Chronic B-cell stimulation may be a predisposing event in the early pathogenesis of the acquired immunodeficiency syndrome (AIDS)-related lymphoma (ARL). ARL-derived immunoglobulin (Ig) genes are significantly diversified from germline, suggesting that antigenic stimulation via Ig receptors may occur prior to malignant transformation. We have evaluated 6 ARL-derived antibodies for binding to human immunodeficiency virus (HIV) and cell surface epitopes. Five cases expressed IgM, and 1 case expressed IgG. Expressed V genes were significantly diversified (3%-15%) from known germline V genes. A non-Ig producing mouse myeloma cell line was transfected with expression vectors containing the lymphoma-derived V genes. By enzyme-linked immunosorbent assay and Western blot assay, the lymphoma-derived Ig's showed no reactivity against HIV recombinant proteins. Also, no specific HIV reactivity was observed by flow cytometry with lymphoma-derived Ig's against the T-cell line infected with T-tropic HIV-1 or peripheral blood mononuclear cells infected with M-tropic HIV strains, indicating lack of binding to native HIV epitopes. However, 2 of the lymphoma-derived Ig's (ARL-7 and ARL-14) bound strongly to non–HIV-infected cells of various tissue origins. Thus, these findings suggest that the transformed B cells of AIDS-associated lymphomas may not arise from the pool of anti-HIV specific B cells but, rather, may develop from B cells responding to other antigens, including self-antigens.
Considerable evidence from clinical and experimental studies supports the notion of chronic stimulation of B cells in human immunodeficiency virus (HIV) infection.1-4 For example, peripheral blood B cells from HIV-infected individuals display markedly increased spontaneous proliferation and immunoglobulin secretion, associated with monoclonal and oligoclonal paraproteins.3,4The mechanism(s) responsible for this B-cell hyperactivity is not known. Possibly, alterations in cytokine profiles as a consequence of decreased CD4-positive T cells could lead to a shift in the balance of negative and positive modulators of B-cell activation. For example, the induction of cytokines, such as interleukin-6, tumor necrosis factor-α, and others, within the course of HIV infection may support the cellular dysregulation and the development of neoplastic disease.5,6 However, B-cell hyperactivity is observed early in infection, and polyclonal activation may be inversely correlated with decreased numbers of CD4-positive cells.7 8
Another possibility for the mechanism involved in maintaining the chronic B-cell hyperactivity is that the virus is a direct stimulator of B-cell proliferation. In this regard, evidence exists that HIV itself induces B-cell activation and proliferation.1,4,9,10For conventional antigen recognition and activation, B cells recognize and bind antigen via surface immunoglobulin generated by variable region gene utilization of the heavy (VH) and light (Vκ/λ) genes. By this mechanism, HIV might initiate a proliferative response by B cells with specificity for viral envelope proteins. However, several lines of evidence argue against this as the only mechanism by which HIV stimulates B-cell proliferation. First, while a significant proportion of immunoglobulin-producing cells from patients with acquired immunodeficiency syndrome (AIDS) secrete antibodies with specificity for HIV viral proteins, many do not.11Secondly, serum immunoglobulins from HIV-infected individuals exhibit specificities for both HIV-associated proteins and nonviral determinants, including autoantigens.7,8,10-24 In fact, as many as 20% to 50% of the immunoglobulins spontaneously produced in vitro by peripheral blood lymphocytes may be specific for HIV epitopes.2 25
To test the hypothesis that HIV-specific triggering of B cells is involved in lymphomagenesis, we cloned the AIDS-related lymphoma (ARL)–derived immunoglobulin genes into a plasmid construct that allowed us to express these antibodies in a nonimmunoglobulin-secreting mouse myeloma cell line. The secreted antibodies did not bind to HIV-related epitopes by use of various experimental approaches, including enzyme-linked immunosorbent assays (ELISA) employing recombinant HIV proteins, Western blotting of HIV-infected cell lysates, or flow cytometry of HIV-infected T cells. Interestingly, 2 of 6 ARL-derived immunoglobulins bound strongly to hematopoietic and nonhematopoietic cell-associated epitopes (for example, HIV-unrelated), which have not been characterized further. Although a lymphoma-derived B-cell line with anti-gp160 reactivity has been reported previously,26 the results of our current study suggest that HIV-specific B-cell activation may not play a significant role in lymphoma development. On the other hand, self-reactivity along with other factors, such as cytokine imbalances,5,6 concomitant Epstein-Barr virus (EBV) or Kaposi's sarcoma–associated herpesvirus infection,27 28 deregulated oncogene expression, and B cell-endothelial cell interactions, may contribute to lymphoma development in HIV-infected individuals.
Materials and methods
Patient samples
Immunoglobulin-producing transfectomas were established from 6 previously characterized EBV-negative ARLs.29 The histology of 4 cases was of the small noncleaved cell type, and 2 cases were of the diffuse large-cell type. Table 1 shows the VH and VL usage of these B-cell tumors and their corresponding homology to germline sequences.
Cells and antibodies for FACS analysis
Sup-T1, a non-Hodgkin's T-cell lymphoma cell line30 and A1953 (Sup-T1 cells infected with HIV-1 III-B) were kindly provided by Dr J. Hoxie. Other human cell lines used include REH, a pro-B cell line (ATCC CRL 8286); K-562, an erythroleukemia cell line (ATCC CCL 246); HeLa, an epithelioid cell line (ATCC CCL 2); U-87 MG, a glial cell line (ATCC HTB 14); PC-3, a prostate adenocarcinoma cell line (ATCC CRL 1435); and EA.hy.926, an endothelial cell line, kindly provided by Dr Cora-Jean.31Thymocytes from fresh thymus and peripheral blood mononuclear cells (PBMCs) from normal donors were separated by the standard Ficoll-Hypaque gradient technique. Purified T-cell populations were separated from PBMC by the rosetting method. Lysis of sheep erythrocytes was performed by incubation with Tris-ammonium-chloride, pH 7.65, at 37°C for 5 minutes. In some assays, freshly isolated PBMCs were resuspended at a concentration of 1 × 106 to 2 × 106 cells/mL in RPMI 1640 media containing 10% fetal bovine serum (FBS), 1% Q (glutamine), 1% penicillin/streptomycin, and 5 μg/mL of phytohemagglutinin (PHA). After 48 hours of incubation at 37°C with 5% carbon dioxide, cells were washed to eliminate PHA and resuspended at a concentration between 0.2 × 106 to 0.4 × 106 cells/mL in media containing 5 U/mL of interleukin-2, just before being infected with HIV-1 Ba-L32or with HIV-1 SF162.33 Supernatants from HIV-1 infected PBMC cultures were tested for the presence of p24 using the HIV-1 p24 ELISA kit (NEN, Life Science Products Inc, Boston, MA). Infected PBMCs were used to perform fluorescence-activated cell sorter (FACS) analysis after 8 days of being infected with HIV-1 Ba-L or HIV-1 SF162, when supernatants from these cultures reached a p24 level of over 100 pg/mL and 200 pg/mL, respectively. Samples with uninfected PHA-stimulated PBMC were also run as controls. Monoclonal mouse antibodies used for flow cytometry included anti-CD3-FITC, anti-CD3-PE, anti-CD19-FITC, anti-CD14-PE/CD45-FITC (Becton Dickinson Immunocytometry Systems, San Jose, CA), and anti-HLA-ABC (Immunotech, Marseille, France). The human isotype (IgM-κ) control antibody was either 5-1G2 or 2D3, both monoclonal anti-cardiolipin antibodies developed in our laboratory, or was the antibody produced by our transfectoma control, transformed with the expression vectors carrying its original V genes encoding for Fc (rheumatoid factor) specificity (Table 2). The monoclonal antibody (mAb) 71-31, a human IgG—λ reactive against p24 and other HIV-1 III-B core proteins,34 or the rabbit polyclonal anti-p24 antibody from the HIV-1 p24 ELISA kit (NEN, Life Science Products) were used to differentiate infected versus noninfected permeabilized T cells. A rabbit polyclonal anti-mouse IgG3 (Zymed, San Francisco, CA) was used as the isotype control for the rabbit anti-p24. The human mAb 6-F (IgM—κ), also developed in our laboratory, was used as a positive control for binding to cytoplasmic determinants. Goat anti-mouse IgG-FITC, goat F(ab′)2 fragments anti-human IgG/IgM (heavy and light [H&L]) biotin-labeled (Jackson ImmunoResearch Laboratories Inc, West Grove, PA), and goat F(ab′)2 fragments anti-rabbit IgG-FITC (Sigma, St. Louis, MO) were used as secondary antibodies. Streptavidin-FITC (Zymed) was used for samples stained with biotin-conjugated antibodies.
Polymerase chain reaction
Monoclonal polymerase chain reaction (PCR) containing lymphoma-derived VDJ sequences were generated and cloned into the pBluscript II KS (+) phagemid (Stratagene, La Jolla, CA) as previously reported.29 Because lymphoma tissue often contains normal lymphocytes in addition to tumor tissue, we obtained, in some of the cases, PCR products with more than 1 primer combination. However, when the amplified products were size-separated on a 10% polyacrylamide gel, the intense and sharp tumor-related band could easily be distinguished from the diffuse bands of different sizes corresponding to the polyclonal lymphocytes in the lymph node specimen. All monoclonal PCR products and some of the PCR products showing diffuse bands were reamplified with the nested JHbio primer and directly sequenced. Although the sequence analysis of the monoclonal bands represented a single nucleotide sequence, the diffuse polyclonal bands represented, as expected, multiple sequences within the third complementary determining region (CDR3).29Restriction sites for ClaI, SalI and NotI were introduced into primer sequences to enable subsequent cloning of PCR products.
Cloning of PCR products
PCR-amplified rearranged IgH and IgL genes were gel-purified using the Micropure DNA purification kit (Amicon, Beverly, MA). IgH amplification products were digested with 10 U ClaI and 10 U SalI (Promega, Madison, WI) and ligated with T4 ligase (Gibco BRL, Gaithersburg, MD) into the pRTM expression vector, kindly provided by Dr Kipps.35 IgL amplification products were digested with 10 U NotI (New England Biolabs Inc, Beverly, MA) and 10 U ClaI, and were ligated into the pSV2 vector.35 IgH-pRTM ligation products were introduced into XL-1 Blue Escherichia coli strain (Stratagene) and IgL-pSV2 ligation products into the JM110 strain (New England Biolabs) by electroporation (Gene Pulser, Bio-Rad Laboratories, Hercules, CA). Settings of 200 Ω, 25 microfarad (μF), and 1.8 kV were applied, and 0.2-cm cuvettes (Bio-Rad) were used. Plasmid DNA was extracted from single colonies using the QIAGEN-tip 100 kit (QIAGEN, Chatsworth, CA) as recommended by the manufacturers. All vectors were sequenced to confirm that the desired inserts were cloned. Nucleotide sequencing was performed by an automatic sequencer (Perkin Elmer, Norfolk, CT).
Transfection of eukaryotic cell line
A total of 10 μg of pRTM vector, containing the IgH insert, and 10 μg of pSV2 vector, containing IgL insert, were linearized with 20 U of PvuI (Pharmacia Biotech, Piscataway, NJ). Transfection of these vectors into the P3-X63-Ag8.653, a nonsecreting mouse myeloma cell line,36 was performed by electroporation (960 μF and 0.4 kV) using 0.4-cm cuvettes (Bio-Rad). Transfected cells were incubated in RPMI 1640 containing 20% FBS, 2% Q, and 1% penicillin/streptomycin. After 48 to 72 hours, neomycin (G418; Gibco BRL) was added to a final concentration of 1 mg/mL, and cells were transferred into 96-well tissue culture plates. Cells growing under neomycin selection were checked for immunoglobulin production by ELISA, and the best secretors were further expanded. Table3 shows characteristics of the obtained transfectomas.
Purification of ARL antibodies
ARL-derived antibodies contained in the culture supernatant from each transfectoma were purified using goat anti-human IgM agarose columns (Sigma), eluted with 0.1 mol/L glycine/HCl containing 0.02% NaN3, pH 2.5, and immediately neutralized to pH 7 with 0.5 mol/L phosphate buffer, pH 8. Eluates obtained were further dialyzed with nitrocellulose membranes (Collodion Membranes, Schleicher & Schuell Inc, Keene, NH). The purity of the concentrated antibodies was checked on 12% acrylamide gels under denaturing conditions. Proteins were transferred to 0.2-μm PDVF membranes (Bio-Rad). The Western blots containing the heavy and light chains were incubated with goat F(ab′) fragments anti-human IgM/IgG (H&L) biotin-labeled, followed by streptavidin/horseradish peroxidase, and developed by the ECL-Western blotting detection kit (Amersham International, Buckinghamshire, England). The size of the bands was compared to standard molecular weight markers to verify that the transfectomas secreted both heavy and light chains of the appropriate molecular weight.
ELISA for detecting immunoglobulin production by the transfectomas
MaxiSorp plates (Nunc Inc, Naperville, IL) were coated with 100 μL/well of goat anti-human IgM (Sigma) (2 μg/mL in phosphate-buffered saline (PBS)). After 3 washes with PBS containing 0.1% Tween 20, pH 7.3, wells were blocked with PBS containing 0.5% dry milk and 0.01% thimerosal, pH 7.0, for 1 hour at 37°C. Supernatants (100 μL) were added to the wells and incubated for 1 hour at 37°C. After 3 washes, 100 μL/well of goat anti-human κ chain labeled with alkaline phosphatase (Sigma) was added and incubated for 1 hour at 37°C. Plates were washed, developed with P-Nitrophenyl Phosphate (Sigma), and read (OD 410 nm) by an ELISA microplate reader (Dynatech Laboratories Inc, Chantilly, VA). The use of the “sandwich” anti-μ/ARL-Ab/anti-κ allowed us to discard clones transfected with only 1 of the 2 desired V genes.
Anti-HIV reactivity
ELISA kit for determining anti-HIV reactivity (Abbott HIV AB HIV-1/HIV-2 3.0) was used as recommended by the manufacturers (Abbott Laboratories, Santa Clara, CA). For the Western blot assays, we used the Novapath HIV-1 Immunoblot Kit (Bio-Rad). The Novapath kit is an in vitro qualitative assay for the detection of antibodies to individual proteins of HIV-1. Specificities of these antibodies can be inferred from the position of bands on the nitrocellulose strips. The kit tested for the presence of the immunoglobulins specific for the following HIV proteins or glycoproteins: gag (p18, p24, p55), pol (p51, p65, p32), env (gp120, gp160, gp 41-43). Because the kit is designed to detect only IgG isotype (the secondary antibody provided is a goat anti-human IgG-AP), we substituted the provided secondary antibody for a goat anti-κ—AP antibody (Sigma) that would also detect IgM antibodies.
Flow cytometric analysis
Aliquots of 5 × 105 to 1 × 106 cells were incubated with ARL-derived antibodies and diluted in PBS containing 2% FBS, at 4°C, for 30 minutes. The cells were washed and incubated with goat F(ab′) fragments anti-human IgG/IgM (H&L) biotin-labeled, for 30 minutes, and washed before streptavidin-FITC was added. After the final washing, cells were resuspended in PBS containing 2% FCS and 0.9% paraformaldehyde prior to analysis. To differentiate HIV-infected versus noninfected T-cell lines, aliquots of cells were permeabilized using a previously described technique.37 The human mAb 71-31 was used as a positive control for HIV-infected T cells.
Autoantibody assays
Anti-cardiolipin antibody assay.
The ELISA procedure was performed as described previously.38 Cardiolipin (Avanti Polar Lipids Inc, Alabaster, AL) was diluted 1:100 in absolute ethanol. A MaxiSorp plate was coated with the antigen (50 μL/well) and left uncovered at 4°C overnight. The next day, after 3 washes with PBS containing 0.1 g/L CaCl2, plates were blocked with PBS containing 0.1 g/L CaCl2, 3% BSA, and 10% FBS, pH 7.2 (blocking buffer), for 1 hour at 37°C. After washing, ARL-derived antibodies and controls were added to the wells, diluted in PBS containing 0.1 g/L CaCl2 and 10% FBS, pH 7.2 (diluting buffer), and incubated for 1 hour at 37°C. Wells were washed before adding goat anti-human heavy- and light-chain alkaline phosphatase (Southern Biothechnology Associates Inc, Birmingham, AL) (1:500 dilutions) for 1 hour at 37°C. After a final washing, samples were developed and read (O.D. 410 nm) as described above.
Anti-ssDNA ELISA.
DNA (Spermidine trihydrochloride) (Sigma) stock solution was prepared as previously described.39 Immulon 2 plates (Dynatech) were coated with 2.5 μg/50 μL per well of Poly-L-Lysine (Sigma), diluted in ddH2O, and left at room temperature for 30 minutes. Plates were washed 3 times with PBS and coated with 50 μL of single-stranded (ss)DNA, 2.5 μg/mL in autoclaved triethanolamine-buffered saline (TBS), pH 7.4, and left at room temperature for 2 hours. DNA was denatured (95°C for 5 minutes) and chilled rapidly before use. After 3 more washes, wells were blocked with Poly-L-Glutamine (Sigma) and diluted in ddH2O (2.5 μg/50 μL) for 2 hours at room temperature. Two more washes were performed before coating the plate with TBS (50 μL/well) for storage overnight at 4°C. The following day, plates were washed 3 times with PBS containing 0.1% Tween 20, pH 7.3 (washing solution), and blocked with PBS containing 0.5% dry milk and 0.01% thimerosal, pH 7.3 (blocking buffer), for 1 hour at 37°C. ARL-derived antibodies and controls were incubated for 1 hour at 37°C. After 3 washes, the secondary antibody (the same used for anticardiolipin antibodies [ACLA]-ELISA) was incubated for 1 hour at 37°C. Plates were developed and read (O.D. 410 nm) as described above.
Anti-i/I and Pr2 blood cell binding assay.
Results
Lack of binding to HIV-1 proteins by ARL-derived immunoglobulin
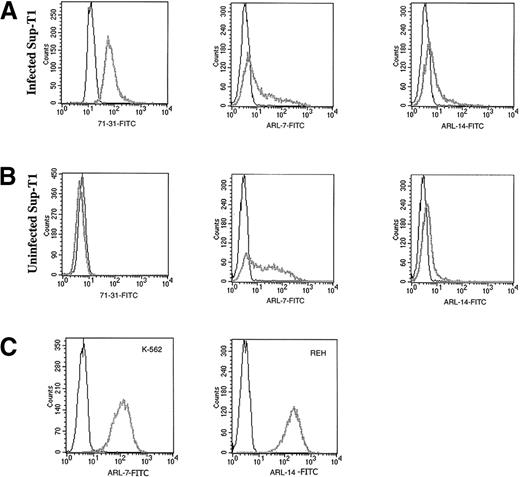
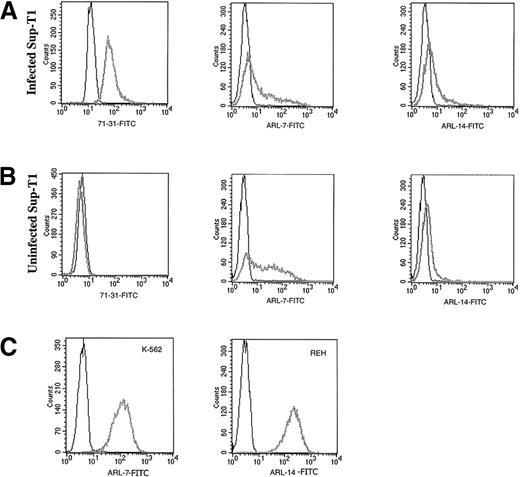
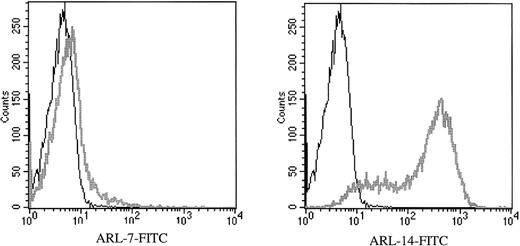
Transfectomas expressing the VH and VL genes of 6 EBV-negative ARL were evaluated for binding to HIV-related epitopes. No binding was detected to recombinant HIV proteins as measured by ELISA (Table 2) or to immunoblotted HIV peptides (data not shown). To evaluate the binding of ARL-derived immunoglobulins to native HIV proteins, we performed flow cytometric assays using a T-cell line infected with HIV-1 III-B or PBMC infected with HIV-1 Ba-L or HIV-1 SF162. To confirm productive HIV infection of cells used for FACS analysis, we used permeabilized cells because the 71-31 mAb with anti-p24 specificity or the rabbit polyclonal anti-p24 antibody only recognize cytoplasmic HIV epitopes. No specific HIV reactivity was observed by flow cytometry with lymphoma-derived immunoglobulins against the T-cell line infected with T-tropic HIV-1 or peripheral blood mononuclear cells infected with M-tropic HIV strains (data not shown). However, 2 of the 6 ARL-derived immunoglobulins (ARL-7 and ARL-14) bound to both infected as well as uninfected nonpermeabilized T cell lines (Figure 1). The binding pattern of ARL-7 and ARL-14 to nonpermeabilized cells indicated that these antibodies bound to surface, non–HIV-related epitopes.
The binding of ARL antibodies to HIV-infected and uninfected lymphocytes.
Human antibody 71-31 (IgG-λ) was used as a positive control to identify HIV-infected cells. F(ab′) fragment specific for human IgG/IgM (H&L) biotin-labeled was used as the secondary reagent, and streptavidin-FITC was used as the tertiary reagent. X axis represents the intensity of fluorescence and Y axis the number of events. Panel A shows binding of 71-31, ARL-7, and ARL-14 antibodies to the cytoplasmic (71-31) and surface (ARL-7 and ARL-14) antigens of infected cells (thick gray line) in comparison to isotype control (thin line). Panel B shows binding of the same antibodies to uninfected cells. Panel C shows binding of ARL-7 and ARL-14 antibodies to K-562, an erytholeukemia cell line, and REH, a pro-B cell line, respectively (thick gray line), in comparison to isotype controls (thin line).
The binding of ARL antibodies to HIV-infected and uninfected lymphocytes.
Human antibody 71-31 (IgG-λ) was used as a positive control to identify HIV-infected cells. F(ab′) fragment specific for human IgG/IgM (H&L) biotin-labeled was used as the secondary reagent, and streptavidin-FITC was used as the tertiary reagent. X axis represents the intensity of fluorescence and Y axis the number of events. Panel A shows binding of 71-31, ARL-7, and ARL-14 antibodies to the cytoplasmic (71-31) and surface (ARL-7 and ARL-14) antigens of infected cells (thick gray line) in comparison to isotype control (thin line). Panel B shows binding of the same antibodies to uninfected cells. Panel C shows binding of ARL-7 and ARL-14 antibodies to K-562, an erytholeukemia cell line, and REH, a pro-B cell line, respectively (thick gray line), in comparison to isotype controls (thin line).
Binding of ARL-7 and ARL-14 to purified T lymphocytes from PBMC
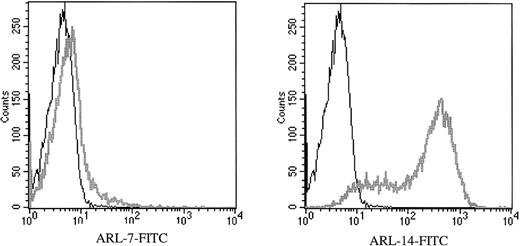
To determine binding of ARL-derived immunoglobulin to primary human cells, we performed flow cytometry using purified peripheral T lymphocytes, the purity being more than 95% CD3-positive T cells. We found that ARL-7 bound weakly to peripheral T cells, with 5% of cells binding more strongly to ARL-7 than the isotype control. In contrast, ARL-14 bound to most peripheral T cells, with 86% of cells demonstrating reactivity compared with the isotype control (Figure 2). ARL-7 also bound to 62% of thymocytes isolated from fresh thymus (data not shown).
ARL-7 and ARL-14 binding to the surface of peripheral blood T lymphocytes.
Peripheral blood T lymphocytes were isolated as described in “Materials and Methods.” F(ab′) fragment specific for human IgG/IgM (H&L) biotin-labeled was used as the secondary reagent, and streptavidin-FITC was used as the tertiary reagent. The histograms show CD3-gated cells. X axis represents the intensity of fluorescence and Y axis the number of events.
ARL-7 and ARL-14 binding to the surface of peripheral blood T lymphocytes.
Peripheral blood T lymphocytes were isolated as described in “Materials and Methods.” F(ab′) fragment specific for human IgG/IgM (H&L) biotin-labeled was used as the secondary reagent, and streptavidin-FITC was used as the tertiary reagent. The histograms show CD3-gated cells. X axis represents the intensity of fluorescence and Y axis the number of events.
Binding of ARL-7 and ARL-14 to different cell lines
Next, we wished to evaluate whether the cell-surface binding of ARL-7 and ARL-14 was restricted to T cells. Thus, we performed flow cytometric assays with both antibodies using a panel of hematopoietic and nonhematopoietic cells. The binding of these antibodies and their isotype control (expressed as mean channel fluorescence intensity) to the different human cells tested is shown in Table4. We observed that ARL-7 bound to a broad spectrum of cell types, while ARL-14 bound to a smaller number of cell types. ARL-7 bound most strongly to the K-562 erythroleukemic cell line (mean channel fluorescence 218 ± 4.2), whereas ARL-14 bound most strongly to the REH pro-B cell line (mean channel fluorescence 221 ± 4). Some cell lines were analyzed only once and, thus, no SD is noted. Interestingly, for peripheral blood T lymphocytes we tested multiple donors, and a wide range of binding was observed, suggesting inter-donor variability.
Correlating surface binding of ARL-7 with cell cycle
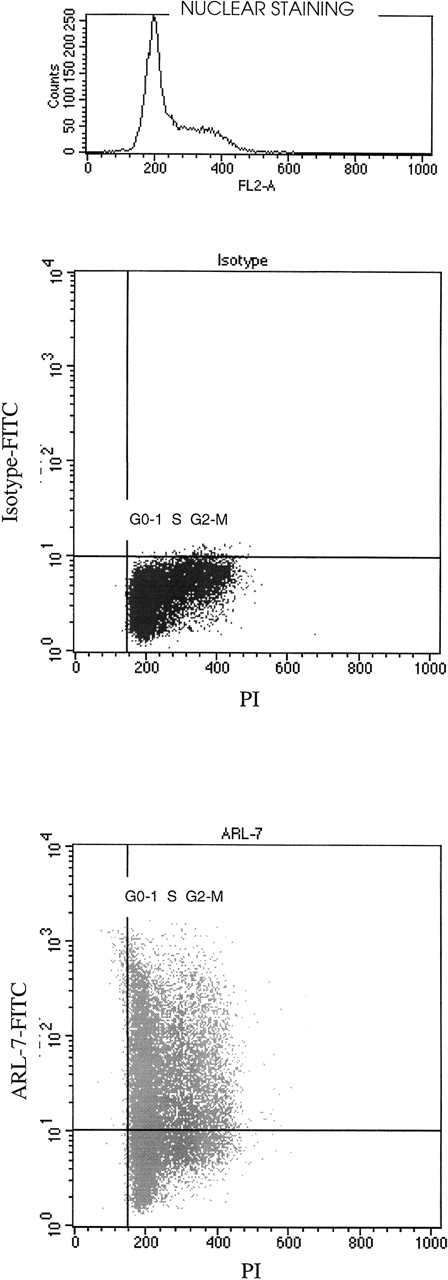
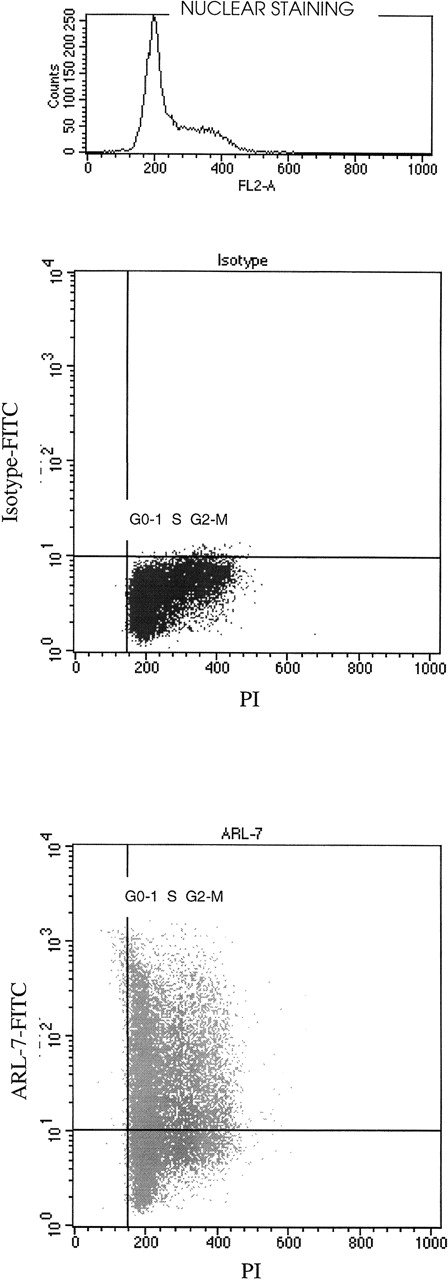
We noted variation in binding of the ARL-7 antibody to T-cell lines (range, 23%-56%), peripheral blood T cells (only 5%), and thymocytes (62%). In addition, significant variation in binding was seen within a T-cell line population. We therefore speculated that the ARL-7 target might be cell-cycle related. After surface staining with ARL-7, cells were permeabilized and incubated with propidium iodide (PI) for nuclear staining to define cell populations in different phases of the cell cycle. Figure 3 shows that cell-surface staining with ARL-7 did not vary between cells of different stages of cell cycle, indicating that the antigen to which ARL-7 bound was not related to a specific phase of the cellular cycle.
The binding of ARL-7 to surface antigens in relation to cell cycle.
REH cells were first incubated with ARL-7 antibody or isotype control, then fixed, permeabilized, and stained with propidium iodide (PI) before analysis. The upper dot blot shows the nuclear staining with PI (X axis) versus surface staining with isotype-FITC (Y axis), and the lower dot blot shows PI nuclear staining versus ARL-7-FITC surface staining. The cell cycle phases are shown as G0-1(gap 0-1), S (synthesis) and G2-M (gap 2-mitosis).
The binding of ARL-7 to surface antigens in relation to cell cycle.
REH cells were first incubated with ARL-7 antibody or isotype control, then fixed, permeabilized, and stained with propidium iodide (PI) before analysis. The upper dot blot shows the nuclear staining with PI (X axis) versus surface staining with isotype-FITC (Y axis), and the lower dot blot shows PI nuclear staining versus ARL-7-FITC surface staining. The cell cycle phases are shown as G0-1(gap 0-1), S (synthesis) and G2-M (gap 2-mitosis).
Binding of ARL antibodies to commonly tested auto-antigens
Because 2 of 6 ARL-derived antibodies showed strong reactivity to cell surface determinants suggestive of autoreactivity, we speculated that perhaps the remaining ARL-derived antibodies might have specificity to self-antigens. However, we could not demonstrate binding of the 6 lymphoma-derived immunoglobulins to cardiolipin (Table5). Only 1 ARL-derived immunoglobulin had weak reactivity to ssDNA (Table 6). The lack of binding to ssDNA and cardiolipin suggests that these IgM antibodies are less likely to be polyreactive. Additionally, the antibodies did not bind to i/I autoantigens on red blood cells (data not shown).
Discussion
Several laboratories have carried out in vitro and in vivo analyses to document B-cell hyperactivity during the course of HIV infection. In a previous study, we have provided evidence for early B-cell activation preceding lymphoma development.29 We were able to identify an expanded B-cell clone in the bone marrow of an HIV-infected individual 3 years prior to its progression to clinical lymphoma. These studies suggested that B-cell hyperactivity might predispose certain B cells to lymphomagenesis. In view of the significant degree of somatic diversification of immunoglobulin genes expressed by ARL, we speculated that an antigen-driven B-cell process may play an important role in ARL pathogenesis. Because serum paraproteins with specificity for various HIV epitopes exist in HIV-infected individuals, it has been questioned whether HIV-specific B-cell activation might predispose to lymphoma development. To examine for reactivity to HIV epitopes, we expressed lymphoma-derived immunoglobulin VH and VL genes from 6 ARL in a mammalian-expression system. This approach was necessary because ARL is not associated with paraproteins that are clearly tumor-related, as is the case in plasmacytic B-cell tumors. First, we used ELISA and Western blotting assays to investigate for HIV reactivity. Because none of the tumor-related immunoglobulins bound to HIV proteins in these assays, we used immunocytometry that would detect native HIV epitopes. The tumor-related antibodies were negative for HIV reactivity by this method as well, thus suggesting that HIV reactivity of ARL-derived immunoglobulin is uncommon. This finding therefore does not support our initial hypothesis that interaction between HIV and surface immunoglobulin often predisposes HIV-specific B cells to lymphomagenesis. However, it is possible that HIV may bind to and activate B lymphocytes via nonimmunoglobulin receptors in a CD4-dependent or CD4-independent manner.42 For example, HIV proteins have been shown to bind to cell surface determinants, such as galactosyl ceramides, CD26 antigen, tryptase TL2, and adhesion molecules.40,41 43
Interestingly, we observed strong reactivity of 2 of 6 tumor-related antibodies for cell surface determinants present on cells of different lineages. Autoantibody specificities to cell surface determinants on a wide variety of hematopoietic and nonhematopoietic cells are frequently detectable in serum of HIV-infected individuals. Moreover, the incidence of these serum autoantibodies parallels with the progression of HIV infection.7 8 While the remaining tumor-related immunoglobulins in the present study did not show autoreactivity, it is still possible that they are reactive to other self-antigens that were not tested for.
Autoantibodies to other, better defined, ubiquitous self-antigens, such as i/I, actin, myosin, ssDNA, and cardiolipin, also are often detectable in the serum of HIV-infected individuals.10,11,16-24,44 Indeed, 2 ARL-derived cell lines with anti-i/I specificity have been previously reported.45 Several studies have suggested that B-cell autoreactivity to cell surface determinants (for example, anti-i/I, anti-Pr2) in B-cell lymphoma development may play a role in non-HIV infected individuals, including patients with congenital immunodeficiency syndromes.40 46 Thus, it is possible that autoreactivity to cellular components of the immune system may contribute to immune dysregulation and lymphoma development.
In the course of our investigations, we observed that ARL-derived B cells may have auto-reactivity to cell surface determinants. It has been speculated that auto-reactivity may contribute to lymphoma development in general. This speculation is based on the documentation that B cells derived from lymphoid neoplasms,40,41,47,48including a few cases of ARL,26,40,41,45 47-49 are autoreactive. However, additional studies are necessary to define the mechanisms by which autoreactivity might predispose B cells to lymphomagenesis.
Acknowledgment
To Darlisha Williams, who provided the M-tropic–infected and the uninfected PBMCs.
Supported by National Institutes of Health grant P50HL54 516.
Reprints:Leslie E. Silberstein, Departments of Medicine and Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 284 John Morgan Building, 36th and Hamilton Walk, Philadelphia, PA 19104-6082; e-mail: silbersl@mail.med.upenn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.