Abstract
X-linked thrombocytopenia with thalassemia (XLTT; Online Mendelian Inheritance in Man [OMIM] accession number 314050) is a rare disorder characterized by thrombocytopenia, platelet dysfunction, splenomegaly, reticulocytosis, and unbalanced hemoglobin chain synthesis. In a 4-generation family, the gene responsible for XLTT was mapped to the X chromosome, short arm, bands 11-12 (band Xp11-12). The maximum lod score possible in this family, 2.39, was obtained for markers DXS8054 and DXS1003, at a recombination fraction of 0. Recombination events observed for XLTT and markers DXS8080 and DXS8023 or DXS991 define a critical region that is less than or equal to 7.65 KcM and contains the gene responsible for the Wiskott-Aldrich syndrome (WAS; OMIM accession number 301000) and its allelic variant X-linked thrombocytopenia (XLT; OMIM accession number 313900). Manifestations of WAS include thrombocytopenia, eczema, and immunodeficiency. In WAS/XLT the platelets are usually small, and bleeding is proportional to the degree of thrombocytopenia. In contrast, in XLTT the platelet morphology is normal, and the bleeding time is disproportionately prolonged. In this study no alteration in the WAS gene was detected by Northern blot or Western blot analysis, flow cytometry, or complimentary DNA dideoxynucleotide fingerprinting or sequencing. As has been reported for WAS and some cases of XLT, almost total inactivation of the XLTTgene-bearing X chromosome was observed in granulocytes and peripheral blood mononuclear cells from 1 asymptomatic obligate carrier. The XLTT carrier previously found to have an elevated :β hemoglobin chain ratio had a skewed, but not clonal, X-inactivation pattern favoring activity of the abnormal allele. Clinical differences and results of the mutation analyses make it very unlikely that XLTT is another allelic variant of WAS/XLT and strongly suggest that X-linked thrombocytopenia mapping to band Xp11-12 is a genetically heterogeneous disorder.
In 1977, Thompson et al1 described a family segregating X-linked thrombocytopenia with thalassemia (XLTT; Online Mendelian Inheritance in Man [OMIM] accession number 3140502). This rare disorder is characterized by moderate thrombocytopenia, splenomegaly, reticulocytosis, and unbalanced hemoglobin (Hb) chain synthesis with elevated HbA2 and HbF, which resembles β-thalassemia minor. The morphology of platelets and red cells in XLTT is essentially normal as are platelet factor 3 levels, clot retraction, and platelet aggregation. However, platelet dysfunction is evidenced by disproportionately prolonged bleeding times, and affected males manifest petechiae, epistaxis, and easy bruising. Some female carriers also exhibit globin synthesis imbalance and/or mild reticulocytosis.
Several disorders associated with thrombocytopenia have been localized to the X chromosome.2,3 Chronic idiopathic intestinal pseudoobstruction (CIIP; OMIM accession number 3000482) is a recessive disorder of gastrointestinal motility that maps to the X chromosome, long arm, band 28 (band Xq28).4 Some CIIP-affected males have chronic thrombocytopenia with large platelets.5,6 The phenotypic spectrum of X-linked dyskeratosis congenital (DKC; OMIM accession number 3050002) includes pigment and nail abnormalities, mucosal leukoplakia, increased risk for malignancies, atresia of the lacrimal ducts, testicular atrophy, anemia, and elevated fetal hemoglobin in addition to thrombocytopenia.7,8 DKC is caused by mutations in the gene encoding dyskerin (DKC1; OMIM accession number 3001262) at band Xq28.9 A syndrome of immunodeficiency with increased immunoglobulin M (IgM) (HIGM1; OMIM accession number 3082302) at band Xq26 is primarily a T-cell disorder caused by mutations in the CD40 ligand gene.10 The thrombocytopenia that may develop in this disorder is likely a manifestation of autoimmunity. Paroxysmal nocturnal hemoglobinuria (PNH), an acquired clonal disorder of multipotential hematopoietic stem cells manifested by complement-mediated hemolysis, results from mutations in the gene at chromosome band Xp22.1, which encodes the Class A phosphatidylinositol glycan (PIGA) gene.11,12 In PNH there is deficient biosynthesis of the glycosylphosphatidylinositol (GPI) anchor and decreased surface expression of multiple GPI-anchored proteins.13,14 It has been suggested that a germline mutation resulting in defects in this biosynthetic pathway would be lethal.15 But it is possible that certain portions of the gene would tolerate a relatively conservative mutation, and this might lead to a modified phenotype.
Wiskott-Aldrich syndrome (WAS) and X-linked thrombocytopenia (XLT) form a spectrum of disorders resulting from mutations in the WAS protein (WASP) gene at band Xp11-12.16-23 Patients with classic WAS develop severe immunodeficiency, thrombocytopenia, and eczema including a propensity to develop autoimmune disorders and malignancies, while patients with the mildest cases of XLT develop isolated thrombocytopenia. We performed a study to localize the gene for XLTT and to determine whether it represents an allelic variant of another thrombocytopenia syndrome previously described.
Materials and methods
Patients
The family with XLTT was extensively evaluated in 1977.1Average platelet counts of 3 affected males, individuals III-5, III-7, and IV-3 (Figure 1), were 77, 76, and 72, respectively (all counts × 109/Lj; normal range, 55 to 90). Platelet survival time measured in 2 of the men was shortened to 3.4 days from the normal range of 8-12 days. Bleeding times were markedly prolonged in all 3 men (greater than 30 minutes); however, platelet aggregation studies with adenosine diphosphate, epinephrine, collagen, and thrombin were all normal. Electronic sizing and smear evaluation revealed occasional platelet enlargement in individual IV-3 but normal platelet volumes in individuals III-5 and III-7. The α:β chain ratios were 1.5 to 1.8 in individuals III-5 and III-7, respectively, and 1.58 in individual II-4, an obligate carrier. The HbA2 level was mildly elevated at 3.8%-4.4% (normal range: 1.8%-3.4%). Hemoglobin chain synthesis was balanced in all other heterozygotes studied (individuals III-3, IV-6, and IV-8). Unlike the β-thalassemia trait, red cell morphology in affected individuals was normal.
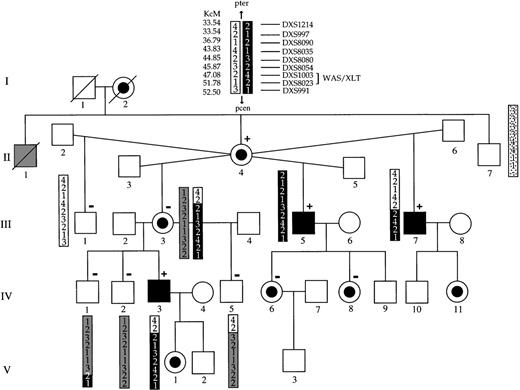
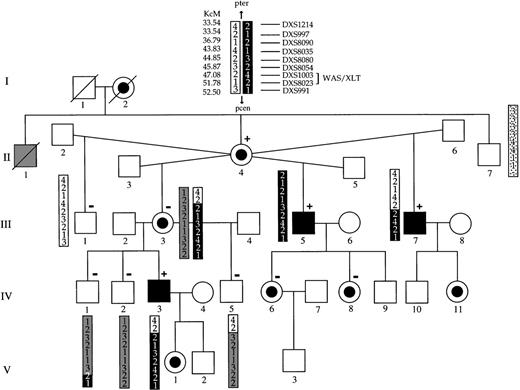
Pedigree and haplotypes of the family with XLTT.
Although individual II-1 was not examined, his history of bleeding and bruising with minimal trauma strongly suggest that he was affected. The portion of the chromosome containing the XLTT gene is shown in black. Previously, α:β globin chain ratios were studied in some members. Individuals in whom an altered hemoglobin chain ratio was detected are denoted by a + sign, and individuals with a normal ratio are denoted by a − sign.
Pedigree and haplotypes of the family with XLTT.
Although individual II-1 was not examined, his history of bleeding and bruising with minimal trauma strongly suggest that he was affected. The portion of the chromosome containing the XLTT gene is shown in black. Previously, α:β globin chain ratios were studied in some members. Individuals in whom an altered hemoglobin chain ratio was detected are denoted by a + sign, and individuals with a normal ratio are denoted by a − sign.
DNA and RNA analyses
DNA was extracted from leukocytes or Epstein-Barr virus–transformed (EBV-transformed) B-lymphoblastoid cell lines and was amplified by polymerase chain reaction (PCR) in a programmable thermal controller (PTC-100; MJ Research, Watertown, MA) as previously described.24 Primers (Research Genetics, Huntsville, AL) used in the study spanned the X chromosome at distances ranging from 5-14 KcM, and 1 primer of each pair was end labeled with γ32 adenosine 5′-triphosphate (ATP) by a T4 kinase reaction.
As previously described,25 RNA isolation was performed using a single-step method and (Trizol; Gibco-BRL, Gaithersburg, MD), and reverse transcriptase–PCR (RT-PCR) was performed with a system kit (SuperScript Preamplification System, Gibco-BRL).
WAS complementary DNA (cDNA) was amplified in 1 fragment using previously reported primers W-2 and W-1708c21 and PCR (Expand High Fidelity PCR System, Gibco-BRL). PCR products were purified by agarose gel electrophoresis. To screen the amplified WAS cDNA for mutations, we modified the dideoxynucleotide fingerprinting (ddF) method originally designed by Sarkar et al26 as previously described21(fmol DNA Cycle Sequencing System; Promega, Madison, WI). The resulting PCR products were electrophoresed in 5% polyacrylamide nondenaturing gel at 4°C.
To sequence WAS, cDNA was PCR amplified using 4 overlapping primer sets (Table 1) and purified (Nucleospin Extraction Kit; Clontech, Palo Alto, CA). Sequencing was performed on fluorescence-labeled DNA (ABI PRISM BigDyeTerminator Cycle Sequencing Ready Reaction kit; PE Biosystems, Foster City, CA), and electropherograms (Model 373 DNA sequencer; Applied Biosystems) were generated according to manufacturer's instructions. The same primer sets used for PCR amplifications were also used as sequencing primers.
Protein analysis
For fluorescence-activated cell sorting, 2 × 106EBV-transformed B-lymphoblastoid cells from individual III-5 and from a normal control were fixed in 1 mL 4% paraformaldehyde and phosphate-buffered saline (PBS) for 15 minutes at room temperature. The cells were then washed twice with PBS and permeabilized with 0.1% Triton X-100/Tris-buffered saline (tris[hydroxymethyl] aminomethane–buffered saline [TBS]) for 5 minutes at room temperature. Cells were washed twice and resuspended in 100 μL PBS containing 1% fetal calf serum and mouse anti-WASP monoclonal antibody (mAb) 3F3.A5 (gift of Dr David Nelson, National Institutes of Health, Bethesda, MD) at a final concentration of 5 μg/mL for 20 minutes on ice. The cells were washed again and then stained with fluorescein isothiocyanate–conjugated (FITC-conjugated) antimouse IgG1 mAb at a dilution of 1:500 for 20 minutes on ice. The cells were then washed and analyzed on a fluorescence-activated cell sorter (FACS) flow cytometer (FACScan; Becton-Dickinson, San Jose, CA).
Western blot analysis
EBV-transformed cells from affected individuals, III-5 and IV-3, a positive control, and a negative control were suspended at 2.5 × 107 cells/mL in lysis buffer containing 200 μg/mL phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, and 5 μg/mL leupeptin and kept on ice for 30 minutes. The protein concentration in each lysate was determined by a protein assay kit (Bio-Rad, Hercules, CA). From each sample, 23 μg total protein was electrophoresed through a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a membrane (PVDF Immobilon-P; Millipore, Bedford, MA). The membrane was incubated with anti-WASP antibody 503 (1:2000) and anti-Actin antibody (1:500) (Sigma, St. Louis, MO) for 1.5 hours at room temperature. After washing twice with 0.05% TBS Tween/PBS, the membrane was incubated with horseradish peroxidase–conjugated goat antirabbit IgS (Biosource International, Camarillo, CA) at a concentration of 1:1000 for 45 minutes at room temperature. The membrane was then washed 4 times, and the results were visualized by an enhanced chemiluminescent method (Pierce, Rockford, IL).
X-inactivation analysis
DNA extracted from polymorphonuclear and mononuclear cells was separated by centrifugation through a discontinuous gradient (Ficoll/Hypaque, Sigma) and evaluated for methylation patterns at the human androgen receptor alpha (HUMARA) locus by a modification of the method published by Allen et al.27 Genomic DNA (1 μg) was digested overnight at 37°C in 40-mL volumes containing 80 unitsHpaII at a concentration of 50 units/mL (New England Biolabs, Beverly, MA) and 10 units RsaI (Pharmacia Biotech, Uppsala, Sweden) or RsaI alone. This solution (4 μL) was PCR amplified in 20-μL volumes containing 200 μmol/L dNTP (a′-deoxynucleoside 5′-triphosphate) and 0.5 units polymerase (AmpliTaq, Perkin Elmer) at a final concentration of 3.7 mmol/L magnesium dichloride (MgCl2) in addition to 0.38 μmol/L each of the forward and reverse primers end labeled with 0.016 MBq (0.45 μCi) γ32ATP.
The following thermal profile was used: initial denaturation for 3 minutes at 94°C; 30 cycles each of 45 seconds at 94°C, 30 seconds at 65°C, and 30 seconds at 72°C; and a final 6-minute incubation at 72°C. Amplified products were electrophoresed for 4 hours at 70 W through 6% polyacrylamide gels containing 7 mol/L urea and 30% formamide. Dried gels were autoradiographed, and the relative band intensities in theRsaI/HpaII- and RsaI-digested lanes were quantitated by a scanning densitometer (Hoefer GS300 with GS370 software; Hoefer Scientific Instruments, San Francisco, CA). Clonal female DNA was included to evaluate completeness of HpaIIdigestion.
Linkage analysis
Power analysis and 2-point linkage analyses were performed with the SLINK and MLINK subprograms of the LINKAGE package version 5.0,28 as implemented in FASTLINK for DOS (version 3.0P).29 X-linked recessive inheritance with a gene frequency of 0.0001 for the disease allele was assumed for XLTT. Allele sizes and frequencies and the order of the marker loci were obtained from the Genome Database [GDB].30 Sex-averaged map distances are described in Broman et al31 and are available from the Marshfield website.32 When possible, allele sizes on the autoradiographs were standardized by comparison to DNAs from CEPH families 1331 and 1347.33
Results
Mapping the XLTT syndrome to band Xp11-12
Simulation studies suggested that a maximum lod score of 2.39 at a recombination fraction of θ = 0 could be obtained with the samples available. For an X-linked disorder, a lod score of 2 provides evidence at approximately P = .05 for linkage. Genotypes were then evaluated for polymorphic markers spanning the X chromosome (Table2). A lod score of 2.39 was obtained at θ = 0 for markers DXS8054 and DXS1003. After locating this region of interest, finer scale mapping was performed, and haplotypes were constructed. Recombination events were observed for the XLTTgene and markers DXS8080 and DXS8023 or DXS991 (Figure 1). Because carrier III-3 is homozygous for DXS8023, the proximal breakpoint could not be more specifically placed. These recombinations define a critical region that is less than or equal to 7.65 KcM on band Xp11-12. This region contains the gene responsible for WAS.
Exclusion of the WAS gene as the cause of XLTT
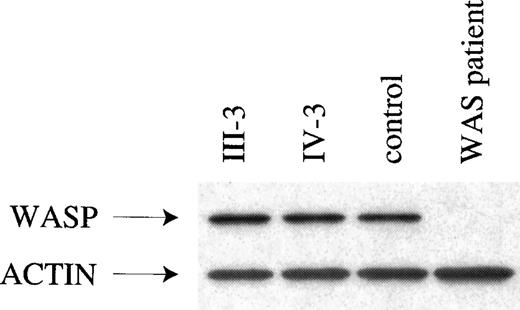
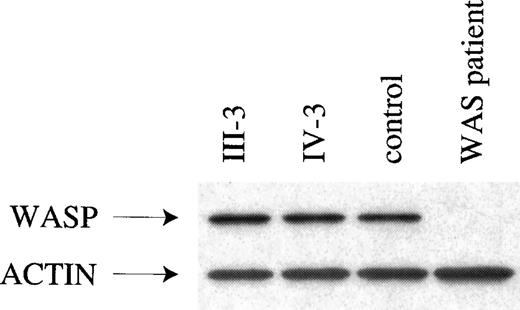
Dideoxynucleotide fingerprinting, which combines single-base dideoxy sequencing and the single-strand conformation polymorphism assay, was used as the initial mutation screen. The dideoxy component detects the substitution, addition, or deletion of the single base, and the single-strand conformation polymorphism component detects changes in other base pairs (bp). The ddF protocol used would have detected mutations affecting nucleotides A and T, and only guanine/cytosine transitions might have been missed. No alterations were seen in the ddF pattern of WAS cDNA from the B cell lines of affected male carriers III-5 and IV-3. Exons 1-12 were then sequenced from cDNA, thereby revealing only the wild type sequence. Finally, WAS from these affected XLTT males was analyzed by Western blot (Figure 2) and FACS (not shown). Western blot analysis revealed that WASP was of normal size, and both assays detected normal amounts of WASP.
Evaluation of WASP by Western blot analysis.
Western blot evaluations are given for the following: WASP and actin from 2 affected males with XLTT, an unrelated normal control, and an individual with classic WAS. The patient with classic WAS carries a nonsense mutation in theWAS gene, which results in absence of detectable WASP.
Evaluation of WASP by Western blot analysis.
Western blot evaluations are given for the following: WASP and actin from 2 affected males with XLTT, an unrelated normal control, and an individual with classic WAS. The patient with classic WAS carries a nonsense mutation in theWAS gene, which results in absence of detectable WASP.
X-inactivation studies in obligate carriers of XLTT
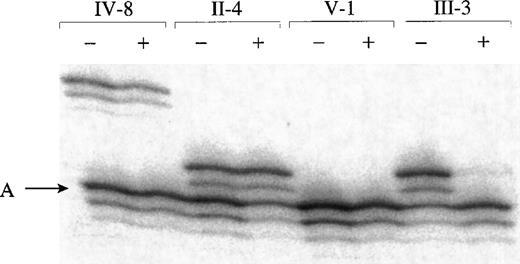
X-inactivation patterns were assessed by evaluating the methylation status of cytosine resides near a highly polymorphic trinucleotide repeat sequence in the HUMARA locus (Figure3). The allele on the inactive X chromosome is highly methylated and resists digestion by methylation-sensitive restriction endonucleases such as HpaII, whereas the allele on the active X chromosome is digested. Therefore, only inactive X chromosomes contribute alleles for the PCR amplification step that follows. In heterozygous women, the relative proportion of inactive maternally- and paternally-derived X chromosomes can be compared. Of the 4 obligate heterozygous females sampled, 1 (carrier V-1) was homozygous for HUMARA and, therefore, could not be evaluated. Marked skewing of the X-inactivation pattern was observed in carrier III-3; the allele carried by affected males was resistant to HpaIIdigestion, which shows that it is uniformly inactivated. The pattern in carrier II-4 was biased in the opposite direction; the ratio was 67:33, favoring activity of the abnormal XLTT gene-bearing chromosome. Skewing was not observed in the X-inactivation pattern of carrier IV-8.
Evaluation of X-inactivation patterns in 4 obligate carriers of XLTT.
DNA digested with RsaI (lanes marked −) or RsaIand HpaII (lanes marked +) was PCR amplified using primers for HUMARA. Only alleles on inactive X chromosomes are amplified followingHpaII digestion. The allele carried by affected males is denoted A (data not shown). HUMARA lies just distal to the minimal region containing XLTT, and there were no recombinations between XLTT and HUMARA in individuals III-3, III-5, or IV-3. Although carriers IV-8 and V-1 were not included in the linkage analysis, they each must have inherited the X chromosome from their affected fathers, without any further recombinations.
Evaluation of X-inactivation patterns in 4 obligate carriers of XLTT.
DNA digested with RsaI (lanes marked −) or RsaIand HpaII (lanes marked +) was PCR amplified using primers for HUMARA. Only alleles on inactive X chromosomes are amplified followingHpaII digestion. The allele carried by affected males is denoted A (data not shown). HUMARA lies just distal to the minimal region containing XLTT, and there were no recombinations between XLTT and HUMARA in individuals III-3, III-5, or IV-3. Although carriers IV-8 and V-1 were not included in the linkage analysis, they each must have inherited the X chromosome from their affected fathers, without any further recombinations.
Discussion
Localization of XLTT to the region of the X chromosome containing the gene responsible for WAS/XLT suggested that XLTT might be another allelic variant of this syndrome. However, there are substantial clinical differences between the XLTT and WAS/XLT syndromes. In XLTT, platelet morphologies are normal and the bleeding times are disproportionately prolonged, whereas in WAS/XLT, the platelets are usually small, and bleeding times are proportional to the degree of thrombocytopenia.23 The failure to find an alteration in WAS DNA, RNA, or protein from XLTT males by a variety of approaches provides strong evidence against inclusion of XLTT in the WAS/XLT spectrum. These data provide evidence for additional genetic heterogeneity of XLT syndromes and suggest that band Xp11-12 contains more than 1 gene involved in platelet production and/or survival.
Out of 4 XLTT obligate carrier women evaluated, 1 manifested some characteristics of XLTT. Because females are mosaics of cells bearing active paternally- and maternally-derived X chromosomes, they are much less likely to show manifestations of X-linked recessive disorders than males. The process of X inactivation occurs early in embryogenesis and is random with respect to the choice of X chromosome in each cell, and the activity status is thereafter stably maintained during mitosis.34 Expression of a variety of X-linked recessive diseases in carrier females35-37 has been attributed to the chance occurrence of markedly skewed X inactivation of the chromosome bearing the normal allele. However, a minority of cases may result from mutation in theXIST gene.38
In contrast, in some disorders a markedly skewed pattern of X inactivation is seen in cells from most carrier females. For instance, the pattern in platelets, granulocytes, and lymphocytes of women carrying the WAS gene is skewed so that essentially all cells have the abnormal X chromosome inactivated.39-43 The situation is less clear for XLT. Some investigators report a skewed pattern in carriers, whereas others find a random pattern in some cell lineages.44,45 The most cogent explanation for the skewed pattern is postinactivation selection that favors cells bearing the normal allele in the active state in tissues in which the gene is expressed.46-48 Female carriers are usually asymptomatic, but females affected with WAS/XLT have been identified, and in these patients, the mutant WASallele was found to be active.36
Unlike WAS, there is not a consistent pattern of selection for the X chromosome bearing the normal XLTT allele. As seen in carriers of the WAS gene, almost total inactivation of the XLTTgene-bearing chromosome was observed in 1 clinically unaffected obligate carrier (carrier III-3). However, a random X-inactivation pattern was found in the other informative unaffected obligate carrier (carrier IV-8). In contrast, a skewed pattern favoring activity of the chromosome bearing the abnormal XLTT allele was demonstrated in the 1 carrier previously shown to have an elevated α:β hemoglobin chain ratio (carrier II-4). Perhaps this individual manifests only mild clinical features of XLTT because a portion of her hematopoietic cells express a normal XLTT product.
Thrombocytopenia, elevated fetal hemoglobin levels, and thalassemia are features of several X-linked disorders. With the exception of WAS, these disorders do not map to the critical region for the XLTTgene. However, information about their structure or function may help in identifying the gene. DKC1 contains 2 TruB pseudouridine synthase motifs, multiple phosphorylation sites, and a carboxy-terminal lysine-rich domain. DKC1 is predicted to be a nuclear protein involved in the cell cycle and in nucleolar function.9,49The CD40 ligand is a type 2 glycoprotein expressed as a homotrimer on the membranes of activated T cells.10WAS encodes a cytoplasmic signal transduction adaptor protein containing proline-rich domains that bind SH3-domain–containing proteins, a pleckstrin homology (PH) domain, and a guanosine 5′-triphosphatase–binding (GTPase-binding) domain.22 PH domains are known to mediate binding of cytoplasmic proteins to membrane phospholipids,50,51 and SH3-domain–containing proteins are involved in protein-protein interactions that mediate signal transduction cascades and may be involved in cytoskeletal regulation.52,53 GTPases function in transcriptional activation, maintenance of the actin cytoskeleton, and cell cycle regulation.54,55 The mechanism of thrombocytopenia in WAS/XLT is not entirely understood, but it appears that the amino-terminal region of WASP is required for platelet production. Mutations in the PH domain result in a phenotype of XLT with predominantly platelet abnormalities and very mild or no symptoms of immune deficiency.22 56
Hereditary persistence of fetal hemoglobin (HPFH) is a genetically heterogeneous condition. Although most cases are associated with point-mutations or deletions in the β-globin cluster,57unlinked loci have been shown to influence HgF levels in both healthy individuals and patients with sickle cell anemia.58-60 One of these factors is the F cell locus (FCP1, OMIM accession number 3054352) at band Xp22.2-22.3.61 62 The gene at this locus is implicated in Swiss-type HPFH, but it has not yet been cloned.
There is precedence for thalassemia to be part of an X-linked syndrome. Although the α-globin locus maps to chromosome 16,63 the ATR-X syndrome, which is characterized by mental retardation and α-thalassemia, is caused by a mutation in the helicase gene XH2 in band Xq13.64 Among other activities, helicases regulate gene expression.65 When mutated, the ATRX gene selectively down-regulates the α-globin locus but not the β-globin locus.66 The study of this family with XLTT suggests that there is an X-linked locus which plays a role similar to the role of ATR-X. It is likely that this locus, possibly coding for a helicase or other regulator of transcription, is mutated in XLTT.
Aside from the WAS gene, the critical region on band Xp11-12 contains at least 30 known genes and many more expressed sequence tags that can be investigated as candidates for XLTT.67 Many of these transcripts are expressed in spleen cells, but it is not known whether the transcripts are tissue specific. The following genes can be omitted from further consideration because they have functions and/or expression patterns unlikely to produce the phenotype of XLTT: MAOA, MAOB, NDP,RGN, KIAA0215, ARAF1, RBM3,KCND1, PFC, SYP, SYN1, CLCN5,AKAP82, PLP2, HADH2, GJA1,FGD1, TRO, and ALAS2. Of potential interest are the genes UHX1, UBE1,PCTK1, TIMP1, and HDAC6 and a number of zinc finger genes.
Ubiquitination of proteins is important for many cellular processes including DNA repair, cell cycle control, and peroxisome synthesis.68 There are 2 genes involved in the ubiquitin system that map to the critical region: UH × 1, a ubiquitin-specific protease,69 and UBE1, the ubiquitin-activating enzyme E1, which catalyzes the first step in ubiquitin conjugation.70,71 The PCTK1 gene is a member of a family of cdc2-related serine/threonine-specific protein kinases.72PCTK1 has wide expression, although it is most highly expressed in testis and brain. UBE1 andPCTK1, which are contiguous genes, both escape X inactivation.73 The gene for the glycoprotein growth factor tissue inhibitor of metalloproteinase 1 (TIMP1) is particularly suitable as a candidate for triggering XLTT. TIMP1 is synthesized in and secreted by platelets and, as suggested by its earlier designation for erythroid potentiating activity (EPA), it belongs to a class of molecules involved in erythropoeisis.74 To date, mutations in the UBE1,UH × 1, and TIMP genes have been implicated in retinal disorders, but given their expression patterns and/or function it is reasonable to speculate that other organ systems may be affected by mutations in these genes.
Several zinc finger protein family genes also lie in this region:ZNF41, ZNF21, ZNF81, ZNF157, andGATA1.75 Zinc fingers mediate transcriptional activity by recognizing and binding to guanine/cytosine-rich sequences in the control regions of a wide variety of cellular genes.76 The effects may be tissue-specific or ubiquitous and are modulated through interactions with cofactor proteins. Given the wide-reaching importance of these transcription factors in development, knockout mutations are often embryonic lethals.77 However, viable transgenic models have been created by limiting expression of the mutation to specific lineages.78 Histone deacetylases also function as regulators of transcription by modulating the nuclear proteins that bind DNA.79 The histone deacetylase 6 gene (HDAC6), which maps to the critical region for XLTT, is a recently cloned serine/threonine protein kinase whose function is not well characterized.80 The 10 genes discussed above will be evaluated in the future for sequence alterations in XLTT. Consensus sequences for functional subportions of the genes involved in the disorders discussed above may aid in identifying additional candidate genes for study.
Acknowledgments
We appreciate the participation of the family afflicted with XLTT, without whose cooperation this work would not have been possible. We are grateful to Dr David Nelson of the National Institutes of Health, Bethesda, MD, for the generous gift of anti-WASP mAb 3F3.A5.
Supported in part by grants CA16448 (W.H.R., M.M., and J.W.), HD17427 (H.D.O.), and 5R37 HL20899 (G.S.) from the National Institutes of Health, Bethesda, MD; grant FY98-418 (H.D.O.) from the March of Dimes Birth Defects Foundation, White Plains, NY; and a grant from the DeJoria Wiskott-Aldrich Research Fund, Beverly Hills, CA (H.D.O.).
Reprints:Wendy H. Raskind, Department of Medicine, Box 357720, University of Washington, Seattle, WA 98195; e-mail:wendyrun@u.washington.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.