Thioredoxin (Trx) is a ubiquitous protein disulfide oxidoreductase with antioxidant, cytokine, and chemotactic properties. Previously, we showed that Trx, in synergy with interleukin 1 (IL-1), IL-2, IL-4, tumor necrosis factor (TNF-), and CD40-ligation induced S-phase entry and mitosis in normal B cells and B-type chronic lymphocytic leukemia (B-CLL) cells. The viability of B-CLL cells stimulated by these protocols is high, and it has been hypothesized that the overexpression of Bcl-2 found in B-CLL protects the cells from apoptosis in vitro and in vivo. In this study, we have analyzed the response of cells derived from 12 samples of patients with B-CLL to recombinant human Trx in spontaneous apoptosis, with special reference to the Bcl-2 expression. Long-term cultures of B-CLL clones showed significantly higher viability when supplemented with human Trx (P = .031), also exemplified with clones surviving more than 2 months. Short-term cultures of B-CLL cells exposed to 1 μg/mL of Trx for 1, 5, or 12 days maintained expression or delayed down-regulation of Bcl-2 compared with control cultures containing RPMI 1640 medium and 10% fetal calf serum only (P = .032, .002, .026, respectively). All B-CLL cells expressed constitutive Trx at varying but low levels, in contrast to adult T-cell leukemias, which overexpress Trx, as previously reported. We found that Trx added to B-CLL cells increased in a dose-dependent fashion the release of TNF-, which has been suggested to be an autocrine growth factor for these cells. In conclusion, we have found that human recombinant Trx induced TNF- secretion, maintained Bcl-2, and reduced apoptosis in B-CLL cells.
B-type chronic lymphocytic leukemia (B-CLL) is the most common leukemia of adults in western countries, accounting for 30% of all cases.1-3 The diagnostic hallmark of B-CLL is an accumulation of monoclonal CD5-positive B cells resting in the G0/G1 stage of the cell cycle that express a limited VH-gene repertoire. Typical B-cell surface antigens are present along with low amounts of surface IgM/IgD.4,5The expression of the proto-oncogene bcl-2 is up-regulated 1.5- to 25-fold in most cases of B-CLL.6,7 The Bcl-2 protein overexpression may explain the relentless successive expansion of the malignant clone despite a minimal proliferating cell fraction. Bcl-2 expression is enhanced by cytokines such as interleukin 4 (IL-4), IFN-α, IFN-γ, basic fibroblast growth factor, and CD6-ligation.8-12 In contrast, glucocorticoids, IgM-ligation, IL-10, or growth factor withdrawal leads to bcl-2down-regulation and bax up-regulation.13,14 High Bcl-2 to Bax ratios were found to protect against apoptosis.12 It is not known by which mechanism B-CLL cells proliferate, but physiological stimulation through membrane receptor-ligation and cytokine ligand-receptor interactions will induce mitogenic responses, although often weak.15-22 One of these cytokines that contributed to S-phase entry and mitosis in synergy with IL-2, IL-4, tumor necrosis factor α (TNF-α), and CD40-ligation was thioredoxin (Trx).19
Trx is a ubiquitous redox-active and multifunctional molecule of Mr 11 800, containing an active site motif with amino acids Cys-X-X-Cys.23-25 Together with the selenoprotein thioredoxin reductase (TrxR) and reduced nicotinamide adenine dinucleotide phosphate (NADPH), it is a potent antioxidant and protein disulfide oxidoreductase.26 Several extracellular cytokine activities, such as IL-1–like factor, B-cell stimulatory factor, IL-2R inducing factor, eosinophil cytotoxicity enhancing factor, and recently, chemotactic factor, have been attributed to Trx.19,27-30 In macrophages, it was found that Trx is a potent costimulus of cytokine expression and release.31 Overexpression of Trx has been observed in neoplastic disease, protecting cells from oxidative stress.23,32 In B-CLL cells, Trx expression was previously found to be low but inducible.33
On the basis of the hypothesis that cognate interaction between B-CLL cells and normal cells within bone marrow34 and peripheral lymphoid organs (eg, TH cells, follicular dendritic cells, and monocytic cells) may initiate overexpression of cytokines, including Trx, contributing to aberrant apoptosis regulation, we wanted to explore, in this study, the role of Trx in regulating Bcl-2 expression and in protecting the B-CLL cells from apoptosis. During the course of the study, we found that Trx-stimulated autocrine TNF-α release in a dose-dependent manner and that B-CLL cells were rescued from spontaneous apoptosis.
Materials and methods
B-CLL cells and patients
Blood was collected in heparinized tubes and diluted with an equal volume of sterile phosphate-buffered saline (pH 7.2). Lymphocytes were separated by density gradient centrifugation (Ficoll-Hypaque, Pharmacia, Uppsala, Sweden). Cells from the interface were carefully collected, washed 3 times with phosphate-buffered saline, and then frozen and stored in liquid nitrogen. Cells from patients I-105, I-40, I-90, I-276, I-397, and I-424 were collected at the University Hospital in Uppsala, Sweden. The other patient samples were collected at the Hematology Clinic, University Hospital, Linköping, Sweden. Table1 shows the data for patients with CLL and the phenotypes of the purified cells, which were analyzed by flow cytometry with the use of Becton Dickinson reagents (San Jose, CA). The majority was B cells, and NK and T cells were below 1% in most B-CLL samples (range 0.02%-2.5%, mean = 0.86%) (Table 1). For comparison, CD19-positive selected samples were analyzed from I-105 patient, showing identical results in Trx stimulation experiments. The ethical committees at the University Hospitals approved this study, including informed written consent from the patients.
Production of recombinant human Trx
Two different preparations of human recombinant full-length Trx were used. A Trx expression plasmid, containing the complementary DNA sequence for human Trx in the MP6 cell line was transferred into anEscherichia coli host, BL21, and purified as previously described, by sonication, ultracentrifugation, and MonoQ liquid chromatography.35 Alternatively, a human trxcomplementary DNA was subcloned into the pACA expression vector, and the overexpressed protein was purified as described36 (also commercially available from IMCO, Stockholm, Sweden). Trx is sensitive to atmospheric oxidation and dimerizes easily. For maintenance of biological activity, recombinant Trx was, therefore, reduced by dithiothreitol-activated beads, ReductacrylTM reagent (Calbiochem Novabiochem, San Diego, CA), and sterilely filtered immediately before addition to cultures.
Analysis of apoptosis
FITC-labeled Annexin-V (Boeringer-Mannheim, Mannheim, Germany) for detection of phosphatidyl serine exposed on the plasma membrane was used, according to the manufacturer's instruction. Detection of apoptosis was also performed by analysis of DNA fragmentation by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) with the use of biotinylated dUTP, according to the manufacturer's recommendation (Boeringer Mannheim). Alternatively, cells were stained with propidium iodide, according to the method of Hotz et al,37 followed by FACS analysis for detection of DNA loss. The viable cell number was assessed by counting cells in triplicates in a Bürker chamber with the use of the trypan blue dye exclusion method.
Flow cytometry and immunofluorescence microscopy
Immunofluorescence staining for microscopy or FACS analysis was performed with 1 × 106 cells, which were washed, fixed in 4% paraformaldehyde, and finally permeabilized with 0.1% saponin, according to the method of Sander et al.38 In some experiments, 70% methanol was used for permeabilization instead of saponin, without affecting Bcl-2 fluorescence intensity. The following antibodies were used: anti–Bcl-2-FITC (clone 124, Dako, Glostrup, Denmark), mouse IgG isotype control FITC-conjugate (Dako), anti-Trx39 monoclonal antibody (mAb), anti-TrxR mAb,40 produced in our laboratory at Department of Biomedicine and Surgery, Division of Cell Biology, Linköping University, Sweden, anti-Bax (P19, Santa Cruz Biotechnology, Santa Cruz, CA), in combination with FITC-labeled goat antimouse Ig, F(ab)'2-fragments (Dako). Positive and negative cell lines for each antigen were included as specificity controls for the different antibodies.
Short-term and long-term cultures of B-CLL
B-CLL cells were cultured in RPMI 1640 medium with 10% fetal calf serum (Gibco, Paisley, Scotland) plus antibiotics (Gibco) at a cell density of 1 × 106 cells/mL at 37°C in 5% CO2 atmosphere. Trx (1 μg/mL) was added into the RPMI 1640 medium containing 10% fetal calf serum. Long-term cultures were fed once a week, inspected microscopically, and determined for viability by trypan blue dye exclusion. Trx was also collected from serum-free, 24-hour culture medium of the MP6 T-cell hybridoma cells41 and used as an alternative source of Trx (25% of MP6 supernatant) in some experiments as indicated. The stimulatory activity of this 24-hour Trx-containing supernatant was completely abolished by a preincubation with Sepharose-anti-Trx beads for 30 minutes at 4°C.19
TNF-, IL-6, and IL-1 determinations by enzyme-linked immunosorbent assay
TNF-α, IL-6, and IL-1β released into B-CLL supernatants were determined by sandwich enzyme-linked immunosorbent assay techniques, using commercially available kits from R&D Systems Inc (Minneapolis, MA) and Mabtech (Stockholm, Sweden).
Statistical analysis
Statistical differences between Trx-treated and nontreated control cultures of B-CLL clones were evaluated pair-wise by using Wilcoxon signed rank sum test. All statistical evaluations were performed with JMP version 3.2.5 (SAS Institute, Cary, NC) software and a Gateway G6-300 PC microcomputer.
Results
Trx delayed Bcl-2 down-regulation and reduced apoptosis in B-CLL cells
Our previous studies on in vitro cultures of B-CLL cells, in which we established several B-CLL cell lines by Epstein-Barr virus transformation, showed that addition of Trx or combinations ofStaphylococcus aureus Cowan I with IL-2/Trx to the cultures considerably increased the chances of obtaining cell lines,22 probably by increasing viability and by allowing the cells to enter the cell cycle.42 In considering the improved cell culture viability, we asked whether exogenous Trx might affect apoptosis and Bcl-2 expression in short-term cultures of B-CLL cells.
First, we analyzed the expression of Bcl-2 after 1, 5, and 12 days of in vitro culture with human Trx added to 12 different B-CLL cell samples. At the time of bleeding (day 0), Bcl-2 was found to be high (median of 12 mean fluorescence intensities (MFI) = 43.7, range 30.6-49.1) in all cells and reduced to half (MFI = 20.4) on culturing in vitro over a 12-day period, in agreement with previously published results.6 We found that growth factor removal already led to Bcl-2 down-regulation after 24 hours in 7 of 12 samples, and, at day 5 and day 12, all B-CLL cultures expressed lower Bcl-2 levels. Trx-treated cell samples showed delayed down-regulation and expressed minor albeit significantly more Bcl-2 protein compared with control medium in 9 of 12 cultured cells (Table 2). The differences were gradually increasing from 4.5%, to 7.0%, and to 11.5% on day 1, day 5, and day 12, respectively. Paired data were evaluated statistically in Wilcoxon signed rank sum test, showing significant differences between the two groups (P values day 1 = .032; day 5 = .002; day 12 = .026).
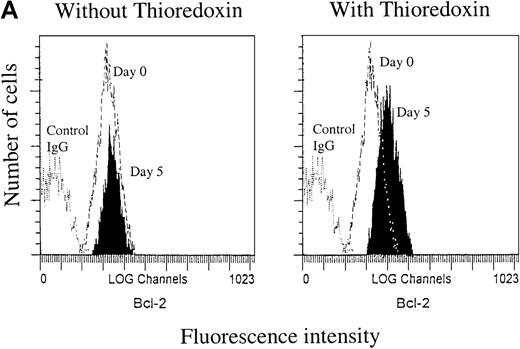
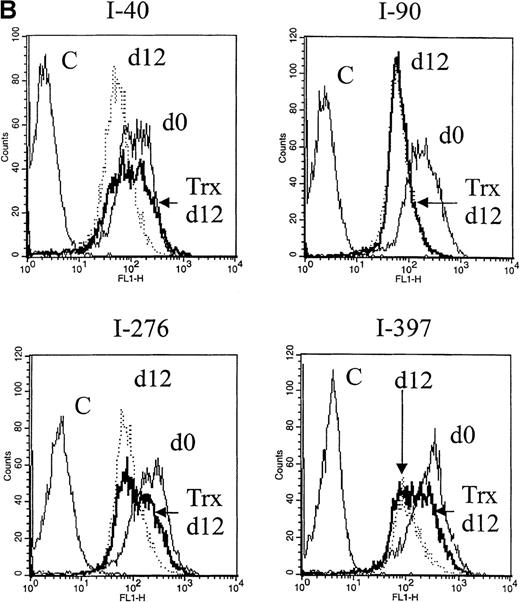
Representative flow cytograms from separate experiments at day 5 are shown in Figure 1A (left panel), showing that the Bcl-2 expression in RD-1 B-CLL cells was not significantly changed after 5 days in the control medium. In cultures exposed to Trx (Figure 1A, right panel), however, Bcl-2 expression was higher compared with control culture with medium only. After 12 days, the Bcl-2 expression levels were reduced to a high extent in control cultures without Trx, but they were less reduced in cultures with Trx (Figure 1B), represented by B-CLL samples I-40, I-276, and I-397. We also assayed for apoptotic events in B-CLL cells by Annexin-V. Comparisons between Trx-treated and medium-cultured cells revealed small but significantly reduced percentages of apoptotic cells (median = 7.0%, n = 12) in the Trx-treated group (P = .031). Apoptosis was also analyzed by the TUNEL method for detection of DNA fragments, which occurs later in the apoptotic process than the phosphatidyl serine expression, as a rule. In one such experiment, 5 of 6 cell cultures showed significantly less DNA fragmentation in the presence of recombinant Trx (1 μg/mL) (data not shown).
Bcl-2 expression.
A. Bcl-2 expression at day 0 and day 5. Right panel: cultures with recombinant thioredoxin (rTrx) 1 μg/mL; left panel: cultures without rTrx. B-type chronic lymphocytic leukemia (B-CLL) cells were obtained from patient RD-1. B. Bcl-2 expression 12 days after onset of cultures with Trx (Trx day 12, thick line) and without Trx (day 12, dotted line) in comparison with Bcl-2 expression at the onset of experiment (day 0, thin line). Flow cytometry analysis was performed as described in Materials and methods section. C = control mouse isotype IgG; counts = cell number; FL1-H = fluorescence intensity.
Bcl-2 expression.
A. Bcl-2 expression at day 0 and day 5. Right panel: cultures with recombinant thioredoxin (rTrx) 1 μg/mL; left panel: cultures without rTrx. B-type chronic lymphocytic leukemia (B-CLL) cells were obtained from patient RD-1. B. Bcl-2 expression 12 days after onset of cultures with Trx (Trx day 12, thick line) and without Trx (day 12, dotted line) in comparison with Bcl-2 expression at the onset of experiment (day 0, thin line). Flow cytometry analysis was performed as described in Materials and methods section. C = control mouse isotype IgG; counts = cell number; FL1-H = fluorescence intensity.
Trx promotes long-term survival of B-CLL cells
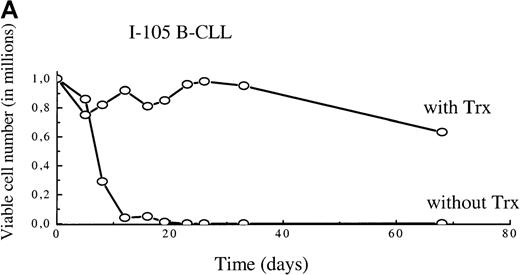
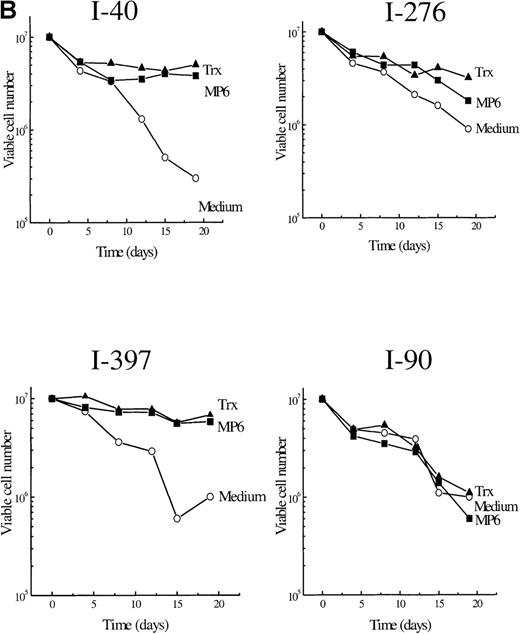
To find out whether the observed delay in Bcl-2 down-regulation and the decrease in apoptosis could be sustained and affect long-term survival, we extended the observation time and supplied exogenous reduced Trx once a week to B-CLL cultures. Figure2A and B show viable cell numbers (survival curves) of 5 different B-CLL cells grown in RPMI 1640 with 10% fetal calf serum with Trx and in their control cultures. The I-105 B-CLL cells were followed for 68 days. Without Trx addition, the cells were all dead after 12 days, in contrast to Trx cultures, which showed <40% reduction in cell numbers after 68 days (Figure 2A). Cells from patients I-40, I-276, I-397, and I-90 (Figure 2B) were followed for 3 weeks. Addition of Trx had a significant survival advantage in 3 of the 4 B-CLL cultures. The percentage of viable cells at day 19 (last day of observation in Figure 2B) was significantly higher in Trx-supplied cultures (P = .031) (Table 3).
A. Viability curves for I-105 cells cultured with and without MP6-thioredoxin.
B. Survival curves for cells in vitro of 4 patients with B-type chronic lymphocytic leukemia with and without recombinant thioredoxin or MP6-thioredoxin.
A. Viability curves for I-105 cells cultured with and without MP6-thioredoxin.
B. Survival curves for cells in vitro of 4 patients with B-type chronic lymphocytic leukemia with and without recombinant thioredoxin or MP6-thioredoxin.
Constitutive Trx expression is low in most B-CLL cells and does not correlate with survival
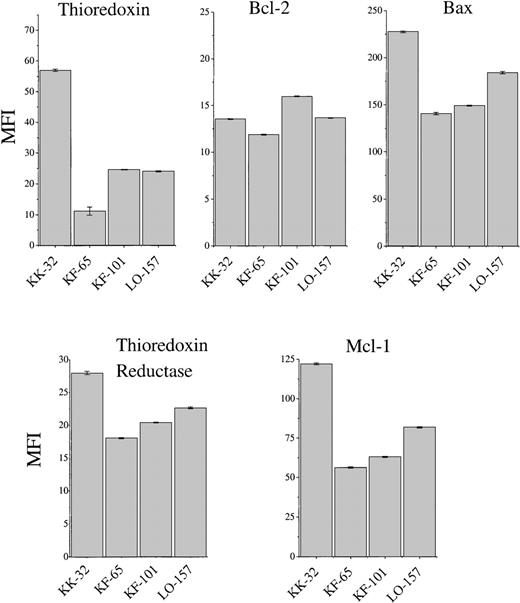
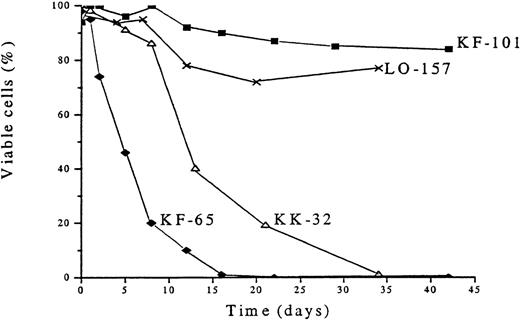
The overexpression of Trx found in human adult T-cell leukemia and certain other malignancies could not be found in B-CLL cells (Figure3), showing fresh B-CLL cell samples (before onset of in vitro culture). In addition to Trx, we analyzed, by flow cytometry, Bcl-2 and the apoptotic protein Bax, which heterodimerizes with Bcl-2, as well as TrxR, which maintains Trx in an active and reduced state, and the anti-apoptotic protein Mcl-1, which was reported to play a role in B-CLL survival.43 The expression of Trx was highly variable (Figure 3). KK-32 showed the highest Trx expression but also the highest Bax expression. This B-CLL clone died rapidly in spontaneous apoptosis medium (Figure4). The long-term survivor KF-101 was high in Bcl-2 but low in Trx expression. In separate cultures, KF-32 cells remained viable for a long time in the presence of 1 μg/mL of recombinant Trx with a similar profile to Trx-treated I-105, I-40, I-276, and I-397 cells shown in Figure 2 (data not shown). Thus, constitutive Trx expression did not correlate with survival nor with Bcl-2 expression. The correlation between high Bcl-2 expression levels (high Bcl-2 to Bax ratios) and degree of survival observed in various B-CLL samples in vitro has been reported by several groups.12,43 44 In this study, KK-32 showed the lowest Bcl-2 to Bax ratio and died rapidly. KK-101, however, expressed the highest Bcl-2 to Bax ratio combined with the highest observed viability after 45 days in vitro (Figure 4).
FACS analysis of thioredoxin, thioredoxin reductase, Bcl-2, Mcl-1, and Bax expression of cells from patients with B-type chronic lymphocytic leukemia (KK-32, KF-65, KF-101, and LO-157).
Mean ± SEM (vertical bars) is shown.
FACS analysis of thioredoxin, thioredoxin reductase, Bcl-2, Mcl-1, and Bax expression of cells from patients with B-type chronic lymphocytic leukemia (KK-32, KF-65, KF-101, and LO-157).
Mean ± SEM (vertical bars) is shown.
Survival profiles of cells of 4 patients with B-type chronic lymphocytic leukemia (B-CLL) after in vitro culturing without thioredoxin.
The B-CLL cells were cultured in RPMI 1640 plus 10% fetal calf serum. Viability was assessed by trypan blue dye exclusion.
Survival profiles of cells of 4 patients with B-type chronic lymphocytic leukemia (B-CLL) after in vitro culturing without thioredoxin.
The B-CLL cells were cultured in RPMI 1640 plus 10% fetal calf serum. Viability was assessed by trypan blue dye exclusion.
Trx induces TNF- secretion in B-CLL in a dose-dependent manner
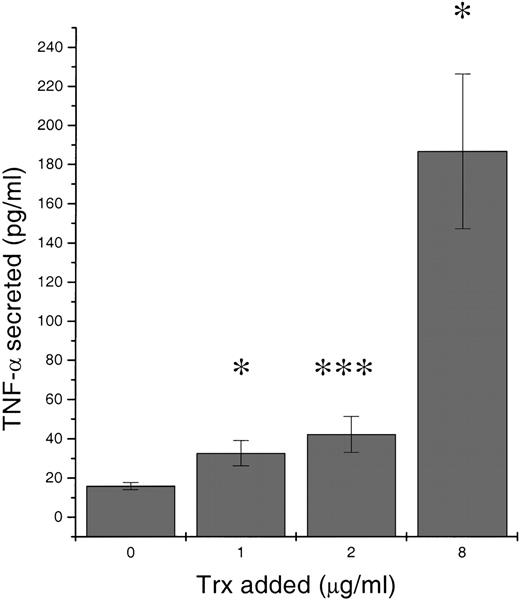
To approach an understanding of the mechanism behind the Trx-induced enhancement of Bcl-2, we investigated Trx-treated B-CLL culture supernatants for the presence of autocrine secreted cytokines. Recombinant Trx (1, 2, and 8 μg/mL) was added to B-CLL LS-109 cells and cultured for 1 day. Figure 5 shows that Trx significantly increased TNF-α release compared with control medium without Trx (P = .023, P = .001,P = .042, respectively). Supernatants from 12 Trx-treated cultures (1 μg/mL) and control cultures were analyzed for TNF-α. Ten of 12 Trx-treated cultures contained more TNF-α than did control cultures. The percentage of TNF-α increase for these Trx-cultures were +269%, +104%, +60%, +25%, +22%, +21%, +13%, +12%, +5%, +3%, -11%, and -17% (median = 17%; P = .008). In addition, IL-1β and IL-6 were found in Trx-stimulated cultures, but no significant difference between stimulated and nonstimulated cultures could be found at the tested concentration of 1 μg/mL of recombinant Trx (data not shown).
Release of tumor necrosis factor (TNF-) in response to increasing amounts of thioredoxin added.
106 B-type chronic lymphocytic leukemia LS-109 cells per mL were cultured for 24 hours in RPMI 1640 with 10% fetal calf serum. Culture supernatants were analyzed in a sandwich enzyme-linked immunosorbent assay for TNF-α. Mean values represent mean of 2 separate cultures each analyzed in triplicates with SEM. Wilcoxon signed rank test P values were calculated. *P < .05, *** P < .001.
Release of tumor necrosis factor (TNF-) in response to increasing amounts of thioredoxin added.
106 B-type chronic lymphocytic leukemia LS-109 cells per mL were cultured for 24 hours in RPMI 1640 with 10% fetal calf serum. Culture supernatants were analyzed in a sandwich enzyme-linked immunosorbent assay for TNF-α. Mean values represent mean of 2 separate cultures each analyzed in triplicates with SEM. Wilcoxon signed rank test P values were calculated. *P < .05, *** P < .001.
Discussion
The main finding of this study is that Trx added to B-CLL cells significantly delayed Bcl-2 down-regulation and diminished the number of apoptotic cells, resulting in prolonged survival. Trx also stimulated autocrine TNF-α release in a dose-dependent manner in these cultures, which may partly explain the augmented viability.
The characteristic overexpression of the anti-apoptotic protein Bcl-2 found in B-CLL in vivo is well documented,6,7 and it is not, as a rule, a result of t (14:18) translocation events, as described for follicular lymphomas. Apoptosis can be mediated by several factors, including oxidative stress.45 Bcl-2 does not appear to influence the generation of oxidative stress mediators (eg, hydrogen peroxide), but it does prevent oxidative damage to cellular constituents, including lipid membranes.46 Several studies12,13 indicate that the mechanism behind the high Bcl-2 level in B-CLL cells involves continuous input of external signals generated via cytokine receptors. Bcl-2 expression, or rather the Bcl-2 to Bax ratio in B-CLL, was up-regulated by IL-4, IFN-α, IFN-γ, TNF-α, basic fibroblast growth factor, IL-8, and CD6-ligation or down-regulated by IL-10.8-12,14,47 Growth factor removal on explantation in vitro causes the external signals to relax, leading to Bcl-2 down-regulation and apoptosis induction as found in this study and by others.12 We found that growth factor removal led to Bcl-2 down-regulation after 24 hours in 7 of 12 samples, and, at day 5 and day 12, all B-CLL cultures expressed lower Bcl-2 levels. Supplementation of recombinant human Trx, however, delayed to some extent the down-regulation of Bcl-2 in 9 of 12 cultures (Table 2) and resulted in a concomitant drop in the percentage of apoptotic cells. After 1 day of Trx treatment, a small but significant difference in Bcl-2 expression was found: 4.5% higher than control, a value that increased to 7% and 11.5% on day 5 and 12, respectively. Also in the Annexin V analysis, small (7%) but significant differences between Trx and control cultures were found. Accumulating day-by-day effects of Trx may select for survival enhancement of Bcl-2–expressing cells and add up to viable cells as seen on day 19 in Figure 2 in which a more evident difference between Trx and control cultures is seen. In view of the biology of CLL and its chronic appearance and many times a slow accumulation of leukemic cells in the blood of the patient, we think that these tiny 1-day differences should not be overlooked.
During the course of this study, we found that Trx induced a dose-dependent release of TNF-α, a known growth factor for B-CLL.17,18 In macrophages, extracellular Trx in synergy with phorbol 12-myristate 13-acetate was previously shown by Schenk et al31 to induce a release of cytokines IL1, IL6, IL8, and TNF. The mechanism by which extracellular Trx acts is largely unknown, but Trx may modulate membrane receptor thiols via membrane localized protein disulfide isomerase. No specific receptor for Trx has been identified hitherto nor has any active uptake of Trx over the plasma membrane been described23,30 (Rosén and Lindquist, unpublished observations). Direct modulation (reduction and activation) by Trx of cystein-rich receptors has been described for the interferon-γ receptor or ICAM-1.48,49 CD5 and CD6 membrane receptors, expressed on B-CLL cells, belong to the cystein-rich scavenging receptor family.50 An alternative explanation is that reduced extracellular Trx may transfer reducing equivalents (electrons) via membrane bound TrxR, thus changing the intracellular reduced or oxidized Trx balance. Intracellular Trx is a direct inhibitor of apoptosis signal-regulating kinase 1 and is a regulator of NF-kappa B activation.51 52
Has the Trx-induced survival of B-CLL cells in this study any in vivo relevance? Since we found that the leukemic B-CLL cells themselves do not overexpress Trx, in contrast to adult T-cell leukemia,28 we considered the possibility that Trx can be supplied by other cells in close contact with the leukemic cells in the patient with B-CLL. Antigen-presenting cells residing in the pseudofollicles of the lymph nodes of patients with B-CLL34may contribute to Trx on the basis of suggestions from findings in mice lymphoid tissue, revealing that interdigitating cells are highly positive for Trx.53 We and others39,40,54(Söderberg A, Sahaf B, Rosén A, et al [submitted]) have found that Trx and TrxR are present in high quantities on plasma membranes of antigen-presenting monocytes or macrophages. From other systems, it is known that bone marrow stromal cells interact closely with pro-B cells and may up-regulate Bcl-2 expression and prevent Bax induction in pro-B cells55 and that bone marrow fibroblasts will rescue plasma cells from apoptosis by sustaining a high Bcl-2 expression.56 Tötterman et al57 have observed that, before recurrence of malignant B-cells, a transient peak of increased T-cell numbers occurs in several patients. These T cells may be potential Trx producers as suggested by our own previous studies. Those studies19 58 showed that a T-helper cell line, MP6, established by fusion between the leukemic cell line Molt4 and normal healthy blood donor T-helper cells, was the most potent Trx producer and the best stimulator of B cells among a wide panel of cell lines tested. We suggest that B-CLL cells maintain Bcl-2 expression and survival in vivo through cell-cell contact with antigen-presenting follicular dendritic cells that overexpress Trx or stimulated T-helper cells that may lead to multiple but low-dose autocrine cytokine release. Some of the B-CLL samples tested in this study contained 1%-2% T cells; therefore, we compared in separate experiments these samples with CD19 positively selected samples (containing <0.2% T cells) for possible influence of T cells on the outcome of Trx treatment. No difference in response was found.
We have shown in this study that the participation of the redox-active molecule Trx is important for B-CLL cell survival in vitro for extensive time periods and that Bcl-2 down-regulation was delayed by Trx additions. Future work will focus on the in vivo cellular source and expression pattern of redox-active proteins and their role in the leukemogenic process.
Acknowledgments
We thank Ms Anita Lönn, Inga-Lill Scherling, and Ms Kerstin Willander for skillful technical assistance. We express our gratitude for help with clinical specimens of patients with B-CLL to Drs Karin Karlsson, Gunnar Juliusson, Claes Malm, and Ilse Christiansen, GSD.
Supported by funds from the Swedish Cancer Association, Stockholm, Sweden, and the Portuguese Foundation for Science and Technology.
Reprints:Prof. Anders Rosén, Department of Biomedicine and Surgery, Division of Cell Biology, University of Linköping, S-581-85 Linköping, Sweden; e-mail: AndRo@mcb.liu.se.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.