A low cation conductance and a high anion conductance are characteristic of normal erythrocytes. In sickle cell anemia, the polymerization of hemoglobin S (HbS) under conditions of low oxygen tension is preceded by an increase in cation conductance. This increase in conductance is mediated in part through Ca++-activated K+ channels. A net efflux of potassium chloride (KCl) leads to a decrease in intracellular volume, which in turn increases the rate of HbS polymerization. Treatments minimizing the passive transport of ions and solvent to prevent such volume depletion might include inhibitors targeting either the Ca++-activated K+ channel or the anion conductance. NS1652 is an anion conductance inhibitor that has recently been developed. In vitro application of this compound lowers the net KCl loss from deoxygenated sickle cells from about 12 mmol/L cells/h to about 4 mmol/L cells/h, a value similar to that observed in oxygenated cells. Experiments performed in mice demonstrate that NS1652 is well tolerated and decreases red cell anion conductance in vivo.
The polymerization of hemoglobin S (HbS) has been shown to be extremely concentration dependent.1 The formation of sickle cells is enhanced when solute is lost, since changes in intracellular salt content are accompanied by the transport of water in the form of an isotonic or near isotonic solution, leading to an increase in the intracellular concentration of hemoglobin.2
When the red blood cells of patients with sickle cell anemia enter areas of the circulation where the oxygen tension is low, the cells lose solute due to increased net transmembrane cation fluxes. At least in part, this is due to an increase in the cation conductance resulting from the opening of Ca++-activated K+ channels. It is not clear that this potassium channel is ever activated under normal physiologic conditions. However, due to an influx of calcium, this pathway is probably activated when sickle cells are deoxygenated.3,4 As a consequence, HbS polymerizes, causing red cells to sickle and to be less deformable, thus impairing their flow through the capillary beds.5 Therapeutic interventions have been centered on the dilution of HbS, either through the stimulation of the synthesis of fetal hemoglobin, HbF,6 or through the inhibition of electrolyte loss through the blockade of Ca++-activated K+ channel currents.3 7-9 The rationale for the latter approach is that polymerization of HbS occurs after a lag time that is extremely concentration-dependent. Even a slight decrease in the rate of solute loss increases this lag time enough to delay the onset of sickling until the cells have passed through the capillaries, thus avoiding occlusion.
The normal human red cell has a high conductance for anions,10 such as a conductance of 25 μS/cm2 for chloride,11 and a very low conductance for cations. As a consequence, increases in potassium conductance can result in a massive loss of salt. Since the passive transport of potassium is dependent on the kinetics of the movement of this ion as well as those of counter-ions,12 inhibition of the anion pathway by application of inhibitors of chloride conductance may represent another approach to controlling the net loss of salt.
It has been shown that decreasing the anion conductance using 4, 4′ diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) lowers the potassium loss after activation of the Ca++-activated K+ channel.13 However, until now, targeting the anion conductance in vivo has not been practical due to the lack of suitable high affinity inhibitors of the red cell anion conductance. A new compound, NS1652, has recently been synthesized and seems promising in this regard.14 Here we describe the effect of this potent reversible inhibitor of conductance on human sickle red cells in vitro and on murine red cells in vivo.
Materials and methods
Reagents
Valinomycin, DIDS, CCCP (carbonylcyanide-m-chloro-phenyl-hydrazone), and NEM (N-methyl-maleimide) were obtained from Sigma (Vallensbokstrand, Denmark) and NS1652 (2-(N′-trifluoromethylphenyl)ureido)benzoic acid) was synthesized at NeuroSearch14 (Ballerup, Denmark). For the in vitro experiments, all chemicals were prepared as stock solutions in DMSO and diluted to the final concentration by direct addition to the cell suspension. The final DMSO concentration was 0.3%, a concentration that affected neither the membrane potentials nor the ion fluxes reported in this study. All basic salts were of analytical grade or higher. Cremophore was from Basis Kemi A/S, Copenhagen. A 5% (w/v) solution prepared in water was used for the injections.
Erythrocytes
Blood from healthy human donors, A/A cells (the authors), or from homozygous sickle cell patients, S/S cells (informed volunteers), were drawn into heparinized vacuum tubes. They were then washed 4 times with unbuffered saline to remove the buffy coat and plasma proteins. Following the last centrifugation, the packed cells were stored on ice in an oxygenated environment until use.
Determination of membrane potential
The CCCP method was used for the determination of membrane potential.15 16 When erythrocytes are suspended in nominally buffer-free solution in the presence of the protonophore CCCP, changes in the membrane potential are reflected by changes in extracellular pH, since protons are kept at equilibrium across the membrane. The membrane potential can thus be estimated from:
Due to the high red cell buffer capacity, the intracellular pH remains constant (a7.2) throughout an experiment and can be estimated as the pH of the solution after lysis with Triton-X-100 at the end of the experiment.
Standard experimental procedures
Ionophore-induced net efflux.
Red cells were washed 3 times in 10 volumes of wash buffer. After the last wash, the cells were transferred to Eppendorf centrifuge tubes, centrifuged for 30 seconds at 20,000× g, and the remaining buffer was aspirated off. The cells were then stored on ice as packed cells (estimated cytocrit 95%). For studies, 100 μL of packed cells were added to 3 mL of experimental solution (2 mmol/L K+, 154 mmol/L Na+, 156 mmol/L Cl−) to give a final cytocrit of 3.2%. CCCP (20 μM final concentration) and test compound were added, and a KCl net-efflux was induced after 1 minute by increasing the potassium conductance, usually by application of 0.1 μM valinomycin.
Potassium net fluxes.
were estimated from changes in the extracellular K+-concentration determined by flame photometry (Radiometer, Copenhagen, Denmark). The instrument was calibrated against standard solutions. Red cell suspensions (100 μL) were added to ice-cold phthalate-containing tubes (400 μL phthalate, 873 μL stop-buffer). The tubes were then inverted several times and centrifuged for 30 seconds at 20,000× g. Using this procedure, the K+ efflux is stopped by isolation of the cells as a pellet below the phthalate oil, whereas the extracellular solution is diluted in the aqueous phase. Potassium was measured against an internal Li-standard of 3.00 mmol/L contained in stop-buffer. Since the standard dilution factor for the flame photometer is 200 and the dilution factor for the phthalate tube samples is 10, the signal is consequently amplified 20 times, resulting in a functional sensitivity of 5 μM.
Conductance.
Assuming zero current conditions at the peak of the ionophore-induced membrane hyperpolarization (dV/dt = 0; IK = −ICl), the K+ or l− chord conductance can be calculated according to:
or
where JK is the K+-efflux, ECl and EK the K+ and Cl− equilibrium potentials, and F is Faradays constant.
Spontaneous efflux.
The buffer contained 149 mmol/L Na+, 2 mmol/L K+, 5 mmol/L MOPS, 5 mmol/L glucose, 0.05 mmol/L Ca++, and 0.1 mmol/L ouabain. This buffer was adjusted to a pH of 7.0 at 38°C. Red cells were suspended at a hematocrit of 10% in the reaction buffer and were pre-equilibrated at 38°C for 30 minutes before addition of NS1652 at time = 0. Deoxygenation by a continuous stream of argon (saturated with water at 38°C) was initiated 105 minutes after the start of the experiment. Samples of 400 μL of the suspension were taken every 15 minutes, transferred to Eppendorf centrifuge tubes, centrifuged for 30 seconds at 20,000×g, and the supernatant was diluted with stop-buffer as described above. Hemolysis was followed by photometric determination of the hemoglobin content of the extracellular solution.
KCl co-transport.
KCl co-transport was induced by incubating a 10% suspension of red cells for 30 minutes with 1 mmol/L NEM in nitrate medium. After incubation, the cells were washed 3 times in 10 volumes of the actual efflux medium before the experiment was started.
In vivoexperiments.
NS1652 was suspended in a carrying vehicle, cremophore (pig-40 hydrogenated castor oil, CAS nr. 61788/85/0), at a concentration of 5 mg/mL. At time zero, an amount corresponding to 1% of animal weight (about 250 μL of suspension) was injected into mice though the tail veins (NMRI strain, 5-6 weeks, from Bomholtgård, Gl, Ry, Denmark). At several time intervals after the injection, the mice were decapitated and the blood collected was collected and centrifuged for 60 seconds. The plasma was removed by aspiration and the packed cells were stored on ice until use. Immediately before measurement, the packed cells were resuspended in 1 volume of ice-cold experimental medium and centrifuged for 30 seconds. A total of 100 μL of packed cells were then immediately transferred to 3 mL medium, and CCCP and valinomycin added. The blood samples were analyzed in random order with respect to the time of decapitation.
Correction for number of binding sites
Determination of inhibition constants for compounds binding to the anion exchange/conductance sites in a suspension of red cells represents a special problem, since the number of binding sites is comparable to the number of molecules of inhibitor. It can be assumed that the number of binding sites on a red cell is 1.2 ∗ 106/cell as determined by binding of 3H-DIDS.17 Since 1 L of packed cells contains 1.1 ∗ 1013 cells, the number of binding sites in 1 L of packed cells equals 1.3 ∗ 1019 sites, corresponding to 21.2 μmol/L packed cells.
Inhibitor constants are determined by a fit to
where [I] is the actual concentration of inhibitor. This concentration can be expressed as:
where IT is the total concentration added, and IB the bound fraction.
Assuming the binding of the inhibitor to follow a standard competitive reaction, the bound amount can be expressed as:
where CT is the concentration of binding sites.
Inserting Equation 3 into Equation 2 leads to:
which after rearranging gives:
The solution of this is:
where the + sign gives physical meaningful results. This expression should be substituted into Equation 1. Under standard experimental conditions, 100 μL cells in 3 mL buffer, CT has a value of 0.6835 μmol/L.
Results
Studies with normal red blood cells
To investigate the properties of the novel compound, NS1652 (Figure1), this compound was tested on human red cells with regard to conductive fluxes. Two different methods were used: (1) determination of the red cell membrane potential as a function of the NS1652 concentration following an induced increase in the potassium conductance and (2) concomitant determination of K+ fluxes.
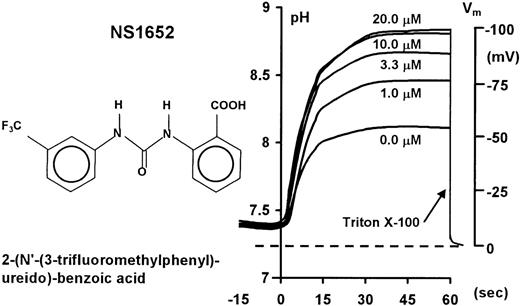
Right panel. Carbonylcyanide-m-chloro-phenyl-hydrazone–mediated pH traces (left axis) and corresponding membrane potentials (right axis) from suspensions of normal erythrocytes (A/A) showing the increasing hyperpolarization due to inhibition of the chloride conductance with increasing concentrations of NS1652 (0, 1.0, 3.3, 10, and 20 μM) under standard experimental conditions.
The valinomycin concentration was 10−7 mol/L. All experiments were stopped by addition of Triton-X-100 to determine the pHi and thereby the corresponding zero membrane potential. Left panel: Chemical structure of NS1652.
Right panel. Carbonylcyanide-m-chloro-phenyl-hydrazone–mediated pH traces (left axis) and corresponding membrane potentials (right axis) from suspensions of normal erythrocytes (A/A) showing the increasing hyperpolarization due to inhibition of the chloride conductance with increasing concentrations of NS1652 (0, 1.0, 3.3, 10, and 20 μM) under standard experimental conditions.
The valinomycin concentration was 10−7 mol/L. All experiments were stopped by addition of Triton-X-100 to determine the pHi and thereby the corresponding zero membrane potential. Left panel: Chemical structure of NS1652.
The red cell potassium conductance was manipulated by addition of either the Ca++ ionophore A23187, which activates the Ca++-activated K+ channel, or by the addition of the K+ ionophore valinomycin. Figure 1 shows typical experimental pH traces obtained with valinomycin-treated cells in the presence of NS1652. Using high concentrations of NS1652, the maximal hyperpolarization approached the calculated equilibrium potential for K+ (−110 mV) and the initial K+ efflux was reduced by more than a factor of 10.
As shown in Figure 1, the hyperpolarization observed increased in a dose-dependent manner. Since this hyperpolarization could be caused both by an increase of gK+ or by a decrease of gCl−, the flux was estimated from the change in the extracellular K+ concentration, and the conductance calculated according to the equations for calculation of the chord conductances. Although NS1652 inhibited the rate of K+efflux, both when valinomycin or A23187 were used, the K+conductance was unaffected. In the case of the Ca++-activated K+ conductance these values were 48.2 ± 1.70 (SD) without NS1652 and 44.0 ± 1.13 (SD) at 10 μM NS1652. Consequently, the hyperpolarization is due to inhibition of the chloride conductance. The Cl− ground conductance was found to be 25 μS/cm2, in accordance with previous estimates obtained with the CCCP technique.11 18This conductance was inhibited by NS1652 with an apparent inhibitor constant, IC50, of 0.62 μM and an insensitive fraction of 0.066, corresponding to a maximal inhibition of a95% (Figure 2A). The data were fitted to a single-site inhibitor equation of the form:
where capital G represents the relative conductance.
(A) The relative Cl− conductance for normal erythrocytes versus the concentration of NS1652.
The solid line represents the best fit to a single-site inhibitor equation with IC50 = 0.62 μM, and an insensitive fraction (GCl([∞])) of 0.066. (B) Effect of NS1652 on valinomycin-induced erythrocyte dehydration. Erythrocytes were suspended at a cytocrit of 10% and incubated with various concentrations of NS1652 or 4,4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) for 2 minutes before addition of 5 ∗ 10−8 mol/L valinomycin. After 5 minutes of valinomycin incubation, the cytocrits were measured. The solid line represents the best fit of the NS1652 data (⧫) to a single-site inhibitor equation. The IC50value was estimated at 1.3 μM, the relative volume after valinomycin incubation in absence of blocker (V[0]) was 74.2%, and the relative volume at saturating concentrations of NS1652 (V[∞] + V[0]) was 93.1%. DIDS data (□) are shown for comparison only.
(A) The relative Cl− conductance for normal erythrocytes versus the concentration of NS1652.
The solid line represents the best fit to a single-site inhibitor equation with IC50 = 0.62 μM, and an insensitive fraction (GCl([∞])) of 0.066. (B) Effect of NS1652 on valinomycin-induced erythrocyte dehydration. Erythrocytes were suspended at a cytocrit of 10% and incubated with various concentrations of NS1652 or 4,4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) for 2 minutes before addition of 5 ∗ 10−8 mol/L valinomycin. After 5 minutes of valinomycin incubation, the cytocrits were measured. The solid line represents the best fit of the NS1652 data (⧫) to a single-site inhibitor equation. The IC50value was estimated at 1.3 μM, the relative volume after valinomycin incubation in absence of blocker (V[0]) was 74.2%, and the relative volume at saturating concentrations of NS1652 (V[∞] + V[0]) was 93.1%. DIDS data (□) are shown for comparison only.
Due to the high permeability of red cells for water, a net salt loss is coupled to loss of cellular water, and an inhibitor of Cl− conductance should be able to restrict the salt as well as the cell volume loss induced by valinomycin. Figure 2B shows the effect of various concentrations of NS1652 on the change in fractional cellular volume (cytocrit) in a suspension of red cells incubated for 5 minutes with valinomycin. Data obtained with DIDS are included for comparison. The fractional volumes at 5 minutes were fitted to an equation of the form:
An IC50 value of 1.3 μM was found for the decrease of volume loss at 5 minutes. Since the hematocrit in the conductance experiments was 3.2% compared to 10% in the experiments on volume loss, and the number of binding sites for the inhibitor are roughly on the same order of magnitude as the number of inhibitor molecules, the IC50 values are of comparable potency at infinite dilution (see “Materials and Methods”).
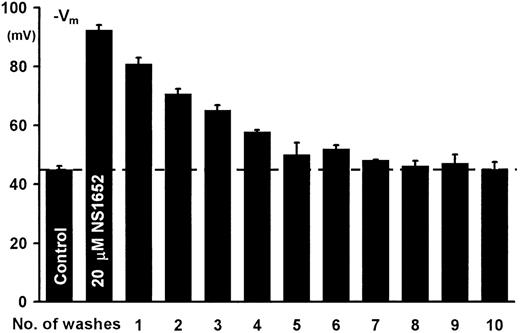
To verify that the effect of NS1652 was reversible, a total of 60 mL of erythrocyte suspension (cytocrit 3.2%) was incubated at 37°C. Initial aliquots of 3 mL were transferred to the experimental chamber for determination of valinomycin-mediated hyperpolarization. NS1652 was added to a concentration of 20 μM and the suspension incubated for 15 minutes. Aliquots of 3 mL were then distributed to centrifuge tubes, and valinomycin-induced hyperpolarization was determined for each pair. The remaining samples were centrifuged at 4500× g for 10 minutes and 2.5 mL of supernatant was replaced by an equal volume of an identical salt solution. After resuspension, the cells were allowed to equilibrate for 5 minutes at room temperature before the next analysis/centrifugation step. The procedure was repeated until complete recovery of the Cl-conductance was obtained. Figure3 shows that NS1652 is a completely reversible inhibitor of the red cell Cl-conductance, since repeated washing restores the valinomycin-induced hyperpolarization to control levels in contrast to the irreversible effect of DIDS.
Reversibility of the NS1652-mediated Cl−conductance block.
From left to right, valinomycin-induced hyperpolarization before addition of inhibitor, in presence of 20 μM NS1652, and washout profiles for NS1652. Error bars show SD for 2 determinations.
Reversibility of the NS1652-mediated Cl−conductance block.
From left to right, valinomycin-induced hyperpolarization before addition of inhibitor, in presence of 20 μM NS1652, and washout profiles for NS1652. Error bars show SD for 2 determinations.
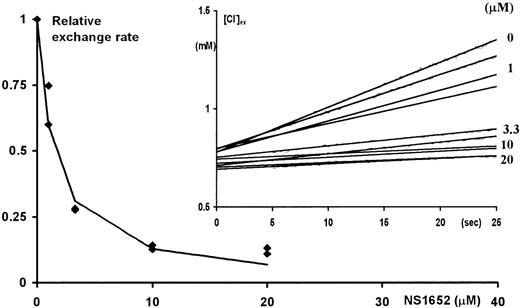
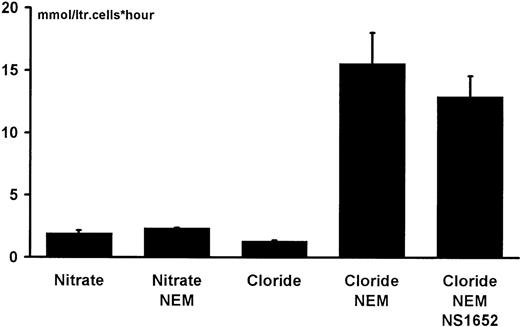
To verify whether NS1652 had an effect on the obligatory anion-exchange mechanism, the effect of the compound was investigated in Cl−/SO42− hetero-exchange experiments at room temperature. In contrast to Cl−/Cl− exchange, which even at room temperature is very fast, the hetero-exchange approach makes it possible to use an extracellular Cl−-sensitive electrode to measure the changes in extracellular Cl−concentrations. The insert in Figure 4shows the extracellular Cl− concentration as a function of time after injecting packed erythrocytes into isotonic low (0.5 mM) Cl−/high SO42− salt solution containing various concentrations of NS1652. The rate of appearance of Cl− in the extracellular phase is clearly slowed by NS1652, indicating an inhibition of the anion exchange process. Figure4 itself shows the initial rate of the Cl effluxes plotted against the concentrations of NS1652. An IC50 value of 1.49 μM was estimated for inhibition of the exchanger. To further characterize its specificity, tests were performed to see if 10 μM NS1652 had an effect on the NEM-induced KCl co-transport. As can be seen from Figure5, the effect, if any, is very small.
NS1652 effect on Cl−/SO42− exchange in A/A erythrocytes.
Initial Cl− fluxes (calculated between 5 and 15 seconds after injection of the cells) versus the concentration of NS1652 (0, 1, 3.3, 10, or 20 μM). At time zero, 100 μL packed cells were transferred to vigorously stirred 3 mL isotonic SO42− exchange solutions containing various concentrations of NS1652. The increase in the extracellular Cl− concentration (insert) was followed by on-line recording of the potential from a calomel-Ag/AgCl electrode pair. The electrodes were calibrated by Cl− standards immediately before and after the exchange experiments. NS1652 had no effect on electrode sensitivity. The extrapolation of the curves to zero intercept at a higher extracellular chloride concentration than 0.5 mmol/L due to the trapped volume of high-chloride medium between the packed cells.
NS1652 effect on Cl−/SO42− exchange in A/A erythrocytes.
Initial Cl− fluxes (calculated between 5 and 15 seconds after injection of the cells) versus the concentration of NS1652 (0, 1, 3.3, 10, or 20 μM). At time zero, 100 μL packed cells were transferred to vigorously stirred 3 mL isotonic SO42− exchange solutions containing various concentrations of NS1652. The increase in the extracellular Cl− concentration (insert) was followed by on-line recording of the potential from a calomel-Ag/AgCl electrode pair. The electrodes were calibrated by Cl− standards immediately before and after the exchange experiments. NS1652 had no effect on electrode sensitivity. The extrapolation of the curves to zero intercept at a higher extracellular chloride concentration than 0.5 mmol/L due to the trapped volume of high-chloride medium between the packed cells.
KCl co-transport in mmol/L cells/h estimated from the changes in the extracellular potassium concentration following suspension of NEM-treated cells
(10% cytocrit) in a low-potassium (2 mmol/L) medium containing 0.1 mmol/L ouabain. The concentration of NS1652 was 10 μM. Bars indicate SD of duplicate experiments.
KCl co-transport in mmol/L cells/h estimated from the changes in the extracellular potassium concentration following suspension of NEM-treated cells
(10% cytocrit) in a low-potassium (2 mmol/L) medium containing 0.1 mmol/L ouabain. The concentration of NS1652 was 10 μM. Bars indicate SD of duplicate experiments.
Studies on sickle red blood cells
Dose-response experiments were performed using red blood cells obtained from homozygotes for HbS to verify whether NS1652 was as effective an inhibitor of the Cl conductance in oxygenated as well as deoxygenated sickle erythrocytes as in normal cells after application of valinomycin. Furthermore, experiments were performed to demonstrate whether NS1652 was able to lower the spontaneous cellular salt loss, which occurs when a suspension of sickle cells is deoxygenated. Dose response experiments are shown in Figure 6. The uninhibited Cl conductances were identical for both oxygenated and deoxygenated cells and were in the normal range. NS1652 inhibited the conductance of both oxygenated and deoxygenated sickle cells with potencies close to the values found for normal red cells.
Inhibition of Cl− conductance in sickle (S/S) erythrocytes by NS1652.
Dose-response curves showing the effects of NS1652 on the Cl− conductance in deoxygenated (▪) as well as oxygenated (⧫) sickle cells. A suspension of erythrocytes (cvf = 30%) was deoxygenated in a humidified argon atmosphere for 2 hours. The cells were then packed and stored on ice in a tightly sealed argon-filled vial until use; 100 μL deoxygenated, packed cells were quickly transferred to 3 mL deoxygenated experimental salt solution. The suspension was kept under argon throughout the experiment. The oxygenated cells were handled in parallel, but exposed to the normal atmosphere. Experimental details are otherwise as in Figure 1.
Inhibition of Cl− conductance in sickle (S/S) erythrocytes by NS1652.
Dose-response curves showing the effects of NS1652 on the Cl− conductance in deoxygenated (▪) as well as oxygenated (⧫) sickle cells. A suspension of erythrocytes (cvf = 30%) was deoxygenated in a humidified argon atmosphere for 2 hours. The cells were then packed and stored on ice in a tightly sealed argon-filled vial until use; 100 μL deoxygenated, packed cells were quickly transferred to 3 mL deoxygenated experimental salt solution. The suspension was kept under argon throughout the experiment. The oxygenated cells were handled in parallel, but exposed to the normal atmosphere. Experimental details are otherwise as in Figure 1.
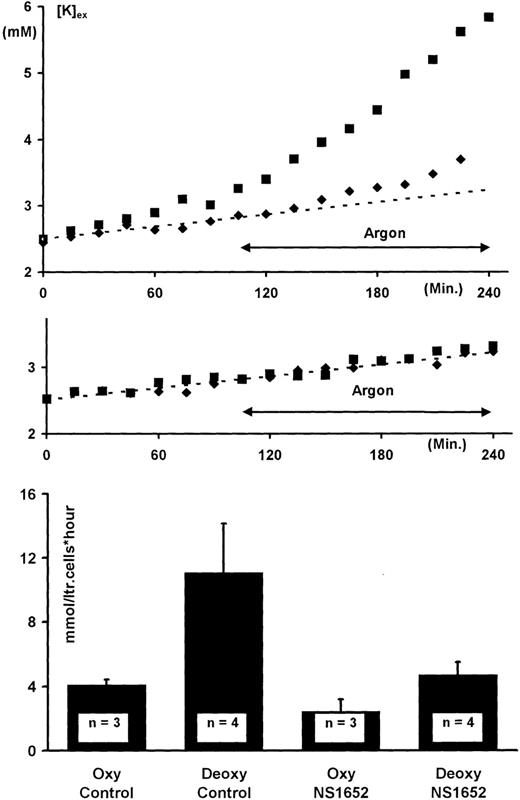
Figure 7 shows the effect of NS1652 on the passive K+ efflux from normal and sickle erythrocytes under oxygenated as well as deoxygenated conditions. The basal K+efflux from healthy cells is insensitive to deoxygenation, which can be seen from the middle panel of Figure 7, showing a linear increase in the extracellular K+ concentration, independent of the deoxygenation by the introduction of argon gas. In contrast, the corresponding efflux from sickle cells was increased up to 4-fold by deoxygenation, as demonstrated by the accelerated rise in extracellular K+ (Figure 7, upper panel). This stimulation was largely blocked in the presence of 10 μM NS1652. In summary, as shown by the bar diagram, Figure 7 lower panel, the application of 10 μM NS1652 to sickle cells reduces the K+ loss during deoxygenation to values close to the level found in the oxygenated state. Hemolysis, determined as the increase of hemoglobin in the extracellular solution, progressed linearly during the experiment, probably due to the action of the stirring magnet, and at the end of the experiments was about 1%. The contribution to the extracellular K+ compartment was thus insignificant.
Effect of NS1652 on deoxygenation-induced cation fluxes.
Normal or sickle erythrocytes were suspended (hematocrit = 10%) in an oxygenated, buffered, ouabain-containing (0.1 mmol/L) salt solution for 105 minutes before deoxygenation was initiated by application of a humid stream of argon. Upper panel: The effects of deoxygenation and NS1652 (10 μM) on the net K+ efflux from sickle (S/S) erythrocytes. Middle panel. Similar experiment as in (A) except with normal (A/A) erythrocytes. The extracellular K+concentration (Y-axis, upper and middle panel) was followed as a function of time (X-axis) by flame photometry on samples of the extracellular solution taken every 15 minutes. Control (▪) as well as NS1652-containing suspensions (⧫) were run in parallel. The broken line (middle panel) is the linear regression curve to the data obtained with the normal cells. This line has been superimposed on the data from sickle cells (upper panel). Lower panel: Net potassium effluxes per liter cells per hour, calculated by linear regression to the data points in the linear phases (0-105 minutes for oxygenized cells, 135-240 minutes for deoxygenized cells) of efflux experiments as shown in the panels above. Text boxes indicate the number of experiments; error bars indicate SD.
Effect of NS1652 on deoxygenation-induced cation fluxes.
Normal or sickle erythrocytes were suspended (hematocrit = 10%) in an oxygenated, buffered, ouabain-containing (0.1 mmol/L) salt solution for 105 minutes before deoxygenation was initiated by application of a humid stream of argon. Upper panel: The effects of deoxygenation and NS1652 (10 μM) on the net K+ efflux from sickle (S/S) erythrocytes. Middle panel. Similar experiment as in (A) except with normal (A/A) erythrocytes. The extracellular K+concentration (Y-axis, upper and middle panel) was followed as a function of time (X-axis) by flame photometry on samples of the extracellular solution taken every 15 minutes. Control (▪) as well as NS1652-containing suspensions (⧫) were run in parallel. The broken line (middle panel) is the linear regression curve to the data obtained with the normal cells. This line has been superimposed on the data from sickle cells (upper panel). Lower panel: Net potassium effluxes per liter cells per hour, calculated by linear regression to the data points in the linear phases (0-105 minutes for oxygenized cells, 135-240 minutes for deoxygenized cells) of efflux experiments as shown in the panels above. Text boxes indicate the number of experiments; error bars indicate SD.
In vivo murine experiments
The acute toxicity and the functional pharmacokinetics of NS1652 were tested by intravenous administration to mice. In prior in vitro experiments (not shown) it was found that, although the chloride conductance of murine red cells was somewhat higher than that of human red cells, the pattern of inhibition was identical. The compound showed no acute toxicity at doses up to at least 250 mg/kg (single doses, 3 days' survival), and there were no behavioral side effects observed. Toxicology experiments were performed in rats as well: In these animals, no effects were observed on blood pressure or heart rate (data not shown). Figure 8 shows the valinomycin-induced hyperpolarization obtained with suspensions of murine erythrocytes isolated at various times after injection of NS1652 (50 mg/kg). Injection of the cremophore was without effect on the standard hyperpolarization obtained after 1 minute. However, after NS1652 injection, the cells hyperpolarized to about −90 mV, indicating a block >90% of the Cl− conductance. The NS1652 effect declined fairly steeply after the injection, and the Cl− conductance normalized after 2 hours. It should be noted that the observed values are somewhat lower than the actual in vivo values due to the wash (see Figure 3), and do not necessarily exactly mirror the in vivo conductances. The experiments are qualitative rather than quantitative with regard to the feasibility of in vivo inhibition of the red cell anion transport system.
Transient in vivo inhibition of the murine erythrocyte Cl− conductance.
The mice were intravenously dosed with NS1652 (50 mg/kg) or vehicle (5% W/v cremophore) at time 0. At 1, 5, 30, and 120 minutes after injections, 3 mice were killed and their blood collected in heparin and immediately centrifuged. The vehicle-injected animals were killed after 1 minute. The packed erythrocytes were separated from the plasma and stored on ice until use (1-2.5 hours). Erythrocytes from uninjected control animals were processed similarly. Immediately before analysis, the packed cells were resuspended in 1 vol experimental salt solution and centrifuged, and 100 μL were transferred to 3 mL experimental solution for recording of hyperpolarization induced by a fixed valinomycin concentration (5 ∗ 10−7 mol/L). The individual blood samples were analyzed at random. ○ indicates control animals; □ indicates cremophore-injected animals; ⧫ indicates NS1652-injected animals. n = 3 for each group. Data are means ± SD. Broken line indicates the mean of control and cremophore-injected animals.
Transient in vivo inhibition of the murine erythrocyte Cl− conductance.
The mice were intravenously dosed with NS1652 (50 mg/kg) or vehicle (5% W/v cremophore) at time 0. At 1, 5, 30, and 120 minutes after injections, 3 mice were killed and their blood collected in heparin and immediately centrifuged. The vehicle-injected animals were killed after 1 minute. The packed erythrocytes were separated from the plasma and stored on ice until use (1-2.5 hours). Erythrocytes from uninjected control animals were processed similarly. Immediately before analysis, the packed cells were resuspended in 1 vol experimental salt solution and centrifuged, and 100 μL were transferred to 3 mL experimental solution for recording of hyperpolarization induced by a fixed valinomycin concentration (5 ∗ 10−7 mol/L). The individual blood samples were analyzed at random. ○ indicates control animals; □ indicates cremophore-injected animals; ⧫ indicates NS1652-injected animals. n = 3 for each group. Data are means ± SD. Broken line indicates the mean of control and cremophore-injected animals.
Discussion
It is generally accepted that sickle cells respond to deoxygenation with increased net cation fluxes, leading to dehydration. It is probable, however, that more than 1 pathway is involved in this process. Mechanisms attracting attention as possible major pathways are the sodium/potassium pump acting on sodium/potassium leaks induced by deoxygenation,19 the KCl co-transport system,20,21 and the Ca++-activated K+ channel.3
The Ca++-activated K+ channel has recently been the target of successful symptomatic treatment aimed at reducing cell shrinkage by reducing the K+ efflux through the use of clotrimazole as inhibitor.8 Since this pathway involves the transport of a cation, it is dependent on a concomitant transport of counterions. In principle, it should be possible to obtain the same effect by a down-regulation of the anion conductance.
The present experiments demonstrate the feasibility of volume control of red cells using the new red cell anion conductance inhibitor NS1652, which has been shown to selectively decrease the chloride conductance. Comparison of the corrected IC50 observed for NS1652 inhibition of the chloride conductance on normal red cells with that of other conductance inhibitors demonstrates that it is a powerful agent, with an IC50 lower than that found for DIDS for reversible inhibition.18 Contrary to DIDS, the long-term action of NS1652 is reversible, as shown by washout experiments. The potency of NS1652 for inhibition of the chloride conductance is paralleled by its potency for reducing the rate of solute loss in the presence of valinomycin, and it should be noted that the latter experiment was performed without CCCP, ruling out interference from this compound.
Parallel experiments performed on sickle cells showed that these cells had a chloride conductance identical to the one found for healthy human red cells, in accordance with previous reports.22 Identical IC50 values for inhibition of the chloride conductance by NS1652 were found. As in the case of DIDS, a fraction of about 5% seems to be insensitive to NS1652. Furthermore, it has been shown that the anion exchange pathway in sickle red cells seems to be unaffected by this condition.22 Thus it seems reasonable to assume that the properties of sickle cells with regard to conductive anion transport are identical to normal red cells. Furthermore, it has been shown that NS1652 has little if any effect on the NEM-induced KCl co-transport, which supports the notion that the mechanism behind the reduction in net salt loss from deoxygenated sickle red cells is a block of the chloride conductance.
Even using a high potency inhibitor of the chloride conductance, however, it is not certain that an appreciable effect on the salt and concomitant solute loss can be attained. This is based on the fact that the increase in the K+ conductance estimated on the basis of the flux acceleration for deoxygenated sickle cells is rather modest. Based on theoretical arguments it has been shown that if the increased efflux is due to a slight increase of the cellular cation conductance in a homogeneous population, only an extremely high degree of inhibition of the anion pathway suffices to make the anion pathway rate limiting, and thus capable of lowering the volume loss.12 If, however, an apparent small increase in potassium conductance (mean conductance) is the result of a considerable increase in only a short time or in a small subpopulation, inhibition of the anion conductance leads to a proportional decrease of the net efflux. Experiments have been presented indicating that a population of identical cells can behave in a nonhomogeneous fashion; the calcium entry, which activates the K+ channel, is a stochastic all-or-none process.23 This means that the small increase in mean conductance is based on a full activation of a small fraction of the cells at a time leaving the rest unaffected.
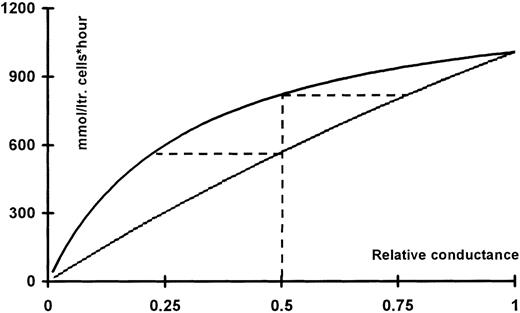
Assuming maximal activation of the Ca++-activated K+ channels at about 10% (37°C24 and 150 channels per red cell25 with a single-channel conductance of 10 pS26) results in a potassium conductance of 85 μS/cm2. This estimate corresponds nicely with the value of 62 μS/cm2 determined directly using an experimental setup of the same type as that used for the present experiments.27 Using a chloride conductance of 25 μS/cm2 11 and equilibrium potentials of −15 and −95 mV, the fluxes at a given level of inhibition of either the Cl−- or the K+conductance can be calculated as described above (see Figure9). It is apparent from Figure 9 that, given this mode of operation, inhibition of the conductive chloride fluxes is more efficient at a given degree of inhibition than inhibition of the potassium conductance at the same level. The broken lines indicate that a 50% inhibition of the potassium conductance results in the same flux as 25% inhibition of the chloride conductance. Correspondingly, a 50% inhibition of the chloride conductance gives the same flux reduction as an 80% block of the potassium conductance.
KCl fluxes calculated as function of the relative conductance for potassium (upper trace) or chloride (lower trace).
For a given curve, the conductance of the co-ion is assumed to have its ground value. The ground values used for normalization were: gK 85 μS/cm2 and gCl 25 μS/cm2. The corresponding equilibrium potentials were taken to be −95 and −15 mV, respectively. The horizontal broken lines originating from the 50% level intercept the flux curve for the co-ion at a value that would result in the same level of flux reduction.
KCl fluxes calculated as function of the relative conductance for potassium (upper trace) or chloride (lower trace).
For a given curve, the conductance of the co-ion is assumed to have its ground value. The ground values used for normalization were: gK 85 μS/cm2 and gCl 25 μS/cm2. The corresponding equilibrium potentials were taken to be −95 and −15 mV, respectively. The horizontal broken lines originating from the 50% level intercept the flux curve for the co-ion at a value that would result in the same level of flux reduction.
The interpretation supported by the present work is that NS1652 seems to be a rather specific inhibitor for anion exchange and conductance, having no effect on basal cation limited fluxes, the KCl co-transporter, or the Ca++-activated K+channel. Nonetheless, 10 μM of NS1652 is able to reduce a spontaneous efflux of about 11 mmol/L cells/h from deoxygenized sickle red cells by at least 50% (see Figure 7, lower panel, in this case also in the absence of CCCP). Assuming that an 11 mmol/L cells/h transport is due to pathways involving the movement of ions, the corresponding mean potassium conductance can be calculated to be about 0.15 μS/cm2. Using this value, even a 95% inhibition of the chloride conductance would result in a far lower effect.
It has thus been demonstrated, in accordance with existing evidence, that at least part of the increase in net fluxes occurring when sickle cells are deoxygenated results from ion movement. Furthermore, it has been shown that a possible target for a symptomatic treatment of sickle cell anemia is moderation of red cell volume loss by inhibition of anion conductance. This method represents an alternative to inhibition of the Ca++-activated potassium channel. In many respects, NS1652 shows properties that may be desirable for a compound with the potential for symptomatic treatment of sickle cell anemia using the anion conductance as target. However, our work has centered on the use of this compound to demonstrate the principle, rather than on the application as a pharmaceutical. Future work will need to be performed to address this possibility.
Acknowledgments
We thank Henrik Olesen for the supply of sickle erythrocytes; Inge Hüttel for performing the animal experiments; and Søren Johansen for expert assistance with the in vitro experiments.
Reprints:Poul Bennekou, The August Krogh Institute, University of Copenhagen, Universitetsparken 13, 2100 Denmark; e-mail:pbennekou@aki.ku.dk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 2. (A) The relative Cl− conductance for normal erythrocytes versus the concentration of NS1652. / The solid line represents the best fit to a single-site inhibitor equation with IC50 = 0.62 μM, and an insensitive fraction (GCl([∞])) of 0.066. (B) Effect of NS1652 on valinomycin-induced erythrocyte dehydration. Erythrocytes were suspended at a cytocrit of 10% and incubated with various concentrations of NS1652 or 4,4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) for 2 minutes before addition of 5 ∗ 10−8 mol/L valinomycin. After 5 minutes of valinomycin incubation, the cytocrits were measured. The solid line represents the best fit of the NS1652 data (⧫) to a single-site inhibitor equation. The IC50value was estimated at 1.3 μM, the relative volume after valinomycin incubation in absence of blocker (V[0]) was 74.2%, and the relative volume at saturating concentrations of NS1652 (V[∞] + V[0]) was 93.1%. DIDS data (□) are shown for comparison only.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1842.005a37_1842_1848/6/m_bloo00537002x.jpeg?Expires=1765086925&Signature=0NCXv~r2aLp9UQbkgCjSlCwkyQmlYW6pSL4y2Zrf3a3I5UNeNOR5M2DbrTAHSVs5zLlIk81AYvnFIx9XxdEYe3dOrrcvRz0WPLD4BtSoX-FM9FZ5ip5MhT4067uYjJgE-r94HIP~qetYtcJVlq-VfdpuB8X39w7IMQcC~ADyZeK0xQytztYPpC197KOO5iP3yY99U-ahoJb7ehYeIODALlipOalQS3b2XUviBgXFlnutuIzmd-QRvsqFaEJSlei-A~qr7ywta99LA9wWFYHFfbCj8TbFHrBu58ZUf2MLB73tXqpUz6MUFSWLWqGOmNiCVUWh0TDVIQoSDSh8TuJIXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)






![Fig. 2. (A) The relative Cl− conductance for normal erythrocytes versus the concentration of NS1652. / The solid line represents the best fit to a single-site inhibitor equation with IC50 = 0.62 μM, and an insensitive fraction (GCl([∞])) of 0.066. (B) Effect of NS1652 on valinomycin-induced erythrocyte dehydration. Erythrocytes were suspended at a cytocrit of 10% and incubated with various concentrations of NS1652 or 4,4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) for 2 minutes before addition of 5 ∗ 10−8 mol/L valinomycin. After 5 minutes of valinomycin incubation, the cytocrits were measured. The solid line represents the best fit of the NS1652 data (⧫) to a single-site inhibitor equation. The IC50value was estimated at 1.3 μM, the relative volume after valinomycin incubation in absence of blocker (V[0]) was 74.2%, and the relative volume at saturating concentrations of NS1652 (V[∞] + V[0]) was 93.1%. DIDS data (□) are shown for comparison only.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/5/10.1182_blood.v95.5.1842.005a37_1842_1848/6/m_bloo00537002x.jpeg?Expires=1765353510&Signature=38CJ83D5qMS0fPQOrXLMpqSTFATnO94aG2leBvzzxh7HU~wlZ0lzTvbHkNfSFHFYDGyo6PevAZW16gkIBwyUgZWQ6CvKFpSlRzfPgeLoqAYLBERGAsrLF7RJ9lg5Wixe8w0L8Q7zw7o43n0O2Q1jHgfhmQrDL-mOZE3FMcpdXog2zL3yCk0aGnsTo5jf3jmRe14rmmsp7s1Fd~CNIQRamxBo~0aah6tFKM1vXVIWkZLG1ZbHGPuK9mny8bKd1vR-Yl5dw2GDVZLliosIeVmQr4CIHJEJ3SHf3KgMUlBxh2TElF93Z~1Tmy6y6wMLq3eq8mijn2X7PhCXjmqqczB9rw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)