The myelomagenic capacity of clonotypic myeloma cells in G-CSF mobilized blood was tested by xenotransplant. Intracardiac (IC) injection of NOD SCID mice with peripheral cells from 5 patients who had aggressive myeloma led to lytic bone lesions, human Ig in the serum, human plasma cells, and a high frequency of clonotypic cells in the murine bone marrow (BM). Human B and plasma cells were detected in BM, spleen, and blood. Injection of ex vivo multiple myeloma cells directly into the murine sternal BM (intraosseus injection [IO]) leads to lytic bone lesions, BM plasma cells, and a high frequency of clonotypic cells in the femoral BM. This shows that myeloma has spread from the primary injection site to distant BM locations. By using a cellular limiting dilution PCR assay to quantify clonotypic B lineage cells, we confirmed that peripheral myeloma cells homed to the murine BM after IC and IO injection. The myeloma progenitor undergoes self-renewal in murine BM, as demonstrated by the transfer of human myeloma to a secondary recipient mouse. For 6 of 7 patients, G-CSF mobilized cells from patients who have minimal disease, taken at the time of mobilization or after cryopreservation, included myeloma progenitors as identified by engraftment of clonotypic cells and/or lytic bone disease in mice. This indicates that myeloma progenitors are mobilized into the blood by cyclophosphamide/G-CSF. Their ability to generate myeloma in a xenotransplant model implies that such progenitors are also myelomagenic when reinfused into patients, and suggests the need for an effective strategy to purge them before transplant.
Multiple myeloma (MM) is an incurable cancer of the blood and bone marrow. Although the symptoms in myeloma result primarily from the plasma cells that colonize the bone marrow (BM), molecular analysis indicates the presence of circulating members of the malignant clone.1-9 With single cell and in situ RT-PCR analysis, clonotypic B cells have been demonstrated to be present at high frequency in the blood of myeloma patients.10-13 These B cells persist after autologous transplantation but may be depleted by allogeneic transplant.11 Drug-resistant clonotypic MM B cells12,14 are highly abnormal in their expression of the stem cell marker CD34,10 and recent work suggests that highly enriched hematopoietic progenitor populations include clonotypic cells.15 16 However, there is as yet no direct evidence identifying myeloma progenitor(s).
Many laboratories have shown that clonotypic cells are mobilized by G-CSF, 15, 17-24 but their significance is unknown. Reinfusion of MM progenitors mobilized by G-CSF is likely to contribute to the reemergence of MM. After transplant, clonotypic cells persist in circulation11;25 suggesting that they are a clinically significant component of the malignant clone and may participate in the relapse that ultimately befalls nearly all autologous transplant patients.26-28 Even purification of hematopoietic progenitor cell fractions may not eradicate all malignant cells from the autograft 10-16 Operationally, the measure of a malignant progenitor is its ability to regenerate the malignancy. In practical terms, this means that a xenotransplant model of human myeloma is desirable to assess the extent to which, if any, mobilized blood collections include myeloma progenitors able to repopulate the patient with malignant disease.
NOD SCID mice are the most immunodeficient of the SCID variants,29 are the most supportive host for normal and malignant human stem cells,30,31 and B-cell differentiation occurs in the absence of supplemental growth factors.31Most SCID mouse myeloma models use MM cell lines.32-35 In 2 studies, fresh human MM BMC survived in conventional SCID mice, but no colonization of the mouse BM was detected.36,37 Ex vivo MM cells or human MM cell lines colonize human fetal bone implanted subcutaneously in SCID mice, but do not migrate to the mouse BM.38 39 A clinically relevant model of primary MM requires migration of introduced MM cells to and from the BM.
Injection of cancer cells into the left cardiac ventricle allows injected cells to bypass the lungs and flow directly to the BM.40-42 This route of injection should detect MM cells able to traffic from the blood to the BM. However, malignant spread of MM also involves migration from one bone site to another. Here, we describe the use of intracardiac (IC) injection of human MM cells into immunodeficient NOD SCID mice to measure traffic from blood to the BM, and the use of intraosseus (IO) injection into the sternum to measure the ability to exit the bone and home to new skeletal locations. For these NOD SCID models, murine T cells are absent and human T cells do not engraft.31-44 Thus, a cell-mediated attack against engrafted MM cells, or control by T-cell immunoregulation, is not possible.
In this study, we show that peripheral cells from myeloma patients with aggressive disease, and G-CSF mobilized blood cells from myeloma patients with minimal disease after remission induction chemotherapy include myeloma cells able to engraft human myeloma to the BM of NOD SCID mice, as measured by phenotypic, pathologic, and molecular analysis. On the basis of their ability to generate progeny in the murine microenvironment, we have termed the engrafting cells, MM progenitors. By using a cellular limiting dilution assay to quantify cells with clonotypic transcripts, we show that engrafted myeloma cells are frequent in the murine BM. The engrafting myeloma progenitor from patients with aggressive disease self-renews in the murine BM as measured by its ability to transfer the human myeloma clone to a secondary recipient mouse.
Materials and methods
Patients
Blood, pleural effusion, or G-CSF mobilized leukaphereses were obtained from 11 myeloma patients (Table1), after informed consent and ethics approval. Blood was taken from 5 patients with aggressive myeloma, all of whom had plasma cells in the peripheral blood. Pleural effusion cells were taken from 1 myeloma patient. G-CSF mobilized blood was from 7 myeloma patients with minimal disease after 4 monthly cycles of vincristine, doxrubicin, and dexamethasone (VAD), and in whom there was > 75% decrement of paraprotein and 5% or less bone marrow plasma cells. Four G-CSF mobilized bloods were freshly obtained at the time of apheresis, and 3 were obtained after cryopreservation, at the time of reinfusion/transplant. All 7 patients proceeded to high-dose melphalan and autotransplantation after VAD. For patient 5, samples were taken in minimal disease and again during posttransplant relapse. Mononuclear cells were purified over Ficoll-Hypaque (Pharmacia, Dorval, QB, Canada).
Mice
NOD/LtSz-SCID (NOD SCID) mice30 were bred and maintained in microisolators in barrier conditions. At 6 to 8 weeks of age, animals were whole body irradiated with 300 to 340 cGy and injected with patient-derived MNC. After the mice were killed, BM from the femurs, spleen, and sometimes blood cells were collected for molecular and/or phenotypic analysis. The sternum and the humeri were processed for histologic analysis, and the carcasses examined by high resolution radiographs. For some mice, serum was collected. For phenotypic analysis, red blood cells were lysed using Intraprep (Coulter, Hialiah, FL) before staining. Intracardiac (IC) injection: As described by Arguello et al,40-42 mice were inoculated with ex vivo human cells by injection into the left cardiac ventricle. Briefly, mice were anesthetized with methoxyflurane and injected with 2 to 5 × 106 cells in 0.1 mL medium, through the second intercostal space. Preliminary experiments using dye confirmed that the injection site was the left ventricle. Intraosseus (IO) injection: To inject ex vivo human cells directly into bone sites, mice were anesthetized as previously described and the chest washed and shaved. An incision was made through the skin directly over the sternum. The sites of injection were at the third, fourth, or fifth intercostal spaces. Two to 5 × 105 cells were injected in 10 μL of medium. Slow pressure was applied to the needle plunger until the sternum was punctured, after which pressure was immediately released to avoid exit through the other side. The skin was then rejoined and the mouse allowed to recover. Injection of dye confirmed that the sternal BM was injected and that the dye was localized as expected.
Engrafted mice were killed when they developed symptoms of disease, including lethargy, hunched posture, failure to eat and drink normally, and for some mice, hind limb paralysis. A small proportion of engrafted mice were killed earlier to monitor disease progression.
Antibodies
Immunofluorescence
Mouse cells were treated with Intraprep (Coulter, Hialeah, FL) to lyse red blood cells, and stained with the above mAbs in 2-color immunofluorescence.14 Samples were analyzed on a FACSsort (Becton Dickinson). Files of 10 000 to 20 000 cells were collected and analyzed with Lysis II software.
ELISA (enzyme-linked immunosorbent assay) assay for human light chain
The 96-well microtiter plates ((Flow Laboratories, McLean, VA) were coated with 50 μg per well of 2 mg/mL goat F(ab)2antihuman Ig(IgM+IgG+IgA, H+L) (Southern Biotechnology) and blocked with 20% FCS. Serum samples were added at serial 2-fold dilutions for 60 minutes at 37°C, washed and bound human Ig detected with goat antihuman kappa-horseradish peroxidase (HRP) or goat antihuman lambda-HRP (Southern Biotechnology). After washing, ATBS substrate was added (Sigma, St Louis, MO) and color development read at 405 nm. A standard curve was constructed with purified human immunoglobulin. All reactions always included normal mouse serum, and all sera were assayed for both light chain types.
Histology
Sternum, humerus, and spleen tissue were removed at death for routine histologic examination, as well as any obvious tumor masses. No x-ray guided sampling was performed. Tissue was fixed in neutral buffered formalin and/or B-5, decalcified in RDO (BioGenex, San Ramon, CA), followed by routine paraffin embedding and sectioning. Routine morphology was assessed with hematoxylin and eosin staining. Immunohistochemistry was performed with a streptavidin/HRP-biotin method (Dako, LSAB+) using diaminobenzadine as the chromogen. Antihuman kappa and lambda light chain antibodies were obtained from Dako (Glostrup, Denmark) (polyclonal rabbit) and used at a 1/8000 dilution.
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR was as previously described11 using RNA purified from MM-engrafted mouse spleen or BM cells, or from normal NOD SCID mouse control tissues. Primers to histone11 amplified both human and mouse transcripts. Primers to β2-microglobulin (CCAGCAGAGAATGGAAAGTC, GATGCTGCTTACATGTCTCG) were specific for human transcripts and did not detect mouse B2 microglobulin transcripts. Consensus primers for human IgH VDJ were to FR1, FR2, and Jh and were previously described.11 Patient-specific primers were to the CDR2 and CDR3.11 These were identified from plasma cells at the time of diagnosis and confirmed to be expressed by > 80% of individual plasma cells from that patient as measured using single cell and in situ RT-PCR,11 and specificity was confirmed.11 Amplification using patient-specific CDR2/CDR3 primers occurred only for RNA from mice engrafted with the appropriate MM patient, and not for RNA from mice engrafted with an unrelated patient or an uninjected mouse. The detection of clonotypic transcripts used PCR with CDR2/CDR3 primers for 35 cycles. For engrafted mice in which clonotypic transcripts were not detected in the initial PCR reaction, a nested PCR strategy was used, in which the first step was amplification with FR1/Jh primers for 35 cycles and then a second stage PCR with CDR2/CDR3 primers for 35 cycles.
Cellular limiting dilution RT-PCR
To quantify the number of cells that expressed clonotypic IgH transcripts, we used a cellular limiting dilution method previously described.11 Briefly, cells were deposited at defined cell numbers (3-fold dilution series from 100 to 1 cell per tube) into PCR tubes containing lysis buffer for reverse transcription and nested PCR using VH family/Jh primers for the first stage and CDR2/CDR3 primers for the second stage PCR. The entire RT-PCR was performed in the same tube, the amplified product run on a gel and the product detected using ethidium bromide staining. Tubes in which the correct sized product is amplified are those that contained at least 1 clonotypic cell.
Results
Peripheral cells from myeloma patients with aggressive disease engraft human myeloma to NOD SCID mice
Blood or pleural effusion cells from patients with aggressive myeloma, defined as the presence of leukemic plasma cells, were injected into mice using intravenous (IV, patient 1) or intracardiac injection (IC, patients 1-5). Mice were sublethally irradiated to facilitate engraftment and growth of human cells. Usually, no engraftment occurred after IV injection. However, pleural effusion cells from patient 1 did engraft after IV injection. Table2 summarizes the measures used to detect engraftment of mice by human cells. Of the aggregate 48 mice that were injected by any route, 34 developed evidence of disease (71%) with overall morbidity, hind leg paralysis, and/or boney changes, including easily fractured long bones and macroscopic loss of red marrow (Table2). Mice injected IC with cells from each of the 5 patients developed symptoms at 2 to 5 months after injection. Mean overall latency of end stage disease for the cells from this set of patients ranged from 63 to 131 days (Table 2). For patient 1, the mean latency for mice injected IV (111 ± 61 days) or IC (119 ± 50 days) was shorter than that for mice injected IO (186 ± 46 days, see below).
Human B lineage cells are detectable in the bone marrow (BM) of mice engrafted with peripheral myeloma cells
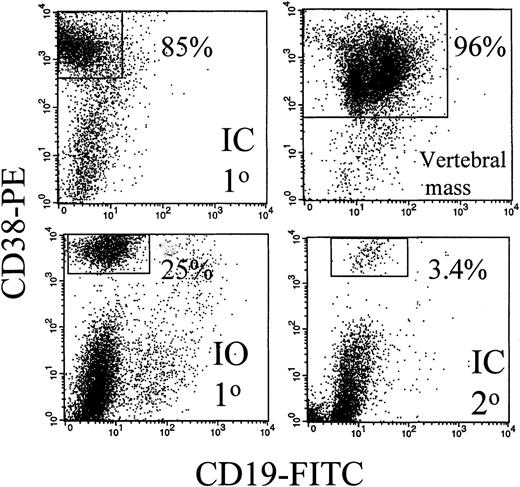
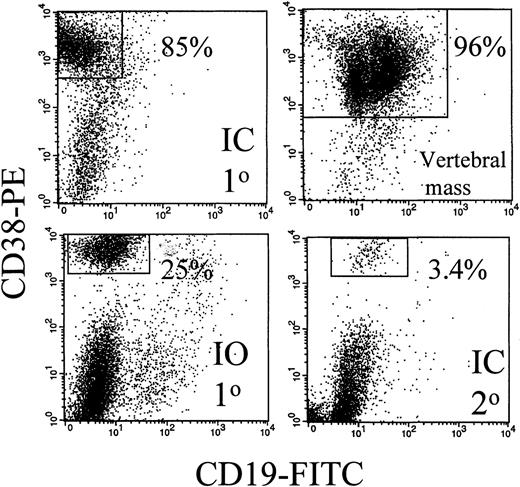
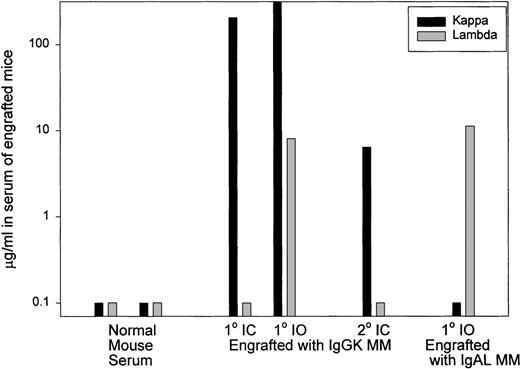
Expression of human CD19 and CD38 identified human B and plasma cells, as shown by the representative dot plots of Figure1. After IC injection, CD38hiplasma cells comprised 85% of the murine BMC (Figure 1). A large vertebral mass also was predominantly CD38hi plasma cells (Figure 1). Table 3 shows that after IC injection, human B and plasma cells colonize the BM, leading to fragile bones, lack of erythropoiesis, and lytic bone lesions characteristic of human myeloma (see below). In MM patients, plasma cells comprise 5% to ∼ 90% of BMC.8-11 In MM-engrafted mice, the proportion of B and plasma cells in blood and BM was comparable, with 0.3% to 87% plasma cells of total BMC. Human B lineage cells were also found in spleen and peripheral blood. For 6 animals, representing engraftment by cells from 3 different MM patients, large skeletal masses of plasma cells were detected on autopsy. Overall, engrafted human cells were detectable in 36 of 51 animals analyzed by flow cytometry (71%). However, flow cytometry may not detect all engrafted myeloma cells because it depends on retention of characteristic phenotypic markers after engraftment. For example, in cells from 1 mouse, no phenotypic expression of human CD45, CD19, or CD38 was detected, but extensive plasma cell infiltration of the BM was evident by histology, and clonotypic transcripts were detectable from BM and from a large CD38− plasma cell tumor, indicating extensive human engraftment (not shown). Confirming the presence of human B lineage cells, human light chain was detected in 10 of 15 sera from these MM-engrafted mice. In most cases the light chain was monotypic. However, in some mice, the non-MM light chain was also detectable, presumably indicating engraftment of normal B cells. Representative sera are shown in Figure 2.
The BM and a vertebral mass from MM-engrafted mice includes human CD38hi plasma cells.
Mouse BM was removed from the femurs, the red cells lysed, and the remaining cells stained with CD38 and CD19 mAbs in 2-color immunfluoresence. Representative dot plots from 3 different mice and from 1 vertebral tumor are shown. No CD38hi cells were detected in uninjected control mice (not shown).
The BM and a vertebral mass from MM-engrafted mice includes human CD38hi plasma cells.
Mouse BM was removed from the femurs, the red cells lysed, and the remaining cells stained with CD38 and CD19 mAbs in 2-color immunfluoresence. Representative dot plots from 3 different mice and from 1 vertebral tumor are shown. No CD38hi cells were detected in uninjected control mice (not shown).
Human light chain is detectable in serum from MM-engrafted mice.
Representative analysis of sera from control mice, mice injected IC with human myeloma, a mouse injected IO with human myeloma, and from a secondary recipient mice injected with BM from primary MM-engrafted mice was analyzed by ELISA for human kappa and lambda light chain. The black histograms represent expression of kappa light chain and gray bars represent expression of lambda light chain. Normal mouse serum was from uninjected mice.
Human light chain is detectable in serum from MM-engrafted mice.
Representative analysis of sera from control mice, mice injected IC with human myeloma, a mouse injected IO with human myeloma, and from a secondary recipient mice injected with BM from primary MM-engrafted mice was analyzed by ELISA for human kappa and lambda light chain. The black histograms represent expression of kappa light chain and gray bars represent expression of lambda light chain. Normal mouse serum was from uninjected mice.
The presence of transcripts for β2 microglobulin and IgH confirms engraftment of human B cells after IC injection
Human β2 microglobulin and IgH VDJ transcripts, detected using consensus FR2/Jh primers, were found in the BMC from engrafted mice engrafted but not in control uninjected mice (Figure3A). Sixteen of 17 mice tested, which had been engrafted IC with cells from the 5 aggressive myelomas, had human β2 microglobulin and IgH VDJ RNA transcripts. Overall, human β2 microglobulin and IgH VDJ transcripts were more readily detected in BM than in spleen (not shown). In situ hybridization for Epstein-Barr virus (EBV) on sections of bone that had large numbers of plasma cells was negative, indicating that the engrafted human B lineage cells were not EBV-transformants (not shown).
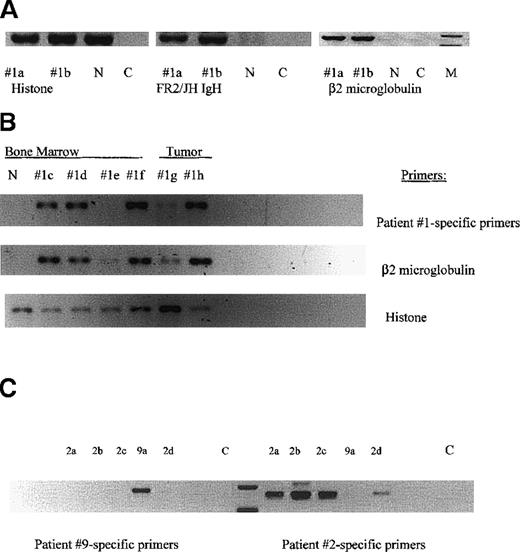
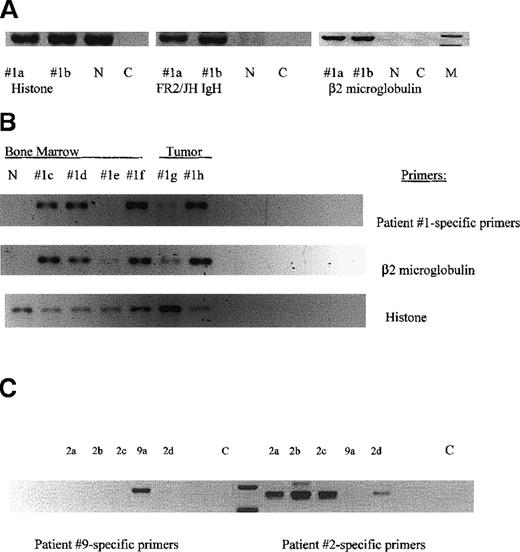
MM-engrafted mice have BM cells and spleen cells expressing human β2-microglobulin, IgH VDJ and clonotypic transcripts.
Each lane represents femoral bone marrow or vertebral tumor from an individual mouse. The numerical designation indicates the patient from whom the injected cells were obtained. (A) Bone marrow (femur) from mice engrafted with cells from patient 1. RNA from the indicated tissues was amplified in RT-PCR using the indicated primers. Histone primers detect both human and mouse transcripts. Primers for human β2 microglobulin detect only human transcripts. n = uninjected NOD SCID: C = water control. (B) Clonotypic transcripts in RNA of engrafted mice. A second set of tissues from individual mice, were tested for histone, β2 microglobulin and clonotypic transcripts in nested RT-PCR using first primers to FR2/Jh in the first stage and patient 1-specific primers for CDR2/CDR3 in the second stage. Each lane represents femoral BM, or a vertebral tumor from an individual mouse injected with cells from patient 1. (C) Patient-specific primers are specific. Patient-specific primers for patients 2 and 9 were tested in a crisscross specificity experiment. Each lane represents femoral BM cells from an individual mouse injected with either cells from patient 2 or 9. RNA from each mouse was amplified in nested RT-PCR using FR2/Jh primers, followed by CDR2/CDR3 primers specific for either patient 2 or 9. Because the PCR products are patient-specific, the size of the product varies with each individual patient.
MM-engrafted mice have BM cells and spleen cells expressing human β2-microglobulin, IgH VDJ and clonotypic transcripts.
Each lane represents femoral bone marrow or vertebral tumor from an individual mouse. The numerical designation indicates the patient from whom the injected cells were obtained. (A) Bone marrow (femur) from mice engrafted with cells from patient 1. RNA from the indicated tissues was amplified in RT-PCR using the indicated primers. Histone primers detect both human and mouse transcripts. Primers for human β2 microglobulin detect only human transcripts. n = uninjected NOD SCID: C = water control. (B) Clonotypic transcripts in RNA of engrafted mice. A second set of tissues from individual mice, were tested for histone, β2 microglobulin and clonotypic transcripts in nested RT-PCR using first primers to FR2/Jh in the first stage and patient 1-specific primers for CDR2/CDR3 in the second stage. Each lane represents femoral BM, or a vertebral tumor from an individual mouse injected with cells from patient 1. (C) Patient-specific primers are specific. Patient-specific primers for patients 2 and 9 were tested in a crisscross specificity experiment. Each lane represents femoral BM cells from an individual mouse injected with either cells from patient 2 or 9. RNA from each mouse was amplified in nested RT-PCR using FR2/Jh primers, followed by CDR2/CDR3 primers specific for either patient 2 or 9. Because the PCR products are patient-specific, the size of the product varies with each individual patient.
Monotypic human plasma cells infiltrate the murine BM
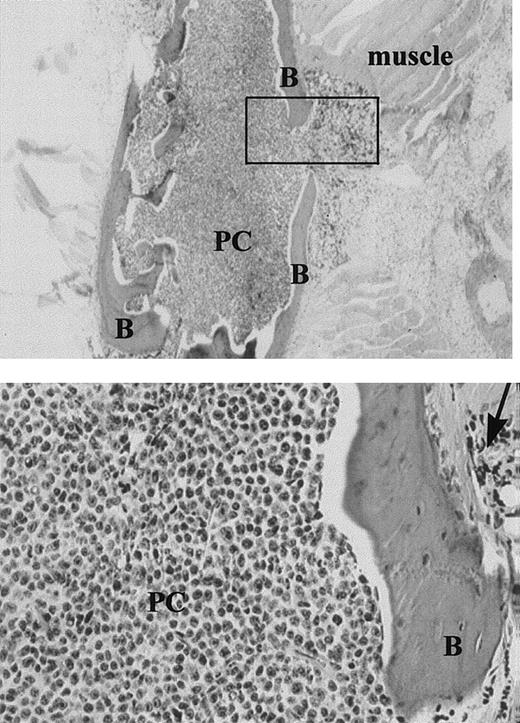
On autopsy, many MM-engrafted mice had easily fractured or spongy bones, and for a large proportion, the BM appeared white. A small proportion of the injected mice developed hind leg paralysis. Human plasma cells were shown by immunohistochemistry to fill the mouse BM (Figure 4). For those tissues analyzed by flow cytometry as in Figure 1, other bones from the same animal, or sections of the vertebral mass, were histologically identified as plasma cells. Infiltrating plasma cells were monotypic, with restricted human light chain expression (not shown). For 9 of 11 IC-injected mice analyzed, morphologically detected monotypic plasma cells were seen in the BM.
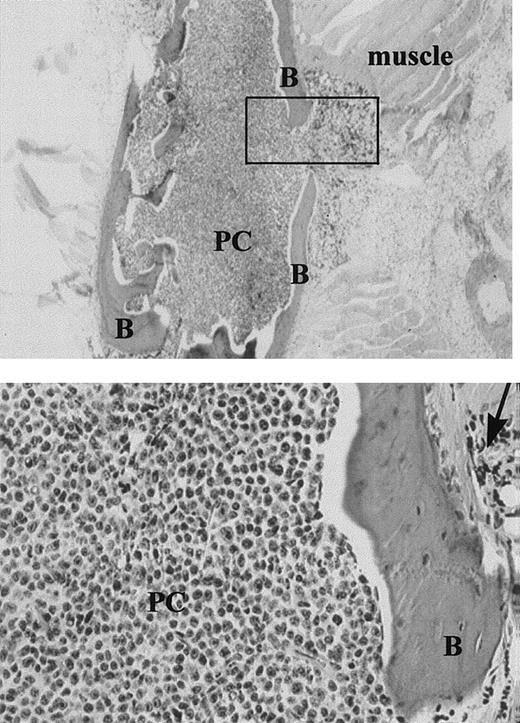
Expansile bone lesion in the sternum of an MM-engrafted mouse injected IC with ex-vivo human MM cells (× 20).
Upper panel: The box indicates the expansile bone lesion. B = bone, PC = human plasma cells. Lower panel: Higher power view of the expansile lesion (× 100), arrow = expansile lesion. Sequential sections were stained with antihuman kappa chain, or antihuman lambda. All the plasma cells, which were derived from a patient with IgK myeloma, were strongly positive for kappa. None stained with antilambda.
Expansile bone lesion in the sternum of an MM-engrafted mouse injected IC with ex-vivo human MM cells (× 20).
Upper panel: The box indicates the expansile bone lesion. B = bone, PC = human plasma cells. Lower panel: Higher power view of the expansile lesion (× 100), arrow = expansile lesion. Sequential sections were stained with antihuman kappa chain, or antihuman lambda. All the plasma cells, which were derived from a patient with IgK myeloma, were strongly positive for kappa. None stained with antilambda.
Intra-osseus injection of aggressive MM cells results in MM engraftment in distant BM sites
For 3 MM patients with aggressive disease, peripheral cells were injected directly into the mouse sternum, a rich site of hematopoiesis, to detect intraskeletal traffic. For mice with IO injection of MM cells to the sternum, the distribution of monotypic human B lineage cells provided strong evidence of exit from the BM to colonize the blood and distant BM sites. IO-injected mice developed disease symptoms with a latency of 186 ± 41 days (patient 1, 5 mice), or 26 and 35 days (patient 2, 2 mice) and 88 days (patient 3, 1 mouse). Phenotypically identified B lineage cells were detected in IO-injected mice at levels comparable to those in IC-injected mice (Table 3). Figure 1 shows that for 1 representative IO-injected mouse, CD38hi plasma cells comprised 25% of murine femoral BMC. This indicates that MM cells had migrated from the sternum to the femur. After IO injection, human light chain of the appropriate type was detectable in mouse serum (Figure 2). Myeloma cells grew at the site of injection as indicated by the presence of morphologically identified monotypic plasma cells in the sternum for 3 of 6 mice analyzed. However, distant bone sites were also colonized. After injection to the sternum, the femur became engrafted with human cells, as detected by β2-microglobulin and IgH transcripts, comparable to the results shown in Figure 3, and by the presence of lytic bone lesions (see below). Like the IC-injected mice, the IO-injected mice developed easily fractured bones, and a macroscopic absence of red marrow.
MM-engrafted mice innoculated IC or IO exhibit lytic bone lesions
To determine whether MM-engrafted mice displayed the osteolytic bone lesions, animals were radiographed after they were killed. Consistent with the fragile bones observed in many mice, lytic lesions were often detectable in the long bones, vertebral bodies, or in the skull. Overall, for those mice radiographed, 9 of 14 mice injected IC with aggressive myeloma cells had lytic bone lesions, as did 4 of 7 IO-injected mice. However, no lytic lesions were detectable at week 20 for 8 sublethally irradiated but unengrafted mice, or for 4 normal control mice (not shown). Radiograph images in Figure5A show lytic bone lesions in vertebral bodies and the tibia for IC-injected mice. Figure 5B shows lytic lesions in the skull, vertebral bodies, and tibia from IO-injected mice, showing colonization of distant bone sites. For 3 mice, a bone having evidence of lytic lesions by x-ray analysis was dissected, fixed, and sectioned. For all 3, the affected area was seen to be infiltrated with monotypic human plasma cells (not shown).
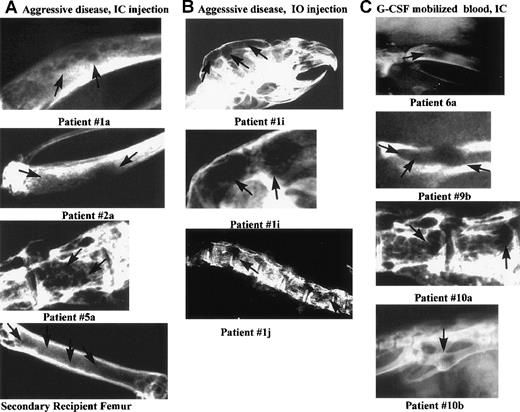
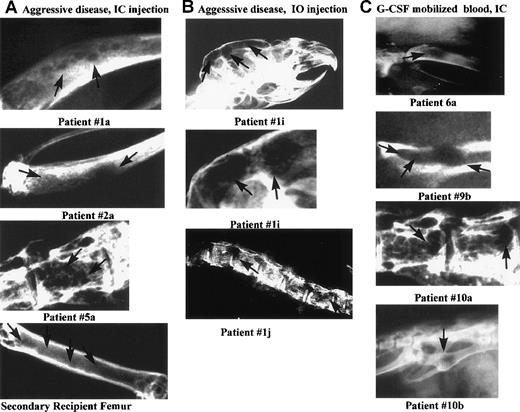
Lytic bone lesions in the skull, tibia, pelvis, or spine of 10 MM-engrafted mice.
Carcasses of 56 engrafted mice killed at preterminal disease stages, and 12 control mice, were radiographed using mammography. Ten representative radiographs of bones from MM-engrafted mice that had lytic lesions are shown here. The comparable bones from either irradiated or normal control mice did not have detectable lesions (not shown). Each panel is labeled with the source of the engrafting cells and the route of injection. Arrows indicate lytic lesions. The panels are labeled with the patient number from whom the engrafting MM cells were derived (A) Diseased bones (tibias or vertebral body) from mice engrafted IC with aggressive MM cells, or from a secondary recipient mouse (femur). (B) Diseased bones from mice injected IO with aggressive myeloma cells (skull or spine). The middle panel is an enlarged image of the skull lesions. (C) Diseased bones from mice engrafted IC with G-CSF mobilized blood cells (tibia, pelvis, vertebral body, pelvis).
Lytic bone lesions in the skull, tibia, pelvis, or spine of 10 MM-engrafted mice.
Carcasses of 56 engrafted mice killed at preterminal disease stages, and 12 control mice, were radiographed using mammography. Ten representative radiographs of bones from MM-engrafted mice that had lytic lesions are shown here. The comparable bones from either irradiated or normal control mice did not have detectable lesions (not shown). Each panel is labeled with the source of the engrafting cells and the route of injection. Arrows indicate lytic lesions. The panels are labeled with the patient number from whom the engrafting MM cells were derived (A) Diseased bones (tibias or vertebral body) from mice engrafted IC with aggressive MM cells, or from a secondary recipient mouse (femur). (B) Diseased bones from mice injected IO with aggressive myeloma cells (skull or spine). The middle panel is an enlarged image of the skull lesions. (C) Diseased bones from mice engrafted IC with G-CSF mobilized blood cells (tibia, pelvis, vertebral body, pelvis).
Clonotypic human B lineage cells in MM-engrafted mice
For each individual myeloma patient, the malignant clone is uniquely characterized by the IgH VDJ gene rearrangement of the BM plasma cells. All plasma cells and the majority of circulating B cells in myeloma patients express transcripts encoding the same IgH VDJ rearrangement.10 11 For most patients described in Table 1, the clonotypic IgH VDJ rearrangement was identified, its specificity for the patient confirmed, and the sequence validated as clonotypic by showing its expression in > 80% of autologous plasma cells. Figure3B shows that for 4 different mice engrafted IC with cells from patient 1, but not from a normal mouse, clonotypic IgH VDJ transcripts were detected in the BM from 3 of 4 mice and in vertebral tumors from 2 mice (section B, row 1). For most mice, clonotypic sequences were detectable in a first stage PCR reaction, suggesting a high copy number of target transcripts. For 1 mouse with no detectable clonotypic transcripts, human engraftment was weakly detected, based on the presence of β2-microglobulin transcripts (section B, row 2, 1e). The amplification of histone transcripts indicates the presence of intact mRNA in all samples (section B, row 3). Figure 3C shows clonotypic transcripts in the BM of mice engrafted with cells from patient 2, as well as demonstrating the specificity of the patient-specific RT-PCR. Primers specific for patient 9 amplify product only from the mouse engrafted with patient 9, whereas primers specific for patient 2 amplify product only from mice engrafted with cells from patient 2.
Clonotypic myeloma cells are frequent in BM of MM-engrafted mice
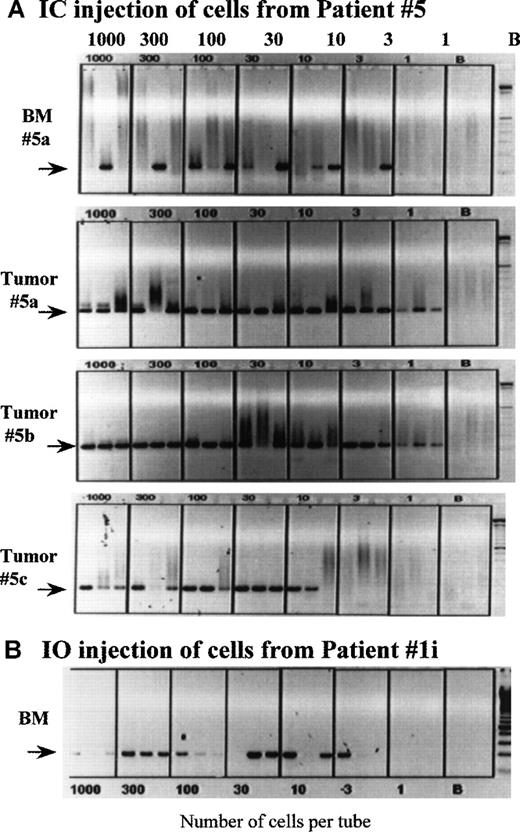
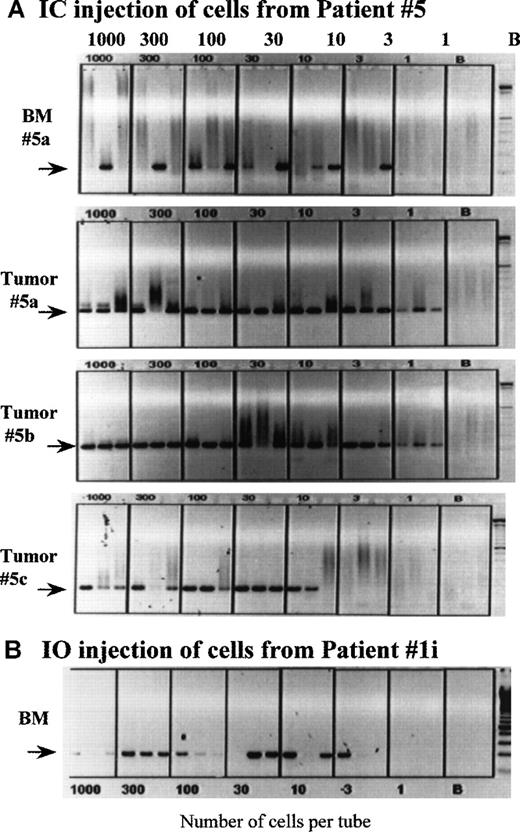
To determine the frequency of clonotypic cells in mice, we used a cellular limiting dilution assay. BMC and spleen cells from engrafted mice were deposited at limiting dilution into PCR tubes, followed by RT-PCR with patient-specific primers to detect clonotypic transcripts. A representative dilution series for an IC-injected mouse (Figure6A) and for an IO-injected mouse (Figure6B) are shown. All tubes contained intact mRNA as indicated by the presence of detectable histone transcripts (not shown). For the IC-injected mouse, all 3 tubes that had 10 cells per tube, and 1 of 3 tubes with 3 cells per tube are positive for clonotypic transcripts, a frequency of about 12%. Figure 7A also shows the very high frequency of clonotypic cells in vertebral plasma cell masses from 3 mice injected IC with PBMC from patient 5. For the IO-injected mouse, the frequency of clonotypic cells in BMC from the femur was about 22% (2 of 3 tubes at 3 cells per tube were positive or at least 2 clonotypic cells per 9 cells analyzed), indicative of migration from the sternum to the femur. For both mice, the frequency of clonotypic cells in spleen was about 100-fold higher in BM than in spleen (not shown). As shown in Table 4A, high frequencies were obtained from mice engrafted IC with cells from patient 1 and patient 5. For other patients, clonotypic transcripts were seen only in RNA from aliquots of 106 BM cells (a frequency of at least 1/106), or a limiting dilution series was not available. The clonotypic cells were detectable in mice at relatively high frequency at about 3 to 6 months after injection, indicating persistence of the myeloma clone for prolonged periods.
Clonotypic cells are frequent in the BM of MM-engrafted mice.
Mice engrafted IC with cells from patient 5 (A), and a mouse injected IO into the sternum with cells from patient 1 (B) were killed at the time of end stage disease, followed by harvest of the femoral bone marrow. The number of clonotypic cells engrafting the femurs was determined by a cellular limiting dilution RT-PCR, as described in “Materials and Methods.” In a replicate dilution series, primers to histone were used to determine the number of tubes having intact mRNA. For patient 5, tumors from 3 different engrafted mice were dispersed through a sieve and the cells deposited at limiting dilution into PCR tubes; the phenotypic analysis of the tumor from mouse 5a is shown in Figure 1. Because the PCR is a direct lysis method, aliquots having 1000 cells per tube sometimes exhibit less PCR product that tubes having fewer cells, presumably because of cellular proteins compromising the PCR reaction. The extent to which this occurs appears to depend on the source of cells being deposited into the tubes.
Clonotypic cells are frequent in the BM of MM-engrafted mice.
Mice engrafted IC with cells from patient 5 (A), and a mouse injected IO into the sternum with cells from patient 1 (B) were killed at the time of end stage disease, followed by harvest of the femoral bone marrow. The number of clonotypic cells engrafting the femurs was determined by a cellular limiting dilution RT-PCR, as described in “Materials and Methods.” In a replicate dilution series, primers to histone were used to determine the number of tubes having intact mRNA. For patient 5, tumors from 3 different engrafted mice were dispersed through a sieve and the cells deposited at limiting dilution into PCR tubes; the phenotypic analysis of the tumor from mouse 5a is shown in Figure 1. Because the PCR is a direct lysis method, aliquots having 1000 cells per tube sometimes exhibit less PCR product that tubes having fewer cells, presumably because of cellular proteins compromising the PCR reaction. The extent to which this occurs appears to depend on the source of cells being deposited into the tubes.
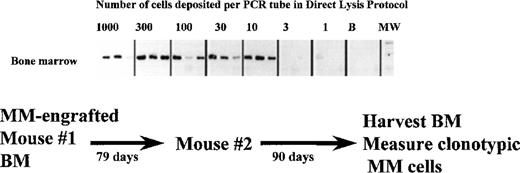
Self-renewal of MM stem cells in primary host mice, as measured by their ability to transfer MM to a naive secondary host.
A NOD SCID mouse was injected with aggressive myeloma cells from patient 1. When this primary host developed end stage disease at day 79, it was killed and the BM harvested. The BMC were then injected into a second naive NOD SCID recipient (secondary host) to determine whether or not MM stem cells had been regenerated while resident in the primary host BM. The frequency of clonotypic cells in the secondary recipient was determined using the cellular limiting dilution assay. 1000 to 1 BMC from the secondary host were deposited in a 3-fold series into PCR tubes for direct lysis and RT-PCR. The BMC had intact mRNA as measured by the amplification of histone transcripts in separate aliquots of 1 to 1000 cells.
Self-renewal of MM stem cells in primary host mice, as measured by their ability to transfer MM to a naive secondary host.
A NOD SCID mouse was injected with aggressive myeloma cells from patient 1. When this primary host developed end stage disease at day 79, it was killed and the BM harvested. The BMC were then injected into a second naive NOD SCID recipient (secondary host) to determine whether or not MM stem cells had been regenerated while resident in the primary host BM. The frequency of clonotypic cells in the secondary recipient was determined using the cellular limiting dilution assay. 1000 to 1 BMC from the secondary host were deposited in a 3-fold series into PCR tubes for direct lysis and RT-PCR. The BMC had intact mRNA as measured by the amplification of histone transcripts in separate aliquots of 1 to 1000 cells.
Self-renewal of the myeloma clone occurs in MM-engrafted mice
If the myeloma clone self-renews after engraftment into mice, myeloma stem cells should be generated in the bone marrow. To determine whether this was the case, BMC from the femora of primary MM-engrafted mice were injected IC into an irradiated secondary mouse, and the frequency of clonotypic cells in this secondary recipient was analyzed at 2 to 3 months after inoculation. Figure 1 shows that 3.4% of murine BMC in a secondary recipient were CD38hi plasma cells. Analysis of human light chain in serum of the secondary mice indicates the presence of human kappa light chain (the monoclonal Ig type) (Figure 2) but not lambda light chain, suggesting the presence of the human myeloma clone. For 4 secondary mice, clonotypic transcripts were detected in purified RNA from BMC (not shown), or lytic lesions were detected in the bones. Figure 5 shows multiple lytic lesions in the femur of a secondary recipient mouse. To determine the frequency of clonotypic cells colonizing the secondary recipient, a limiting dilution analysis was performed for BM harvested from the secondary recipient (Figure 7). The frequency of clonotypic cells in BM after the secondary transfer was about 10%. The frequency in spleen was < 0.03% (not shown). For other secondary mice, clonotypic cells were not detected in the limiting dilution assay, even though they were seen in purified RNA, indicating a frequency of between 1/103 to 1/106 in BM. This experiment shows that the myeloma stem cell homes to, and self-renews in the BM, as measured by its ability to engraft the same myeloma clone into a secondary recipient.
G-CSF mobilized blood cells include myeloma progenitors
G-CSF mobilized blood cells were injected into mice to detect progenitors capable of transferring the myeloma clone. A total of 49 mice were injected with mobilized blood cells from 7 myeloma patients (Table 2). No plasma cells were detectable in the circulating blood of any patient on clinical screening. All 7 patients had minimal disease at the time of mobilization. Clonotypic transcripts were detectable in RNA purified from aliquots of the injected cells for the 6 evaluable patients. After IC injection of mobilized cells from 3 of 7 patients, mice developed disease symptoms with a latency of 103 to 157 days (Table 2). Few human cells were detectable in injected mice by using flow cytometry. For 4 of 5 G-CSF mobilized blood engraftments, human light chain was detected in 6 of 9 mice tested, but it did not always correlate with the expected mIg type, suggesting at least some Ig originated from normal B cells. On radiograph, numerous lytic bone lesions were apparent. Fifteen of 29 mice (52%) injected with mobilized cells from all 6 patients tested, had lytic bone lesions (Table 2). Figure 5C shows representative radiographs from mice engrafted with cells from patients 6 and 9 (fresh G-CSF mobilized blood) and from patient 5A (cryopreserved G-CSF mobilized blood), indicating that an MM progenitor is present in mobilized blood, and that it survives cryopreservation. Overall, evidence of MM engraftment was detected in at least 2 injected mice for G-CSF mobilized cells from 6 of 7 patients (Table 2).
Clonotypic transcripts are detectable in mice engrafted with G-CSF mobilized blood
Mobilized blood cells include normal hematopoietic progenitors, as well as putative malignant progenitors. We found that 13/35 mice tested had human β2 microglobulin transcripts (37%), and 18 of 35 had detectable IgH VDJ transcripts (51%), indicating engraftment by human B cells. With patient-specific primers to the CDR2/CDR3 of the myeloma IgH VDJ, 10 of 35 mice tested (29%), from 3 of 6 patients for whom the clonotypic sequence was available, had clonotypic transcripts (Table 2) in BM and/or in spleen. For most of these mice, a nested second stage PCR reaction was required to detect clonotypic sequences, suggesting a low copy number of the target transcripts. Table 4B reports the frequency of clonotypic cells in mice that had been injected with mobilized blood cells from patients 7, 9, and 11. The patient-specific PCR product from mice engrafted with patient 9 was sequenced and found to be identical with the sequence derived from BM plasma cells of patient 9, verifying the accuracy of the PCR. For mobilized blood from patient 9, engraftment of clonotypic cells was detectable at 4 weeks after injection and persisted for at least 3 months (Table 4). Overall, the frequency of clonotypic cells appeared to be lower than that found after injection of aggressive myeloma cells (compared with Table 4A). However, for mice injected with G-CSF mobilized blood cells and having clonotypic frequencies about 1% or higher, a conservative estimate is that at the time of death at least 5 × 106clonotypic cells are present (based on an estimate of about 5 × 108 total lymphocytes in the mouse). These must have arisen from the 2 to 5 × 106 unfractionated G-CSF MNC originally injected. Assuming that myeloma stem cells are likely to be relatively infrequent in G-CSF mobilized blood, this suggests that considerable clonal expansion of malignant progenitors has occurred in these mice.
Discussion
This work shows the ability of peripheral cells from myeloma patients having either aggressive or minimal disease to engraft human myeloma into the BM of NOD SCID mice, as measured by molecular, pathologic, and phenotypic analysis. Further, self-renewal of the engrafted human myeloma clone occurs within the murine BM microenvironment, as identified by molecular analysis of clonotypic IgH VDJ transcripts. Of particular significance is the presence of malignant progenitors in fresh aphereses or in cryopreserved G-CSF mobilized blood cells, identified by their ability to engraft the myeloma clone into mice. Even though at the time of mobilization, these myeloma patients had minimal disease after initial therapy with VAD, the mobilized cells used for hematopoietic rescue after high-dose chemotherapy include myeloma progenitors at a frequency of at least 1 per 2 to 5 × 106 MNC. This observation strengthens the case for purging of malignant cells before autologous transplant by showing the presence among G-CSF mobilized apheresis cells of generative cells able to transfer myeloma to a new host. Myeloma progenitors survive cryopreservation, based on our observation that aliquots of thawed aphereses taken at the time of transplant retain the ability to engraft human myeloma to immundeficient mice. However, the experiments do not identify myeloma progenitors, beyond showing by their progeny that they are present among peripheral cells during stages of minimal disease, as well as during aggressive myeloma progression.
The use of IC and IO injection methods, provides an experimental model to test the ability of myeloma cells to home from the blood to the BM, (IC) and from the BM through the blood to distant BM sites (IO). This work shows that extraskeletal peripheral cells from patients with aggressive disease include members of the malignant clone that are able to migrate in both directions. The ability of human plasma cells to colonize the mouse BM, and to give rise to boney changes, lytic lesions, and hind limb paralysis after IC or IO injection to the sternum, indicates that in these models, the mouse BM is, or has became, a supportive environment for human MM cells. Overall, this work confirms the 2-way traffic by the myeloma clone, to and from the BM. For MM progenitors present during aggressive disease, the transfer of human myeloma from a primary to a secondary recipient mouse shows that the original engrafting myeloma stem cell is able to regenerate itself in the murine BM microenvironment. However, we cannot distinguish whether human myelomagenesis occurred through MM progenitor cell support from murine stromal cells and growth factors, or alternatively through colonization of the murine BM by human microenvironmental cells.
For mice engrafted with MNC from G-CSF mobilized blood, the appearance of disease symptoms was less evident than for mice engrafted with cells from patients with aggressive disease, and the extent of engraftment appeared less extensive. In addition, clonotypic cells derived from G-CSF mobilized blood appeared more likely to home to the murine spleen than did the progeny of aggressive myeloma cells. Engraftment of clonotypic cells was detected in about a third of injected mice (29%, from 3 of 6 patients for whom testing was possible), but lytic bone lesions were found for about half of the mice injected with G-CSF mobilized blood, after engraftment of mobilized cells from all 6 patients tested. The lower frequency of clonotypic cells after engraftment of mobilized blood may reflect the generation of progeny having a lower copy number of IgH transcripts than do the late stage B and plasma cells likely to be present during aggressive disease, leading to less sensitive detection of engrafted clonal cells. The relatively high incidence of lytic bone disease, coupled with the relatively infrequent detection of clonotypic cells, may suggest the presence in mobilized blood of an earlier stage myeloma progenitor than that present in peripheral tissues of patients with aggressive or terminal myeloma. This idea is supported by work showing unusual stem-like B cells having clonotypic transcripts are present within the hematopoietic progenitor cell fraction of mobilized blood.16 At the terminal stages of myeloma, selective pressure may have favored the emergence of aggressive plasma cell clones. If so, this might allow more active disease progression than was seen for engrafted MM progenitors from mobilized blood. The extent to which the mouse BM microenvironment is altered by the engrafting myeloma cells is unknown. However, the ability of myeloma progenitors from aggressive or minimal disease to undergo clonal expansion, as measured molecularly and by detection of lytic bone lesions, shows that the murine BM microenvironment is a supportive one. We observed long-term generation of clonotypic MM cells, because clonotypic engraftment was detectable as early as 1 month after transplant and as late as 5 months.
The work here describes a mouse model of human myeloma in which ex vivo human myeloma cells colonize the bone marrow, self-renew, and cause lytic bone disease. This model, when fully characterized, will provide an ideal tool for determining the effects of treatment on members of the MM clone, the susceptibilities of generative components to clinical interventions, and the mechanisms of malignant spread. Because myeloma progenitors appear to engraft and generate progeny in these model systems, they may allow identification of those MM subsets from blood and BM with MM progenitor potential. In the murine microenvironment, T cells are absent and the antigenic environment is likely to bear little resemblance to that of the patient. Valuable insights into immunoregulation of the myeloma clone,46 if such immunoregulation occurs, may be gained by comparing transplant of the myeloma clone into mice with development and expansion of the same myeloma clone after autologous transplantation, where T cells and the antigenic environment are relatively unchanged before and after transplant. In addition to providing a tool to evaluate the biology of myeloma, ultimately, these model systems may also provide a preclinical model for testing of novel therapies.
The potential clinical impact of G-CSF mobilized MM cells has not previously been evaluated. Although mobilized blood cells include a myeloma-engrafting cell as measured in NOD SCID mice, the consequences of reinfusing the same clonotypic B lineage cells to the patient are unknown. The presence of myeloma progenitors in autografts may explain why syngeneic transplants result in comparatively improved survival,47 because syngeneic grafts are genetically identical but tumor-free. It seems safest, given the results reported here, to assume that the myeloma progenitor cells detected in mice have equivalent or better generative potential in an autologous microenvironment and are likely sources of disease regeneration after transplant. If clinical eradication of all malignant cells could be achieved with ablative therapy, then reinfusion of a “clean” transplant gains in importance. Our results suggest that autologous transplants may be a significant source of myeloma progenitor cells. Effective purging strategies may prolong patient survival after transplant. The xenograft model described here provides a system within which to identify myeloma progenitor cells during minimal and aggressive stages of human disease, and for preclinical characterization of MM progenitor cell susceptibility to novel treatment strategies.
Acknowledgments
Mr Dan McGinn and Ms Juanita Wizniak provided skilled assistance. Dr John Dick generously provided breeding pairs of NOD SCID mice. We thank Dr Nick Nation Agnieszka Szczepek, Dr Brian Taylor, Yvon DeMossiac, Dr Tim Terry, Cheryl Penrice, and Adele Andriashek for their help.
Funded by the Medical Research Council of Canada.
Reprints:Linda M. Pilarski, Cross Cancer Institute, 11560 University Ave, Edmonton, AB T6G1Z2, Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.