowed direct access to the underlying CAM.
Filter disks of No. 1 filter paper (Whatman Inc, Clifton,NJ) were soaked in 3 mg/mL cortisone acetate (Sigma, St Louis, MO) in a solution of 95% ethanol and water and subsequently air-dried under sterile conditions. FGF2 was used to grow vessels on the CAMs of 10-day-old chick embryos. Sterile filter disks adsorbed with FGF2 dissolved in PBS at 1 μg/mL were placed on growing CAMs. At 24 hours, control vehicle or test compounds were added to CAMs topically or by intravenous injection.
A range of 0 to 25 μg in 25 μL of HK recombinant domains or synthetic HK peptides 10 to 200 μM or 25 μL of saline was applied to the saturated filter 24 hours later and was applied to the stimulated CAMs. At least 10 embryos were used per treatment group. Each experiment was performed at least 3 times. Thus, the mean ±SEM is based on 30 separate observations.
Microscopic analysis of CAM sections
CAM tissue directly beneath FGF2-saturated filter disk was resected from embryos treated 48 hours prior with compounds or controls. Tissues were washed 3 times with PBS. Sections were placed in a 35-mm Petri dish (Nalge Nunc, Rochester, NY) and examined under an SV6 stereomicroscope (Karl Zeiss, Thornwood, NY) at 50 × magnification. Digital images of CAM sections adjacent to filters were collected using a 3-CCD color video camera system (Toshiba America, New York, NY) and analyzed with Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). The number of vessel branch points contained in a circular region equal to the area of a filter disk (angiogenesis index) was counted for each section. Percent inhibition data are expressed as the quotient of the experimental value minus the negative control value divided by the difference between the positive control value and the negative control value.
Molecular modeling
We modeled domain 5 after the homologous protein hisactophilin. The atomic coordinates of the hisactophilin protein (Brookhaven Database File: 1HCE) were obtained and, based on the sequence alignment shown below, used to model the D5 domain of HK. None of the gaps shown in the alignment violate the hydrophobic core of the protein. In fact, all of the sequence deletions are easily accommodated by the surface-exposed loops of the protein. The HK-specific side chain replacement on the hisactophilin template were used to create the new model of D5, as previously described by Jameson.26 27 After replacing the side chains, the model was subjected to alternating rounds of molecular mechanics (energy minimizations) and molecular dynamics (energy-dependent stimulations of molecular motion). The modeling was performed using the Biopolymer module from the Sybyl computational chemistry suite of programs (Tripos and Assoc, St Louis, MO) on a Silicon Graphics OCTANE computer. A Connolly Surface was calculated for the nuclear magnetic resonance (NMR)-based structure using a hypothetical sphere with a radius of 0.28 nm (twice the radius of a water molecule). An electropotential gradient has been superimposed on the surface of the protein and the surface exposure in terms of solvent accessibility calculated.
Results
To test the hypothesis that HK fragments could modulate angiogenesis, we tested sequences from domains 3 and 6 and the entire domain 5 for their ability to inhibit endothelial cell proliferation (Table 1). A peptide from domain 3 (T255-Y280) was selected because it bound to platelets8 and contained a binding sequence to neutrophils.14 Domain 5 (GST-K420-S513) was selected because it contains sites for binding to platelets, neutrophils, and endothelial cells. A 30–amino acid sequence from domain 6 (S565-P594) was used because it contains the information for binding to PK. GST-D5 gave 100% inhibition of proliferation at 0.27 μM, while the peptide from D3 and D6 showed no inhibition at concentrations in excess of 100-fold.
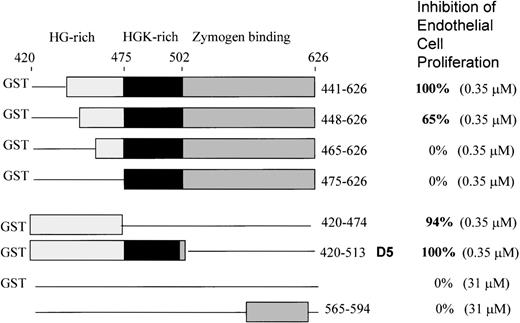
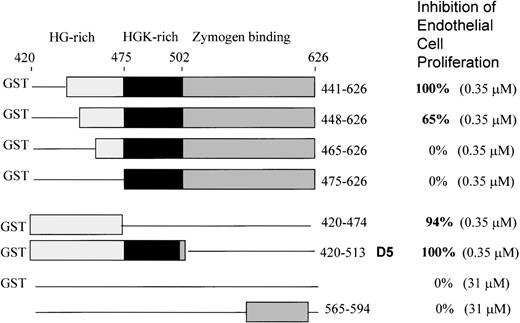
To further delineate the amino acid sequence(s) responsible for inhibition of endothelial cell proliferation, we used 6 deletion mutants of the light chain previously constructed,12 shown in Figure 1. All are fused to GST at the N-terminal end. All were tested at the same concentration (0.35 μM). GST domain 5 (K420-S513) inhibited 100 ± 0%, as previously shown. We found that the mutant 441-626 gave 100 + 0% inhibition of endothelial proliferation at 0.35 μM (Figure 1). Removal of amino acids 442-447 from the N-terminal end in mutant 448-626 resulted in less inhibitory activity (65 + 8%), while mutant 465-626 showed 0 + 0% inhibition and mutant 475-626 displayed 0 + 0% inhibition. GST420-474 (94 ± 6%) and GST420-513 (GST-D5) (100 ± 0%) showed virtually complete inhibition. Thus, the sequence H441-D474 is the smallest deduced sequence containing the full inhibitory activity of HK D5. Neither GST (0 + 0%) nor a peptide from D6 565-594 (0 + 0%) had inhibitory activity at 100-fold the concentration of GST-D5.
Schematic structures of the HK deletion mutants of D5 and D6 and inhibition of endothelial proliferation.
The HG-rich (light gray) and HGK-rich (black) regions of D5 and D6 (dark gray) are shown. Deletions are represented by a line. Mutants are fused to glutathione-S-transferase (GST) at the N-terminal. GST without HK sequence is indicated by a line. The bottom construct is a synthetic peptide from D6.
Schematic structures of the HK deletion mutants of D5 and D6 and inhibition of endothelial proliferation.
The HG-rich (light gray) and HGK-rich (black) regions of D5 and D6 (dark gray) are shown. Deletions are represented by a line. Mutants are fused to glutathione-S-transferase (GST) at the N-terminal. GST without HK sequence is indicated by a line. The bottom construct is a synthetic peptide from D6.
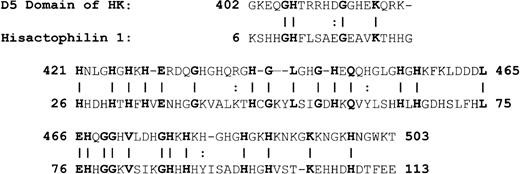
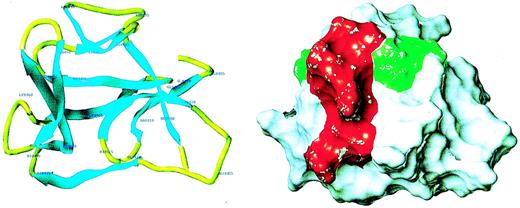
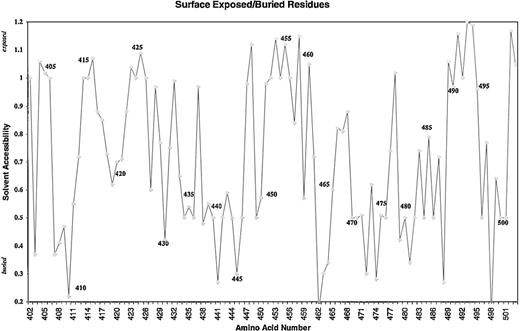
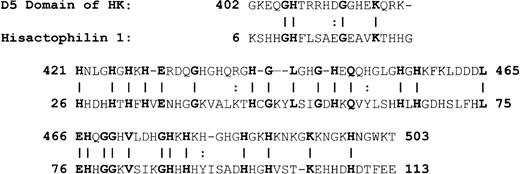
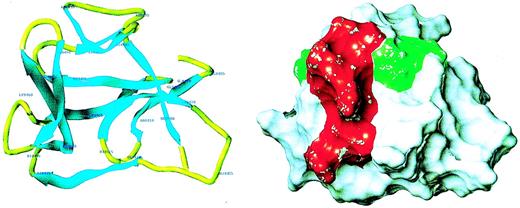
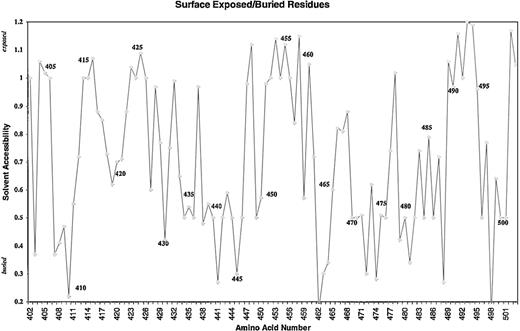
Because of the potential involvement of the D5 domain of HK in angiogenesis, we searched the known databases for a suitable template on which to base a homology-modeled structure. The D5 domain of HK shows 30.6% identity and 40.5% homology (identity + similarity) with a histidine-rich actin binding protein, hisactophilin 1, fromDicytostellium discoideum (Figure2). The NMR solution structure for the hisactophilin 1 was determined several years ago28 29 and found to display a 3-dimensional structure very similar to interleukin-1β and FGF2. By analogy, the D5 of HK might also resemble these proteins. The structure of the homology model of HK D5 is shown in Figure 3. The ribbon representation of HK D5 shown in Figure 4A indicates that the core is composed of β sheets ending in β turns, which represent surface-exposed loops. The solvent accessibility parameters were calculated as a measure of buried and exposed residues. Figure 4 is a plot of the surface exposure of each of the residues in the D5 domain.
Alignment of HK D5 and hisactophilin.
A vertical line equals identity; 2 dots represent similarity.
Alignment of HK D5 and hisactophilin.
A vertical line equals identity; 2 dots represent similarity.
Homology model of HK D5.
(A) Ribbon diagram of model. The amino acid residues are labeled. (B) Surface properties of model. The red area, which subsumes the amino acids 440-455, is adjacent to the green area, amino acids 483-485.
Homology model of HK D5.
(A) Ribbon diagram of model. The amino acid residues are labeled. (B) Surface properties of model. The red area, which subsumes the amino acids 440-455, is adjacent to the green area, amino acids 483-485.
Surface exposure plot of the amino acid residues of D5.
Solvent accessibility is plotted against the amino acid.
Surface exposure plot of the amino acid residues of D5.
Solvent accessibility is plotted against the amino acid.
Five peptides were selected, each of which displayed surface loops that could potentially participate in protein-protein interactions (Figure5). Each was studied as a function of concentration from 0.01 μM to 100 μM for its ability to inhibit endothelial cell proliferation, and the IC50 (concentration to yield 50% inhibition) ± SEM was calculated and compared to GST-D5 (amino acids 420-513). Peptide 440-455 (IC50 = 0.11 ± 0.08 μM) was almost as potent as GST-D5 (IC50 = 0.05 ± 0.02 μM). The sequence shows the highest electropotential of the Connolly surface (Figure 4B). Because it is unlikely that buried residues partake in the binding activities, it seems probable, from the surface exposure plot (Figure4), that the observed activity in peptide 440-455 maps to its carboxy-terminal end. Two of the other active D5 peptides, 475-485 (IC50 = 1.1 ± 0.5 μM) and 483-497 (IC50 = 2.8 ± 0.9 μM) overlap between residues 483-485 (Figure 4B). This conclusion is consistent with the finding that peptide 486-502 has an IC50 of 55 ± 15 μM. As can be seen from the surface exposure plot in Figure 5, residue 486 is relatively buried. The residues 483-485 form part of a surface patch that is adjacent to the surface presented by residues 440-455 (Figure4B) and may represent a secondary interaction site.
Inhibition of endothelial cell proliferation by synthetic peptides derived from D5 by molecular modeling.
The concentration to yield 50% inhibition (IC50) is plotted on a log scale for each peptide and GST-D5.
Inhibition of endothelial cell proliferation by synthetic peptides derived from D5 by molecular modeling.
The concentration to yield 50% inhibition (IC50) is plotted on a log scale for each peptide and GST-D5.
We also tested the peptides from D3, GST-D5, the peptides derived from molecular modeling of D5, as well as the D6 peptide for their ability to inhibit endothelial migration to a higher concentration of vitronectin in the lower compartment of a Boyden chamber (Table2). HK D3 is a region that inhibits the binding of HK to neutrophils14 and platelets.30Even at 139 μM, it only inhibits migration to vitronectin 10% with an IC50 > 50 μM. It should be noted that the D3 peptide T255-Y280 is a linear, nonoptimized peptide of 26 amino acids, which may account for the relatively high concentrations needed. A control peptide (D3-control) with random amino acids on each side of a small binding region inhibited 5% and thus did not differ from the D3 peptide. In contrast, HK D5 is highly inhibitory of migration to vitronectin (85%) at 0.20 μM, with an IC50 = 0.12 μM. In contrast to the results for proliferation, in which peptide 440-455 was the most active and peptides 475-485 and 483-497 had more modest activity, only peptide G486-K502 gave inhibition of migration. This finding suggests that more than 1 region accounts for the inhibitory effect of D5 (Table 2). In D6, a 30-mer peptide from the binding site for PK gave 31% inhibition at 0.2 μM with an IC50 = 0.5 μM.
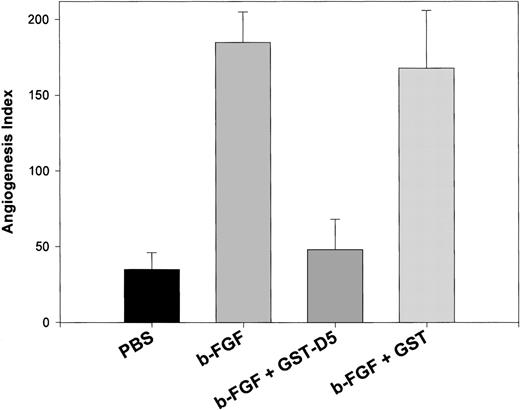
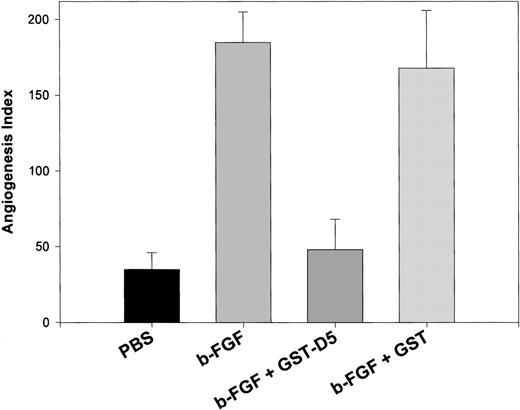
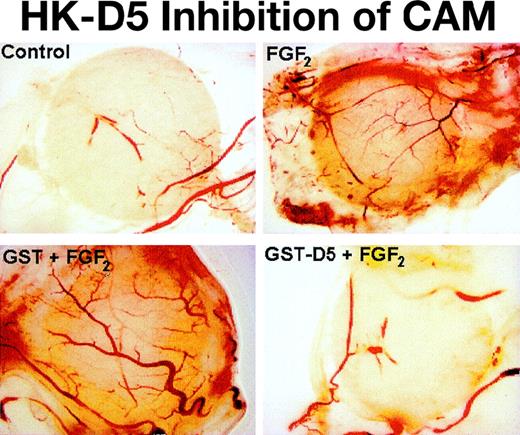
To ascertain whether HK peptides inhibited angiogenesis in vivo, we used CAM. We had tested the HK D5 (GST-K420-S513), which inhibited both endothelial cell migration (85%) and proliferation (100%) at 0.20 μM. At the same concentration, it inhibited new vessel formation after CAM stimulation by FGF2 97% (Figure6). In contrast, GST failed to inhibit FGF2-stimulated angiogenesis (Figure 6). Importantly, HK D5 also inhibited a CAM stimulated with both FGF2 and VEGF 95% (data not shown). In Figure 7, representative photomicrographs of neovascularization by FGF2 and its inhibition by GST-D5, but not GST, are shown. Interestingly, HKa but not HK was equally potent to GST-D5 (data not shown).
Inhibition of angiogenesis in CAM stimulated by FGF2 by GST D5.
The difference between control (PBS)- and FGF2-stimulated angiogenesis index in CAM is highly significant (P < .01). The difference between FGF2 + GST-D5 and FGF2 + GST D5 is highly significant (P < .01).
Inhibition of angiogenesis in CAM stimulated by FGF2 by GST D5.
The difference between control (PBS)- and FGF2-stimulated angiogenesis index in CAM is highly significant (P < .01). The difference between FGF2 + GST-D5 and FGF2 + GST D5 is highly significant (P < .01).
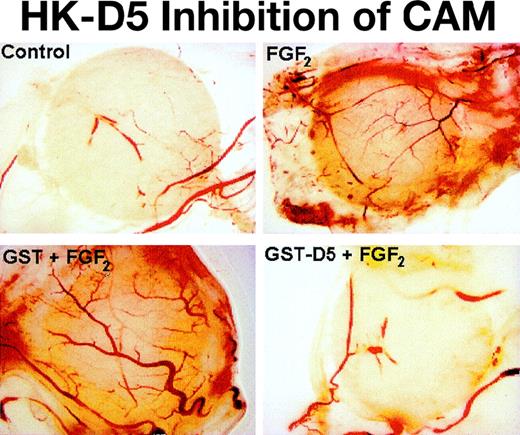
Photomicrograph of CAM: inhibition by HK D5 and HKa.
Few vessels are seen when CAM is incubated with a disk containing control (PBS). When the CAM is incubated with a disk containing GST (0.2 μM), no differences from control are observed (not shown). Many new vessels are seen with stimulation by FGF2. When the CAM is incubated with FGF2 and GST (0.2 μM), no differences from FGF2 alone are observed. The formation of new vessels by FGF2 + GST-D5 (0.2 μM) is markedly decreased compared to FGF2 alone and does not differ from control (original magnification × 40).
Photomicrograph of CAM: inhibition by HK D5 and HKa.
Few vessels are seen when CAM is incubated with a disk containing control (PBS). When the CAM is incubated with a disk containing GST (0.2 μM), no differences from control are observed (not shown). Many new vessels are seen with stimulation by FGF2. When the CAM is incubated with FGF2 and GST (0.2 μM), no differences from FGF2 alone are observed. The formation of new vessels by FGF2 + GST-D5 (0.2 μM) is markedly decreased compared to FGF2 alone and does not differ from control (original magnification × 40).
Finally, we tested the ability of the most potent peptide in inhibiting endothelial proliferation to inhibit angiogenesis in vivo, and we compared it to GST-D5. GST-D5 (50.0 μg) inhibited 85.2 ± 12.9%, and peptide 440-455 (41.2 μg) inhibited 79 ± 6.9%. To define the specificity of the inhibition, we prepared 2 altered peptides. The first peptide, 440 mutant, had the sequence scrambled to change the relative positions of the histidine residues with an amino acid sequence of HGGLGHGHGHEQHQGLGHG-amide. The second peptide, 440AA, had 2 alanines replacing the 2 glutamines, 449 and 450, in 440-455. Peptide 440 mutant (21.5 μg) only inhibited 29.4 ± 16.9%, while peptide 440AA (21.5 μg) showed no detectable inhibition. Thus, the sequence in 440-455 seems to be specific not only for proliferation but for in vivo angiogenesis.
Discussion
We have shown that domain 5 (GST420-513) of HK inhibits endothelial cell proliferation at nanomolar concentrations, while sequences from D3 that bind to endothelial cells and domain 6 that bind PK fail to inhibit cell proliferation when tested at concentrations over 100-fold higher. Using deletion mutants of GST-D5, we mapped the required region to H441-D474. To design peptides, we used a molecular model based on the NMR structure of hisactophilin 1, which has a 3-dimensional structure similar to the angiogenic protein FGF. We selected 5 peptides that subsumed surface residues and obtained a peptide 440-455 with an IC50 of 100 nM, which was only 3-fold less potent than the entire domain 5 (IC50 = 30 nM) in inhibiting proliferation. These concentrations are in the same range of potency as that reported for angiostatin (IC50 = 10-100 nM).31 The antiangiogenic activity of a fragment of a proangiogenic plasma protein is a common feature of both kininostatin (HK D5)/HK and angiostatin/plasminogen and suggests a similar negative feedback mechanism. Although D5 was also a potent inhibitor of migration toward vitronectin (IC50 = 120 nM), only 1 peptide from the 5 we chose could reproduce this effect, and this required a higher concentration than GST-D5, suggesting that a different region was required for migration than for proliferation. The ability of different peptides from D5 to inhibit migration and proliferation suggests that D5 could inhibit angiogenesis by more than 1 mechanism. Further, we showed that GST-D5, which inhibits both proliferation and migration, inhibited neovascularization of the chicken CAM induced by FGF2. GST had no effect, but HKa was equally as potent as GST-D5. Finally, we showed that peptide 440-455 was not only potent but specific in inhibiting angiogenesis, because a scrambled peptide had little inhibitory action and a substituted peptide showed no inhibition.
Our initial hypothesis that HKa was involved in regulating angiogenesis stemmed from our observation that HKa, but not HK, formed a complex with soluble uPAR19 and that the binding of HKa to endothelial cells was blocked by soluble uPAR. The penetration of cells through extracellular matrix is a requirement for angiogenesis.21,22 The expression of cell surface plasmin activity is essential for promoting cell migration through extracellular matrix32 and depends on proUK, uPAR, plasminogen, and plasminogen-activator inhibitor type-1 (PAI-1). The expression of cell surface urokinase activity requires initial binding to a specific, high-affinity cellular receptor, uPAR,1 a 55 000-kd protein5 expressed on the surfaces of monocytes, endothelial cells,33 and most tumor cells.32This receptor has been cloned34 and revealed to be a glycophosphatidylinositol (GPI)-anchored protein,35consisting of 3 approximately 90–amino acid repeats of domains 1, 2, and 3. The urokinase binding site of the receptor is contained within uPAR domain 1 residues 1-135.5 The uPAR plays a critical part in initiating cell migration and enhancing adhesion and regulatory proteolysis on the cell surface and by increasing the activation of cell-associated plasminogen. Cell-bound plasmin is relatively protected from inhibition by its natural inhibitors36 and therefore may effectively enhance degradation of the extracellular matrix components, such as fibronectin, laminin, and, indirectly, collagen by its capacity to activate matrix collagenases.
Over the past several years, uPAR has been regarded as playing a critical role in the mediation of angiogenesis. Bovine endothelial cell migration induced by basic fibroblast growth factor (FGF2) was shown to be mediated by uPA.37Physiologic angiogenesis is dependent in vivo on the expression of uPA and PAI-1.38 Human endothelial cells in vitro depend on plasminogen activators and fibrin for repair-associated angiogenesis.39 Both the proteolytic and angiogenic properties of endothelial cells are stimulated concordantly by FGF2.40 Invasion of a CAM is enhanced in the presence of cell-associated urokinase. Moreover, metastasis of human prostate carcinoma cells in mice is prevented by transfection of an inactive urokinase mutant. Finally, the expression of uPAR is increased in migrating endothelial cells.41 These observations support the role of urokinase and uPAR in angiogenesis.
The initiating mechanism by which uPAR-bound proUK becomes activated to an active enzyme is central to the expression of cell surface proteolytic activity. The binding of proUK to uPA induces a conformational change in the zymogen, which endows it with proteolytic activity.42 Alternatively, plasmin can cleave pro-UK to UK,43 but this reaction is a positive feedback and does not explain the mechanism of initiation. Recent studies from our laboratory20 and others44 have defined a third mechanism of proUK activation, which could be an initiating reaction. Expression of plasmin activity on the endothelial cell surface is significantly increased following the binding of HK and PK (Figure8). Our studies have demonstrated that following its HK-dependent binding to endothelial cells, PK is activated to kallikrein by cell-associated cysteine protease and subsequently promotes the activation of cell-bound proUK to uPA.20 However, a negative control mechanism may be enhanced by cleavage of proUK to uPA, because the single chain proUK bound to uPAR is more resistant to inhibition by PAI-1 than uPA.45 In vivo, the relative importance of these mechanisms of cell-associated plasmin formation is not clear at present.
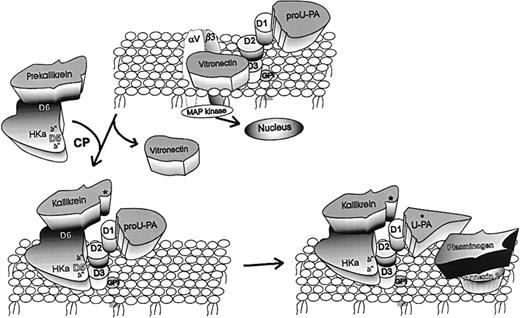
Mechanisms underlying antiangiogenic activity of HKa.
Vitronectin (VN) is pictured bound to both the integrin (αvβ3) and the urokinase receptor (uPAR) by domains 2 and 3 (D2, D3). In this tentative hypothesis, HKa is pictured as binding by D5 to domains 2/3 of uPAR. By doing so, HKa displaces VN from the same domains of uPAR, thus acting as an antiadhesive ligand. In addition, prekallikrein (PK), which circulates in complex with HK or HKa bound to D6, is recruited to the endothelial surface. PK is converted to kallikrein (K) by an endothelial cell cysteine protease (CP). K then cleaves pro-uPA to uPA. Plasminogen (PG) bound to 1 of its receptors, such as annexin 2, is converted by uPA to plasmin (PN). Thus, HKa can augment cell-bound plasmin-dependent proteolysis to facilitate endothelial cell migration. Finally, pro-uPA is known to stimulate endothelial cell signal transduction, probably by the association of uPAR with integrins such as αvβ3. It remains to be shown that the action of HKa to inhibit cell proliferation is by a similar mechanism.
Mechanisms underlying antiangiogenic activity of HKa.
Vitronectin (VN) is pictured bound to both the integrin (αvβ3) and the urokinase receptor (uPAR) by domains 2 and 3 (D2, D3). In this tentative hypothesis, HKa is pictured as binding by D5 to domains 2/3 of uPAR. By doing so, HKa displaces VN from the same domains of uPAR, thus acting as an antiadhesive ligand. In addition, prekallikrein (PK), which circulates in complex with HK or HKa bound to D6, is recruited to the endothelial surface. PK is converted to kallikrein (K) by an endothelial cell cysteine protease (CP). K then cleaves pro-uPA to uPA. Plasminogen (PG) bound to 1 of its receptors, such as annexin 2, is converted by uPA to plasmin (PN). Thus, HKa can augment cell-bound plasmin-dependent proteolysis to facilitate endothelial cell migration. Finally, pro-uPA is known to stimulate endothelial cell signal transduction, probably by the association of uPAR with integrins such as αvβ3. It remains to be shown that the action of HKa to inhibit cell proliferation is by a similar mechanism.
HK binds to uPAR domains 2 + 3, leading to the assembly of a complex consisting of uPAR, proUK, HK, and PK on the endothelial cell surface, which could be one of the initiating mechanisms for the activation of proUK and, subsequently, plasminogen to plasma (Figure 8). We hypothesize that this complex may facilitate the invasion of endothelial cells through the basement membrane and extracellular matrix, a critical step in angiogenesis. Our observation that HKa binds to a site within domains 2 + 3 of uPAR, and the binding is competed for by vitronectin, indicates that HKa may possess antiadhesive properties, because uPAR is recognized as an adhesion receptor for vitronectin. The inhibition of adhesion of tumor cells to vitronectin by HKa was noted by Asakura et al.17 By displacing vitronectin from uPAR at focal contact sites, HKa may facilitate cellular migration, which could facilitate angiogenesis.
The similar activity of HKa and domain 5 is consistent with our previous observations that the cleaving of HK to HKa enhances its coagulant activity10 and causes major conformational changes as judged by circular dichroism.46 Electron microscopy reveals that domain 5 is more exposed after cleaving of HK to HKa.47 The effects of D5 may not be due entirely to binding to uPAR, because HK or HKa binds to other receptors on endothelial cells such as cytokeratin 148 or the receptor for the globular head of Clq.49 Indeed, we have postulated that these receptors may exist in a complex on the endothelial cell surface.50
The major unexpected finding of these studies was the potent inhibition by GST-D5 and the HK peptide 440-455 of endothelial cell proliferation. It should be noted that the latter peptide is more potent by an order of magnitude than the 2 peptides, 475-485 and 483-497, that subsume the endothelial binding site mapped by Hasan et al.15 Although uPAR has no cytoplasmic domains, it is physically associated with integrins51 and facilitates β2-integrin adhesion of human monocytes.52 Thus, uPAR signaling through an endothelial integrin such as αvβ3 can be initiated by uPAR occupancy by HKa (Figure 8), and inhibition of proliferation could well be a consequence. Whether either cytokeratin 1 or the receptor for the globular head of C1q is involved in cell signaling is not known. Further studies, which are ongoing, will determine whether MAP kinase or other components in the signaling cascade are affected by binding of HKa to endothelial cells or to uPAR.
Acknowledgments
We thank Seema Mohamed for conducting the angiogenesis assays. We appreciate the skillful preparation of the manuscript and some of the figures by Rita Stewart and the preparation of additional figures by Robin Pixley, PhD. This work was supported by grants from NIH P01 HL56914 and R01 CA63938 to R.W.C.
Reprints:Robert W. Colman, Sol Sherry Thrombosis Research Center, Temple University School of Medicine, 3400 North Broad St, Philadelphia, PA 19140; e-mail: colmanr@astro.temple.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.