A D-methionine–containing peptide, Trp-Lys-Tyr-Met-Val-D-Met-NH2 (WKYMVm), featuring a unique receptor specificity was investigated with respect to its ability to activate neutrophil effector functions. The peptide was found to be more potent than the N-formylated peptide N-formyl-Met-Leu-Phe (fMLF) at inducing neutrophil chemotaxis, mobilization of neutrophil complement receptor 3 (CR3), and activation of the neutrophil NADPH-oxidase. The fact that binding of fML[3H]F was inhibited by both fMLF and WKYMVm suggests that N-formyl peptide receptor (FPR) is shared by these peptides. However, the neutrophil response induced by the WKYMVm peptide was insensitive to the fMLF antagonists, cyclosporin H, and Boc-FLFLF that specifically block the function of the FPR. These results suggest that even though WKYMVm may bind FPR the cells are activated preferentially through a receptor distinct from the FPR. Using transfected HL-60 cells expressing either the FPR or its neutrophil homologue FPRL1, also referred to as LXA4R because it has been shown to bind lipoxin A4, we show that WKYMVm is about 300-fold more active at mobilizing intracellular calcium through FPRL1 than through FPR. The WKYMVm activates FPRL1-expressing cells in a cyclosporin H-independent manner with an EC50 of around 75 pmol/L, whereas it activates FPR-expressing cells with an EC50 of around 25 nmol/L. The observation that exudated cells are primed in their response to WKYMVm suggests that FPRL1/LXA4R like FPR is stored in mobilizable organelles.
The human neutrophils play a key role in the innate immune response to infection. They act at inflammatory sites, which they reach after targeting and extravasation from the peripheral blood stream. The extravasation process is induced by different chemoattractants.1-3 These chemoattractants are recognized by G-protein–coupled cell surface receptors, and the second messengers generated by the dissociated Gα and Gβγsubunit complex activate downstream effector molecules.4,5On interaction with the chemoattractants, the neutrophil cytoskeleton is reorganized6,7 to promote cell migration (chemotaxis) and new receptors are subsequently recruited from the granules8 to the cell surface. In addition, chemoattractants have been found to activate the neutrophil NADPH-oxidase resulting in the production of large amounts of toxic oxygen radicals (superoxide anion and hydrogen peroxide9,10). The precise mechanism by which such activation is induced and regulated remains unclear and multiple intracellular signaling pathways have been implicated.2,11,12 However, the degree of activation is dependent on the identity of the agonist and to what extent the receptors involved in the activation process are expressed on the cell surface.13-15
By screening a number of random oligopeptide sequences, Seo and coworkers16,17 recently identified a new group of peptides with a unique receptor specificity. These are hexapeptides with the consensus sequence XKYX(P/V)M that bind to a G-protein–coupled receptor in several leukocyte cell lines, resulting in activation of phospholipase C.16,17 Substitution of the L-methionine at the carboxy-terminus with a D-methionine markedly increased the potency of the peptides17 and the D-methionyl–containing peptide was shown to activate the NADPH-oxidase in peripheral blood neutrophils, provided that the cell cytoskeleton was first disrupted by cytochalasin B.
The aim of this study was to further investigate the biologic activities of the hexapeptide Trp-Lys-Tyr-Met-Val-D-Met-NH2(WKYMVm) in relation to the function of human neutrophils. We found that the peptide is chemotactic for neutrophils, that it induces secretion of granule constituents and subsequently mobilization of adhesion molecules to the cell surface, and that it is a potent activator of the NADPH-oxidase. The receptor activated by WKYMVm was found to be identical to the formyl peptide-like receptor, FPRL1, earlier shown to recognize lipoxin A4 and being referred to as the lipoxin A4 receptor.3 18
Materials and methods
Isolation of human neutrophils
Exudated neutrophils were harvested from skin chambers placed on unroofed skin blister lesions on the volar surface of the forearms of healthy human volunteers as previously described.19 In each experiment, 2 chambers with 3 0.6-mL wells covering the lesions were used. The chambers were filled with autologous serum, and the neutrophils were allowed to accumulate in the chambers for 24 hours. More than 95% of the cells harvested from the chambers were neutrophils.
Blood neutrophils were isolated from heparinized whole blood (obtained from the same person as was carrying the skin chambers) or from buffy coats from healthy blood donors, using dextran sedimentation and Ficoll-Paque gradient centrifugation.20 All cells were washed and resuspended (1 × 107/mL) in Krebs-Ringer phosphate buffer containing glucose (10 mmol/L), Ca++ (1 mmol/L), and Mg++ (1.5 mmol/L) (KRG, pH 7.3). The isolation procedure used allows for cells to be isolated with minimal mobilization effects.21
Neutrophils radiolabeled with 51Cr], using the technique described by Gallin and co-workers,22 were used in transmigration experiments.
Peptides and peptide receptor antagonists
The hexapeptide WKYMVm was synthesized and high-performance liquid chromatography purified by Alta Bioscience (University of Birmingham, UK). The formylated peptides formylmethionyl-leucyl-phenylalanine (fMLF) and formylmethionyl-leucyl-phenylalanyl-lysine (fMLFK) as well as N-t-Butoxycarbonyl-phenylalanine-leucyl-phenylalanine-leucyl-phenylalanine (Boc-FLFLF) were from Sigma Chemical Co (St Louis, MO). Cyclosporin H was kindly provided by Novartis Pharma (Basel, Switzerland). The peptides/receptor antagonists were dissolved in dimethyl sulfoxide to 10−2 mol/L and stored at −70°C until use. Further dilutions were made in KRG. The fML[3H]F (56 Ci/mmol) was purchased from DuPont-New England Nuclear (London, UK).
Receptor binding
Receptor binding of fML[3H]F was determined by centrifugation of cells through an oil layer as described earlier.23 In short, cells were allowed to bind fML [3H]F (final concentration, 2 × 10−8 mol/L) in the presence or absence of nonradioactive peptides (fMLF or WKYMVm) for 15 minutes at 4°C. These cell suspensions were then placed on a mixture of dibutylphtalate and dinanylphtalate (10:3 v/v) in 250 μL Eppendorf tubes. The cells were separated from nonbound peptide by centrifugation (9000 × g for 30 seconds), and the tips of the tubes containing the cells were cut off and put into scintillation vials.
Neutrophil chemotaxis
A modified version of the technique described by Agace and coworkers24 was used to determine neutrophil chemotaxis.25 In short, epithelial cells (KB cells) were cultured in Dulbecco's modified Eagle's medium (GIBCO, UK) supplemented with 200 mmol/L L-glutamine, fetal calf serum, and 100 mg/mL penicillin/streptomycin. Subcultivation was performed twice a week and cells used in the experiments were never older than 25 passages. The KB cells were suspended in culture medium (0.7 × 105 cells/200 μL) and seeded on inverted Transwell inserts (Costar, Cambridge, MA; 12 mm membranes with a pore diameter of 3 μm). The cells were allowed to attach onto the membrane and after 2 days of incubation the cell layers were confluent.
The cell monolayers were inverted and transferred to new 12-well culture plates with a lower reservoir (1.5 mL) containing an attractant. The [51Cr]-labeled neutrophils (0.5 mL containing 106 cells) were added on top of the Transwell filters, and transepithelial migration was quantified by measuring the radioactivity accumulating in the lower reservoir after a 2-hour incubation period. The maximal amount of radioactivity recovered in the lower compartment (achieved with the highest concentration of attractant) was between 25% and 30% of that added to the upper compartment, and the cell-associated radioactivity was never below 98% (determined by centrifugation of the cells in the lower compartment).
Neutrophil chemotaxis was also determined with the agarose technique as described earlier.26
Neutrophil NADPH-oxidase activity
The NADPH-oxidase activity was determined using a luminol/isoluminol-enhanced chemiluminescence (CL) system.27 The CL activity was measured in a 6-channel Biolumat LB 9505 (Berthold Co. Wildbad, Germany), using disposable 4-mL polypropylene tubes with a 0.90-mL reaction mixture containing 1 to 2 × 105 neutrophils. The tubes were equilibrated in the Biolumat for 5 minutes at 37°C, after which the stimulus (0.1 mL) was added. The light emission was recorded continuously. To quantify intracellularly and extracellularly generated reactive oxygen species, respectively, 2 different reaction mixtures were used. Tubes used for measurement of extracellular release of superoxide anion contained neutrophils, horseradish peroxidase (HRP; a cell impermeable peroxidase; 4U), and isoluminol (a cell impermeable CL substrate; 2 × 10−5 mol/L).27 By a direct comparison of the superoxide dismutase (SOD) inhibitable reduction of cytochrome C and SOD inhibitable CL, 7.2 × 107 cpm were found to correspond to a production of 1 nmol of superoxide (a millimolar extinction coefficient for cytochrome C of 21.1 was used; details about the CL technique are given by Lundqvist and Dahlgren27). Tubes used for measurement of intracellular generation of reactive oxygen species contained neutrophils, SOD (a cell impermeable scavenger for O2−; 50 U), catalase (a cell impermeable scavenger for H2O2; 2000 U), and luminol (a cell permeable CL substrate; 2 × 10−5 mol/L).
Mobilization of complement receptor 3
Mobilization of subcellular organelles was followed by measuring the exposure of complement receptor 3 (CR3) on the neutrophil surface. Cells were labeled with phycoerythrin-conjugated monoclonal antibodies specific for CD11b (Becton Dickinson Clone 12 cat.no 347550; 10 μL to a cell pellet of 106 cell), and examined by FACScan (Becton Dickinson, Mountain View, CA).28
Subcellular fractionation
Subcellular fractionation was performed by the method described by Borregaard et al.29 In short, neutrophils were resuspended in relaxation buffer and disintegrated by nitrogen cavitation (Parr Instrument Company, Moline, IL). The postnuclear supernatant was centrifuged on a Percoll gradient of 14 mL solution with a density of 1.05 g/mL, overlaying 14 mL of a solution with a density of 1.12 g/mL. The gradients were collected in 1.5 mL fractions by aspiration from the bottom of the centrifuge tube and the localization of subcellular organelles in the gradients was determined by marker analysis of the fractions. Alkaline phosphatase (ALP) activity was measured by hydrolysis of p-nitrophenyl phosphate (PNPP; 3.5 mmol/L) at pH 10.5 in a sodium-barbital buffer. Subcellular fractions were added to a buffer solution with or without Triton X-100 (0.2% final concentration) and containing the substrate; the samples were then incubated at 37°C for 45 minutes, hydrolysis of the substrate was determined as an increase in absorbance at 410 nm, and the mobilization of the secretory vesicles was determined as a loss in ALP latency (difference in ALP activity in the presence and absence of detergent30). Vitamin B12-binding protein was determined with the cyanocobalamin technique.31 Gelatinase was measured using an enzyme-linked immunosorbent assay (ELISA) method (the antibodies used were a kind gift from Drs Lars Kjeldsen and Niels Borregaard, Copenhagen) and myeloperoxidase (MPO) was determined either by an ELISA assay or from the 472-nm peak of reduced-minus-oxidized difference spectra.29 32
Neutrophil priming
Two different protocols were used for mobilization of neutrophil subcellular organelles to the cell surface. The first cell population was merely incubated at 22°C for 60 minutes without additive.21 32 The second cell population was subjected to stimulation by the calcium ionophore ionomycin. After preincubation of the neutrophils at 37°C for 5 minutes, ionomycin (5 × 10−7 mol/L final concentration) was added and the incubation was continued for 5 minutes. The cell populations were then sedimented by centrifugation and the supernatants were collected for marker analysis (described above). The pellet was suspended in KRG, washed once to remove any prestimulating agent, resuspended to 1 × 107 cells/mL in KRG or relaxation buffer, and put on ice until use.
Stable expression of formyl peptide receptor and formyl peptide-like receptor in HL-60 cells
The pEF-FPR and pEF-FPRL1 expression plasmids were constructed in the following way: The cDNA sequence encoding a FLAG-tagged version of formyl peptide receptor (FPR) was excised from pcDNA3.1 by Nhe I and Xba I, and that encoding FPRL1 was excised by Xba I from CDM8. Inserts were ligated into the pEF-neo plasmid cleaved by Xba I.33Transfection of HL-60 cells was performed by electroporation with a Bio-Rad Gene Pulser apparatus, according to a slightly modified version of the technique described by Tonetti et al.34 In brief, 20 μg of supercoiled plasmid DNA in TE 10 mmol/L Tris-HCl,1 mmol/L EDTA, pH 8.0 were mixed with 107 cells in 0.5 mL of phosphate-buffered sucrose (272 mmol/L sucrose, 7 mmol/L Na2HPO4, pH 7.4). Cells were electroporated with a pulse of 250 V for 18 to 20 ms. Control cells were transfected in the same conditions with the pEF-neo plasmid encoding CXCR2 (the IL-8 receptor). After electroporation, cells were allowed to recover in 20 mL of culture medium for 48 hours before selection in a medium containing G418 (1 mg/mL). The G418 resistant transfected clones were obtained by limited dilution into 24-well microtiter plates and positive clones were identified by their ability to mobilize intracellular calcium on addition of N-formyl-Met-Leu-Phe-Lys (1μmol final concentration) and IL-8 (20 nmol), respectively.
Determination of changes in cytosolic calcium
HL-60 cells at the density of 1 to 3 × 106cells/mL were washed with phosphate-buffered saline (PBS). The cell pellets were resuspended at a density of 2 × 107cells/mL in RPMI medium without phenol red containing 0.1% bovine serum albumin (BSA) and loaded with 2 μmol Fura 2-AM for 30 minutes at 37°C. Cells were then diluted with 2 volumes of the same medium without BSA, washed once in KRG, and resuspended in RPMI medium without phenol red at a density of 2 × 107 cells/mL. Calcium measurements were carried out with a SPEX FluoroMAX fluorescence spectrophotometer (SPEX Industries, Stanmore, UK) with an excitation wavelength of 340 nm, an emission wavelength of 505 nm, and slit widths of 5 and 10 nm, respectively. Maximal and minimal fluorescence were determined in the presence of Triton X-100 and EGTA/Tris-HCl, respectively. Intracellular calcium concentrations were calculated using the following formula: [Ca++] = Kd(F−Fmin)/(Fmax−F) where the Kd for Fura-2 equals 224 nmol/L.
Results
Hexapeptide Trp-Lys-Tyr-Met-Val-D-Met-NH2 induces neutrophil chemotactic activity
The ability of WKYMVm to induce chemotaxis in human neutrophils was investigated using epithelial cells grown on polycarbonate filters as a matrix over which transepithelial gradients of potential chemoattractants were formed. We found that neutrophils migrated through the filter and epithelial cell layer when WKYMVm was present in the lower compartment (Figure1). The peptide was, thus, found to be chemotactic with a potency exceeding that of fMLF.
Chemotactic activity induced by WKYMVm.
Monolayers of KB cells growing on Transwell filters were inverted and transferred to plates with a lower reservoir containing WKYMVm (closed symbols) or fMLF (open symbols). The [51Cr]-labeled neutrophils were added on top of the filters, and transepithelial migration was quantified by measuring the radioactivity accumulating in the lower reservoir after a 2-hour incubation period. Abscissa, agonist concentration; ordinate, transmigration expressed as percentage of cells recovered in the lower compartment. Each symbol represents the mean ± SD of 3 experiments and the values obtained using 10−8 mol/L of the peptides differ significantly (P < .05; paired Studentt test).
Chemotactic activity induced by WKYMVm.
Monolayers of KB cells growing on Transwell filters were inverted and transferred to plates with a lower reservoir containing WKYMVm (closed symbols) or fMLF (open symbols). The [51Cr]-labeled neutrophils were added on top of the filters, and transepithelial migration was quantified by measuring the radioactivity accumulating in the lower reservoir after a 2-hour incubation period. Abscissa, agonist concentration; ordinate, transmigration expressed as percentage of cells recovered in the lower compartment. Each symbol represents the mean ± SD of 3 experiments and the values obtained using 10−8 mol/L of the peptides differ significantly (P < .05; paired Studentt test).
A chemotactic response measured as transmigration through a cell layer may be influenced by the epithelial (or endothelial) matrix formed in or on the polycarbonate filter, we could, however, verify the chemotactic activity of WKYMVm using the underagarose technique (data not shown) in which the neutrophils experience the attractant without any influence from the epithelial cells.
Hexapeptide Trp-Lys-Tyr-Met-Val-D-Met-NH2 induces NADPH-oxidase activity in neutrophils
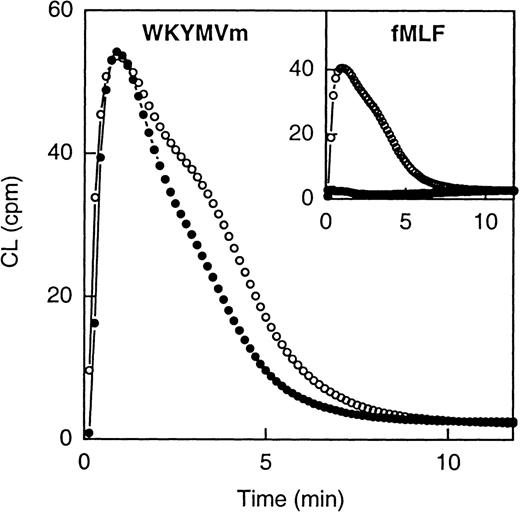
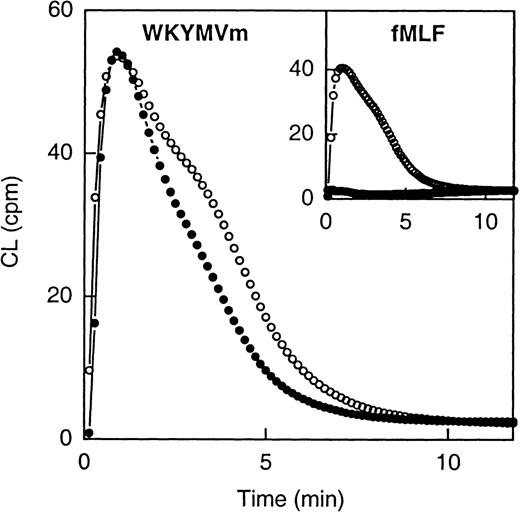
Both WKYMVm and fMLF were able to induce superoxide anion production in human neutrophils (Figure2). The time courses of the 2 responses were very similar, but the EC50 values differed, WKYMVm being the more potent activator having an EC50 of 2 × 10−9 mol/L compared with 5 × 10−8 mol/L for fMLF.
Neutrophil NADPH-oxidase activity induced by WKYMVm and fMLF.
The figure shows the time courses of the neutrophil CL responses induced by WKYMVm (1 × 10−7 mol/L) and fMLF (1 × 10−7 mol; inset), respectively, in the absence (open symbols) or presence (closed symbols) of Boc-FLFLF (1 × 10−5 mol/L). Abscissa, time of study (minute); ordinate, cellular production of superoxide anion expressed in CL units (Mcpm = 106 cpm).
Neutrophil NADPH-oxidase activity induced by WKYMVm and fMLF.
The figure shows the time courses of the neutrophil CL responses induced by WKYMVm (1 × 10−7 mol/L) and fMLF (1 × 10−7 mol; inset), respectively, in the absence (open symbols) or presence (closed symbols) of Boc-FLFLF (1 × 10−5 mol/L). Abscissa, time of study (minute); ordinate, cellular production of superoxide anion expressed in CL units (Mcpm = 106 cpm).
A very low level of intracellular NADPH-oxidase activity was induced by both WKYMVm and fMLF (not shown).
Hexapeptide Trp-Lys-Tyr-Met-Val-D-Met-NH2–induced mobilization of neutrophil complement receptor 3
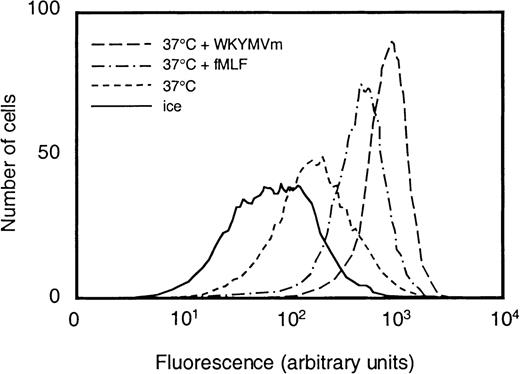
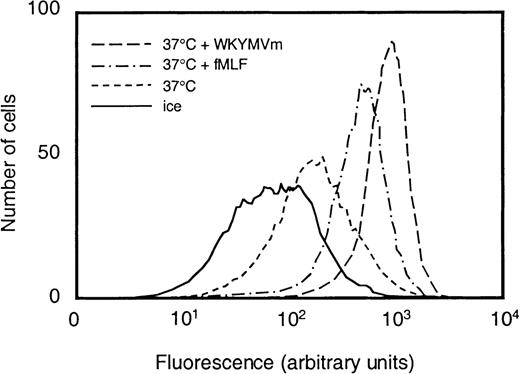
We examined to what extent WKYMVm could induce neutrophil granule mobilization by measuring the exposure of CR3 on the cell surface. Treatment with the hexapeptide at concentrations from 1 × 10−12 to 1 × 10−7mol/L increased the amount of CR3 on the cell surface (shown for 10−7 mol/L in Figure 3). The EC50 value for WKYMVm was 5 × 10−11 mol/L, whereas the corresponding value for fMLF was 2 × 10−9 mol/L.
Surface exposure of CR3 in neutrophils stimulated with fMLF or WKYMVm.
Control neutrophils (kept on ice or at 37°C for 10 minutes) and cells that were stimulated with WKYMVm (1 × 10−7mol/L) or fMLF (1 × 10−7 mol/L), respectively, at 37°C for 10 minutes were paraformaldehyde fixed, incubated with phycoerythrin-conjugated antibodies directed against CR3, and analyzed by flow cytometry. Abscissa, intensity of fluorescence; ordinate, number of cells.
Surface exposure of CR3 in neutrophils stimulated with fMLF or WKYMVm.
Control neutrophils (kept on ice or at 37°C for 10 minutes) and cells that were stimulated with WKYMVm (1 × 10−7mol/L) or fMLF (1 × 10−7 mol/L), respectively, at 37°C for 10 minutes were paraformaldehyde fixed, incubated with phycoerythrin-conjugated antibodies directed against CR3, and analyzed by flow cytometry. Abscissa, intensity of fluorescence; ordinate, number of cells.
Characteristics of the receptor activated by hexapeptide Trp-Lys-Tyr-Met-Val-D-Met-NH2
The cellular responses induced by WKYMVm are in many ways similar to those induced by fMLF. To determine whether WKYMVm acts through a novel receptor or whether it interacts with a unique binding site on the fMLF receptor, we performed receptor-binding analyses and desensitization experiments.
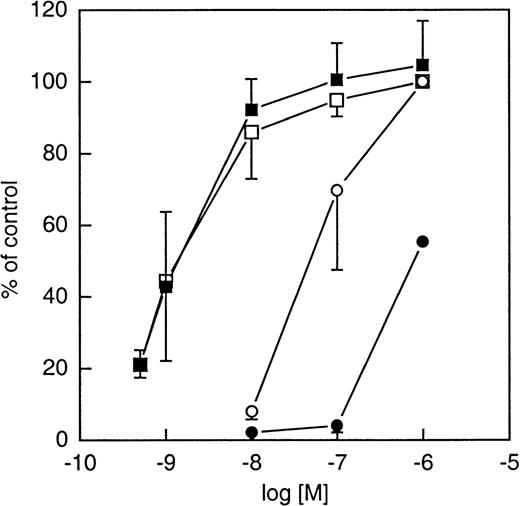
The fMLF antagonists cyclosporin H (CsH) and Boc-FLFLF are known to block the fMLF-evoked responses35,36 (for a review see Ye and Boulay3). As illustrated in Figure4, CsH blocked the response induced by fMLF but had no effect on the response induced by WKYMVm, regardless of the concentration of agonist used. Similar results were obtained with the Boc-FLFLF. The differential effect of the Boc-peptide on the WKYMVm- and fMLF-evoked NADPH-oxidase activation is shown in Figure 2. Although these results suggest that fMLF and WKYMVm act through different receptors, both peptides were able to inhibit fML[3H]F binding to neutrophils, suggesting that fMLF and WKYMVm, nevertheless, share the same receptor (see Figure 5). This paradox can be solved if one considers the existence of a receptor that is activated primarily of WKYMVm.
Effects of cyclosporin H (10−6 mol/L) on the neutrophil NADPH-oxidase activity induced by fMLF (circles) and WKYMVm (squares).
The neutrophils were incubated with (closed symbols) or without (open symbols) cyclosporin H for 5 minutes and then stimulated with different concentrations of the agonists. The respiratory burst activity is expressed in percentage of the activity induced by 10−6 mol/L of the respective peptide in the absence of cyclosporin H and each symbol represents the mean value (± SD) calculated from 5 experiments.
Effects of cyclosporin H (10−6 mol/L) on the neutrophil NADPH-oxidase activity induced by fMLF (circles) and WKYMVm (squares).
The neutrophils were incubated with (closed symbols) or without (open symbols) cyclosporin H for 5 minutes and then stimulated with different concentrations of the agonists. The respiratory burst activity is expressed in percentage of the activity induced by 10−6 mol/L of the respective peptide in the absence of cyclosporin H and each symbol represents the mean value (± SD) calculated from 5 experiments.
Effects of WKYMVm on fML[3H]F binding to neutrophils.
The radiolabeled formylpeptide fML[3H]F (final concentration, 2 × 10−8 mol/L) was allowed to bind in the absence or presence of increasing concentrations of nonlabeled WKYMVm or fMLF and the amount of cell-associated radioactivity was determined after removal of nonbound peptide. The results are expressed as percent inhibition induced by the peptides. The amount of peptide bound in the absence of nonlabeled peptide was 28 ± 6 fmol/106 cells. The figures represent the mean values (± SD) of 4 experiments.
Effects of WKYMVm on fML[3H]F binding to neutrophils.
The radiolabeled formylpeptide fML[3H]F (final concentration, 2 × 10−8 mol/L) was allowed to bind in the absence or presence of increasing concentrations of nonlabeled WKYMVm or fMLF and the amount of cell-associated radioactivity was determined after removal of nonbound peptide. The results are expressed as percent inhibition induced by the peptides. The amount of peptide bound in the absence of nonlabeled peptide was 28 ± 6 fmol/106 cells. The figures represent the mean values (± SD) of 4 experiments.
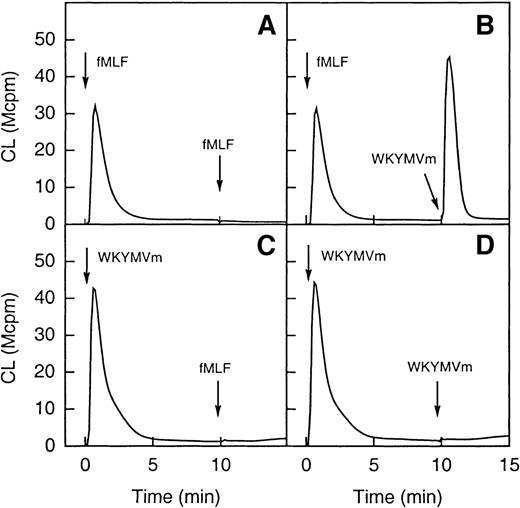
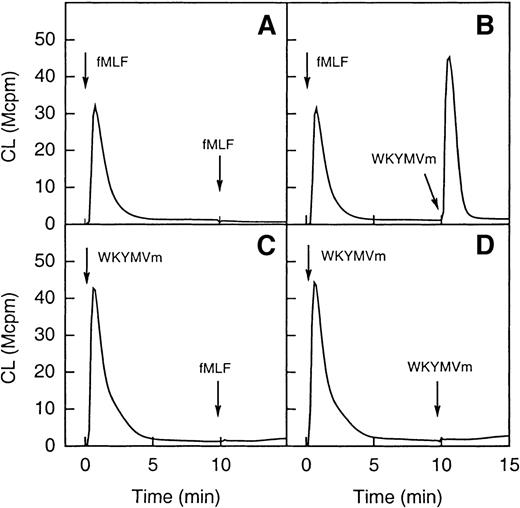
To test this hypothesis, desensitization experiments were performed. On binding of fMLF to its receptor, the occupied receptor is phosphorylated37 and interacts with the cytoskeleton.38-40 Cells are subsequently desensitized and unable to generate oxidase-activating signals through the same receptor. As illustrated in Figure 6 (A and B), after a first stimulation with fMLF, cells were unable to generate a second burst of superoxide when challenged 10 minutes later with fMLF. A robust superoxide production was, however, observed when they were challenged with WKYMVm. When cells were first stimulated with WKYMVm, neither fMLF nor the WKYMVm peptide was able to induce a second burst of superoxide (Figure 6C and D). These results strongly support the hypothesis that the 2 peptides act through different receptors.
Desensitization of neutrophil superoxide production.
In the upper part of the figure (A and B) the cells were first activated with fMLF (10−7 mol/L) and this was then followed (10 minutes later) by a restimulation with the same concentration of either fMLF (A; homologous desensitization) or WKYMVm (B; no desensitization). In the lower part of the figure (C and D) the cells were first activated with WKYMVm (10−7 mol/L) and this was then followed (10 minutes later) by a restimulation with the same concentration of either WKYMVm (C; homologous desensitization) or fMLF (D; heterologous desensitization). The arrows indicate the times for addition of an agonist and the curves are from a representative experiment. Abscissa, time of study (minute); ordinate, cellular production of superoxide anion expressed in CL units (Mcpm = 106 cpm).
Desensitization of neutrophil superoxide production.
In the upper part of the figure (A and B) the cells were first activated with fMLF (10−7 mol/L) and this was then followed (10 minutes later) by a restimulation with the same concentration of either fMLF (A; homologous desensitization) or WKYMVm (B; no desensitization). In the lower part of the figure (C and D) the cells were first activated with WKYMVm (10−7 mol/L) and this was then followed (10 minutes later) by a restimulation with the same concentration of either WKYMVm (C; homologous desensitization) or fMLF (D; heterologous desensitization). The arrows indicate the times for addition of an agonist and the curves are from a representative experiment. Abscissa, time of study (minute); ordinate, cellular production of superoxide anion expressed in CL units (Mcpm = 106 cpm).
Peptide-induced mobilization of intracellular Ca++ in transfected HL-60 cells expressing the formylpeptide receptor or its homolog (LXA4R/FPRL1).
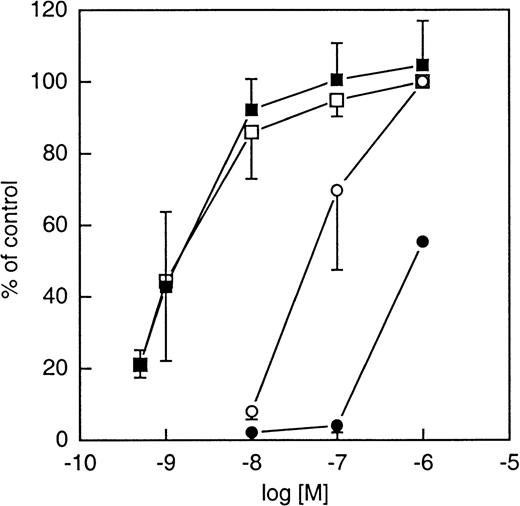
In addition to the high-affinity N-formyl peptide receptor (FPR), human neutrophils express a structurally related receptor originally known as FPRL1. Despite its high degree of sequence similarity with the FPR (69%), FPRL1 binds fML[3H]F with low affinity (Kd > 400 nmol/L) (reviewed in Ye and Boulay3). More recently FPRL1 has been shown to bind lipoxin A4 with high affinity and it is consequently referred to as LXA4R.18To test whether FPRL1/LXA4R was able to interact with WKYMVm, either FPR, FPRL1/LXA4R, or CXCR2 (control cells) was stably expressed in HL-60 cells, a cell line of myeloid origin that does not express these receptors when undifferentiated. As illustrated in Figure 7A (inset), the formylpeptide induced a rise in intracellular calcium in FPR expressing cells with an EC50 of around 5 nmol/L, whereas WKYMVm had an EC50 of around 25 nmol/L. This indicates that FPR is a shared receptor for the 2 peptides. Interestingly, when WKYMVm was assayed with FPRL1/LXA4R–expressing cells, it induced a rise of intracellular calcium with an EC50 around 75 pmol/L, whereas concentrations of the formyl peptide higher than 500 nmol/L were necessary to mobilize the same level of intracellular calcium (Figure 7B, inset). The CsH was found to be inactive on FPRL1/LXA4R–expressing cells (not shown). The fMLFK was found to be a better agonist for FPRL1 than the prototypical peptide fMLF, which was unable to trigger calcium mobilization with concentrations lower than 1 μmol/L (data not shown). Thus, WKYMVm is at least 6 000-fold more potent than the formyl peptide at stimulating the FPRL1/LXA4R. Neither of the 2 peptides induced a calcium increase in transfected HL-60 cells expressing CXCR2.
Changes in cytosolic calcium in undifferentiated HL-60 cells transfected with either the FPR or the lipoxin A4receptor (LXA4R/FPRL1).
Undifferentiated HL-60 cells expressing either FPR or LXA4R/FPRL1 were loaded with 2 μmol Fura-2 and analyzed with respect to the rise in intracellular [Ca++] mediated by the application of peptides. (A) FPR-expressing HL-60 cells have a resting value [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 150 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−7 mol/L; open circles) or WKYMVm (final concentration, 10−7 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. (B) HL-60 cells expressing LXA4R/FPRL1 have a resting [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 300 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−6 mol/L; open circles) or WKYMVm (final concentration, 10−9 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. For both figures, abscissa, time of study; ordinate, concentration of [Ca++]i.
Changes in cytosolic calcium in undifferentiated HL-60 cells transfected with either the FPR or the lipoxin A4receptor (LXA4R/FPRL1).
Undifferentiated HL-60 cells expressing either FPR or LXA4R/FPRL1 were loaded with 2 μmol Fura-2 and analyzed with respect to the rise in intracellular [Ca++] mediated by the application of peptides. (A) FPR-expressing HL-60 cells have a resting value [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 150 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−7 mol/L; open circles) or WKYMVm (final concentration, 10−7 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. (B) HL-60 cells expressing LXA4R/FPRL1 have a resting [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 300 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−6 mol/L; open circles) or WKYMVm (final concentration, 10−9 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. For both figures, abscissa, time of study; ordinate, concentration of [Ca++]i.
Neutrophil priming
The WKYMVm peptide was able to activate the superoxide anion-generating NADPH-oxidase also in exudated human neutrophils. The peptide was added to neutrophils harvested from a skin chamber (exudated cells) and the response in these cells was compared with that induced in neutrophils isolated from peripheral blood. The WKYMVm (as well as fMLF) induced a pronounced extracellular release of superoxide anion in the exudated neutrophils, and these cells were primed compared with the peripheral blood cells (the increase being 346% ± 194% and 612% ± 365% for WKYMVm and FMLF, respectively; mean peak values ± SD, n = 3). The isolation procedure that we use allows for cells to be isolated from peripheral blood with minimal mobilization effects.21 It can, however, not be excluded that the low degree of vesicle mobilization associated with any neutrophil isolation protocol41 affects the cellular response to the WKYMVm peptide, and subsequently, the responsiveness of the peripheral blood cells may be overestimated.
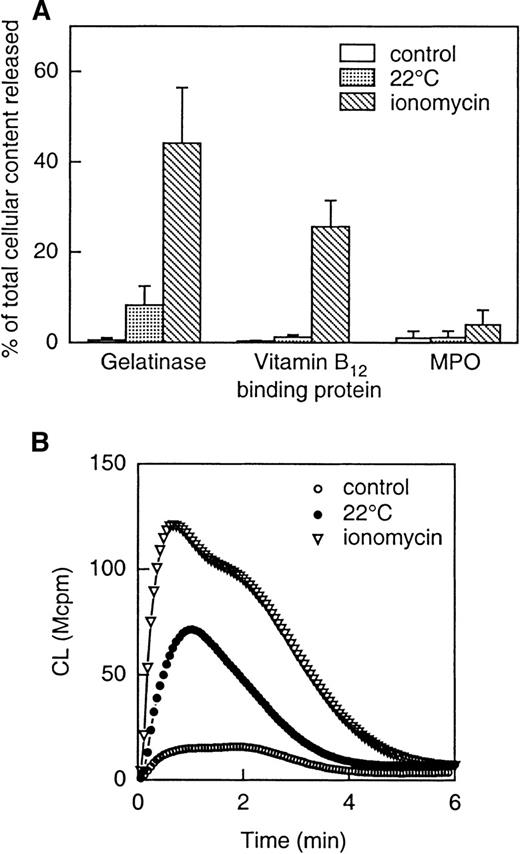
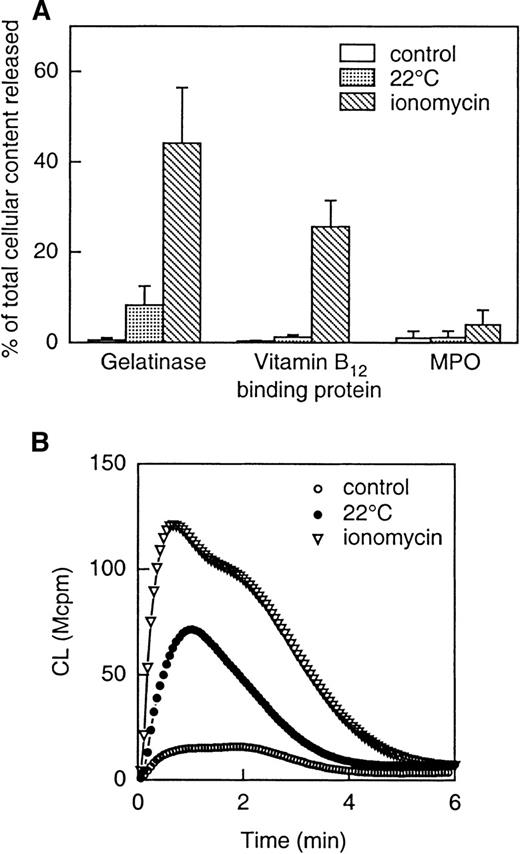
To examine the extent to which mobilization of intracellular granules (and subsequently granule-stored receptors) to the cell surface might explain the increased responsiveness of the exudated neutrophils to WKYMVm, mobilization of vesicles and granules was induced in vitro in peripheral blood neutrophils (see “Methods” for details). The secretory vesicles were mobilized by storage of neutrophils,21,42 ie, by incubating the cells at room temperature for 1 hour. During this process, the secretory vesicles supply the plasma membrane with new surface components, determined as a loss in latency of the alkaline phosphatase activity (not shown). In addition, a slight release of gelatinase from the gelatinase granules was seen in these cells (Figure 8A). The neutrophils stored for an hour were found to be primed when stimulated by WKYMVm (Figure 8B). Further release of gelatinase, along with part of the specific granule content of vitamin B12-binding protein was achieved by stimulating the neutrophils with ionomycin (Figure 8A). Such ionomycin-treated cells were further primed with respect to the NADPH-oxidase activity when stimulated by 2 × 10−9 mol/L WKYMVm (Figure 8B). At this concentration, WKYMVm is unable to mobilize intracellular calcium through FPR (see inset Figure 7A). The fact that an intracellular calcium mobilization is a prerequisite for chemoattractant receptor-mediated oxidative burst43 44 further strengthens our conclusion that the burst induced by WKYMVm does not arise from the activation of FPR.
Release of granule constituents induced by storage of cells (at 22°C) or by ionomycin stimulation (A) and effect of the different priming protocols on NADPH-oxidase activity induced by WKYMVm (B).
The upper part (A) shows the release into the medium of markers for gelatinase granules (gelatinase), specific granules (gelatinase and vitamin B12-binding protein) and azurophil granules (MPO). Neutrophils were treated as follows: (1) incubated on ice (control); (2) incubated at 22°C for 1 hour; (3) incubated with ionomycin (5 × 10−7 mol/L) for 5 minutes at 37°C. The values are given as percent released marker of the total amount in control cells. Data are given as mean ± SD, n = 4. The lower part (B) shows superoxide production induced by WKYMVm (2 × 10−9 mol/L) in neutrophils treated as follows: (1) incubated on ice (control; open circles); (2) incubated at 22°C for 1 hour (closed circles); (3) incubated with ionomycin (5 × 10−7 mol/L; open triangles) for 5 minutes at 37°C. The cells were washed and resuspended in KRG, and superoxide production was followed with the CL technique. Abscissa, time of study (minute), ordinate, and cellular production of superoxide anion are expressed in CL units (Mcpm = 106 cpm).
Release of granule constituents induced by storage of cells (at 22°C) or by ionomycin stimulation (A) and effect of the different priming protocols on NADPH-oxidase activity induced by WKYMVm (B).
The upper part (A) shows the release into the medium of markers for gelatinase granules (gelatinase), specific granules (gelatinase and vitamin B12-binding protein) and azurophil granules (MPO). Neutrophils were treated as follows: (1) incubated on ice (control); (2) incubated at 22°C for 1 hour; (3) incubated with ionomycin (5 × 10−7 mol/L) for 5 minutes at 37°C. The values are given as percent released marker of the total amount in control cells. Data are given as mean ± SD, n = 4. The lower part (B) shows superoxide production induced by WKYMVm (2 × 10−9 mol/L) in neutrophils treated as follows: (1) incubated on ice (control; open circles); (2) incubated at 22°C for 1 hour (closed circles); (3) incubated with ionomycin (5 × 10−7 mol/L; open triangles) for 5 minutes at 37°C. The cells were washed and resuspended in KRG, and superoxide production was followed with the CL technique. Abscissa, time of study (minute), ordinate, and cellular production of superoxide anion are expressed in CL units (Mcpm = 106 cpm).
Discussion
We found the D-methionine–containing hexapeptide WKYMVm to be a potent agonist in terms of chemotactic activity, capability to mobilize neutrophil granules/vesicles, and ability to induce a neutrophil respiratory burst response. This peptide was first identified by Seo and coworkers17 as a molecule that activates PLC in several leukocyte cell lines. The details of the structure-activity relationship of the peptide was determined, and it was found that Met6 was critical for activity. This was illustrated by the fact that when Met6 was either eliminated or replaced by glycin, the resulting peptides were inactive.17 The peptide also depends highly on the presence of a D-methionine at its C-terminus (introduction of an L-type amino acid, replacing the D-Met, increases the EC50 value 100-fold17; our own unpublished observation). Seo and coworkers17 suggested that WKYMVm is recognized by a unique receptor. Here we bring this issue further and determine the identity of this receptor. Cyclosporin H abrogated the fMLF induced cellular response, whereas the WKYMVm-induced response was unaffected. Although CsH is completely unrelated to the N-formyl peptide, it is a potent antagonist of N-formyl peptide binding to the FPR and of functional responses triggered by fMLF,35 and our results, thus, support the suggestion that the WKYMVm peptide activates neutrophils through a receptor different from the FPR. Nevertheless, the mechanisms of activation downstream of the receptors are very similar for these 2 peptides.
After the cloning of FPR from an HL-60 cDNA library,45,46 a gene encoding a human FPR-like receptor (FPRL1) was isolated.47 48 This receptor is expressed also in human neutrophils and shares a high degree of amino acid identity with the FPR, particularly in the signaling cytoplasmic domain. The FPR and FPRL1 are thus likely to transduce the same signals downstream of the receptor but to be activated by different ligands. Here we identify the WKYMVm peptide as a potent activator operating through the FPRL1.
Earlier studies suggest that FPRL1 functions as a neutrophil receptor for the lipoxygenase-derived eicosanoid lipoxin A4,18,49 and the receptor may thus also be termed LXA4R. FPRL1 has recently been shown to recognize also 2 synthetic peptides derived from HIV-1 (a leucine zipper-like domain of the HIV-1 envelope gp 41 and a sequence from the V4-C4 region of gp 120, respectively) and serum amyloid A (SAA).50-52The latter is an acute phase protein exhibiting chemotactic activity for neutrophils as well as monocytes. The biologic activities of LXA4 have been summarized in 2 recent reviews,53,54 and in relation to neutrophils, lipoxin A4 has been suggested to be “anti-inflammatory” as it downregulates for example expression of integrin receptors49 and secretion of granule constituents.55 Although lipoxin A4 does not induce any of the biologic activities in neutrophils shown to result from WKYMVm stimulation, it was reported to chemotactically activate monocytes through FPRL1,56 suggesting differential activation of second messengers in monocytes and neutrophils. The molecular background for the differences in functional responses (and by that in signaling) induced in different cell types during occupation of FPRL1 with an activating (ie, WKYMVm or SAA in neutrophils) or an inhibiting (ie, lipoxin A4 in neutrophils) agonist, respectively, is beyond the scope of this report, but an intriguing subject for future studies.
Although it is clear that fMLF and WKYMVm activate neutrophils through different receptors (FPR and FPRL1/LXA4R, respectively), it is obvious that WKYMVm binds also to the FPR. This is based on the following: Cross desensitization was obtained with cells desensitized with WKYMVm, ie, the fMLF response was downregulated by WKYMVm (Figure5). Furthermore, transfected HL-60 cells (used as expression vehicles) expressing FPR were activated by both peptides. The most direct interpretation of these results is that WKYMVm binds not only to FPRL1/LXA4R on the neutrophils but also to FPR and that this binding results in desensitization to fMLF. It should, however, be mentioned that cross (heterologous) desensitization has been described for other chemoattractants known to act through different receptors.57-60 Whether heterologous desensitization is involved also in WKYMVm-induced cross desensitization remains to be determined.
The reason for neutrophils to recognize D-methionine–containing peptides is not as obvious as the reason for recognizing bacterial-derived N-formylated peptides.61 Natural proteins normally include only L-amino acids, however, some exceptions exist: the cell wall of gram-negative bacteria contains D-alanine and a number of small oligopeptides made as antibiotic agents by a variety of micro-organisms containing D-amino acids.62,63 The WKYMVm receptor may thus have evolved to recognize and find microbial intruders; however, it is appropriate to add a reservation to this hypothesis as several natural ligands (earlier mentioned) completely unrelated to our synthetic peptide have been described to bind to FPRL1. A reservation as to whether fMLF is the natural ligand for the FPR can also be made.64 65
We show that the WKYMVm peptide is a very potent activator of the neutrophil NADPH-oxidase in exudated cells. These cells are primed, not only in response to the hexapeptide, but to various inflammatory mediators, chemoattractants, and other ligands.19,23,66-69 The primed response achieved through extravasation may be a regulatory mechanism, granting a cellular response (such as release of toxic oxygen radicals) only at sites where it is functional and necessary, eg, in inflamed or infected areas. During the extravasation process, a number of different inflammatory mediators are generated,70,71 and the receptors for mediators such as IL-8 and C5a are downregulated in exudated cells.70 The fact that exudated cells are primed (and not downregulated) with respect to the NADPH-oxidase activity induced by WKYMVm as well as by fMLF indicates that natural agonists for FPR and FPRL1/LXA4R are not generated during the propagation of an aseptic inflammation. Although the molecular mechanism(s) responsible for induction of the primed state is unclear,72-74 the exposure of new receptors is an attractive model as molecular basis for an augmented response. We know from an earlier study75 that intracellular organelles are mobilized to the cell surface during extravasation, resulting in an increased exposure of various receptors, including the FPR.68,76 The molecular background to the primed response to WKYMVm may, thus, be the result of an increased exposure of LXA4R/FPRL1, but a definite proof of that will have to await determination of this receptor's precise subcellular localization. That the cellular response increased with increasing granule mobilization is an additional fact supporting receptor up-regulation as a probable cause of the primed response to WKYMVm, and these results also indicate that the receptors are stored in the secretory vesicles and possibly also in the other mobilizable organelles, a distribution that parallels that of FPR.75 77
In summary, we show that the EC50 values (which allow for a direct comparison of the potency of neutrophil activators in a given functional assay) for WKYMVm are lower than those for the widely studied formylated peptide fMLF, suggesting that WKYMVm is the more potent neutrophils activator of the two. Our study also demonstrates several important functional features of the WKYMVm peptide: (1) it is a new chemoattractant; (2) it mobilizes intracellular stores containing the integrin receptor for complement factor iC3b; (3) it is a potent activator of the NADPH-oxidase, especially in exudated neutrophils; and (4) the peptide has a neutrophil receptor, FPRL1, that structurally and functionally resembles the N-formyl peptide receptor (FPR). WKYMVm is a cheap, easy to handle, and very potent activator of FRPL1/LXA4R that will undoubtedly be of great help for screening of antagonists for this receptor.
Acknowledgment
The skillful technical assistance of Lisbeth Björck is gratefully acknowledged.
The work of the Swedish group was supported by the Swedish Medical Research Council, the King Gustaf V 80-Year Foundation, the Fredrik and Ingrid Thuring Foundation, the Lars Hierta Foundation, the Anna-Greta Crafoord Foundation for Rheumatological Research, the Vårdal Foundation, and The Swedish Society of Medicine. The work of the French group was supported by grants from the Commissariat à l'Energie Atomique, the Centre National de la Recherche Scientifique (CNRS), and the University Joseph Fourier.
Reprints:Claes Dahlgren, The Phagocyte Research Laboratory, Department of Medical Microbiology and Immunology, Guldhedsgatan 10, S-413 46 Göteborg, Sweden; e-mail:claes.dahlgren@microbio.gu.se.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Chemotactic activity induced by WKYMVm. / Monolayers of KB cells growing on Transwell filters were inverted and transferred to plates with a lower reservoir containing WKYMVm (closed symbols) or fMLF (open symbols). The [51Cr]-labeled neutrophils were added on top of the filters, and transepithelial migration was quantified by measuring the radioactivity accumulating in the lower reservoir after a 2-hour incubation period. Abscissa, agonist concentration; ordinate, transmigration expressed as percentage of cells recovered in the lower compartment. Each symbol represents the mean ± SD of 3 experiments and the values obtained using 10−8 mol/L of the peptides differ significantly (P < .05; paired Studentt test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1810.005k06_1810_1818/6/m_bloo00506001x.jpeg?Expires=1769097263&Signature=swDC1u0iZ8rKrXdwRJxUZv6IMpV-f24KXz-VBZ6wKMOq7MyF-7aU7U24oM7ItDoaP1xcrG~npJoVfKAmj6OgqNw1~zwenz2Ux8ag1MOqC2GwPnkcRnGvPS~FWsj1j0U~BmuyA082vBvuFQzR0yMHr79ItktPazMP5jB-hhQISG15dTldyYlvN-dYjWb-tnjsFSt5w5tuYfven50M7S62MJwXZCO3sNhfx0swTO7ADmXt4u6gPuNAVC8uq6RJokgRTBY8ZfQgcTpZEjLXsezJJQpMJDwZ6TRy-gFdxr3oZ5U6UE1dhYHWf2qwsgbT8mkeInzK5K0Pqxelldx9TruRCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effects of WKYMVm on fML[3H]F binding to neutrophils. / The radiolabeled formylpeptide fML[3H]F (final concentration, 2 × 10−8 mol/L) was allowed to bind in the absence or presence of increasing concentrations of nonlabeled WKYMVm or fMLF and the amount of cell-associated radioactivity was determined after removal of nonbound peptide. The results are expressed as percent inhibition induced by the peptides. The amount of peptide bound in the absence of nonlabeled peptide was 28 ± 6 fmol/106 cells. The figures represent the mean values (± SD) of 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1810.005k06_1810_1818/6/m_bloo00506005x.jpeg?Expires=1769097263&Signature=oVzjJ~hWFu~~huqRM9DcM8zSOe5zUlsmJie3tEewH9xXphsR4C9ZaocVuuIY0SZjqP13vrkDS0COrIzUnhjm5fPB2QdwkOl-ZjHqbvNjE21MicvBocKt4wPqXPPyCBGF6t~IiYz1gq907Vi2iyzkMymO3c96ubRU6sB50Q2ChaWhOTeaqqx8Gy3lq-7QCussyJF2jbrKf6K9Bssg002q4uPe8pWN9k2F9wUK4Ssyo8RrRLlhTiiXJ7mF6ckxSJKKWyp1ceR6nJW05fdwaYlcDh6UNTpfxCk~DI4~Lcb4Vyw5uFKE2H-To~VFDTqbKYATC9S6wPOvtM55QSozRRswYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Changes in cytosolic calcium in undifferentiated HL-60 cells transfected with either the FPR or the lipoxin A4receptor (LXA4R/FPRL1). / Undifferentiated HL-60 cells expressing either FPR or LXA4R/FPRL1 were loaded with 2 μmol Fura-2 and analyzed with respect to the rise in intracellular [Ca++] mediated by the application of peptides. (A) FPR-expressing HL-60 cells have a resting value [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 150 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−7 mol/L; open circles) or WKYMVm (final concentration, 10−7 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. (B) HL-60 cells expressing LXA4R/FPRL1 have a resting [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 300 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−6 mol/L; open circles) or WKYMVm (final concentration, 10−9 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. For both figures, abscissa, time of study; ordinate, concentration of [Ca++]i.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1810.005k06_1810_1818/6/m_bloo00506007ax.jpeg?Expires=1769097263&Signature=is3c5Yw0yZpRBOc1Kc7YojmwOPN0EkYrjkrBX07mdaEow6lo7hNZC4j-z8aU-kQOnsUTixCgZ68HuSin99zE6NqROEYZV8tRlPvuMK6WhYqFVCuegohwdcg4VudWA5ydBA-1k1e-gAf-CfVwM6qWjAuJMNVf1BeP7uDxeHsZFoaW5L08g8B4fIEfl3VELOJkP2Yc6AlazmMYidQ1haZ5JhaCYP7EaVpugGHY4KKQNk8N~LYzkCRlJM6rbsaCZun41XIcAeYkwcScCaM1RY48GT7ftXDxbVi-PwXBuf6nGLlcvy3IyyS4OTG5HbjskTzGcEVXfkXSw5IyXOXUfeqs1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Changes in cytosolic calcium in undifferentiated HL-60 cells transfected with either the FPR or the lipoxin A4receptor (LXA4R/FPRL1). / Undifferentiated HL-60 cells expressing either FPR or LXA4R/FPRL1 were loaded with 2 μmol Fura-2 and analyzed with respect to the rise in intracellular [Ca++] mediated by the application of peptides. (A) FPR-expressing HL-60 cells have a resting value [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 150 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−7 mol/L; open circles) or WKYMVm (final concentration, 10−7 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. (B) HL-60 cells expressing LXA4R/FPRL1 have a resting [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 300 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−6 mol/L; open circles) or WKYMVm (final concentration, 10−9 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. For both figures, abscissa, time of study; ordinate, concentration of [Ca++]i.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1810.005k06_1810_1818/6/m_bloo00506007bx.jpeg?Expires=1769097263&Signature=YIxFB4ZrDbCYH~ejaqJzl7BQUhZ3m5WJMrG0el4nTMEFONDDu8G7cRjnep6x9z2cDIO40E716oQdRkuqvN0Pv9uydlcfRBDsayTcDaMvipfwwu2gbEJnhTqkPf8c-Tll~vwxkx4AENvwNvlZ0-3xq3GkgHgbjnHu-ML9cK--GEQ7bsmM1hauCDlzBeZfEKJZ3f4hYnTLSZmNywsSEhtYXepTRGQaH0cjnp3mpYVBUfyl2-MiSVnP33WFoOijvpksxrpyfbPeSTHmeyUumufHY5lYK5AgoEVXxrxUsAmyKWknuvGfmvf55FV0lC0RvKyWOcfTXbrh~Nv1cAfgI6779A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Chemotactic activity induced by WKYMVm. / Monolayers of KB cells growing on Transwell filters were inverted and transferred to plates with a lower reservoir containing WKYMVm (closed symbols) or fMLF (open symbols). The [51Cr]-labeled neutrophils were added on top of the filters, and transepithelial migration was quantified by measuring the radioactivity accumulating in the lower reservoir after a 2-hour incubation period. Abscissa, agonist concentration; ordinate, transmigration expressed as percentage of cells recovered in the lower compartment. Each symbol represents the mean ± SD of 3 experiments and the values obtained using 10−8 mol/L of the peptides differ significantly (P < .05; paired Studentt test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1810.005k06_1810_1818/6/m_bloo00506001x.jpeg?Expires=1769167981&Signature=RBQpotb2qnlu-wTDfWyblAUkeTCDbE6a5-1PCyXsMbCz-DKh-tImx1g9RwzgCOl401CkrQd-mcmn529B2t27PoTIE7hBphZg-TDnZknHNbu2Ffja1zOMUe2-eW3vrXBQE8mQeQWk6imfgej481-o26MlnipnWJ9hCw2mOmDd6oUpWJbByz4LsUfY3Lo5hurXPGn0Usd3pRSF42pD1V9m4diocIc3TQLal3QsKWtHIiNaj2j14KFV5QgUhaWy7wAFeWA8zcfQ6L5mALM-u3NoC6yGASPzjNpg7yPy70p6tlRlyU~v6WC0oLO8ytCt7yIxN7UdDIa9mPuyGa8lwshsuQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effects of WKYMVm on fML[3H]F binding to neutrophils. / The radiolabeled formylpeptide fML[3H]F (final concentration, 2 × 10−8 mol/L) was allowed to bind in the absence or presence of increasing concentrations of nonlabeled WKYMVm or fMLF and the amount of cell-associated radioactivity was determined after removal of nonbound peptide. The results are expressed as percent inhibition induced by the peptides. The amount of peptide bound in the absence of nonlabeled peptide was 28 ± 6 fmol/106 cells. The figures represent the mean values (± SD) of 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1810.005k06_1810_1818/6/m_bloo00506005x.jpeg?Expires=1769167981&Signature=n3n3XiE2uo3bP8JaVr52unt4j4GjZWOKi66wnhRNY826tigBOR03evGbIEcHneIWrZmNxIxLi205NYZ7En9Ehpf1qhNsYT5EYUwcuYiFpZ5PvZsJYLlMz8lA9CBUTY1qkZt7NTUEi9QLcILjDOB-rsMBoU8HfDpdzWR9u0PsjkgQjnkKDhQ~XxS2vl-1On5kzEq9B8w~1SDTMQ4IFGAMj4wRpDk6C1NkjSrnk8S-AjkiJtpq6aqk4DxDTV4aQ2r-Oe6fE1HutWVggy6WrRayLsILta7sMiLSZJWcpC5XhqlvejkdTG46-U9--BZ2LAWQc70DxXTUyUQt-NscW9omAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Changes in cytosolic calcium in undifferentiated HL-60 cells transfected with either the FPR or the lipoxin A4receptor (LXA4R/FPRL1). / Undifferentiated HL-60 cells expressing either FPR or LXA4R/FPRL1 were loaded with 2 μmol Fura-2 and analyzed with respect to the rise in intracellular [Ca++] mediated by the application of peptides. (A) FPR-expressing HL-60 cells have a resting value [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 150 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−7 mol/L; open circles) or WKYMVm (final concentration, 10−7 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. (B) HL-60 cells expressing LXA4R/FPRL1 have a resting [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 300 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−6 mol/L; open circles) or WKYMVm (final concentration, 10−9 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. For both figures, abscissa, time of study; ordinate, concentration of [Ca++]i.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1810.005k06_1810_1818/6/m_bloo00506007ax.jpeg?Expires=1769167981&Signature=Syet9aEebShFXnP4RWy3xx~2hnZmTFr0KfmG4U4diSJtEFa0IzxA9iVUHKMSWzTuJZjBO~zANb5NtDt3wkWfO0P7Pv77D0rJKzLM3yApfLYA9KJd4lEQCAfhGa8gUAd~DRyXvJcSB7eSycHwZrtSCXYJLPAKnhQQ6ZchV9GdBwbuJ-2MmcnYS44O7MiaiGomGLKmbuVQgX3A7cn6pdTmU1tpNzkHLpCO8CyHBAv2Qtmarqt84s4Nz2Rx~RTMEN7X4GXOOAfcg-lsDqPmDEb1ybGLnzn3LIFaA6VWn8t4b-V0bmaUriqUHLOumRd1ofpKl-7GDAVqOLiqVdUUbmZHnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Changes in cytosolic calcium in undifferentiated HL-60 cells transfected with either the FPR or the lipoxin A4receptor (LXA4R/FPRL1). / Undifferentiated HL-60 cells expressing either FPR or LXA4R/FPRL1 were loaded with 2 μmol Fura-2 and analyzed with respect to the rise in intracellular [Ca++] mediated by the application of peptides. (A) FPR-expressing HL-60 cells have a resting value [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 150 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−7 mol/L; open circles) or WKYMVm (final concentration, 10−7 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. (B) HL-60 cells expressing LXA4R/FPRL1 have a resting [Ca++]i around 75 nmol/L. A concentration of [Ca++]i around 300 nmol/L was reached 15 to 20 seconds after addition of fMLFK (final concentration, 10−6 mol/L; open circles) or WKYMVm (final concentration, 10−9 mol/L; closed circles).Inset: The [Ca++]i increase (peak value in percent of maximal value) induced by different concentrations of fMLFK (open circles) and WKYMVm (closed circles), respectively. For both figures, abscissa, time of study; ordinate, concentration of [Ca++]i.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1810.005k06_1810_1818/6/m_bloo00506007bx.jpeg?Expires=1769167981&Signature=49KcZR0zh1mn4NS~cgGIv-gBaWK26d4b3pWtl1jBQ4e7UUJkaJ2Xp6i1HCAe5qtNHYn5alySZTfJzpkuIHlU1z1FjcAQ-kuSJR9InLglCXQzIY3zdm8AMQqR9pdyVE-jGbV6PPIUo2U8sQ2zakywuX2Lk-Jp66Qbp5o8QH9TVHzafaEBV2Re9EWHUR9xC5m99-ZP~8wN~3u0GyW32NqctgraHl-~aysnEN5n5fx87LR5oZp2LNOv7d~dvpGu7eYNoiD0s8ZAoqsjcS9MiqL0A24xjpjz1j0pIBQ7xuEDSAGJI8qVZnQ6OnXdFCWd11CRwShBbwcX-o5npkKKU5-Zgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)