The role of the cellular immune response in human T-cell leukemia virus type I (HTLV-I) infection is not fully understood. A persistently activated cytotoxic T lymphocyte (CTL) response to HTLV-I is found in the majority of infected individuals. However, it remains unclear whether this CTL response is protective or causes tissue damage. In addition, several observations paradoxically suggest that HTLV-I is transcriptionally silent in most infected cells and, therefore, not detectable by virus-specific CTLs. With the use of a new flow cytometric procedure, we show here that a high proportion of naturally infected CD4+ peripheral blood mononuclear cells (PBMC) (between 10% and 80%) are capable of expressing Tax, the immunodominant target antigen recognized by virus-specific CTLs. Furthermore, we provide direct evidence that autologous CD8+ T cells rapidly kill CD4+ cells naturally infected with HTLV-I and expressing Tax in vitro by a perforin-dependent mechanism. Consistent with these observations, we observed a significant negative correlation between the frequency of Tax11-19-specific CD8+ T cells and the percentage of CD4+ T cells in peripheral blood of patients infected with HTLV-I. Those results are in accordance with the view that virus-specific CTLs participate in a highly efficient immune surveillance mechanism that persistently destroys Tax-expressing HTLV-I-infected CD4+ T cells in vivo.
Human T-cell leukemia virus type I (HTLV-I), which belongs to the HTLV-BLV subfamily of retroviruses, infects an estimated 10 million people worldwide.1 Unlike human immunodeficiency virus, HTLV-I causes no disease in a majority of infected subjects (asymptomatic carriers). However, approximately 2%-3% develop an aggressive T-cell malignancy, adult T-cell leukemia/lymphoma, and another 2%-3% develop a disabling chronic inflammatory disease, involving the central nervous system (HTLV-I-associated myelopathy/tropical spastic paraparesis; HAM/TSP), the eyes, the lungs, or the skeletal muscles.2
HTLV-I shares with other retroviruses the three main genomic regions ofgag, pol, and env, but, unlike most leukemia viruses, it has an additional region called pX that codes for two transcriptional regulatory proteins, the Tax and Rex proteins.2 These proteins are the homologues of the Tat and Rev proteins of human immunodeficiency virus.3 The Rex protein stabilizes viral messenger RNAs (mRNAs) and regulates their splicing and transport. The Tax protein is of central importance in virus dynamics because, as well as transactivating viral transcription, it is thought to drive host-cell proliferation.2 Furthermore, Tax is the dominant target antigen recognized by HTLV-I-specific cytotoxic T lymphocytes (CTL) in most responding individuals.4-8 Thus, the Tax protein is at the center of both efficient HTLV-I replication and the host attack on the virus.
The risk of HAM/TSP disease is positively correlated with the magnitude of the proviral load in the blood.9 It is, therefore, important to identify the host factors that determine the magnitude of the proviral load in vivo. In this regard, the role of the immune response in HTLV-I infection is still not clear. A high frequency of circulating Tax-specific CTLs can be found in a majority of HTLV-I-infected individuals.4-8 However, controversy exists over whether this strong CTL response causes or prevents HAM/TSP.10,11 Our recent immunogenetic data favor the possibility that a strong HTLV-I-specific CTL response indeed reduces proviral load and protects against HAM/TSP.12 However, direct evidence that Tax-specific CTLs are able to eliminate HTLV-I-infected cells in vivo has not been obtained. In addition, if HTLV-I-specific CTLs play a role in the reduction of the proviral load, a large proportion of infected cells should be capable of expressing at least the Tax protein, the dominant target antigen recognized by CTLs. This interpretation seems to conflict with the observation that HTLV-I provirus is transcriptionally silent in a high proportion of T-cell clones derived from infected patients,13,14 which suggests that HTLV-I might be latent in most infected peripheral blood mononuclear cells (PBMCs) in vivo. Consistent with this observation, with the use of conventional techniques, Tax protein expression cannot be detected in freshly isolated PBMCs, and, indeed, the existence of a serum factor that represses HTLV-I transcription in vivo has been postulated.15,16 However, the low frequency of Tax expression in fresh PBMCs could be the consequence of an efficient immune surveillance mechanism mediated by the host cellular immune response.11 12 Therefore, to understand the role of HTLV-I-specific CTLs in vivo, it is necessary to determine the proportion of infected PBMCs that are capable of expressing at least the Tax protein and to characterize qualitatively and quantitatively their interactions with autologous HTLV-I-specific CD8+ T lymphocytes.
To date, HTLV-I-specific CTLs have been characterized, using as target cells either leukemic cells4 or transformed cell line treated with peptides or infected with recombinant viruses.6,7,17 In this study, we used a sensitive flow cytometric technique to study intracellular Tax protein expression in naturally infected PBMCs18 of patients with HAM and asymptomatic carriers of HTLV-I. Our observations suggest that Tax-specific CTLs play an important role in reducing the frequency of Tax-expressing CD4+ T lymphocytes in vivo.
Materials and methods
Patients and cells
MT-2 cells were cultured in RPMI 1640 medium (Gibco, Paisley, UK) supplemented with 10% fetal calf serum (FCS) (Sigma, Dorset, UK), 2 mmol/L glutamine (Gibco), 100 IU/mL penicillin (Gibco), and 100 μg/mL streptomycin (Gibco).
PBMCs were obtained from 8 patients with a clinical diagnosis of HAM/TSP, 5 asymptomatic carriers, and 1 normal individual. PBMCs were isolated on Histopaque®-1077 (Sigma) density gradient and washed three times with phosphate buffered saline (PBS). Anti-CD8 or CD4 paramagnetic beads (Miltenyi Biotec Ltd, Surrey, UK) were used according to the manufacturer's instructions to deplete or enrich the respective PBMC subpopulation. Cells were cultured in RPMI 1640 medium supplemented with 10% FCS, 2 mmol/L glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. Alternatively, whole blood was directly cultivated after being diluted in an equivalent volume of RPMI 1640 medium supplemented with 2 mmol/L glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin. PBMCs were then isolated from cultivated blood as described above. In some experiments, 20 nmol/L of Concanamycin A (CMA) (Sigma) was added to the culture medium.
Immunofluorescence
Concomitant detection of Tax, CD4, and CD45RO.
After being harvested, cells were fixed in PBS containing 2% paraformaldehyde (Sigma) for 20 minutes and resuspended in PBS at 4°C until use. Fixed cells were washed with PBS containing 7% of normal goat serum (Sigma) and incubated with PC5-labeled anti-CD4 and FITC-labeled anti-CD45RO monoclonal antibodies (MAb) (Beckman Coulter, Bedfordshire, UK) for 15 minutes at room temperature. The cells were then washed and permeabilized with PBS containing 0.1% Triton X-100 (Sigma) for 10 minutes at room temperature. Permeabilized cells were washed and resuspended in PBS/7% normal goat serum containing an anti-Tax MAb (Lt-4)19 or an isotype control MAb (IgG3) (Southern Biotechnology Associates, Birmingham, AL) for 20 minutes at room temperature. The cells were then washed twice and resuspended in PBS/7% normal goat serum containing FITC-labeled goat F(ab')2 anti-mouse IgG3serum (Southern Biotechnology Associates) for 20 minutes at room temperature. Finally, the cells were washed twice and analyzed by flow cytometry on a Coulter EPICS® XL (Beckman Coulter).
Concomitant detection of Tax and p24 (HTLV-I gag antigen).
The cells were processed as described above, but an anti-p24 MAb (MAB8817: IgG1) (Chemicon International, Temecula, CA) was used in addition to the Lt-4 MAb. The anti-p24 MAb was then detected with a RPE-labeled goat F(ab')2 anti-mouse IgG1 serum (Southern Biotechnology Associates).
Concomitant detection of Tax and cell mortality.
After being harvested, the cells were incubated for 10 minutes in the presence of 5 μg/mL of propidium iodide (Sigma), then washed twice with PBS, fixed in PBS containing 2% paraformaldehyde for 20 minutes, and resuspended in PBS at 4°C until use. Fixed cells were then washed with PBS/7% normal goat serum and processed as described above to detect the Tax protein.
Quantification of tax mRNA
RNA was isolated from PBMCs with the use of a High Pure RNA Isolation Kit (Boehringer Mannheim, Lewes, UK). Complementary DNA (cDNA) was then synthesized with the use of 1st Strand cDNA Synthesis Kit for RT-PCR AMV (Boehringer Mannheim). Polymerase chain reactions (PCRs) were carried out in 50 μL containing cDNA prepared from 10 ng of RNA with primers for HTLV-I Tax or human beta-actin. Tax primers were 5′-TCG CTG CCG ATC ACG ATG CGT TTC C-3′ and 5′-AAC ACG TAG ACT GGG TAT CC-3′. Human beta-actin primers were 5′-AAG AGA GGC ATC CTC ACC CT-3′ and 5′-TAC ATG GCT GGG GTG TTG AA-3′. Cycle conditions for amplification of the tax sequence were one cycle at 95°C for 5 minutes followed by 45 cycles at 95°C for 60 seconds, 58°C for 75 seconds, 72°C for 90 seconds, and one final cycle at 72°C for 10 minutes. Cycle conditions for amplification of the beta-actin sequence were one cycle at 95°C for 5 minutes followed by 30 cycles at 95°C for 60 seconds, 58°C for 75 seconds, 72°C for 90 seconds, and one final cycle at 72°C for 10 minutes. In preliminary experiments, the number of cycles was varied between 25 and 50 to determine the appropriate number of cycles for a semiquantitative PCR (data not shown).
Quantification of proviral load
To measure the proviral load, DNA was extracted from 2 × 106 PBMCs by the proteinase K method. Replicate serial dilutions of the DNA were amplified with the use of a nested PCR technique that reliably detected a single copy of HTLV-I Tax proviral DNA in DNA from 105 cells.20 The proviral DNA titer was calculated from the Poisson distribution of negative samples at the cut-off dilution. Interassay variability (0.3 log10) was determined by repeated testing of a random selection of patient samples.
Detection of Tax11-19-specific CTL and CD4+ cells
In the chronically activated CTL response to HTLV-I, several peptides derived from the immunodominant Tax protein are restricted byHLA*A02.17Tax11-19 is a dominant A*02-restricted epitope.4 Analysis of PBMCs for the presence of Tax11-19-specific CTLs was, therefore, performed by the use of fluorescent-labeled tetramers ofHLA-A*0201 + β2microglobulin + Tax11-19peptide.12 21 PBMCs were incubated with Tax11-19 tetramer at 37°C for 30 minutes and anti-CD4 antibody on ice for 30 minutes. The cells were then washed three times in ice-cold PBS, fixed in 1% paraformaldehyde for 30 minutes at 4°C, and analyzed by flow cytometry on a Coulter EPICS® XL (Beckman Coulter).
Results
Tax protein expression in PBMCs isolated from infected patients
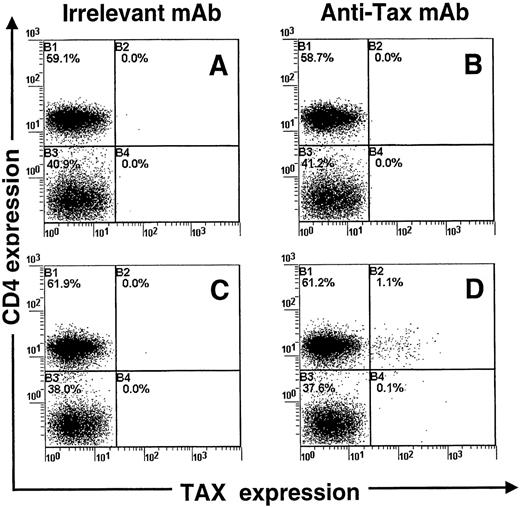
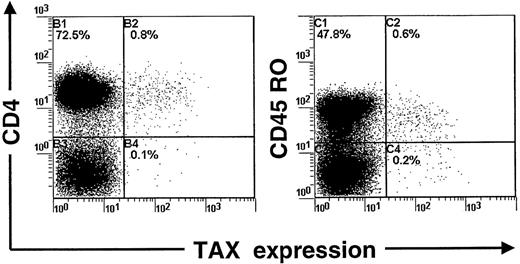
We devised a sensitive flow cytometric assay to detect intracellular Tax protein expression. Preliminary experiments were done with the use of the MT-2 cell line, which is chronically infected with HTLV-I. Those experiments indicated that 98% of MT-2 cells expressed high levels of the Tax protein (data not shown). We next checked whether this new procedure could detect the expression of Tax in PBMCs isolated from patients infected with HTLV-I. PBMCs were isolated from blood samples and harvested directly or after 24 hours in vitro culture (in the absence of interleukin-2 or mitogen). After being harvested, cell samples were fixed and processed to detect concomitantly Tax and CD4 expression by flow cytometry. Figure 1shows that a small fraction of CD4+ lymphocytes express detectable levels of the Tax protein after 24 hours of cultivation. In contrast, no expression of Tax was observed in freshly isolated PBMCs (Figure 1). The use of an isotype control MAb (Figure 1) confirmed the specificity of the detection. Moreover, uninfected PBMCs cultivated in similar conditions for 24 hours remained negative for Tax expression (data not shown). Three-color analysis indicated that Tax-expressing CD4+ lymphocytes were also positive for the CD45RO differentiation antigen (Figure 2). This result is in accordance with Richardson et al18 who observed a similar phenotype of HTLV-I-infected cells in vivo. In addition, we assayed PBMCs for the concomitant expression of Tax and p24 (HTLV-I Gag antigen) after in vitro cultivation for 24 hours. Because Tax is a powerful transactivator of viral transcription, Tax-positive cells should express other viral proteins, including p24. The analysis confirmed that a large proportion (51%) of Tax-positive PBMCs also expressed the p24 protein after culture for 24 hours. In contrast, only 1.9% of Tax-negative PBMCs were positive for the p24 protein.
Concomitant detection of Tax and CD4 antigens in peripheral blood mononuclear cells isolated from a patient infected with human T-cell leukemia virus type I.
Cells were cultivated for 24 hours (C, D) or analyzed fresh (A, B). The Tax protein was detected with the Lt-4 monoclonal antibody (B, D), and an irrelevant monoclonal antibody was used as a control isotype (A, C). One representative experiment of 3 is shown.
Concomitant detection of Tax and CD4 antigens in peripheral blood mononuclear cells isolated from a patient infected with human T-cell leukemia virus type I.
Cells were cultivated for 24 hours (C, D) or analyzed fresh (A, B). The Tax protein was detected with the Lt-4 monoclonal antibody (B, D), and an irrelevant monoclonal antibody was used as a control isotype (A, C). One representative experiment of 3 is shown.
Concomitant detection of Tax, CD4, and CD45 antigens in peripheral blood mononuclear cells isolated from a patient infected with human T-cell leukemia virus type I and cultivated for 24 hours.
One representative experiment of 3 is shown.
Concomitant detection of Tax, CD4, and CD45 antigens in peripheral blood mononuclear cells isolated from a patient infected with human T-cell leukemia virus type I and cultivated for 24 hours.
One representative experiment of 3 is shown.
Those results demonstrate that flow cytometry can be used to specifically detect intracellular Tax expression in PBMCs isolated from infected patients. With the use of this procedure, it is, therefore, feasible to quantify the proportion of naturally infected CD4+ lymphocytes that are capable of expressing the Tax protein and to determine whether this expression is under the control of Tax-specific CTLs or not.
Time course study of Tax expression in PBMCs isolated from infected patients
The time course of Tax expression was determined in PBMCs isolated from both asymptomatic carriers and patients with HAM/TSP. PBMCs were cultivated for various times, harvested, and then processed to detect concomitantly Tax and CD4 expression by flow cytometry. Figure3A and Table 1show the evolution of the percentage of Tax-positive PBMCs at 0, 6, 12, 24, and 48 hours. Tax expression reaches a maximum at 6-12 hours and then decreases by 50% during the next 12-36 hours. In contrast to Tax expression, the percentage of CD4+ lymphocytes remained constant throughout the time course (data not shown). This constant percentage indicates that the observed changes in Tax expression over the time could not be due to a dramatic change in the proportion of the different PBMC subpopulations. In each patient, the comparison of Tax positivity with the proviral load indicated that a large fraction (from 10% to 80%) of infected cells were able to express the Tax protein at a given time point (Table 1).
Time course study of Tax protein (A) or messenger RNA (B) expression in peripheral blood mononuclear cells (PBMCs) isolated from asymptomatic carriers of human T-cell leukemia virus type I (HTLV-I) (HAP, HAW), from patients with HTLV-I-associated myelopathy/tropical spastic paraparesis (TBA, TAN), or from an uninfected individual (UA).
PBMCs were cultivated for various times, harvested, and then processed for the detection of Tax antigen (A: HAP, TBA) or mRNA (B: HAW, TAN, UA) expression. As a control, beta-actin messenger RNA expression was also investigated. One representative experiment of 3 is shown.
Time course study of Tax protein (A) or messenger RNA (B) expression in peripheral blood mononuclear cells (PBMCs) isolated from asymptomatic carriers of human T-cell leukemia virus type I (HTLV-I) (HAP, HAW), from patients with HTLV-I-associated myelopathy/tropical spastic paraparesis (TBA, TAN), or from an uninfected individual (UA).
PBMCs were cultivated for various times, harvested, and then processed for the detection of Tax antigen (A: HAP, TBA) or mRNA (B: HAW, TAN, UA) expression. As a control, beta-actin messenger RNA expression was also investigated. One representative experiment of 3 is shown.
To confirm the increase followed by a decrease in Tax-positive cells, the expression of tax mRNA was investigated by the use of a semiquantitative reverse PCR. As a control, the expression of beta-actin mRNA was also investigated. For this experiment, PBMCs were cultivated for 0, 24, and 48 hours and then treated as described in “Materials and Methods” for PCR analysis. As shown in Figure 3B, constitutive expression of the tax mRNA was detected without cultivation of the PBMCs. The highest level of tax mRNA was observed at 24 hours after cultivation, and then it decreased at 48 hours. In contrast, the level of beta-actin mRNA remained constant throughout the time course.
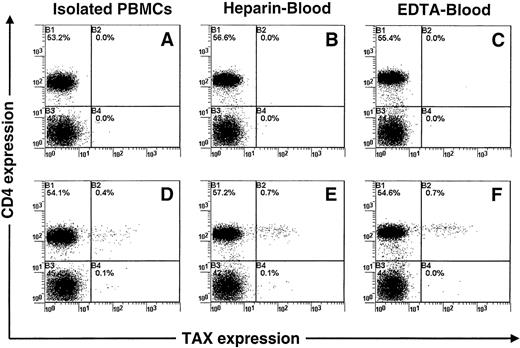
Flow cytometric and PCR analyses indicate that the level of Tax expression in cultivated PBMCs increases rapidly to reach a maximum during the first 12 hours and then decreases during the next 36 hours. The observed increase in Tax expression was not the consequence of a nonspecific mitogenic activation of infected cells by FCS. Indeed, a higher increase in Tax expression (Figure4) was observed in PBMCs cultivated in whole blood (autologous human serum) than in PBMCs cultivated in the presence of FCS. A similar increase in Tax expression was observed both in heparin- and EDTA-treated blood (Figure 4). This experiment also excluded the possibility that the observed increase in Tax expression is due to the removal of a serum factor that represses the expression of viral protein in vivo.15 16
Concomitant detection of Tax and CD4 antigens in peripheral blood mononuclear cells (PBMCs) isolated from a patient infected with human T-cell leukemia virus type I (TAR).
PBMCs were isolated from blood either before or after cultivation for 12 hours. Either heparin or EDTA was used as anticoagulant. The Tax protein was detected with the Lt-4 monoclonal antibody (D, E, F), and an irrelevant monoclonal antibody was used as a control isotype (A, B, C). One representative experiment of 3 is shown.
Concomitant detection of Tax and CD4 antigens in peripheral blood mononuclear cells (PBMCs) isolated from a patient infected with human T-cell leukemia virus type I (TAR).
PBMCs were isolated from blood either before or after cultivation for 12 hours. Either heparin or EDTA was used as anticoagulant. The Tax protein was detected with the Lt-4 monoclonal antibody (D, E, F), and an irrelevant monoclonal antibody was used as a control isotype (A, B, C). One representative experiment of 3 is shown.
Presence of CD8+ lymphocytes decreases the frequency of Tax expression
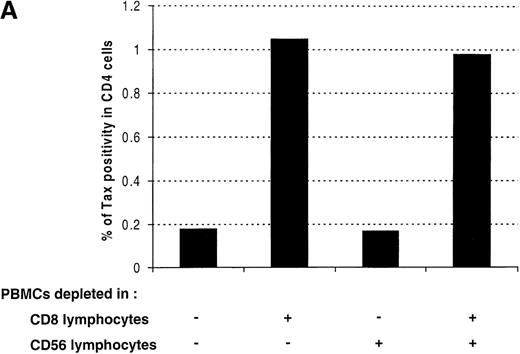
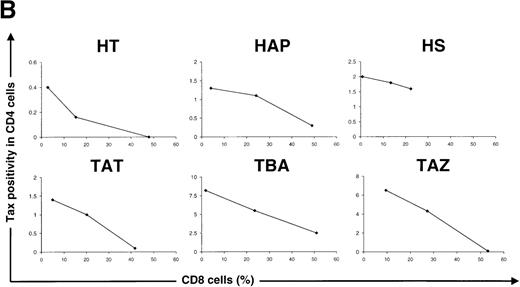
A freshly activated anti-Tax CTL activity has been identified in the blood of the majority of patients infected with HTLV-I.4-7The activity of these Tax-specific CTLs could be responsible for the decrease in the percentage of Tax-expressing cells observed between 12 and 48 hours of cultivation (Figure 3 and Table 1). To test this hypothesis, PBMCs were selectively depleted of CD8+ lymphocytes and cultivated for 24 hours. As a control, PBMCs were also depleted of CD56+ lymphocytes. Figure 5A shows that the depletion of CD8+ lymphocytes is associated with an increase in the percentage of Tax expression in CD4+ lymphocytes. In contrast, the depletion of CD56+ lymphocytes has no significant effect on the frequency of Tax expression. A similar effect of CD8+ lymphocyte depletion on Tax expression has been observed in three asymptomatic carriers of the virus and in 3 patients with HAM/TSP. Furthermore, within a single patient (HT) the results were quantitatively reproducible: the same results have been obtained in 3 independent experiments, using blood samples of the same patient taken at different times (data not shown). We also determined the effect of an artificial enrichment of autologous CD8+ lymphocytes. The results of these experiments are presented in Figure 5B and shows that, for each patient, Tax expression was reduced in a dose-dependent manner when the frequency of CD8+ lymphocytes was increased. These results demonstrate that the presence of CD8+ lymphocytes is associated with a reduction in the frequency of Tax expression in CD4+ lymphocytes.
Effect of CD8+ lymphocytes on the frequency of Tax expression in CD4+ lymphocytes.
(A) Peripheral blood mononuclear cells (PBMCs) were isolated from an asymptomatic carrier (HT) and cultivated for 24 hours either directly or after depletion of CD8+ or CD56+ lymphocytes, or both. The cells were then harvested and processed to perform the concomitant detection of Tax and CD4 antigens. One representative experiment of 3 is shown. (B) PBMCs were isolated from 3 asymptomatic carriers and from 3 patients with HTLV-I-associated myelopathy/tropical spastic paraparesis depleted of CD8+ cells and then cultivated for 24 hours after re-addition of a different number of autologous CD8+ cells. Tax positivity in CD4+ lymphocytes was then plotted as a function of the percentage of CD8+ lymphocytes in each culture. HTLV-I = human T-cell leukemia virus type I.
Effect of CD8+ lymphocytes on the frequency of Tax expression in CD4+ lymphocytes.
(A) Peripheral blood mononuclear cells (PBMCs) were isolated from an asymptomatic carrier (HT) and cultivated for 24 hours either directly or after depletion of CD8+ or CD56+ lymphocytes, or both. The cells were then harvested and processed to perform the concomitant detection of Tax and CD4 antigens. One representative experiment of 3 is shown. (B) PBMCs were isolated from 3 asymptomatic carriers and from 3 patients with HTLV-I-associated myelopathy/tropical spastic paraparesis depleted of CD8+ cells and then cultivated for 24 hours after re-addition of a different number of autologous CD8+ cells. Tax positivity in CD4+ lymphocytes was then plotted as a function of the percentage of CD8+ lymphocytes in each culture. HTLV-I = human T-cell leukemia virus type I.
Effect of CD8+ lymphocytes on the frequency of Tax expression in autologous or heterologous CD4+ lymphocytes
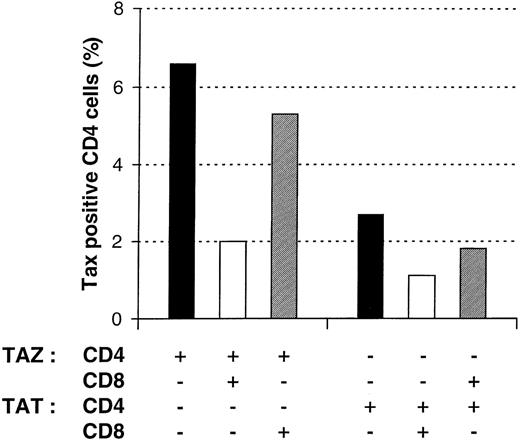
To characterize the mechanism by which CD8+ T lymphocytes reduce Tax expression in PBMCs, we next determined whether CD8+ lymphocytes were able to reduce Tax expression in heterologous CD4+ lymphocytes. For this purpose, CD4+ and CD8+ lymphocytes from 2 different patients (TAZ, TAT), respectively HLA-A*2402/2601, B*5101/52 011, Bw4, Cw*1202/1402 and HLA-A*0201/6801, B*1503/3501, Cw*0202/1601, were isolated. CD4+ lymphocytes from each patient were cultivated for 24 hours in the absence or in the presence of autologous or heterologous CD8+ lymphocytes. As shown in Figure6, the reduction of Tax expression in CD4+ lymphocytes only occurred significantly in the presence of autologous CD8+ lymphocytes. In contrast, heterologous CD8+ lymphocytes only slightly decreased the frequency of Tax expression. This observation is compatible with a dominant major histocompatibility complex class I restricted mechanism of cellular cytotoxicity.22
Effect of CD8+ lymphocytes on the frequency of Tax expression in autologous or heterologous CD4+ lymphocytes.
Various combinations of purified CD4+ and CD8+ lymphocytes of two patients with HTLV-I-associated myelopathy/tropical spastic paraparesis (TAZ and TAT) were cultivated for 24 hours and then processed for the concomitant detection of Tax and CD4 antigens. One representative experiment of 2 is shown. HTLV-I = human T-cell leukemia virus type I.
Effect of CD8+ lymphocytes on the frequency of Tax expression in autologous or heterologous CD4+ lymphocytes.
Various combinations of purified CD4+ and CD8+ lymphocytes of two patients with HTLV-I-associated myelopathy/tropical spastic paraparesis (TAZ and TAT) were cultivated for 24 hours and then processed for the concomitant detection of Tax and CD4 antigens. One representative experiment of 2 is shown. HTLV-I = human T-cell leukemia virus type I.
CD8+ lymphocytes kill Tax-expressing cells by a perforin-dependent mechanism
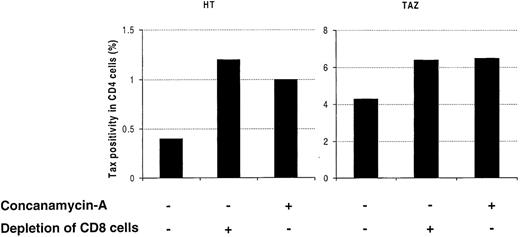
Cytotoxic CD8+ lymphocytes can mediate their antiviral effect through perforin-dependent lysis of infected cells.23 To further determine the mechanism by which CD8+ lymphocytes reduce Tax expression, PBMCs from 2 patients were cultivated for 24 hours with or without 20 nmol/L of CMA, an inhibitor of the perforin-dependent cytotoxic pathway.23 As shown in Figure7, the presence of CMA increased the frequency of Tax expression to that caused by the depletion of CD8+ lymphocytes. In contrast to Tax expression, the percentage of CD4+ lymphocytes remained constant in cultures treated in the presence or in the absence of CMA (data not shown).
Effect of Concanamycin A (CMA) on the frequency of Tax expression in CD4+ lymphocytes.
Peripheral blood mononuclear cells (PBMCs) were isolated from an asymptomatic carrier (HT) and from a patient with HTLV-I-associated myelopathy/tropical spastic paraparesis (TAZ), cultivated with or without CMA for 24 hours, and then processed for the concomitant detection of Tax and CD4 antigens. The effect of CMA was compared with the effect of CD8+ lymphocyte depletion. One representative experiment of 3 is shown. HTLV-I = human T-cell leukemia virus type I.
Effect of Concanamycin A (CMA) on the frequency of Tax expression in CD4+ lymphocytes.
Peripheral blood mononuclear cells (PBMCs) were isolated from an asymptomatic carrier (HT) and from a patient with HTLV-I-associated myelopathy/tropical spastic paraparesis (TAZ), cultivated with or without CMA for 24 hours, and then processed for the concomitant detection of Tax and CD4 antigens. The effect of CMA was compared with the effect of CD8+ lymphocyte depletion. One representative experiment of 3 is shown. HTLV-I = human T-cell leukemia virus type I.
The reduction of Tax expression by a perforin-dependent mechanism has two implications. First, the level of mortality in Tax-positive cells should be higher than that found in Tax-negative cells. Second, the presence of CMA should specifically reduce this higher level of mortality in Tax-positive cells. To test these predictions, PBMCs isolated from 2 infected patients were cultivated in the presence or in the absence of 20 nmol/L of CMA for 24 hours, harvested, and incubated for 10 minutes with propidium iodide, which labels dead cells. Samples were then fixed and processed to detect concomitantly Tax and propidium iodide by flow cytometry. Table 2 shows that, in the absence of CMA, the level of mortality in Tax-expressing cells was indeed more than tenfold higher than the Tax-negative population. As predicted, in the presence of CMA, the level of mortality dropped in Tax-expressing cells, whereas it slightly increased in the Tax-negative population.
To confirm that CD8+ lymphocytes were responsible for the increase in mortality in the Tax-positive population, CD4+ lymphocytes were purified and cultivated for 24 hours in the absence or in the presence of increasing numbers of CD8+ lymphocytes. Cells were then harvested, incubated for 10 minutes with propidium iodide, fixed, and processed to detect concomitantly Tax and propidium iodide by flow cytometry. The analysis showed that the level of mortality in Tax-expressing CD4+ cells increased from 7.8% in the absence of CD8+ cells to 10.3%, 17.3%, and 20.4% when 5%, 12.5%, and 25% of CD8+ cells were added to the culture, respectively. Those results provide strong evidence that CD8+ lymphocytes selectively kill Tax-expressing CD4+ lymphocytes in vitro.
Frequency of Tax11-19-specific CD8+ T cells is negatively correlated with the percentage of CD4+ T cells in peripheral blood
Persistent elimination of Tax-expressing CD4+ T cells by the abundant Tax-specific CTLs might have a significant impact on the CD4+ T cell population in vivo, especially in patients with HAM/TSP who have a high frequency of infected CD4+ T cells. To test this hypothesis, we determined the frequency of Tax11-19-specific CD8+ lymphocytes and the percentage of CD4+ cells in fresh PBMCs isolated from 19 patients with HAM/TSP who wereHLA-A*02 positive. Tax11-19-specific CD8+ lymphocytes were detected by using fluorescent-labeled tetramers ofHLA-A*0201 + β2microglobulin + Tax11-19peptide.12 21 The analysis showed a significant negative correlation (2-tailed P = .015; Spearman rank correlation) between the frequency of Tax11-19-specific CTLs and the percentage of CD4+ cells. This result is consistent with the view that Tax-specific CTLs participate in an immune surveillance mechanism that persistently destroys and removes Tax-expressing infected CD4+ T cells in vivo.
Discussion
The role of the cellular immune response in HTLV-I infection is not fully understood. A persistently activated CTL response to HTLV-I is found in the majority of infected individuals.4-8 However, several observations paradoxically suggest that HTLV-I is transcriptionally silent in most infected cells13 14 and, therefore, not detectable by virus-specific CTLs.
The characterization of the immune response against HTLV-I has been complicated by the difficulty of identifying cells that are naturally infected in vivo, the CD4+ lymphocytes.18 In this study, we report a new flow cytometric approach that allows the detection of the immunodominant T-cell antigen in HTLV-I infection, the Tax protein (Figure 1). The induction of viral protein expression in some PBMCs after long-term cultivation in vitro has been known for a long time.15,16 However, it has been difficult to demonstrate Tax protein expression in freshly isolated PBMCs, although tax mRNA has been detected in a proportion of individuals infected with HTLV-I.24-26 We report here that a large proportion of HTLV-I-infected PBMCs (10%-80%) isolated from infected patients become positive for Tax protein only after 6 hours of cultivation in vitro (Figure 3 and Table 1). Most naturally infected cells in vivo are, therefore, capable of expressing the Tax protein and are consequently detectable by Tax-specific CTLs. In addition, the use of flow cytometry allowed the concomitant detection of Tax and cell surface markers. The majority of cells expressing Tax were characterized as CD4+ CD45RO+ lymphocytes (Figure 2), in accordance with Richardson et al18 who observed a similar phenotype in HTLV-I-infected cells in vivo.
This technique also allowed us to quantify the possible interactions between Tax-specific CD8+ CTLs and their target cells. With the use of PBMCs isolated from both asymptomatic carriers and patients with HAM/TSP, we observed a negative correlation between the frequency of CD8+ T lymphocytes and the frequency of Tax expression (Figure 5). Several observations indicate that this effect is the consequence of a selective killing of Tax-expressing cells by CD8+ T lymphocytes. First, CMA, an inhibitor of perforin-dependent cytotoxicity,23allowed the frequency of Tax expression to increase to a similar extent as CD8+ lymphocyte depletion (Figure 7). Second, CMA reduced the level of mortality observed in Tax-positive cells but had the opposite effect in Tax-negative cells (Table 2). Third, the mortality rate in Tax-positive cells increased in a dose-dependent manner with the frequency of CD8+ T lymphocytes in culture. Finally, CD8+ T lymphocytes were capable of reducing Tax expression in autologous but not heterologous CD4+ T cells, consistent with a major histocompatibility complex-restricted mechanism of cytotoxicity (Figure 6). These results provide evidence that CD8+ lymphocytes rapidly kill autologous Tax-expressing CD4 T cells by a perforin-dependent mechanism.
The results presented in this study raise the possibility that HTLV-I-specific CTLs participate in an immune surveillance mechanism that destroys and removes Tax-expressing CD4+ lymphocytes in vivo. If this mechanism occurs, the persistent elimination of Tax-expressing CD4+ T cells by the abundant Tax-specific CTLs might have a significant impact on the CD4+ T cell population in vivo. Indeed, we observed, in 19 patients infected with HTLV-I, a significant negative correlation between the percentages of CD4+ lymphocytes and Tax11-19-specific CTLs in vivo. Moreover, Jeffery et al11 12 have recently shown that the common major histocompatibility complex class I type, HLA-A*02, is associated with both protection from HAM/TSP disease and a significant reduction in provirus load in asymptomatic carriers of HTLV-I. This observation is also consistent with the view that HTLV-I-specific CTLs eliminate HTLV-I-infected cells in vivo. Furthermore, the existence of this immune surveillance mechanism might explain why a short in vitro incubation (6 hours) of PBMCs is associated with a significant increase in the percentage of Tax-positive cells (Figure 3 and Table 1). The observed increase in Tax expression was neither due to the removal of a serum factor repressing the expression of viral protein nor to a mitogenic activation by FCS (Figure 4). On the contrary, Tax expression was even higher when CD8+ lymphocytes were depleted and was reduced in a dose-dependent manner when the frequency of CD8+ lymphocytes was increased (Figure 5). We suggest, therefore, that the observed increase in Tax expression during short-term culture results from the longer average lifespan of infected CD4+ T cells in vitro. In vivo, the cells are efficiently mixed, and an infected CD4+ cell is likely to be killed shortly after it starts to express Tax. In vitro, the cells are relatively sedentary, so an infected CD4+ T cell can express Tax on average longer before it is destroyed by CTLs. Tax-specific CTLs could, therefore, play an important role in reducing the frequency of Tax-expressing CD4+ T cells in vivo. This effect could be responsible for the low frequency of Tax expression in fresh PBMCs. However, we cannot exclude the possibility that another mechanism suppresses HTLV-I gene expression in peripheral blood.
It is still not known whether HTLV-I-specific CTLs are only protective or also cause bystander tissue damage. However, a dual role of virus-specific CTLs has been demonstrated in lymphocytic choriomeningitis virus27 and influenza28 virus infections. Influenza-specific CTLs have been shown to confer protection against low-dose viral challenge but exacerbate viral pathology and cause mortality at high viral dose.28 In HTLV-I infection, a high proviral load might similarly increase the probability that virus-specific CTLs cause bystander tissue damage in the central nervous system. Consistent with this suggestion, an accumulation of both Tax-expressing CD4+ and CD8+ lymphocytes is found in active central nervous system lesions of patients with HAM/TSP.29-31 In addition, Kubota et al32 demonstrated that a high frequency of PBMCs isolated from patients with HAM/TSP and cultivated in vitro produce large quantities of pro-inflammatory cytokines, including IFN-γ and TNF-α, which are neurotoxic. Therefore, in addition to their ability to eliminate Tax-expressing cells, HTLV-I-specific CTLs might also produce pro-inflammatory cytokines in situ and consequently contribute to tissue damage of the central nervous system. Further experiments must be done to characterize more precisely the role of both HTLV-I-specific CTLs and infected cells in HAM/TSP pathogenesis.
In conclusion, the results presented in this study provide evidence that virus-specific CTLs may participate in an immune surveillance mechanism in vivo that persistently destroys and removes HTLV-I-infected cells in peripheral blood. The efficiency of this immune surveillance mechanism may be of prime importance in the reduction of HTLV-I replication in vivo.
Acknowledgments
We thank the staff members and blood donors of the Kagoshima Red Cross Blood Center and of St. Mary's Hospital. The authors would like to thank Rebecca Asquith for helpful comments on the manuscript. E. Hanon is a senior research assistant of the Fonds National Belge de la Recherche Scientifique (F.N.R.S.).
Supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research (OPSR) (Japan), the Wellcome Trust (UK), the Royal Society (UK), and the Fords National Belge de la Recherche Scientifique (Belgium).
Reprints:Charles Bangham, Department of Immunology, Imperial College School of Medicine, St Mary's Campus, Norfolk Place, W21PG, London, United Kingdom; e-mail: c.bangham@ic.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.