Streptokinase activates platelets, limiting its effectiveness as a thrombolytic agent. The role of antistreptokinase antibodies and proteases in streptokinase-induced platelet activation was investigated. Streptokinase induced localization of human IgG to the platelet surface, platelet aggregation, and thromboxane A2production. These effects were inhibited by a monoclonal antibody to the platelet Fc receptor, IV.3. The platelet response to streptokinase was also blocked by an antibody directed against the cleavage site of the platelet thrombin receptor, protease-activated receptor-1 (PAR-1), but not by hirudin or an active site thrombin inhibitor, Ro46-6240. In plasma depleted of plasminogen, exogenous wild-type plasminogen, but not an inactive mutant protein, S741A plasminogen, supported platelet aggregation, suggesting that the protease cleaving PAR-1 was streptokinase-plasminogen. Streptokinase-plasminogen cleaved a synthetic peptide corresponding to PAR-1, resulting in generation of PAR-1 tethered ligand sequence and selectively reduced binding of a cleavage-sensitive PAR-1 antibody in intact cells. A combination of streptokinase, plasminogen, and antistreptokinase antibodies activated human erythroleukemic cells and was inhibited by pretreatment with IV.3 or pretreating the cells with the PAR-1 agonist SFLLRN, suggesting Fc receptor and PAR-1 interactions are necessary for cell activation in this system also. Streptokinase-induced platelet activation is dependent on both antistreptokinase-Fc receptor interactions and cleavage of PAR-1.
Streptokinase (Sk) is a bacterial protein that is used as a thrombolytic agent in the treatment of myocardial infarction.1,2 It is a plasminogen activator that has no intrinsic protease activity. Rather, in blood it forms an equimolar complex with the zymogen plasminogen, which is an active protease. The active complex is a plasminogen activator, converting further plasminogen to the fibrinolytic enzyme, plasmin.3
Due to its bacterial origin, Sk is antigenic. Low levels of antibodies directed against Sk are detectable in the general population, presumably due to previous streptococcal infections.4Following administration of Sk, the level of antibodies to Sk rises dramatically in most patients, peaking at about 2 weeks and may remain elevated for up to 4 years.5,6 Anti-Sk antibodies are presumed to cause allergic reactions during Sk treatment and may neutralize the activity of Sk, necessitating the administration of a high standard dose of Sk to ensure effective thrombolysis in most patients.7 Antibodies to Sk are also capable of activating platelets8,9 and this may explain how thrombolytic therapy with Sk results in marked platelet activation in vivo, as measured by the production of the platelet-derived eicosanoid thromboxane (TX) A2.10 This platelet activation is functionally important because it delays arterial reperfusion and contributes to early reocclusion.11
Platelets are activated through a low-affinity receptor for IgG on the platelet surface (FcγRII, CD 32) by immobilized immunoglobulin,12 aggregates of IgG,13 or cross-linking of the Fc receptor.14 High concentrations (5000 U/mL) of Sk and anti-Sk IgG cause platelet activation in an Fc receptor-dependent manner.15 Alternatively, proteolytic agents can initiate cellular signaling and activation by cleaving protease-activated receptors (PARs). The original protease receptor, designated PAR-1, was cloned from a megakaryocytic cell line and is present on platelets.16 It belongs to the family of cell surface receptors with 7 transmembrane domains and coupled to G-proteins. PAR-1 has an extended extracellular amino terminus, which includes a site recognized and cleaved by thrombin. This cleavage generates a new amino-terminal sequence on the receptor, which acts as a tethered ligand and activates the receptor.16 Peptides as short as 5 amino acids in length corresponding to the tethered ligand sequence (starting SFLLRN.) activate intact PAR-1 at micromolar concentrations. Other proteases, such as trypsin,16cathepsin G,17 and granzyme A18 activate PAR-1 also, albeit at higher concentrations than thrombin.
Several other PARs have been cloned.19-21 PAR-2 is cleaved by low concentrations of trypsin, but not by thrombin. Its physiologic role is unknown. PAR-3 is activated by thrombin, but appears to be more important in mouse than human platelets.20 Recently a fourth receptor, PAR-4, has been cloned from mouse22 and human21 tissues, and mediates thrombin-induced platelet activation in both species. It has a lower affinity for thrombin than PAR-1 or PAR-3,22 requires higher concentrations of soluble peptide ligand to induce platelet activation,23 and has been postulated to act as a “standby” protease receptor.22 Moreover, in man the platelet effects of thrombin appear to be mediated predominately by PAR-1; only at high concentrations and when PAR-1 activation has been inhibited is platelet activation by thrombin dependent on PAR-4.23
Recent evidence has shown that plasmin can cleave PAR-1 at the site resulting in receptor activation. However, cleavage at other sites in the receptor's extracellular N-terminal domain predominate, resulting in desensitization of the receptor to subsequent challenge with thrombin.24,25 We have previously shown that Sk-induced platelet activation was mediated by a protease,10 although others found immunologic mechanisms predominate.9 This study examines the mechanism of Sk-induced platelet activation, particularly, the interaction between PAR-1 cleavage and anti-Sk antibodies in inducing this response.
Materials and methods
Materials
Sepharose 4B, Sk, adenosine diphosphate (ADP), hirudin, thrombin, human plasminogen, plasmin and α2-antiplasmin were purchased from Sigma (Poole, UK). Monoclonal antibody IV.3 was purchased from Medarex Inc (Annandale, NJ). Monoclonal antibodies SPAN 12 and WEDE 15 were purchased from Immunotech (Marseille, France). Monoclonal antibody 2/389 was a kind gift from the late Dr Stuart Stone, Department of Biochemistry, Cambridge University.
Platelet preparation
Blood was drawn from human volunteers, free from aspirin and other drugs likely to affect platelet function for 10 days. Blood was drawn into 10% final volume of 3.8% sodium citrate. Platelet-rich plasma (PRP) was prepared by centrifugation of whole blood at 150g for 10 minutes. Aggregation was assayed by light transmission in a final volume of 500 μL (PAP-4 aggregometer, Biodata, Horsham, PA). Inhibitory monoclonal antibodies were preincubated in PRP for 2 minutes before addition of agonists. Aliquots of PRP were frozen in liquid nitrogen for subsequent assay of TXA2 formation by enzyme-linked immunoassay for the stable metabolite, TXB2(Assay Designs, Ann Arbor, MI). For some experiments, PRP was depleted of plasminogen by passing it over a column of lysine immobilized on Sepharose 4B. To this was added 50 μL of 8 μM native plasminogen, plasminogen inactivated by mutation of its active site (S741A plasminogen), or buffer in a final volume of 500 μL.
Antibody purification
Anti-Sk antibodies were purified from plasma as follows. Plasma from a normal donor was incubated overnight with 1 g Protein A-Sepharose 4B at 4°C. Sepharose beads were washed in phosphate-bufferred saline (PBS), bound antibody was eluted with glycine HCl (0.1 M, pH 3) and neutralized with Tris HCl buffer (1 M, pH 8). In some cases this IgG was concentrated to 0.1 of the original volume for use in platelet aggregation, by centrifugation through a porous membrane with a molecular weight cut-off of 30 kd (Centricon-30 concentrator, Amicon Inc., Beverly, MA). PRP was treated with ADP and Sk in the presence of buffer alone or IgG purified from different donors. To specifically purify anti-Sk antibodies, Sk was immobilized on activated CH Sepharose 4B (Pharmacia Biotech AB, Uppsala, Sweden), according to the manufacturer's instructions. IgG obtained from plasma as above was incubated overnight with Sk-Sepharose at 4°C and specific anti-Sk antibody eluted and concentrated as described above. Serum from patients recently treated with Sk for myocardial infarction contains high levels of anti-Sk. In some experiments such serum was depleted of Sk-binding antibodies by overnight incubation with Sk immobilized on Sepharose, followed by centrifugation and removal of the supernatant. As a control, aliquots of serum were incubated with bovine serum albumin (BSA) immobilized on Sepharose.
Flow cytometry
Platelet-rich plasma was first treated with the platelet glycoprotein IIb/IIIa antagonist integrelin to prevent aggregation and Sk and ADP added at fully activating concentrations. PRP was then fixed in an equal volume of 0.4% formaldehyde, washed twice in PBS, and incubated at 4°C for 30 minutes with the fluorescein isothiocyanate (FITC)-conjugated secondary antibody (antihuman IgG, Becton Dickinson, San Jose, CA). Platelets were then washed twice and resuspended in formaldehyde for analysis (FACScan flow cytometer, Becton Dickinson).
Synthesis and cleavage of PAR1 peptides
Thrombin receptor peptides LDPRSFLLRNPNDKYEPF (TR18), LDPR (TR4), and SFLLRNPNDKYEPF (TR14) were synthesized on an Applied Biosystems automated peptide synthesiser (model 432A, Norwalk, CT), using a standard solid phase Fmoc (N-[9-fluorenyl] methoxycarbonyl) procedure. All peptides were purified after synthesis using reverse-phase high-performance liquid chromatography (HPLC) and confirmed using electrospray mass spectrometry. The peptide LDPRSFLLRNPNDKYEPF corresponds to the site on PAR-1 recognized and cleaved by thrombin.26 This was incubated at 0.5 mM in PBS with thrombin (10 nM) or a complex formed by incubating 1.0 μM Sk with 0.5 μM plasminogen at room temperature for 30 minutes. Incubations with Sk-plasmin(ogen) were performed in the presence and absence of α2-antiplasmin (2.0 μM). Analysis of the resultant cleavage products was performed by HPLC on a C18 column (Supelco, Poole, UK). Peptides were detected at 215 nm with a SPD-6A UV detector and a C-R3A integrator (Shimadazu, Kyoto, Japan), using a mobile phase of 25% acetonitrile, 75% 5 mM phosphate buffer, pH 7.4, and compared to standard peptides corresponding to cleavage of the parent peptide at the expected site (TR4 and TR14).
Calcium fluorimetry
Human erythroleukemic (HEL) cells were incubated for 30 minutes at 37°C with 4 μM FURA-2-AM (Calbiochem-Novabiochem, Nottingham, UK), before being washed twice in Hank's balanced salt solution containing 1.25 mM Ca++ and finally resuspended in the same buffer. Intracellular Ca++ changes were detected using an LS-50 fluorimeter (Perkin Elmer, Norwalk, CT). Cells were stirred at 37°C in a 500-μL quartz cuvette. FURA-2 fluorescence was measured following excitation at wavelengths 340 and 380 nm, with emission at 510 nm.
Fluorescent microscopy
The HEL cells were pelleted and resuspended in plasma from an individual who exhibited platelet aggregation in the presence of Sk. Cells were treated with no agonist or Sk (1600 U/mL) for 10 minutes in the presence of 0.01% sodium azide to prevent receptor internalization. Treated cells were pelleted and resuspended in Tris-buffered saline (TBS), adhered by centrifugation onto poly-l-lysine-coated slides and fixed in methanol for 7 minutes. Cells were blocked with 2% normal goat IgG in TBS for 30 minutes, incubated with monoclonal antibodies SPAN 12 or WEDE 15 (directed against the cleavage site and hirudin-like site of PAR-1, respectively) at 10 μg/mL in TBS for 45 minutes and stained with goat antimouse IgG conjugated to an Alexa™ 488 fluorescent group (Molecular Probes, Eugene, OR) at 4 μg/mL for 10 minutes. Cells were visualized using a LSM 510 microscope (Carl Zeiss Ltd, Herts, UK) using an argon laser.
Results
Platelet aggregation and TXA2 production
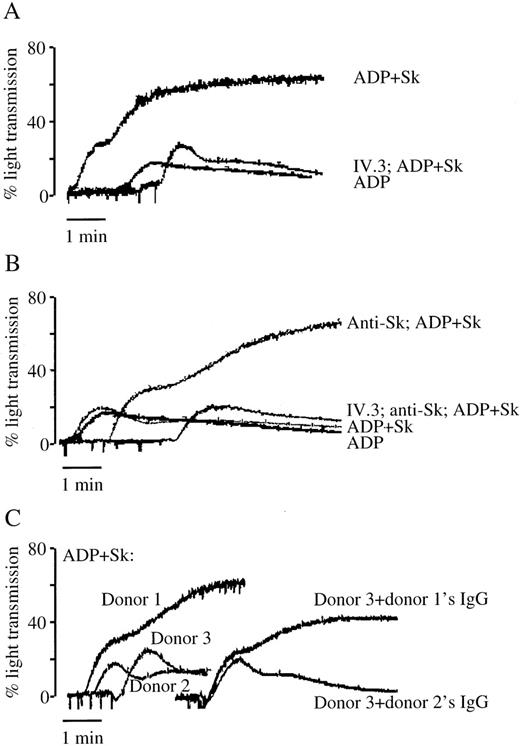
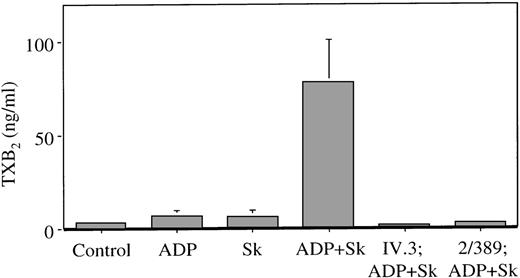
Low concentrations of ADP (0.5-2.0 μM) cause a small and reversible platelet aggregation. In the presence of Sk added to untreated PRP, this is converted to an irreversible platelet aggregation (Figure 1A). The effect can be demonstrated in PRP from some individuals at an Sk concentration of 300 to 500 U/mL, in the range of concentrations achieved in vivo following a standard dose of Sk.27 Sk-induced platelet aggregation was accompanied by increased TXA2 production, measured as its hydrolysis product TXB2, from 3.1 ± 0.8 ng/mL in stirred platelets (no agonist) to 78 ± 22.4 ng/mL in platelets treated with Sk and ADP. Neither ADP nor Sk alone, at the concentrations used, results in significant generation of TXB2 (Figure 2).
The role of antibodies in platelet aggregation to Sk.
(A) Sk (500 U/mL) combined with a subthreshold concentration of ADP (2 μM) results in irreversible platelet aggregation. This is inhibited by 5 μg/mL of the Fc receptor-blocking antibody IV.3. (B) In individuals who fail to aggregate to low concentrations of Sk and ADP, the addition of 50 μL purified anti-Sk antibodies results in an aggregation response. This is also sensitive to inhibition by IV.3. (C) Whole-plasma IgG from a responsive donor (donor 1) but not from a nonresponsive donor (donor 2) can confer sensitivity to Sk on PRP from a third donor. These results are representative of tracings from at least 5 (A and B) or 2 (C) individuals.
The role of antibodies in platelet aggregation to Sk.
(A) Sk (500 U/mL) combined with a subthreshold concentration of ADP (2 μM) results in irreversible platelet aggregation. This is inhibited by 5 μg/mL of the Fc receptor-blocking antibody IV.3. (B) In individuals who fail to aggregate to low concentrations of Sk and ADP, the addition of 50 μL purified anti-Sk antibodies results in an aggregation response. This is also sensitive to inhibition by IV.3. (C) Whole-plasma IgG from a responsive donor (donor 1) but not from a nonresponsive donor (donor 2) can confer sensitivity to Sk on PRP from a third donor. These results are representative of tracings from at least 5 (A and B) or 2 (C) individuals.
Thromboxane production in plasma during Sk-induced platelet aggregation.
Plasma was obtained from platelet aggregations as shown in Figure 1, and assayed for TXB2. Platelets were pretreated with buffer, 5 μg/mL of the Fc receptor-blocking antibody IV.3 or 4 μg/mL of the PAR-1-blocking antibody 2/389 as shown, and challenged with subthreshold concentrations of ADP, 500 U/mL of Sk or both, as shown. Results are mean ± SEM from 3 individuals.
Thromboxane production in plasma during Sk-induced platelet aggregation.
Plasma was obtained from platelet aggregations as shown in Figure 1, and assayed for TXB2. Platelets were pretreated with buffer, 5 μg/mL of the Fc receptor-blocking antibody IV.3 or 4 μg/mL of the PAR-1-blocking antibody 2/389 as shown, and challenged with subthreshold concentrations of ADP, 500 U/mL of Sk or both, as shown. Results are mean ± SEM from 3 individuals.
Involvement of the platelet Fc receptor
The monoclonal antibody IV.3 blocks the binding of IgG antibodies to the Fc receptor found on platelets. Pretreatment of PRP with IV.3 abolished Sk-induced platelet aggregation (see Figure 1A) and TXB2 production (see Figure 2), but had no effect on aggregation to ADP alone. This implicates endogenous antibodies in the plasma in Sk-induced platelet activation. In individuals who did not exhibit platelet aggregation in response to low concentrations of Sk, aggregation to Sk was seen on addition of 50 μL of purified anti-Sk antibodies (Figure 1B). Moreover, a small volume of serum from a patient who previously received Sk and had a high level of anti-Sk antibodies, resulted in Sk-induced aggregation (not shown). These responses were blocked by pretreatment with the antibody IV.3 (see Figure 1B). Incubation of PRP from a nonresponsive donor with whole plasma IgG purified from a responsive donor conferred Sk sensitivity on the PRP, whereas IgG from a nonresponsive donor did not (Figure 1C), indicating that antibodies purified in this way do not of themselves activate platelets.
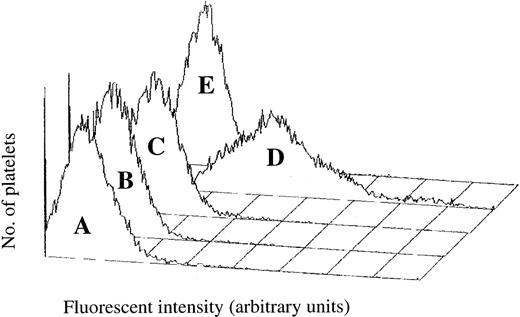
Platelet-rich plasma incubated with an FITC-conjugated monoclonal antibody directed against human immunoglobulin was analyzed using flow cytometry. In the presence of both ADP and Sk, there was a marked increase in human IgG associated with the platelet surface (Figure3). Stimulation of cells by ADP or Sk alone did not enhance human IgG binding. Localization of human IgG to the surface was prevented by pretreatment with the Fc receptor-blocking antibody IV.3, showing that binding of the antibody to the platelet was specific and not due to secretion of IgG by the platelet or to nonspecific adsorption of plasma antibody (see Figure 3). No increase in antibody binding was seen in platelets treated with the PAR-1 agonist SFLLRN (data not shown), indicating that the platelet-associated IgG was not due solely to platelet activation.
Platelet-associated human IgG during Sk-induced platelet aggregation.
Following agonist treatment, platelets were incubated with an FITC-labeled antibody against human IgG and platelet-associated fluorescence measured using flow cytometry. (A) Platelets stirred in the absence of agonist. (B) Subthreshold concentration of ADP. (C) 500 U/mL Sk alone. (D) ADP in the presence of 500 U/mL Sk. (E) Treated with the Fc receptor-blocking antibody IV.3 prior to the addition of Sk and ADP.
Platelet-associated human IgG during Sk-induced platelet aggregation.
Following agonist treatment, platelets were incubated with an FITC-labeled antibody against human IgG and platelet-associated fluorescence measured using flow cytometry. (A) Platelets stirred in the absence of agonist. (B) Subthreshold concentration of ADP. (C) 500 U/mL Sk alone. (D) ADP in the presence of 500 U/mL Sk. (E) Treated with the Fc receptor-blocking antibody IV.3 prior to the addition of Sk and ADP.
Role of PAR-1
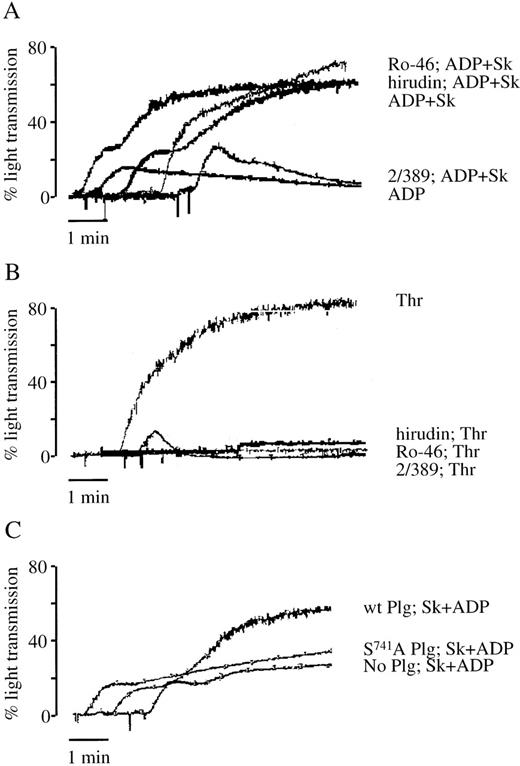
Sk-induced platelet aggregation was prevented by pretreating the platelets with antibody 2/389 raised against a peptide containing the cleavage site of PAR-1 (Figure 4A). The antibody binds to the tethered ligand-binding domain of PAR-1 and prevents PAR-1 cleavage by thrombin (Dr S. Stone, personal communication, July 1995). This antibody also prevented platelet aggregation induced by nanomolar concentrations of thrombin (Figure 4B). Although thrombin-induced platelet aggregation could be prevented by pretreatment with the specific thrombin inhibitor hirudin, platelet aggregation to Sk was unaffected (see Figure 4A and B). Thrombin bound on the platelet surface, or newly generated by the prothrombinase complex, may not be inhibited by hirudin, due to inaccessibility of the anion-binding site. The low molecular weight thrombin inhibitor, Ro46-6240, is a potent, specific inhibitor of thrombin that binds to the active site of the enzyme and not to the anion-binding site.28 Ro46-6240, at a concentration that inhibited platelet aggregation to thrombin, but not to other platelet agonists, had no effect on Sk-induced platelet aggregation (see Figure4A and B). These findings suggest that Sk-induced platelet aggregation is due to the action of a protease distinct from thrombin acting on PAR-1.
The role of thrombin, PAR-1, and plasminogen in Sk-induced platelet aggregation.
Aggregations to ADP and Sk (A) or 0.35 U/mL thrombin (B) were performed without inhibitors or in the presence of the PAR-1-blocking antibody 2/389 (4 μg/mL), hirudin (1 U/mL), or the thrombin active site inhibitor Ro46-6240 (Ro-46) (0.04 μg/mL). (C) Aggregation to ADP and Sk was performed in PRP depleted of plasminogen by passage over a lysine-Sepharose column, to which had been added buffer alone, wild-type plasminogen, or the inactive mutant S741A plasminogen. Each tracing is representative of 2 to 3 experiments.
The role of thrombin, PAR-1, and plasminogen in Sk-induced platelet aggregation.
Aggregations to ADP and Sk (A) or 0.35 U/mL thrombin (B) were performed without inhibitors or in the presence of the PAR-1-blocking antibody 2/389 (4 μg/mL), hirudin (1 U/mL), or the thrombin active site inhibitor Ro46-6240 (Ro-46) (0.04 μg/mL). (C) Aggregation to ADP and Sk was performed in PRP depleted of plasminogen by passage over a lysine-Sepharose column, to which had been added buffer alone, wild-type plasminogen, or the inactive mutant S741A plasminogen. Each tracing is representative of 2 to 3 experiments.
Role of plasminogen
In PRP depleted of plasminogen by passage over a lysine-Sepharose column, Sk at 500 U/mL was unable to induce platelet aggregation. Platelet aggregation was restored by the addition of 0.8 μM native plasminogen. However, when plasminogen rendered inactive by mutation at its active site (S741A) was used at 0.8 μM, no aggregation to Sk was seen (Figure 4C). Sk-induced platelet activation, therefore, requires proteolytically active plasminogen.
PAR-1 cleavage by Sk-plasminogen
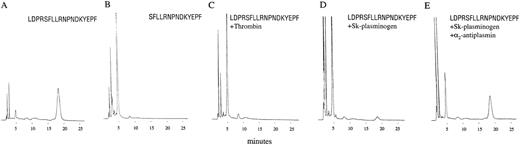
Incubation of a peptide corresponding to the thrombin recognition and cleavage sites of PAR-1 (LDPRSFLLRNPNDKYEPF) with thrombin, resulted in cleavage at the expected site and production of the 2 predicted fragments, LDPR and SFLLRNPNDKYEPF (Figure5A-C). The peptide was also cleaved following incubation with plasmin (not shown) and Sk-plasminogen complex (Figure 5D). Pretreatment with α2-antiplasmin, which inhibits free plasmin, but not plasmin(ogen) complexed with Sk,29 did not prevent Sk-plasminogen cleaving TR18 (Figure5E).
Cleavage of PAR-1 peptide sequences.
(A) The peptide LDPRSFLLRNPNDKYEPF (TR18), corresponding to the uncleaved receptor, elutes as a single peak at 18 minutes. (B) The peptide SFLLRNPNDKYEPF (TR14), corresponding to the receptor following cleavage at the site resulting in receptor activation, elutes as a single peak at 5 minutes. (C) TR18 following 15 minutes incubation with 10 nM thrombin. (D) TR18 following 4 hours incubation with 0.5 μM Sk-plasminogen. (E) TR18 following 4 hours incubation with 0.5 μM Sk-plasminogen and 2 μM α2-antiplasmin. Significant peptide cleavage occurs even in the presence of α2-antiplasmin.
Cleavage of PAR-1 peptide sequences.
(A) The peptide LDPRSFLLRNPNDKYEPF (TR18), corresponding to the uncleaved receptor, elutes as a single peak at 18 minutes. (B) The peptide SFLLRNPNDKYEPF (TR14), corresponding to the receptor following cleavage at the site resulting in receptor activation, elutes as a single peak at 5 minutes. (C) TR18 following 15 minutes incubation with 10 nM thrombin. (D) TR18 following 4 hours incubation with 0.5 μM Sk-plasminogen. (E) TR18 following 4 hours incubation with 0.5 μM Sk-plasminogen and 2 μM α2-antiplasmin. Significant peptide cleavage occurs even in the presence of α2-antiplasmin.
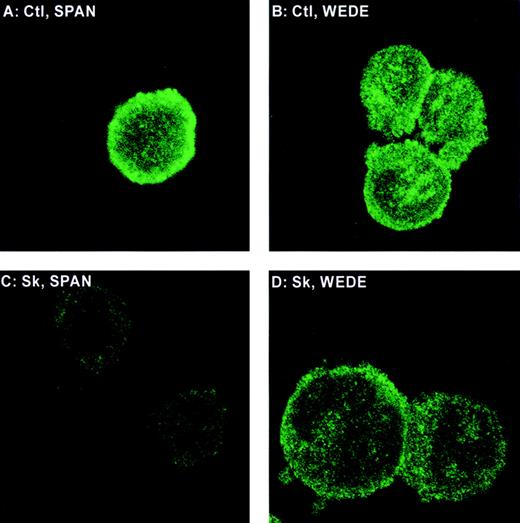
Epitope-specific cleavage of PAR-1 on intact cells
The monoclonal antibody SPAN 12 was raised against an epitope spanning the site on PAR-1 cleaved by thrombin and recognizes the intact receptor only.30 WEDE 15 binds to the hirudin-like site of PAR-1 (removed by plasmin) and recognizes intact and thrombin-cleaved receptors.30 HEL cells incubated with SPAN 12 or WEDE 15 show strong staining at the cell surface (Figure6A and B). Cells treated with Sk in the presence of plasminogen and anti-Sk antibodies showed a substantial reduction in SPAN 12 staining without significant loss of staining of WEDE 15 (Figure 6C and D). Because plasmin cleaves PAR-1 not just at the authentic thrombin cleavage site but also at sites that would delete both the SPAN and WEDE epitopes,25 these data demonstrate that Sk-plasmin(ogen) cleaves PAR-1 primarily at the site that results in receptor activation.
Fluorescent microscopy of PAR-1 on HEL cells.
Cells were stained with the cleavage-sensitive PAR-1 antibody SPAN 12 (A and C) or the cleavage-insensitive PAR-1 antibody WEDE 15 (B and D) and treated with no agonist (A and B) or Sk (C and D) in plasma from an individual whose platelets aggregated to Sk.
Fluorescent microscopy of PAR-1 on HEL cells.
Cells were stained with the cleavage-sensitive PAR-1 antibody SPAN 12 (A and C) or the cleavage-insensitive PAR-1 antibody WEDE 15 (B and D) and treated with no agonist (A and B) or Sk (C and D) in plasma from an individual whose platelets aggregated to Sk.
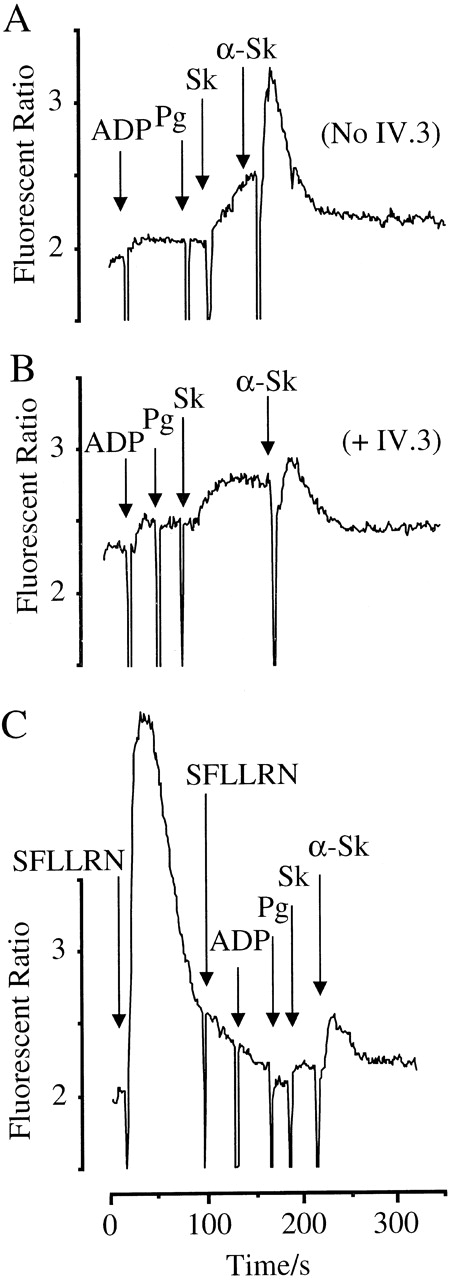
Intracellular calcium responses
The HEL cells mimic platelets in that they express the Fc receptor and PAR-1 (J.P.M. and D.J.F., unpublished observations). HEL cells showed a marked increase in intracellular Ca++ in response to sequential addition of ADP, 1 μM plasminogen, 800 U/mL Sk, and anti-Sk antibodies purified from plasma (Figure7A). Although some rise in Ca++ is elicited in the presence of Sk and plasminogen, this is accelerated by the addition of anti-Sk. As with the platelet activation, this response is substantially blocked by pretreatment with IV.3, implicating antibody-Fc receptor interactions in mediating this effect (Figure 7B). No response was seen to anti-Sk and Sk in the absence of plasminogen (data not shown). Moreover, desensitization of PAR-1 by prior treatment with the agonist SFLLRN substantially reduced the Ca++ response to plasminogen, Sk, and anti-Sk (Figure7C). Thus, activation of both PAR-1 and the Fc receptor is necessary for Sk-induced cell activation.
Activation of HEL cells by ADP, plasminogen, Sk, and anti-Sk antibodies.
Cells were loaded with FURA-2-AM, and Ca++-mobilizing responses measured. Arrows indicate the addition of agonists. (A) Cells were treated with ADP (100 μM), plasminogen (2 μM), Sk (800 U/mL), and anti-Sk antibodies (30 μL). (B) Cells were treated in the presence of 5 μg/mL of the Fc receptor-blocking antibody IV.3. (C) Pretreatment with 12 μM of the PAR-1 agonist SFLLRN, rendering the cells unresponsive to further activation of PAR-1 or to the combination of ADP, plasminogen, Sk, and anti-Sk antibodies.
Activation of HEL cells by ADP, plasminogen, Sk, and anti-Sk antibodies.
Cells were loaded with FURA-2-AM, and Ca++-mobilizing responses measured. Arrows indicate the addition of agonists. (A) Cells were treated with ADP (100 μM), plasminogen (2 μM), Sk (800 U/mL), and anti-Sk antibodies (30 μL). (B) Cells were treated in the presence of 5 μg/mL of the Fc receptor-blocking antibody IV.3. (C) Pretreatment with 12 μM of the PAR-1 agonist SFLLRN, rendering the cells unresponsive to further activation of PAR-1 or to the combination of ADP, plasminogen, Sk, and anti-Sk antibodies.
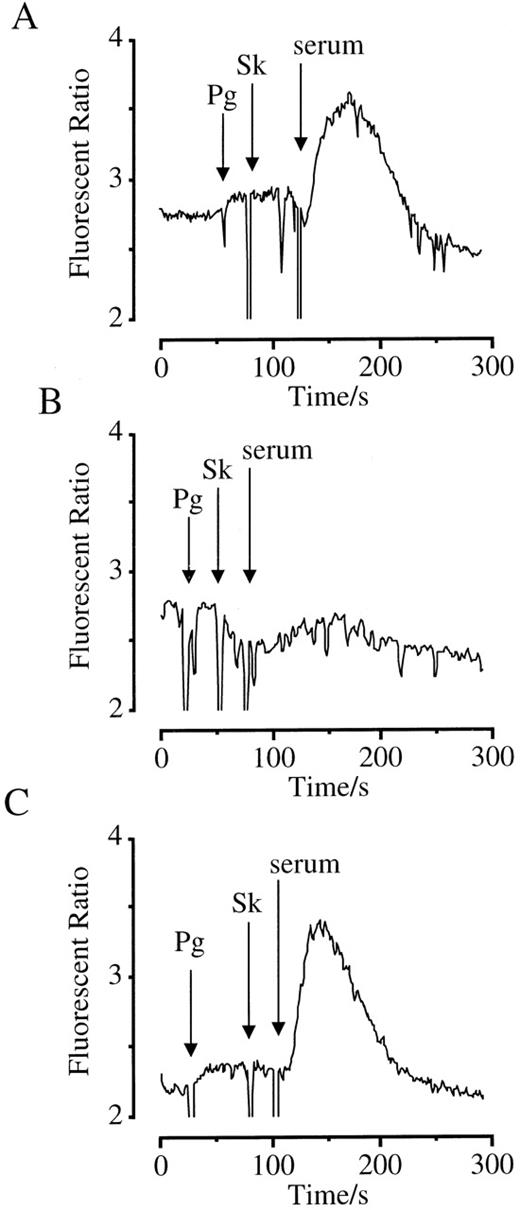
To confirm the specificity of the antibody required to support Sk-dependent cell activation, we used serum from a patient treated with Sk for acute myocardial infarction 1 month before as a source of antibody. Plasminogen and Sk evoked a calcium response in HEL cells only after addition of 10 μL serum (Figure8A). An equivalent volume of serum incubated overnight with Sk-coated Sepharose particles to remove Sk-binding antibodies failed to provoke a calcium response (Figure 8B). In contrast, serum incubated with BSA immobilized on Sepharose supported a calcium response (Figure 8C).
Activation of HEL cells by plasminogen, Sk, and serum from a Sk-treated patient.
Cells were loaded with FURA-2-AM, and Ca++-mobilizing responses measured. Arrows indicate the addition of agonists. (A) Cells were treated with plasminogen (1 μM), Sk (800 U/mL), and serum (10 μL). (B) The serum used was incubated overnight with Sk coupled to Sepharose beads to deplete Sk-binding antibodies. (C) The serum used was incubated overnight with BSA coupled to Sepharose beads as a control.
Activation of HEL cells by plasminogen, Sk, and serum from a Sk-treated patient.
Cells were loaded with FURA-2-AM, and Ca++-mobilizing responses measured. Arrows indicate the addition of agonists. (A) Cells were treated with plasminogen (1 μM), Sk (800 U/mL), and serum (10 μL). (B) The serum used was incubated overnight with Sk coupled to Sepharose beads to deplete Sk-binding antibodies. (C) The serum used was incubated overnight with BSA coupled to Sepharose beads as a control.
Discussion
Streptokinase is widely used in the treatment of coronary thrombosis. Despite lacking protease activity, Sk complexes with plasminogen and generates the plasminogen activator, Sk-plasmin complex. Sk induces reperfusion in the majority of patients presenting with a coronary thrombosis but patency is often delayed31 and is complicated by reocclusion in 15% to 30% of cases.32 Several clinical and experimental studies have shown evidence of platelet activation and thrombosis in patients treated with Sk, and this appears to be more marked than that seen with the nonantigenic tissue-type plasminogen activator.10,33,34The increased platelet activity appears to be functionally important because aspirin greatly enhances the benefit seen with Sk in humans.1 The mechanism for the increased platelet activity is not understood. Although thrombin has been implicated based on measurements of fibrinopeptide A35 and other biochemical measurements,36 neither heparin37,38 nor specific thrombin inhibitors39 influence the response to Sk.
We and others have shown that Sk can induce platelet aggregation and that this was dependent on the presence of antibody.8,10These findings were confirmed in the present study in that Sk at a concentration that is achieved in vivo induced full irreversible aggregation of platelets primed with ADP. This was accompanied by a marked increase in TXA2 formation, as also seen in vivo. Platelet aggregation was abolished by a monoclonal antibody against the platelet Fc receptor. Activation of this receptor alone by cross-linking with immune complexes can cause platelet activation and aggregation.12-14 However, Sk-induced platelet aggregation was also abolished by a monoclonal antibody directed against PAR-1. This antibody prevents cleavage of PAR-1 and thrombin-induced platelet aggregation. Cleavage of the receptor was not due to formation of thrombin because neither hirudin nor Ro46-6240, 2 highly specific thrombin inhibitors, had any effect.
These studies suggest that a protease other than thrombin is responsible for Sk-mediated platelet aggregation. Aggregation depended on the presence of proteolytically active plasminogen, implicating Sk-plasmin(ogen) as the protease responsible for PAR-1 cleavage. Indeed, a PAR-1 peptide was cleaved by Sk-plasminogen at the activating site, although only slowly in solution. This cleavage was not blocked by antiplasmin, suggesting that it was largely as a consequence of the complex rather than free plasmin. Plasmin has been reported to cleave PAR-1 at the thrombin cleavage site and at other sites that delete the tethered ligand domain, preventing receptor activation.24,25 However, we showed in HEL cells that Sk-plasmin is more selective. Thus, the binding of SPAN 12, an antibody to the thrombin cleavage site, was selectively reduced by Sk-plasmin, whereas an antibody to the hirudin-like binding domain (lying in the region putatively cleaved by plasmin) was not. Although PAR-4 expression is detectable by reverse transcriptase-polymerase chain reaction in platelets23 and is a potential target for Sk-plasminogen, Sk-induced platelet activation was prevented by an antibody specific to PAR-1, so PAR-4 is unlikely to be involved. A role for PAR-1 was also seen in HEL cells activated by a combination of Sk, plasminogen, and anti-Sk antibody. These cells contain PAR-1, PAR-4 (J.P.M. and D.J.F., unpublished observations), and the Fc receptor. Pretreating the cells with the PAR-1 agonist SFLLRN to desensitize the receptor reduced the intracellular Ca++ response to Sk.
Thus, 2 distinct signals are required for Sk-mediated platelet activation, the first mediated by the Fc receptor and the second by cleavage of PAR-1. Consistent with a role for Ig-Fc interactions, Sk-induced but not SFLLRN-induced platelet activation targeted IgG to the platelet surface. Moreover, the Ca++ response in HEL cells induced by Sk and plasminogen was accelerated by the addition of anti-Sk antibody and blocked by IV.3. A recent report described antibody-mediated platelet activation in heparin-induced thrombocytopenia (HIT) as dependent on ADP release from the platelet.40 In contrast, the Sk-induced platelet activation described in this report requires exogenous ADP and PAR-1 activation suggesting that signaling through the Fc receptor is not as strong as that seen in HIT. A previous study has shown also that an Sk-plasmin complex devoid of protease activity induced platelet aggregation.8 However, aggregation was induced only in the presence of high concentrations of Sk antibodies and is perhaps similar to what occurs in HIT. In our experiments, Sk-induced platelet activation was always dependent on PAR-1. One possible model is that the Sk antibody localizes the complex to the cell surface allowing a more rapid cleavage of PAR-1. Consistent with this hypothesis, the Sk-plasmin complex is unable to activate a thrombin-sensitive PAR-1 receptor expressed in COS cells, which do not express the Fc receptor (data not shown). The increased surface expression of FcγRII and PAR-1 in partially activated platelets30 41 may explain why ADP sensitizes platelets to Sk.
In summary, activation of platelets by Sk requires both activation of the Fc receptor and cleavage of PAR-1. It is possible that interaction with the Fc receptor provides signals essential for the activation or helps localize the Sk-plasmin complex to the cell membrane where it cleaves PAR-1. This activation of PAR-1 is not susceptible to inhibition by heparin or direct thrombin inhibitors and may explain the limited activity of such treatments during thrombolytic therapy of myocardial infarction.
Acknowledgments
Integrelin was a gift of Dr David Philips, COR Therapeutics (San Francisco, CA). Recombinant plasminogen S741A was a gift of Dr Anthony Brown, British Biotech Pharmaceuticals (Oxford, England). Monoclonal antibody 2/389, directed against the PAR-1 cleavage site was a gift of Dr Stuart Stone (deceased), University of Cambridge. The thrombin inhibitor Ro46-6240 (N-[N4-[(s)-1-amidino-3-piperidinyl]-methyl]N-2(2-naphthalenesulfonyl)-l-asparaginyl]-N-cyclopropylglycine) was a gift of Dr Sebastian Roux, Hoffman-La Roche (Basel, Switzerland).
Supported by grants from the Irish Heart Foundation, the Higher Education Authority of Ireland, and Enterprise Ireland.
Reprints:Desmond J. Fitzgerald, Centre for Cardiovascular Science, Department of Clinical Pharmacology, The Royal College of Surgeons in Ireland, 123 St Stephen's Green, Dublin, 2, Ireland; e-mail: dfitzgerald@rcsi.ie.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.