Severe anemia is one of the most lethal complications in children infected with Plasmodium falciparum. The pathogenesis of this anemia is not completely understood. Experimental data from malaria-infected humans and animal models suggest that uninfected red cells have a shortened life span. This study looked for changes in the red cell surfaces of children with severe malarial anemia that could explain this accelerated destruction. A prospective case-control study was conducted of children with severe P falciparum anemia (hemoglobin of 5 g/dL or lower) admitted to a large general hospital in western Kenya. Children with severe anemia were compared with children who had symptoms of uncomplicated malaria and with asymptomatic children. Cytofluorometry was used to quantify in vitro erythrophagocytosis and to measure red cell surface immunoglobulin G (IgG) and the complement regulatory proteins CR1, CD55, and CD59. Red cells from patients with severe anemia were more susceptible to phagocytosis and also showed increased surface IgG and deficiencies in CR1 and CD55 compared with controls. Red cell surface CD59 was elevated in cases of severe anemia compared with asymptomatic controls but not as compared with symptomatic controls. The surface of red cells of children with severe P falciparum anemia is modified by the deposition of IgG and alterations in the levels of complement regulatory proteins. These changes could contribute to the accelerated destruction of red cells in these patients by mechanisms such as phagocytosis or complement-mediated lysis.
Plasmodium falciparum is responsible for the death of more than 1 million children each year in sub-Saharan Africa. Death is usually due to complications, such as cerebral malaria and severe anemia. In western Kenya, severe anemia due to P falciparum infection is a leading cause of mortality among children younger than 2 years of age. It remains a mystery why, despite the fact that this parasite's primary target is the red blood cell (RBC), severe anemia is seen only in a small proportion of malaria cases.1 The pathogenesis of severe anemia in malaria is not clearly understood, but such factors as malnutrition, iron deficiency,2 bone marrow dysfunction,3 and the level of parasitemia4 are thought to contribute to its manifestation. In addition, there is ample evidence to suggest that the life span of uninfected RBCs is decreased by accelerated destruction.5-8
The mechanism of the increased rate of RBC destruction in malaria is unknown. The presence of circulating monocytes containing phagocytized uninfected RBCs9 suggests that during malaria infection, uninfected cells develop lesions that activate monocytes to capture and engulf them. The nature of the damage that triggers RBC phagocytosis is unclear. We suspect deposition of antibody on the RBC surface. However, RBC surface antibody has not been observed consistently in all studies.10-12 In a study in Thailand,12 for example, no correlation was observed between the number of immunoglobulin G (IgG) molecules on RBCs and the degree of anemia. A possible explanation for the lack of correlation in that study is that only 6 out of 115 patients would qualify as having severe anemia (a packed cell volume of less than 20%) as is known to occur in areas of Africa in which P falciparum is endemic.
Another possible mechanism of RBC destruction is through the activation of complement, which is known to occur in malaria infection.13 RBCs are normally protected from complement activation by the action of surface complement regulatory proteins, such as complement receptor 1 (CR1); decay accelerating factor (CD55); and membrane inhibitor of reactive lysis (CD59). Acquired or inherited deficiencies of these proteins have been implicated in the pathogenesis of some forms of immune hemolytic anemia.14,15 Therefore, complement regulatory proteins may play an important role in protecting uninfected RBCs from destruction during malaria infection. In the study presented here, we used cytofluorometry to determine the susceptibility of red cells to in vitro phagocytosis and to measure red cell surface IgG, CR1, CD55, and CD59 in patients with severe malarial anemia and controls.
Materials and methods
Study design and patient population
Between May and November of 1998, we carried out a case-control study of children admitted to the pediatric ward of the Nyanza Provincial General Hospital in Kisumu, western Kenya. This hospital is the main government referral center for the population living in the malaria holoendemic area of the Lake Victoria basin. Cases of severe anemia were defined as children with asexual P falciparumparasitemia by blood smear and a hemoglobin of 5 g/dL or lower in the absence of clinical evidence of any other infectious process or malignancy. Each case of severe anemia was matched by age ± 2 months and by gender to a symptomatic control (SC) and to an asymptomatic control (AC). Inclusion criteria for SCs were symptoms suggestive of malaria, such as an axillary temperature higher than 37.5°C or, in its absence, 2 of the following complaints voiced by the parent: nausea/vomiting, irritability, poor feeding, myalgias, or headache. The majority (80%) of the children in this group qualified on the basis of an axillary temperature higher than 37.5°C. Children were excluded from this group if there was evidence of complication manifested by respiratory distress, palmar or conjuctival pallor, hypotension, seizures, a hemoglobin number of 5 g/dL, coma, or clinical evidence of any other infectious process.16-18 SCs were identified from the outpatient clinic of the same hospital. Children were included in the AC group if their parents reported that the child was doing well. They were identified from the well baby clinic and from the surrounding community. Additional exclusion criteria applicable to all groups were the inability or unwillingness of the parent or guardian to give consent, the presence of other infection or malignancy, and a history of blood transfusion within 3 months prior to enrollment. All volunteers underwent a standardized history and physical examination upon enrollment; in the cases of severe anemia, these were repeated at 1 month after discharge.
Blood samples and clinical laboratory investigations
After informed consent was obtained from the parent or guardian, 5 mL of blood was collected by venipuncture and aliquoted into ethylenediaminetetraacetic acid (EDTA) and heparin tubes. Determination of complete blood count was done with the use of a hematology analyzer (Coulter, Hialeah, FL). Duplicate thick and thin blood smears were made and stained with Giemsa, and each was read by a different microscopist. The number of asexual-stage parasites in 200 high power fields of the thick smear was reported per 500 white blood cells. An additional 5 mL of blood was obtained from each case prior to and 1 month after hospital discharge. Plasma was obtained from EDTA-anticoagulated whole blood by centrifugation and stored at −70°C until used. Frozen plasma samples were submitted to the Clinical Chemistry Department, the Walter Reed Army Medical Center, Washington, DC, for the measurement of ferritin. Hemoglobin electrophoresis was performed with the use of standard cellulose acetate (Helena Laboratories, Beaumont, TX), and qualitative G6PD measurement was assessed by measuring the conversion of the nonfluorescent glucose-6-phosphate into the fluorescent end product 6-phosphogluconate (Sigma, St. Louis, MO).
Measurement of RBC surface CR1, CD55, CD59, and IgG
Upon arrival at the laboratory, a 20 μL aliquot of EDTA blood was placed in 2 mL of Alsever's buffer (Sigma) and stored at 4°C until fluorescent staining was performed, usually within 48 hours. All assays were performed by personnel who were unaware of the clinical status of the volunteers. Indirect fluorescent staining was done with the use of monoclonal antibodies against red cell surface CR1 (Clone E11, Accurate Chemical Co, New York), CD55 (Clone BRIC-110, Accurate Chemical Co), CD59 (Clone MEM 43, Research Diagnostics Inc, Flanders, NJ), and a secondary goat anti-mouse fluorescein isothiocyanate (FITC)–labeled polyclonal antibody (Becton Dickinson, San José, CA). Irrelevant monoclonal antibodies of the same isotype were used as negative controls (Sigma). For detection of total red cell surface IgG, a FITC-labeled goat anti-human polyclonal F(ab)2 antibody fragment was used (Sigma). Blood samples were stained in 96-well V-bottom polystyrene plates. Primary antibodies were used at a dilution of 1:50 and the secondary antibody was used at a dilution of 1:100 in phosphate-buffered saline (PBS) (pH 7.4) containing 1% bovine serum albumin (BSA). All incubations were carried out in the dark and at room temperature for 30 minutes followed by washing in PBS/1% BSA. After the final incubation, the samples were resuspended in PBS containing 1% paraformaldehyde and were stored in the dark at 4°C until acquisition was performed. Cytofluorometry was performed with the use of a FACScan flow cytometer (Becton Dickinson). Acquisition and analysis were performed with WinFCM and EXPO software packages (Applied Cytometry Systems, Sheffield, UK). Prior to the beginning of the study, the instrument settings were optimized and remained the same throughout the study period. The performance of the instrument was monitored weekly with the use of standard fluorescent beads (Becton Dickinson), according to the manufacturer's instructions. A red blood cell standard normal control (BioErgonomics, White Bear Lake, MN) was stained each day to control for interassay variation. RBCs were gated on the basis of their forward and side scatter characteristics with the use of logarithmic amplification. FL1 fluorescence was measured with the use of logarithmic amplification. The median values from histograms were converted to arbitrary units in a linear scale of 1 to 1024 channels. The values for CR1, CD55, and CD59 were normalized to the mean of the median fluorescence of the red cell standard by use of the following formula:
where Fsc is the corrected median fluorescent channel of the sample, Fs is the uncorrected median fluorescent channel of the sample, MeanFc is the mean of all the median values of the standard control obtained during the study, and Fc is the median fluorescent channel of the control stained in parallel with the sample.
Quantitation of erythrophagocytosis
Cytofluorometry was used to quantify erythrophagocytosis essentially as described.19 Then, 108 washed RBCs from the EDTA tube were labeled with a 2.5 μM solution of the lipophilic fluorescent cell tracking dye PKH-26 (Sigma) for 4 minutes. The reaction was stopped by resuspending the cells in fetal calf serum (FCS) (Life Science Technologies, Rockville, MD) followed by thorough washing with 10% FCS in RPMI 1640. The cells were then resuspended in 1 mL of the same medium. Then, 100 μL of the washed cells were incubated with 106 U-937 monocytic cells (ATCC, Manasas, VA) for 2 hours at 37°C in humidified air containing 5% CO2. The nonphagocytized RBCs were lysed by treatment with hypotonic ammonium chloride buffer (pH 7.2) for 5 minutes at 37°C. Thereafter, the cells were treated at room temperature with 70% ethanol for 20 minutes in order to fix the monocytes and to remove the PKH-26 from any ghosts still adherent to the monocytes. Finally, the cells were resuspended in PBS buffer. Cytofluorometry was performed with the use of a FACScan flow cytometer, and data were analyzed with LYSIS II software (Becton Dickinson). U-937 cells were gated on the basis of their forward and side scatter characteristics with the use of linear amplification. FL2 fluorescence was measured with the use of logarithmic amplification. We counted 10 000 cells and estimated the percentage of fluorescent population, containing ingested RBCs, from the bimodal histogram curve.
Ethical approval and consent
Scientific and ethical approval for this investigation were obtained from the Kenya Medical Research Institute, Nairobi, Kenya, and the Human Subjects Research Review Board, Office of the Surgeon General, US Army, Washington, DC. Informed consent was obtained from each parent or guardian at the time of enrollment.
Data analysis
Statistical analysis was performed with the SPSS software package (SPSS Inc, Chicago, IL). Unless stated otherwise, paired variables containing continuous numeric data were compared by means of the Wilcoxon signed-rank test for 2 related samples. Categorical variables were compared with the use of the Pearson chi-square. All tests were 2-tailed with alpha = 0.05. Continuous numeric data are presented as either medians with ranges or means ± standard deviation (SD).
Results
Demographic and clinical characteristics
A total of 59 potential cases were admitted to the pediatric ward of the Nyanza Provincial General Hospital during the study period. Of these, 42 cases of severe anemia (SA) met the inclusion criteria and were matched to 41 SCs and 40 ACs. Both genders were equally represented in all groups (SA = 50% male; SC = 51.2% male; AC = 50% male). Likewise, there were no differences in the mean age for each group (SA = 17.4 ± 12.5 months; SC = 17.1 ± 12.0 months; AC = 17.0 ± 12.5 months). In each group, 70% or more of the individuals were of Luo ethnic background.
SAs had more signs of acute illness, such as higher temperature, pulse, and respiratory rate than controls (data not shown). Splenic enlargement below the left costal margin was greater in SAs (mean = 3.6 ± 1.8 cm) than in controls (SC = 0.8 ± 1.3 cm; AC = 0.1 ± 0.5 cm) (P < .01). Hemoglobin, parasite density, and ferritin values are summarized in Table1. Whereas all SAs were parasitemic, of the 41 SCs, 29 (70.7%) were parasitemic, and of the 40 ACs, 10 (25%) were parasitemic. There was no significant difference in the parasite density between cases of SAs and SCs (P = .21). Plasma ferritin was elevated in cases (normal range is 12 to 400 ng/mL for males, 10 to 150 ng/mL for females), which is most likely a reflection of their heightened inflammatory state due to activation of the acute-phase response. In contrast, more than 50% of ACs had a low plasma ferritin (lower than 12 ng/mL). These findings suggest that iron deficiency is very prevalent in this population and that ferritin does not adequately reflect iron stores in patients with severe malarial anemia. A total of 9 cases of severe anemia died during the study, 8 in the hospital and 1 after discharge, giving an overall mortality rate of 21.4%. AS hemoglobin phenotype was found in 8 out of 38 (21.1%) ACs compared with 4 out of 39 (10.3%) SAs and 4 out of 38 (10.5%) SCs (P = .18). A low or intermediate level of RBC glucose-6-phosphate dehydrogenase (G6PD) was found in 1 out of 38 (2.6%) SAs, 5 out of 39 (12.8%) SCs, and 9 out of 39 (23.1%) ACs (P = .01).
Cytofluorometry
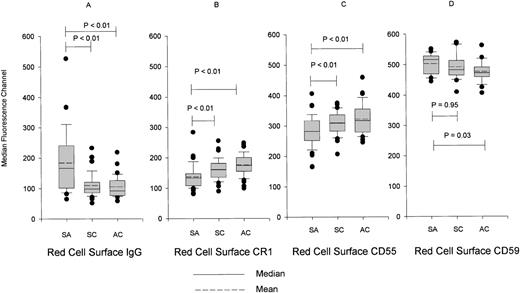
RBCs from patients with severe malarial anemia had more surface IgG upon enrollment than those of controls (Figure 1A). Surface expression of CR1 and CD55 on RBCs from children with severe anemia was lower upon presentation compared with controls (Figures 1B and 1C). The increased surface IgG and the changes in the expression of CR1 and CD55 resolved after treatment with quinine and blood transfusion, suggesting that these abnormalities were acquired as a result of the malaria infection (data not shown). However, we cannot completely exclude the possibility that transfused RBCs contributed to the correction of these abnormalities. Differences were present between cases and SCs in RBC surface IgG, CR1, and CD55 regardless of the parasitemic status of the SCs (data not shown). Surface expression of CD59 was measured in RBCs from 22 volunteers in each group upon enrollment (Figure 1D). Contrary to CR1 and CD55, the surface expression of CD59 was increased in cases of severe anemia compared with ACs (Figure 1D). Furthermore, this increase in CD59 was seen consistently at 2 other time points, prior to discharge and at the 1-month follow-up, indicating that this is unlikely to be a chance finding (data not shown).
Median surface fluorescence of RBCs.
The RBCs were stained for (A) IgG, (B) CR1, (C) CD55, and (D) CD59. RBCs were obtained upon enrollment from cases of severe anemia (SA), symptomatic controls (SC), and asymptomatic controls (AC). Data are presented as box plots with whiskers and outliers. The box represents the interquartile range that contains 50% of values, and the whiskers are lines that extend from the box to the highest and lowest values, excluding outliers. Outliers are cases with values between 1.5 and 3 box lengths from the upper or lower edge of the box. Lines across the box indicate the mean and median.
Median surface fluorescence of RBCs.
The RBCs were stained for (A) IgG, (B) CR1, (C) CD55, and (D) CD59. RBCs were obtained upon enrollment from cases of severe anemia (SA), symptomatic controls (SC), and asymptomatic controls (AC). Data are presented as box plots with whiskers and outliers. The box represents the interquartile range that contains 50% of values, and the whiskers are lines that extend from the box to the highest and lowest values, excluding outliers. Outliers are cases with values between 1.5 and 3 box lengths from the upper or lower edge of the box. Lines across the box indicate the mean and median.
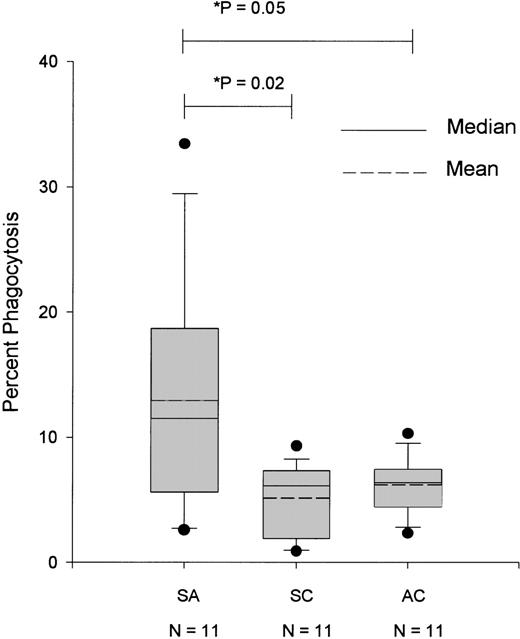
In order to correlate the lesions identified on the surface of RBCs with the extent of their susceptibility to phagocytosis, we labeled the RBCs with the fluorescent dye PKH-26, thus allowing us to detect those monocytic cells that contained engulfed RBCs. Because of the logistical limitations in synchronizing the availability of U-937 cells with the availability of the blood samples, the phagocytosis assay was not performed in all the volunteers. As shown in Figure2, RBCs from patients with severe anemia were more susceptible to phagocytosis compared with RBCs from SCs and ACs. The observed erythrophagocytosis correlated well with the presence of red cell surface IgG (r2 = 0.22,P < .01).
Percentage of U-937 cells with phagocytized RBCs obtained from cases of severe anemia (SA), symptomatic controls (SC), and asymptomatic controls (AC) upon enrollment.
Asterisk indicates t test for unrelated samples with unequal variances.
Percentage of U-937 cells with phagocytized RBCs obtained from cases of severe anemia (SA), symptomatic controls (SC), and asymptomatic controls (AC) upon enrollment.
Asterisk indicates t test for unrelated samples with unequal variances.
Discussion
There is sufficient evidence to suggest that during P falciparum malaria, uninfected RBCs are destroyed at an accelerated rate.5-8 In the present study, we looked for clues of immunologic mechanisms that could account for the premature destruction of red cells in children with severe P falciparum anemia. In these patients, we have identified modifications of the RBCs' surface membranes that consist of the presence of IgG and alterations in the expression of surface CR1, CD55, and CD59. We believe that our analysis supports the conclusion that the surface abnormalities affect both infected and uninfected RBCs because the fluorescence histograms were unimodal and not bimodal, as would be expected from the presence of a separate population of cells with drastically different fluorescence. Furthermore, the surface changes observed are unlikely to come from the sole contribution of infected red cells because more than 80% of volunteers had parasitemias of 4% or less upon enrollment. The median fluorescence that we chose to report is less subject to skewness from the contribution of the small percentage of infected red cells that may have extreme values. It is also noteworthy that we did not find any correlation between the CR1, CD55, CD59, and IgG surface fluorescence and the parasite density.
IgG may come to be deposited on the surface of uninfected RBCs in patients with P falciparum infection as a result of several mechanisms. During the malaria infection, reactive oxygen species (ROS) are produced owing to the effect of tumor necrosis factor or other cytokines.20 Exposure of RBCs to ROS can lead to molecular modifications of the outer cell membrane, as a result of which molecules such as band 3 could become the target of auto-antibodies.21 The host's immunoglobulins may also target malaria antigens attached to the RBC surface. It is equally likely that the antibodies detected are part of immune complexes present on these surfaces.13,22 RBCs coated with antibodies are targeted by resident macrophages of the reticuloendothelial system,23 making this a possible mechanism for some of the phagocytosis observed (Figure 1A). ROS can trigger other RBC surface membrane injuries such as exposure of phosphatidyl serine and/or galactose residues, which induce IgG-independent phagocytosis.19 Regardless of the mechanism of phagocytosis, the consistent observation of splenomegaly in children with severe anemia supports the conclusion that this is an important contributor to the accelerated destruction of RBCs.
In addition to surface antibody, we found alterations in the expression of the complement regulatory proteins CR1, CD55, and CD59 on the RBC surface of patients with severe malarial anemia. The role of these proteins is to protect the surface of host cells from attack by complement.24-27 CR1 is a surface protein that binds C3b in circulating immune complexes, making these complexes available for uptake by macrophages of the reticuloendothelial system.27CR1 also regulates the complement cascade by promoting the inactivation of C3b by factor I. CD55 and CD59 are complement regulatory proteins anchored to the cell surface by phosphatidylinositol.28CD55 prevents the activation of C3 to C3b by accelerating the dissociation of the C3 convertase C4-2a of the classical pathway and C3bBb of the alternative pathway.26 CD59 interferes with the assembly of the membrane attack complex C5b-9.25 The level of expression of these proteins is affected by both genetic and acquired factors. Acquired deficiencies of the RBC complement regulatory proteins are known to occur in various diseases. For example, in the type of hemolytic anemia known as paroxysmal nocturnal hemoglobinuria (PNH), deficiencies in CD55 and CD59 increase the sensitivity of RBCs to complement-mediated lysis.29
CR1 has been recently implicated in rosette formation,30 a phenomenon in which uninfected RBCs bind to infected ones. Strains ofP falciparum that tend to form rosettes have been associated with the occurrence of cerebral malaria.31 Therefore, individuals with low numbers of RBC CR1 living in areas of P falciparum transmission may have a selection advantage owing to the lower risk of cerebral malaria. In the study reported here, CR1 levels were consistently lower in children with severe malarial anemia prior to treatment compared with controls. Because CR1 levels increased following malaria treatment, we believe these changes come about during the infection. However, we cannot rule out a transient correction due to the presence of transfused RBCs. Several other mechanisms could explain how low RBC CR1 and CD55 could come about during malaria infection. CR1 and CD55 are removed from the surface of RBCs during the transfer of immune complexes from cell surface to macrophages as part of the normal process of complement regulation.32 Thus, an acquired deficiency of CR1 and CD55 could occur in association with the formation of circulating immune complexes, as is known to happen in malaria.13 Surface CR1 and CD55 are also known to decrease as RBCs age.33-35 Accelerated RBC aging may be brought about by the combined effect of complement activation and oxidative damage during malaria. It is also possible that a decrease in the number of these molecules may be due to partial or complete cleavage by proteases and/or phospholipases released during schizont rupture. Whatever the mechanism responsible for these changes, it must explain why these changes are more common in patients with severe malarial anemia than in patients with uncomplicated malaria. Additional studies are currently underway in our laboratory to elucidate the mechanism or mechanisms underlying the reductions in CR1 and CD55 in patients with severe malarial anemia.
Unlike CR1 and CD55, RBC surface CD59 was slightly increased in patients with severe anemia compared with ACs. This finding was unexpected and is of unclear clinical significance. It has, however, been shown that CD59 also protects P falciparum–infected red cells from complement-mediated lysis.36 This is also true for normal uninfected RBCs.37 These data, together with ours, suggest that the increase in CD59 may be beneficial to the survival of both infected and uninfected RBCs. We offer 3 possible mechanisms that could explain the increase in CD59 observed in our study. One possibility is that patients with severe malarial anemia may be high expressors of CD59. It is, however, difficult to reconcile the higher expression of CD59 with the presence of severe anemia since the role of CD59 is to protect RBCs from complement-mediated lysis. A second possible explanation is that the apparent increase in the expression of CD59 is the result of reincorporation into the RBC membrane of circulating free CD59 derived from hemolyzed RBCs. This phenomenon has been well described in vitro and has been used to demonstrate the importance of CD59 in reversing the complement susceptibility of PNH red cells.37 A third explanation, and the one we most favor, is that since high levels of RBC surface CD59 may be protective, only those red cells with the highest amount of surface CD59 survive the hemolytic insult; in other words, the lower expressors were destroyed earlier.
It is tempting to postulate that the deficiencies in complement regulatory proteins observed in patients with severe malarial anemia may increase the susceptibility of their red cells to complement-mediated lysis. An added complication of CR1 deficiency is reduced capacity to mop up immune complexes from circulation; these, therefore, become deposited on other cell surfaces and lead to other complications, such as glomerulonephritis.24 The fact that deficiencies in CR1 and CD55 also occur in other conditions characterized by hemolytic anemia and complement activation, such as systemic lupus erythematosus,15 suggests that our findings are of clinical significance.
We do not discount the role that bone marrow dysfunction3,38 and other factors2,4 may play in the pathogenesis of severe malarial anemia. Neither do we ignore the fact that genetic traits such as AS phenotype and G6PD deficiency are protective against severe malaria.39 Our own data, collected in this population, agree with these findings. We demonstrate here that, in addition, the surface of red cells, is modified in individuals with severe malarial anemia by the deposit of surface IgG and by changes in the surface expression of complement regulatory proteins. To the best of our knowledge, this is the first study to link severe malarial anemia to alterations in complement regulatory proteins. We believe that these red cell surface changes are battle scars of the immunologic war against the parasite. These or other lesions may predispose these cells to early destruction by phagocytosis and/or complement activation, thus contributing to the development of anemia. Ironically, therefore, the destruction of much of the red cell mass in malaria may not be due to the direct effect of the parasite but may be the result of the immunologic mechanisms designed to defend the body from this invader.
Acknowledgments
We sincerely thank Alfred Odindo, the late Bonventure Otieno, Solomon Otieno, Fred Onyango, Ramadhan Mtalib, as well as smear readers and the drivers of the Walter Reed Project for their contribution to the successful execution of the study. We are also indebted to the volunteers and their parents for their kind participation.
Supported by the US Army Medical Research and Development Command and an MIM grant from the World Health Organization.
J.N.W. is a senior associate of the National Research Council.
The views of the authors do not purport to reflect the position of the US Department of the Army or the US Department of Defense. The US government has the right to retain a nonexclusive, royalty-free license in and to any copyright covering this paper.
Reprints:José A. Stoute, Unit 64109 Box 401, APO AE 09831-4109; e-mail: stoutej@net2000ke.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.