Three C-C chemokines inhibit human immunodeficiency virus (HIV) entry into macrophages: macrophage inflammatory protein-1 (MIP-1), MIP-1β, and regulated-upon activation, normal T-cell expressed and secreted (RANTES). We studied the ability of placental cord blood mononuclear cells (CBMC) to secrete these C-C chemokines in comparison to adult blood mononuclear cells (ABMC). CBMC had diminished ability to secrete RANTES, as determined by enzyme-linked immunosorbent assay. Secretion of MIP-1 and MIP-1β were similar in CBMC and ABMC. Whereas MIP-1 and MIP-1β secretion were comparable in monocytes and lymphocytes, RANTES was secreted primarily by lymphocytes. Flow cytometric analysis of RANTES expression showed diminished intracellular RANTES expression in cord blood lymphocytes (CBL) compared to adult (peripheral) blood lymphocytes (ABL). A subset analysis of RANTES-producing CBL and ABL demonstrated that RANTES was produced predominantly by CD8+/CD45RO+ cells. CBL had a reduced proportion of CD8+/CD45RO+ cells compared with ABL, which may account for the diminished RANTES secretion by CBMC. These results may be relevant to the pathogenesis of perinatal HIV infection.
Chemokines, a superfamily of polypeptide mediators, are a key component of immune surveillance. The relative positions of the cysteine tandem defines 4 classes of chemokines, the most important of which are the C-C and the CXC chemokines. Chemokines bind to and activate 7-transmembrane domain receptors. The C-C chemokines have a broad spectrum of action and attract monocytes, lymphocytes, eosinophils, basophils, and natural killer cells.1 The C-C chemokines regulate leukocyte trafficking, inflammation, hematopoiesis, antitumor immunity, and human immunodeficiency virus (HIV) infection.1 In contrast, the CXC chemokines predominantly recruit neutrophils. While CXC chemokines, such as interleukin-8 (IL-8), claim a large and long history of experimental investigation, there is a paucity of information on C-C chemokine physiology in neonates.
The C-C chemokine receptor CCR5 is a major fusion coreceptor for HIV infection of macrophages. Three C-C chemokines that are ligands for CCR5 inhibit HIV entry into macrophages2-4: macrophage inflammatory protein-1α (MIP-1α), MIP-1β, and regulated-upon activation, normal T-cell expressed and secreted (RANTES). Individuals with high concentrations of these factors remain uninfected despite repeated exposure to HIV.5 Infants infected with HIV perinatally have a more rapid and fatal course than older children and adults because neonatal macrophages are more readily infected by HIV than are adult blood-derived macrophages.6,7 CD4 and CCR5 expression are similar in cord and adult blood mononuclear phagocytes.6,8 9 It is thus possible that the differences between HIV susceptiblity of neonatal and adult cells are due to differences in the ability to secrete C-C chemokines. We investigated and compared the in vitro abilities of placental cord blood mononuclear cells (CBMC) and healthy adult blood mononuclear cells (ABMC) to secrete the C-C chemokines MIP-1α, MIP-1β, and RANTES.
Study design
Cells
Mononuclear cells were isolated from the umbilical cord blood of healthy term neonates and from the peripheral blood of healthy adult donors by using density-gradient centrifugation of 1500g for 45 minutes over lymphocyte separation medium. Purified monocyte and lymphocyte populations were isolated from the mononuclear cell layer using gelatin-coated flasks, and adherent monocytes were detached with ethylene diamine tetra-acetic acid (EDTA).10The purity of the cell populations thus isolated was >98% as determined by immunofluorescence staining for CD14 and CD3 expression. The cells were incubated in 24-well plates at a density of 1 × 106 cells/mL.
Enzyme-linked immunosorbent assay for C-C chemokines
MIP-1α, MIP-1β, and RANTES secretion in cord and adult blood was compared using 3 groups of cells: CBMC and ABMC, purified cord blood and adult blood lymphocytes (CBL and ABL), and purified monocytes. CBMC, ABMC, CBL, and ABL cells were incubated for 24-96 hours either in the resting state or with phorbol 12-myristate 13-acetate (PMA, 5 ng/mL). Monocytes from cord and adult blood were incubated with or without lipopolysaccharide (LPS, 5 ng/mL) at 37°C with 5% CO2 for 24-96 hours. At the completion of the incubation period, the supernatants were stored at –70°C, and enzyme-linked immunoabsorbent assay (ELISA) was performed in batches, as described by the manufacturer (R&D Systems, Minneapolis, MN).
Intracellular analysis of RANTES expression
CBL and ABL were either unstimulated or stimulated with PMA (5 ng/mL) for 12 hours in the presence of monensin (2 μmol/L) (Golgistop; PharMingen, San Diego, CA) to inhibit protein transport and secretion.11 For 2-color flow cytometry, 2 × 105 CBL or ABL were initially stained with fluorescein-conjugated (FITC-conjugated) monoclonal antibodies (mAbs) to the cell-surface markers CD4, CD8, CD45RA, or CD45RO (PharMingen) at 4°C for 30 minutes. For 3-color flow cytometry, CBL were stained with cychrome-labeled CD4 or CD8 mAbs and FITC-labeled CD45RA or CD45RO antibodies. After washing with 1 × phosphate-buffered saline (PBS), the cells were fixed and permeabilized (Cytofix/Cytoperm solution; PharMingen) according to the manufacturer's instructions. Finally, the cells were incubated with phycoerythrin-conjugated (PE-conjugated) mAb to human RANTES (PharMingen). Control antibodies for the cell-surface markers and RANTES were isotype-matched. Phenotypic analysis was performed on a flow cytometer (EPICS-ELITE; Coulter Electronics, Hialeah, FL).
Statistical analysis
To mitigate the effects of any unrecognized day-to-day variations in sample preparation or culture conditions, each cord blood sample was paired with an adult blood sample that was processed simultaneously and identically. Statistical analysis was done using the Student ttest for paired samples.
Results and discussion
Of the 3 C-C chemokines studied, only RANTES was secreted in the constitutive phase by mononuclear cells and lymphocytes. CBMC and CBL had significantly diminshed ability to secrete RANTES in the constitutive phase, compared with ABMC and ABL, respectively (data not shown). Monocytes did not show significant constitutive expression of any of these chemokines. Even on induction with PMA, RANTES secretion was significantly diminished in CBMC and CBL, compared with PMA-stimulated ABMC and ABL, respectively (Figure1). Study of kinetic changes in RANTES secretion showed that RANTES secretion peaked at 24-30 hours in both CBL and ABL (data not shown). MIP-1α and MIP-1β secretion were similar in PMA-stimulated CBMC and ABMC (Figure 1). Whereas monocytes from cord and adult blood showed similar levels of MIP-1α and MIP-1β secretion, compared with ABL, CBL showed diminished ability to secrete MIP-1α and MIP-1β. MIP-1α and MIP-1β secretion were comparable in monocytes and lymphocytes. However, monocytes were not a significant source of RANTES in adult or cord blood (Figure 1). CBMC comprised 8% to 38% monocytes compared with ABMC, which comprised 4% to 16% monocytes. This finding may account for the relatively enhanced ability of CBMC to secrete MIP-1α and MIP-1β compared to RANTES. Thus, the selective defect in CBMC to secrete RANTES may be due to the immaturity of CBL.
Secretion of C-C chemokines by cord and adult blood cells.
MIP-1α, MIP-1β, and RANTES secretion in cord and adult blood were compared using 3 groups of cells: CBMC and ABMC (n = 15 pairs); purified lymphocytes CBL and ABL (n = 10 pairs); and purified monocytes (n = 6 pairs). CBMC, ABMC, CBL, and ABL were incubated with PMA (5 ng/mL) for 24 hours. Monocytes from cord and adult blood were incubated with LPS (5 ng/mL) for 24 hours. Results are shown as chemokine concentrations in the supernatants (mean ± SEM [standard error of mean]). *Indicates cord versus adult,P < .05.
Secretion of C-C chemokines by cord and adult blood cells.
MIP-1α, MIP-1β, and RANTES secretion in cord and adult blood were compared using 3 groups of cells: CBMC and ABMC (n = 15 pairs); purified lymphocytes CBL and ABL (n = 10 pairs); and purified monocytes (n = 6 pairs). CBMC, ABMC, CBL, and ABL were incubated with PMA (5 ng/mL) for 24 hours. Monocytes from cord and adult blood were incubated with LPS (5 ng/mL) for 24 hours. Results are shown as chemokine concentrations in the supernatants (mean ± SEM [standard error of mean]). *Indicates cord versus adult,P < .05.
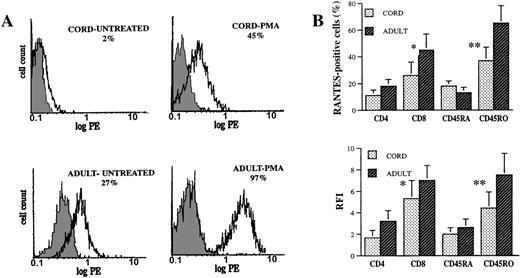
To allow for a more detailed and accurate comparison of a RANTES profile in CBL and ABL, we used flow cytometry to study RANTES expression at the single-cell level. Intracellular expression of RANTES in the resting phase and on stimulation with PMA was significantly diminished in CBL compared to ABL (Figure2A). In addition, constitutive expression of RANTES was seen only in adult PBL. Thus, secretion of RANTES into cell supernatants is concordant with intracellular RANTES expression in lymphocytes, and the diminished ability of CBL to secrete RANTES is not mainly due to defective secretory mechanisms.
Intracellular RANTES expression in CBL and ABL.
CBL and ABL were either untreated or stimulated with PMA (5 ng/mL) for 12 hours, after which staining for RANTES and the cell-surface markers CD4, CD8, CD45RA, and CD45RO was performed as previously described. (A) Flow cytometric RANTES expression in CBL and ABL. The shaded histograms represent control staining with irrelevant mAb, and the open histograms represent cells staining positive for RANTES. Percentages of cells staining positive for RANTES are shown. (B) RANTES expression in lymphocyte subsets (2-color flow cytometry). Results (mean ± SEM) are shown as percentages of cells in each subset expressing RANTES and as relative fluorescence intensity (RFI = mean fluorescence intensity of sample minus mean fluorescence intensity of control/mean fluorescence intensity of control). The results are representative of 6 pairs of cord and adult blood specimens. *Indicates CD8 versus CD4, P < .05. **Indicates CD45RO versus CD45RA, P < .05.
Intracellular RANTES expression in CBL and ABL.
CBL and ABL were either untreated or stimulated with PMA (5 ng/mL) for 12 hours, after which staining for RANTES and the cell-surface markers CD4, CD8, CD45RA, and CD45RO was performed as previously described. (A) Flow cytometric RANTES expression in CBL and ABL. The shaded histograms represent control staining with irrelevant mAb, and the open histograms represent cells staining positive for RANTES. Percentages of cells staining positive for RANTES are shown. (B) RANTES expression in lymphocyte subsets (2-color flow cytometry). Results (mean ± SEM) are shown as percentages of cells in each subset expressing RANTES and as relative fluorescence intensity (RFI = mean fluorescence intensity of sample minus mean fluorescence intensity of control/mean fluorescence intensity of control). The results are representative of 6 pairs of cord and adult blood specimens. *Indicates CD8 versus CD4, P < .05. **Indicates CD45RO versus CD45RA, P < .05.
To further understand the mechanisms involved in the selective defect in RANTES secretion by CBL, we performed subset analysis of RANTES-expressing CBL and ABL using markers for helper and cytotoxic T cells (CD4 and CD8, respectively) and for naive and memory T cells (CD45RA and CD45RO, respectively). Two-color flow cytometry showed that a significantly greater proportion of CD8+/CD45RO+ cells expressed RANTES, compared with the RANTES expression of CD4+/CD45RA+ cells (Figure 2B). Three-color flow cytometry on 3 cord blood specimens showed that CD8+CD45RO+ and CD4+CD45RO+ CBL had significantly higher RANTES expression than CD8+CD45RA+ and CD4+CD45RA+ CBL (data not shown).
We then compared the expression of CD4, CD8, CD45RA, and CD45RO in ABL and CBL. In comparison to ABL, CBL had a reduced proportion of CD8+ cells and CD45RO+ cells. Mean counts are expressed as a percentage of total lymphoid population: CD8+ cells: CBL, 18% ± 5%, and ABL, 30% ± 6% (P < .05); CD45RO+ cells: CBL, 9% ± 2%, and ABL, 60% ± 7% (P < .05). The majority of CBL were CD4+/CD45RA+ cells. Mean counts are expressed as a percentage of total lymphoid population: CD4+ cells: CBL, 42% ± 6%, and ABL, 54% ± 8%; CD45RA+ cells: CBL, 67% ± 8%, and ABL, 12% ± 3% (P < .05).
These figures are consistent with previous studies showing predominance of naive or unprimed CD45RA+ cells and a relative deficiency of cytotoxic/cytolytic CD8+ T cells in cord blood.12-14Whereas CD45RA+ cells are the major source of cytokines, such as IL-2, tumor necrosis factor–α (TNF-α), and interferon–γ (IFN-γ) in cord blood,12 RANTES is secreted mainly by CD45RO+ cells in cord blood. Our results indicate that the reduction of RANTES production in CBL may be due to the relative deficiency of CD8+/CD45RO+ cells.
Consistent with previous reports,15 16 we observed higher total leukocyte counts in cord blood compared with adult blood. We also observed relative neutrophilia and lymphopenia in cord blood. CBL had mean total leukocyte counts of 18.4 ± 5.3 × 103/μL with 17%-30% lymphocytes. ABL had mean total leukocyte counts of 11.4 ± 4.6 × 103/μL with 32%-44% lymphocytes. Thus, absolute lymphocyte counts were only slightly higher in cord blood compared to adult blood. This factor, in addition to the modestly diminished proportion of CD8+ cells and markedly reduced proportion of CD45RO+ cells in cord blood, resulted in lower absolute counts per volume of RANTES-expressing T cells in cord blood compared to adult blood (data not shown).
RANTES is a chemokine with a distinct physiologic role and a unique mode of regulation.1 It is the only chemokine secreted in the constitutive phase by T cells. Our results confirmed this finding in ABL. However, CBL did not show constitutive RANTES expression. RANTES recruits CD45RO+ cells and is the most potent chemoattractant for CD8+ cells.1 Therefore, the ontogeny of RANTES secretion may be relevant to the deficiency of CD8+/CD45RO+ cells in neonates. RANTES acts as an antigen-independent activator of T cells, mediating a spectrum of cellular responses such as calcium channel opening, cytokine release, and IL-2 receptor expression.17It also regulates eosinophil infiltration and activation and induces histamine release from basophils. RANTES plays a key role in allergic inflammation and has been implicated in conditions such as bronchial asthma, chronic eosinophilic pneumonia,18,19 and acute cardiac allograft rejection.20 The reduced incidence of graft-versus-host disease in cord blood transplants14may be in part due to the diminshed ability of neonatal cells to secrete RANTES.
To our knowledge, there have been no previous reports comparing the secretion of MIP-1α, MIP-1β, and RANTES by CBMC and ABMC. MIP-1α, MIP-1β, and RANTES inhibit HIV entry into macrophages.21Of the 3 C-C chemokines, RANTES was the most effective inhibitor of HIV infection of the PM1 cell line (CD4+ cell clone) and human peripheral blood mononuclear cells in vitro.4 Whereas MIP-1α and MIP-1β had no inhibitory effect on HIV infection of human alveolar macrophages when used singly, RANTES by itself inhibited HIV infection in a dose-dependent manner.22
Thus, RANTES plays a key role in modulating HIV infection of mononuclear phagocytes. Suppression of HIV infection of macrophages occurs predominantly due to the combined effect of the 3 C-C chemokines.2,4,5 Hence, a deficiency of RANTES may diminish or neutralize the HIV-suppressive effect of MIP-1α and MIP-1β. In infants with perinatal exposure to HIV, significantly higher levels of RANTES were observed in the sera of HIV-exposed uninfected infants compared with HIV-infected infants, suggesting a potential role for endogenous RANTES secretion in interrupting perinatal transmission of acquired immunodeficiency syndrome (AIDS).23 In HIV-infected infants, RANTES levels correlated with CD8 counts,23 a finding consistent with our results showing that CD8+ cells are important sources of RANTES in cord blood. In addition, RANTES mediates HIV-specific T-cell cytotoxicity via the chemokine receptor CCR3.24 Thus, it is possible that the defect in RANTES secretion by neonatal cells may contribute to the enhanced susceptibility of infants to overwhelming HIV infection and disease.
Acknowledgments
This research is supported by grants MOI RR00040, UO1 AI32921, and R01 MH49981. The authors are grateful to Zanwei Xu and A. R. Reath for technical and editorial assistance.
Reprints:Steven D. Douglas, Division of Immunologic and Infectious Diseases, Abramson Research Building, Room 1208, The Children's Hospital of Philadelphia, 34th Street and Civic Center Blvd, Philadelphia, PA 19104; e-mail: douglas@emailchop.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Secretion of C-C chemokines by cord and adult blood cells. / MIP-1α, MIP-1β, and RANTES secretion in cord and adult blood were compared using 3 groups of cells: CBMC and ABMC (n = 15 pairs); purified lymphocytes CBL and ABL (n = 10 pairs); and purified monocytes (n = 6 pairs). CBMC, ABMC, CBL, and ABL were incubated with PMA (5 ng/mL) for 24 hours. Monocytes from cord and adult blood were incubated with LPS (5 ng/mL) for 24 hours. Results are shown as chemokine concentrations in the supernatants (mean ± SEM [standard error of mean]). *Indicates cord versus adult,P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.715/6/m_bloo00207001x.jpeg?Expires=1768446470&Signature=1A83rd9Dzd9SiOgxlImeWw9Qeg66-JRSgxLgWTjD9JRfUYHJbOqXxyG7n~wFfppAWk2BSxq6LYpVHv6kDstKDyXhVF22WDebgh9oL630fGuZI2Y~V~7SrWpoNg-olWJVYt4QcxzeSL6M3dx1psjSdEL3mz104hcUa-wm9XE2jTZ2XfFq7MDrZ8-MQTiB-Gr9~YgpZyFSkciMluuw7ypR~~7bAFRkQJRstqVYM1QMwJgCip2f8KASw0gerZ5z8nPRAPIil9u-2bKvw2i0w~jZYvW0kT8IUuKrLq1yeSIRHYFmVhMHxBQu79iI6iutQ~zd1B7~A-FEsKbdPGpsmXSb6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)