Abstract
Peripheral blood stem cell (PBSC) transplantation is successful in improving engraftment without increasing acute graft-versus-host disease (GVHD), despite much larger numbers of T cells in unmanipulated PBSCs than in bone marrow grafts. In mouse models and retrospective human studies, granulocyte colony-stimulating factor (G-CSF) therapy has been associated with less acute GVHD. We studied the effect of G-CSF on interferon (IFN)-γ and IL-4 expression in CD4+lymphocytes. CD4+ cells co-cultivated with G-CSF and stimulated with PHA or CD3 monoclonal antibodies showed significant decreases in IFN-γ and increases in IL-4 expression (n = 13;P < .01). G-CSF appeared to have a direct effect on CD4+ cells independent of monocytes present in the culture because purified CD4+ cells exposed to G-CSF, washed, and cocultivated with untreated monocytes demonstrated similar changes in IFN-γ and IL-4 expression, whereas untreated CD4+ cells cocultured with G-CSF–stimulated monocytes behaved as controls. We then studied peripheral blood mononuclear cells (PBMCs) from G-CSF–mobilized PBSC donors. When their PBMCs were cultured with PHA or CD3 monoclonal antibody, the percent of IFN-γ–expressing cells decreased by a mean of 55% and 42%, respectively, whereas the percent of IL-4–containing cells increased by a mean of 39% and 58%, respectively, following G-CSF stimulation. Increased apoptosis of IFN-γ–producing CD4+ cells was not responsible for the shift in TH1/TH2 subsets. G-CSF-R mRNA was present in both CD4+ and CD8+ cells. These results suggest that G-CSF decreases IFN-γ and increases IL-4 production in vitro and in vivo and likely modulates a balance between TH1 and TH2 cells, an effect that may be important in PBSC transplantation.
Use of peripheral blood stem cells (PBSC) for allogeneic bone marrow (BM) transplantation has a number of advantages over conventional BM grafting, including an improved yield of progenitor cells, ease of harvest, and shortened time to achieve engraftment.1,2 Despite a 10-fold increase in the number of CD3+ cells in the apheresis product used for the transplantation of stem cells, patients undergoing PBSC transplantation do not demonstrate an apparent increased incidence or severity of acute graft-versus-host disease (GVHD).3,4 This observation may be accounted for by a number of differences observed in BM and PB stem cells. PBSC obtained from granulocyte colony-stimulating factor (G-CSF)-mobilized donors contain more CD3+CD8−CD4− cells than BM cells5; these cells decrease the severity of acute GVHD in the murine model and are associated with decreased cytotoxic lymphocyte activity in vitro.6 Similarly, monocytes obtained from G-CSF mobilized donors suppress cytotoxic responses and T-cell proliferation when they are cocultivated with T cells.7,8 Recently, G-CSF was shown to polarize the balance within the murine T-lymphocyte subpopulations toward the TH2 cytokine production in vitro.9
CD4+ lymphocytes can be functionally subdivided into TH1 and TH2 cells based on their pattern of cytokine expression.10 TH1 cells can secrete tumor necrosis factor (TNF)-α and IFN-γ, whereas TH2 cells are capable of producing IL-2, IL-4, and IL-10. The balance between these 2 subsets is altered in a number of pathophysiologic conditions and is associated with progression of or protection against some infectious and autoimmune conditions.11-13 Recent results have suggested that acute GVHD is mediated by TH1 lymphocytes and that TH2 lymphocytes may exert a protective effect but may have a role in mediating chronic GHVD.14 In a murine model of GVHD with TH1 and TH2 knock-out mice, TH1 cells mediated the symptoms of severe diarrhea and wasting, ultimately leading to death from acute GVHD15; TH2 cells caused skin disease and were partially effective in antagonizing the effect of TH1 cells. Experiments using the IFN-γ knockout mouse showed significant modification of acute GVHD, confirming the importance of the role of IFN-γ.16
We hypothesized that G-CSF, in addition to its trophic effects on hematopoiesis, may have regulatory effects on T lymphocytes and may modulate immune responsiveness in unmanipulated PBSC grafts and autoimmune hematologic diseases in which it is used. Therefore, in this study we investigated the effects of G-CSF on TH1 and TH2 cell development in mitogen-stimulated human CD4+ cells and studied the mechanisms of G-CSF–mediated changes within the lymphocyte subpopulations. The in vitro findings were correlated with the effects of G-CSF on TH1 and TH2 lymphocyte distribution in PBSC donors receiving G-CSF for stem cell mobilization.
Materials and methods
Stem cell donor selection and studies
Peripheral blood for in vitro studies was obtained from normal volunteers. For studies involving in vivo G-CSF stimulation of normal donors, samples were obtained from normal stem cell donors before and after 5 days of G-CSF treatment (10 μg/kg subcutaneously daily) before apheresis donation. Heparinized blood for cytokine expression studies was obtained on day 1, before the administration of G-CSF, and on day 6. In all cases, informed consent was obtained according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute. PBMC from G-CSF–stimulated donors were separated as described below, and cells were cultured for 48 hours with PHA (1 μg/mL) or anti-CD3 monoclonal antibody (mAb) (0.1 μg/mL). Cells were then surface stained with anti-CD4–phycoerythrin (PE) and intracellularly stained with either anti–IFN-γ-fluorescein isothiocyanate (FITC) or anti–IL-4 FITC as described below.
Cocultivation of G-CSF with T lymphocytes
Peripheral blood mononuclear cells (PBMCs) from normal unstimulated donors were separated using density gradient centrifugation with lymphocyte separation media (Organon, Durham, NC). Cells were washed twice with phosphate-buffered saline (PBS) and resuspended in Iscove's modified medium supplemented with fetal calf serum (FCS; both Life Technologies, Gaithersburg, MD). Cultures of normal unstimulated donor cells were performed at a cell density of 0.5 × 106cells/mL. When appropriate, natural lymphocyte-derived IL-2 (Boehringer Mannheim, Indianapolis, IN) or phytohemagglutinin (PHA; Boehringer Mannheim) were used for stimulation at concentrations of 20 U/mL or 8 μg/mL, respectively. G-CSF was used at a concentration of 100 ng/mL (Amgen, Thousand Oaks, CA). GCSF was added to cultures; either PHA or CD3 mAb were added to cultures 24 hours later to stimulate cytokine expression. Cells were stained and enzyme-linked immunosorbent assay (ELISA) was performed on supernatants 2 days later.
Isolation of CD4+ cells, CD8+cells, and monocytes.
For lymphocyte subset separation, after washing with PBS supplemented with 2% human albumin, cells were applied to either a CD4+or a CD8+ affinity column (R&D Systems, Mineapolis, MN), and the cell fraction was eluted with PBS according to manufacturer's instructions. An aliquot of eluted cells was stained with PE-conjugated anti-CD4 or CD8 HPCA-12 mAb (Becton Dickinson, Mountain View, CA) to assess purity. Monocytes were separated by adhesion to 250-mL polystyrene flasks in the presence of 20% FCS for 2 hours; this was followed by the removal of nonadherent cells and by PBS/2% FCS washes. Adherent cells were detached and resuspended by agitation at 4°C in the presence of 1X Versene (BRL; Life Technologies). The usual purity of the adhesion-separated monocytes ranged between 75% and 85% as determined by the expression of CD14+ antigens by flow cytometry. Cell viability was measured using a standard trypan blue exclusion assay (Life Technologies).
Intracellular staining.
Intracellular staining was performed on density gradient-separated PBMC. Intracellular staining for ICE expression was performed using the Pharmingen Intracellular Staining Kit (Pharmingen). PBMC were stained with FITC-conjugated CD4 mAb and fixed. After membrane permeabilization, cells were stained with PE-conjugated anti-IFN-γ, anti–IL-4 mAb, or appropriate isotypic control mAb (Pharmingen). Permeabilization of cells was controlled using PermaSure reagents (Biosource). Samples were analyzed using an Epics ELITE flow cytometer (Coulter).
Apoptosis assay.
Cultured PBMC were prepared as described above, washed with PBS, and stained with an annexin apoptosis kit (Pharmingen) according to the manufacturer's17 specifications and as previously described. Samples were analyzed using flow cytometry.
Cytokine ELISA
Concentrations of IL-4 and IFN-γ in tissue culture supernatants were measured using commercially available ELISA systems (R&D Systems). All determinations were made in duplicate.
Reverse transcriptase–polymerase chain reaction for detection of G-CSF receptor expression
Peripheral blood mononuclear cells were sorted for CD4+and CD8+ by flow cytometry. Total RNA was extracted using RNA STAT-60 (Tel-Test, Friendswood, TX). Reverse transcription was performed using an oligo d(T)16 primer (RNA CORE KIT; Perkins-Elmer Cetus, Foster City, CA). After reverse transcription, the cDNA was amplified using 5′ and 3′ primer pairs, 5′-AGTACAGTCCTCACCCTGATG-3′, 5′-AAAGTATGCAGATCGCCTGGG-3′ specific for G-CSF receptor.18The following reverse transcription and amplification conditions were used: 42°C for 30 minutes, 99°C for 2 minutes, at 95°C for 1 minute, 55°C for 1 minute, and 72°C for 2 minutes for 40 cycles. PCR products were electrophoresed in 1% agarose gel, and the bands were visualized after staining with ethidium bromide and ultraviolet light exposure.
Results
G-CSF stimulated PBSC donors demonstrate decreases in IFN-γ and increases IL-4 expression in CD4+ cells
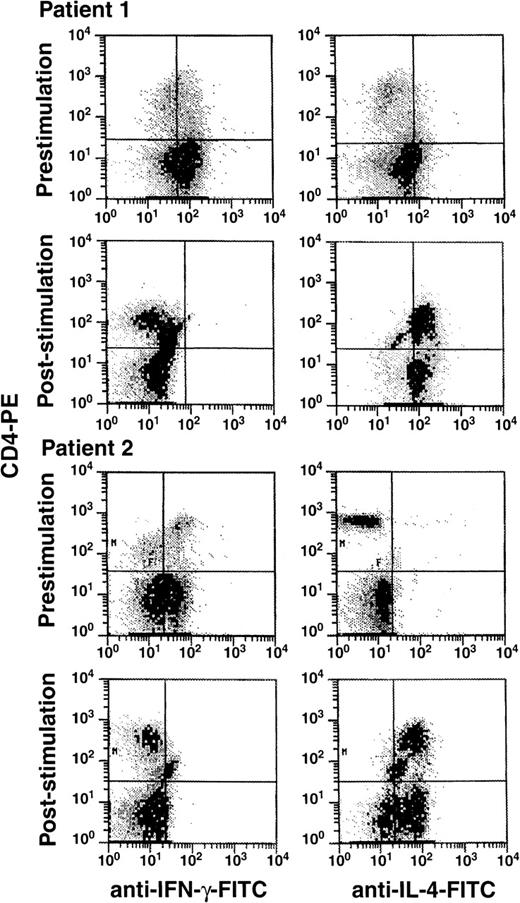
To determine whether G-CSF modulated the cytokine expression pattern of T lymphocytes, we used flow cytometry to examine intracellular cytokine expression in mononuclear cells derived from PBSC of donors receiving G-CSF. Normal donors receiving 10 μg/kg subcutaneously daily for 5 days were studied immediately before and after G-CSF administration for PBSC donation by apheresis. After blood sampling, PBMCs were stimulated for 48 hours with either PHA or CD3 mAb and were subsequently stained for intracellular IL-4 and IFN-γ. Donor CD4+ cells stimulated with G-CSF demonstrated significant changes in cytokine expression (Figures 1and 2). In comparison to pretreatment values, G-CSF increased IL-4 and decreased IFN-γ expression, resulting in a decreased TH1/TH2 ratio (determined by dividing the percentage of cells expressing IL-4 by the percentage of cells expressing IFN-γ). In addition to a decreased proportion of cells expressing IFN-γ and an increased proportion of IL-4–expressing cells, there were characteristic changes in the mean channel fluorescence (MCF) indicating changes in the intracellular cytokine content. IFN-γ and IL-4 MCF was measured on a relative scale (PHA-stimulated normal lymphocytes showed MCF of 5 to 6, whereas isotype controls registered at less than 0.10). CD3 mAb-stimulated CD4+ cells from G-CSF–treated patients showed a mean decrease in MCF of −4.2 for IFN-γ, with a decrease in the percent of cells staining by 42% with a concomitant increase in the number of cells staining for IL-4 by 58% with ΔMCF = +6.0 (n = 10; P < .01). Determination of IL-4 and IFN-γ concentrations in culture supernatants by ELISA resulted in similar changes (Table 1A).
Effects of G-CSF administration on IFN-γ and IL-4 production by peripheral blood lymphocytes.
Eleven normal stem cell donors received G-CSF (10 μg/kg per day) for 5 days. Blood was obtained before and after the administration of G-CSF. PBMC cells were purified by density-gradient centrifugation and were cultured with PHA for 48 hours. After permeabilization, cells were stained for IFN-γ and IL-4 as described in “Materials and methods.” Scattergrams represent log PE (CD4 or CD8) fluorescence activity versus log FITC (IFN-γ and IL-4) fluorescence activity for representative donors (A, B).
Effects of G-CSF administration on IFN-γ and IL-4 production by peripheral blood lymphocytes.
Eleven normal stem cell donors received G-CSF (10 μg/kg per day) for 5 days. Blood was obtained before and after the administration of G-CSF. PBMC cells were purified by density-gradient centrifugation and were cultured with PHA for 48 hours. After permeabilization, cells were stained for IFN-γ and IL-4 as described in “Materials and methods.” Scattergrams represent log PE (CD4 or CD8) fluorescence activity versus log FITC (IFN-γ and IL-4) fluorescence activity for representative donors (A, B).
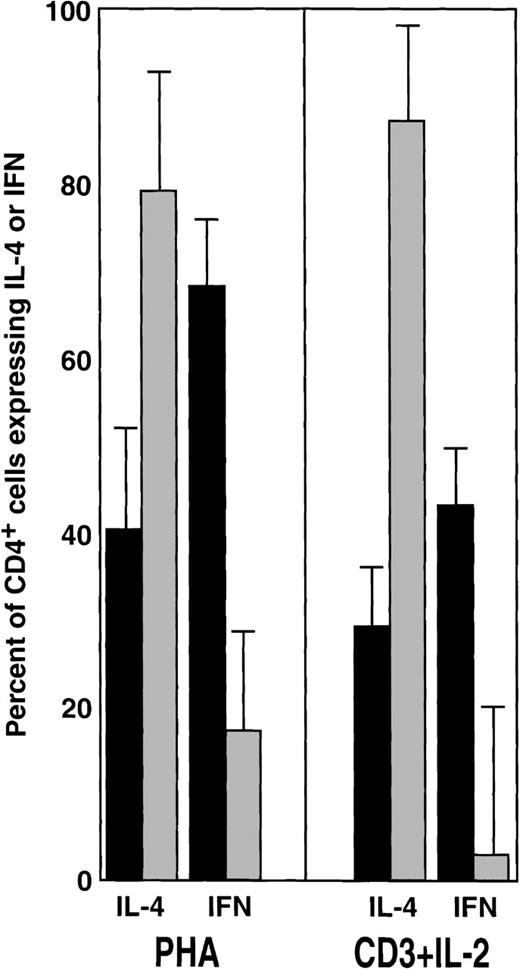
Effects of G-CSF on the percentages of CD4+cells secreting IFN-γ and IL-4.
Summary of the results obtained from 10 PBSC donors, including those exemplified in Figure 1. Bars represent percentages (mean ± SEM) in the IFN-γ and IL-4 expressing CD4+ cells before (black) and after (gray) treatment. Corresponding shifts in the mean fluorescence intensity for IFN-γ and IL-4 are described in the text. Statistical analysis (nonparametric Kruskal–Wallis test) for PHA: IL-4, P < .05; IFN-γ, P < .01. Statistical analysis for CD3 + IL-2: IL-4 P < .01; IFN-γP < .01.
Effects of G-CSF on the percentages of CD4+cells secreting IFN-γ and IL-4.
Summary of the results obtained from 10 PBSC donors, including those exemplified in Figure 1. Bars represent percentages (mean ± SEM) in the IFN-γ and IL-4 expressing CD4+ cells before (black) and after (gray) treatment. Corresponding shifts in the mean fluorescence intensity for IFN-γ and IL-4 are described in the text. Statistical analysis (nonparametric Kruskal–Wallis test) for PHA: IL-4, P < .05; IFN-γ, P < .01. Statistical analysis for CD3 + IL-2: IL-4 P < .01; IFN-γP < .01.
G-CSF decreased the proportion of IL-4 and IFN-γ producing lymphocytes in vitro
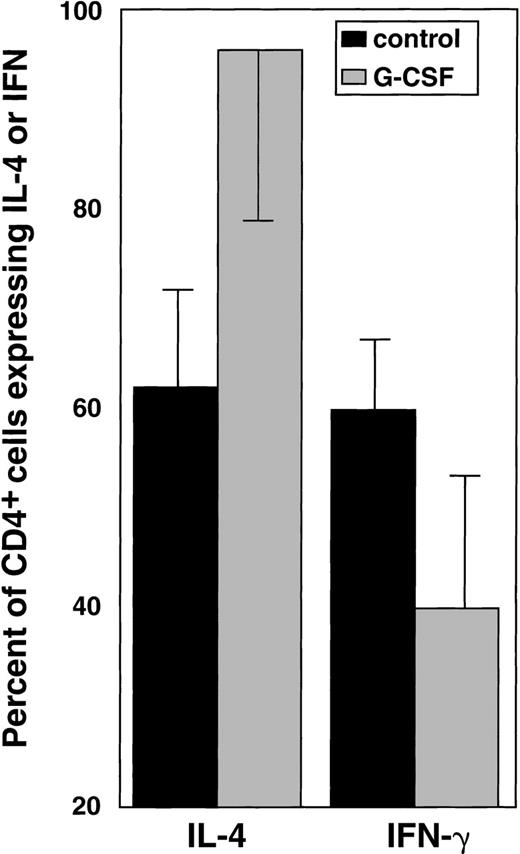
In a subsequent set of experiments, we asked whether the effects of G-CSF observed in PBSC donors could be replicated in vitro. Normal PBMCs were incubated with varying concentrations of G-CSF, then stimulated with IL-2 and CD3 mAb and stained 2 days later with CD4-PE and (after permeabilization) with either IL-4 or IFN-γ. Flow cytometric analysis showed that the number of CD4+ cells expressing IFN-γ decreased, whereas the number of IL-4–producing cells increased in cultures performed in the presence of G-CSF (Figure3). The effect was less pronounced in cells that were not preincubated with G-CSF before stimulation with either PHA or CD3 mAb (data not shown). The culture supernatants were also tested for IFN-γ and IL-4 by ELISA. G-CSF resulted in changes in the cytokine production pattern that paralleled those detected by flow cytometry (Table 1B). The effects of G-CSF were dose dependent (data not shown).
In vitro effects of G-CSF on IFN-γ and IL-4 production.
After exposure to G-CSF and polyclonal stimulation with PHA, IL-4 and IFN-γ expression were determined using flow cytometry as previously described. The columns represent the percentages (mean ± SEM) of cells expressing IFN-γ and IL-4. Summary of 13 experiments performed. Statistical analysis (nonparametric Kruskal-Wallis test) for IL-4:P < .01. Statistical analysis for IFN-γ:P < .01.
In vitro effects of G-CSF on IFN-γ and IL-4 production.
After exposure to G-CSF and polyclonal stimulation with PHA, IL-4 and IFN-γ expression were determined using flow cytometry as previously described. The columns represent the percentages (mean ± SEM) of cells expressing IFN-γ and IL-4. Summary of 13 experiments performed. Statistical analysis (nonparametric Kruskal-Wallis test) for IL-4:P < .01. Statistical analysis for IFN-γ:P < .01.
G-CSF has a direct effect on CD4+ cells
To determine whether the effects of G-CSF on cytokine production by T lymphocytes were directly mediated, we studied the growth factor's effects on purified cell populations contained in PBMC preparations. We compared IFN-γ expression in purified control CD4+ and those treated by G-CSF after PHA stimulation. In the absence of accessory cells, IFN-γ production was not detected in PHA-stimulated CD4+ cells (data not shown).
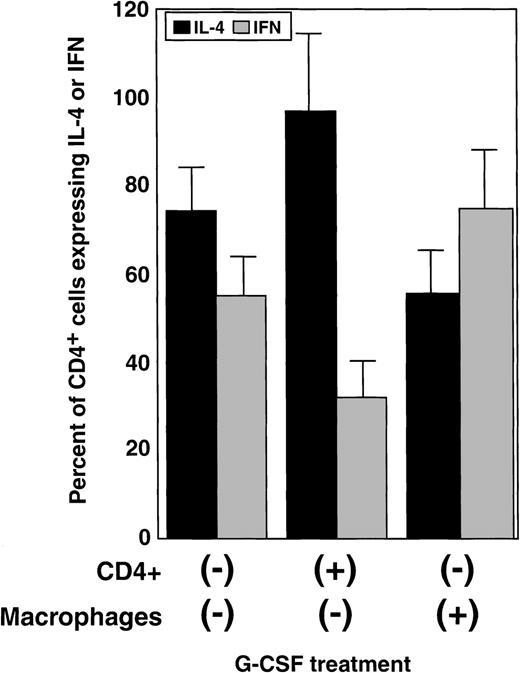
When monocyte preparations, purified CD4+cells, or CD8+ cells (98% pure by flow cytometry) were cultured in the presence of G-CSF, washed, and then mixed—G-CSF–exposed CD4+ cells with untreated monocytes; G-CSF–exposed monocytes with untreated CD4+ cells not treated with G-CSF; G-CSF–exposed CD4+ cells with G-CSF–exposed monocytes—and stimulated for 48 hours with either CD3 mAb or PHA, only CD4+ cells directly exposed to G-CSF showed changes in IFN-γ expression (Figure4 and Table 2). Neither CD4+ cells cultured with G-CSF–exposed monocytes nor CD8+ cells exposed directly or cultured with G-CSF–treated monocytes had altered IFN-γ production.
Direct effects of G-CSF on IFN-γ and IL-4 production by CD4+ T lymphocytes.
Monocytes and CD4+ cells from normal donors were purified to obtain preparations with more than 98% purity as determined by flow cytometry using CD4 and CD14 mAb. Monocytes and CD4+ cells were separately exposed to G-CSF for 24 hours, washed, and mixed together (ratio 1:1). The expression of IFN-γ and IL-4 was measured using an intracellular staining technique and flow cytometry. Bars represent the changes (mean ± SEM) in the percentage of cells expressing IFN-γ (gray) and IL-4 (black) over a control mixture of monocytes and monocytes not exposed to G-CSF (summary of 2 independent experiments).
Direct effects of G-CSF on IFN-γ and IL-4 production by CD4+ T lymphocytes.
Monocytes and CD4+ cells from normal donors were purified to obtain preparations with more than 98% purity as determined by flow cytometry using CD4 and CD14 mAb. Monocytes and CD4+ cells were separately exposed to G-CSF for 24 hours, washed, and mixed together (ratio 1:1). The expression of IFN-γ and IL-4 was measured using an intracellular staining technique and flow cytometry. Bars represent the changes (mean ± SEM) in the percentage of cells expressing IFN-γ (gray) and IL-4 (black) over a control mixture of monocytes and monocytes not exposed to G-CSF (summary of 2 independent experiments).
G-CSF does not decrease TH1/TH2 ratio by increasing apoptosis
The G-CSF-induced modulation pattern of cytokine expression in CD4+ cells could potentially be mediated by the deletion of TH1 cells secreting IFN-γ, as for example by selective apoptosis. Using the annexin technique, we investigated the effects of G-CSF on apoptosis of CD4+ cells. The presence of G-CSF in culture did not affect the numbers of apoptotic cells, as determined by flow cytometry. After 48 hours of culture, a mean of 48% ± 10 TH1 cells and 54% ± TH2 cells stained with annexin (n = 3).
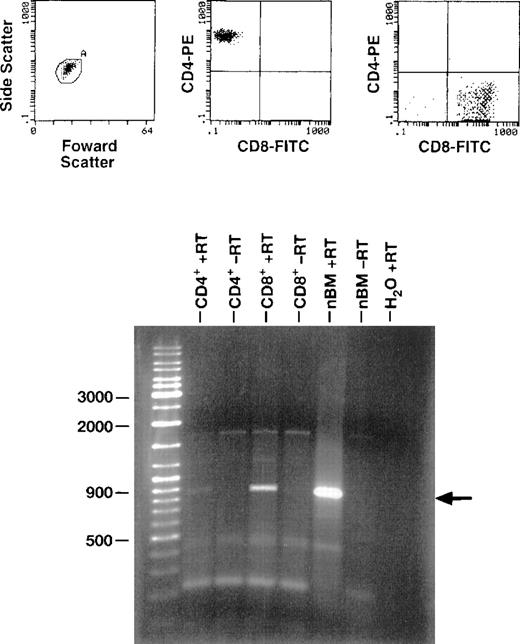
RT-PCR detects GCSF-R m-RNA expression in CD4 and CD8 cells
Peripheral blood mononuclear cells were sorted for CD4+and CD8+ by FACS, resulting in a preparation that was 99.6% and 97% pure, respectively. Total RNA was extracted using RNA STAT-60, and RT-PCR was performed using an oligo d(T)16 primer. After reverse transcription, the cDNA was amplified using primer pairs 5′-AGTACAGTCCTCACCCTGATG-3′ and 5′-AAAGTATGCAGATCGCCTGGG-3′ specific for G-CSF receptor. A band of 900 kbp was present for CD4 and CD8 cells, indicating the molecular presence of G-CSF-R (Figure 5).
Expression of G-CSF mRNA in PB CD4+ and CD8+ cells.
Purified CD4+ and CD8+ cells were derived by sorting PBMCs obtained from a normal healthy control. mRNA was extracted from the cells, and RT-PCR was performed using G-CSFR–specific primers. RT-PCR products were electrophoresed in agarose gel containing ethidium bromide and visualized under ultraviolet light. Unseparated normal bone marrow (nbm) from a normal control was used as a positive control.
Expression of G-CSF mRNA in PB CD4+ and CD8+ cells.
Purified CD4+ and CD8+ cells were derived by sorting PBMCs obtained from a normal healthy control. mRNA was extracted from the cells, and RT-PCR was performed using G-CSFR–specific primers. RT-PCR products were electrophoresed in agarose gel containing ethidium bromide and visualized under ultraviolet light. Unseparated normal bone marrow (nbm) from a normal control was used as a positive control.
Discussion
In this study, we demonstrated that the pretreatment of T cells with G-CSF resulted in diminished IFN-γ and increased IL-4 production when these cells were subsequently subjected to polyclonal stimulation with IL-2, PHA, or CD3 mAb in vitro. In the presence of G-CSF, resting CD4+ cells that expressed low levels of IL-4 produced significant amounts of this cytokine after polyclonal activation. In agreement with in vitro results, we found that when PBSC donors were stimulated with G-CSF for 5 days, their T cells produced less IFN-γ and more IL-4 in comparison to pretreatment levels. This result implies that G-CSF decreased the TH1/TH2 ratio within the CD4+T-cell population. Because monocytes constitute a major population in PBSC harvests, they could in principle mediate the immunomodulatory effects of G-CSF on T lymphocytes. Using cocultivation experiments, we demonstrated that the effects of G-CSF on CD4+ T cells were direct and that monocytes were not required for G-CF–mediated modulation. Although previous results have shown that monocytes undergo apoptosis as a result of exposure to IFN-γ, survival of monocytes in our cultures was improved by their coculture with G-CSF–treated CD4+ cells. This effect may have been caused by the diminished quantities of IFN-γ in G-CSF–supplemented cultures or the survival-promoting effects of G-CSF. G-CF–mediated modulation of the cytokine pattern produced by T cells could be related to apoptosis of a particular T-cell subset (either TH1 or TH2) or their preferential survival. From the lack of changes in the apoptosis rate of lymphocytes stimulated with G-CSF, we concluded that this cytokine exerts its effect on IFN-γ and IL-4 production through modulation rather than the preferential elimination of TH1 cells.
Other investigators7,8,19 have demonstrated that G-CSF–mobilized monocytes exert inhibitory effects on T-cell proliferation and responsiveness. G-CSF–mobilized CD14+cells express significantly lower levels of the costimulatatory molecules B7-2, implying that low levels of B7-2 expression may contribute to the decreasing T-cell responsiveness by providing suboptimal amounts of stimulatory signals.8 The observation that the blockade of B7 with soluble CD28 fusion protein leads to subresponsiveness of the T cells also supports the theory that G-CSF down-modulates the GVHD reactivity of PBSC grafts. Our results are not in contrast with this thesis, and both mechanisms may simultaneously operate in PBSC donors, resulting in an unexpected low rate and intensity of GVHD in recipients of PBSC allografts. Another group20 demonstrated the increased production of both IL-4 and IFN-γ by PBMCs obtained from patients with non-Hodgkin's lymphoma who undergo autologous transplantation compared to normal controls. However, the contributions of the underlying disease, previous chemotherapy, and GCSF in changing cytokine responsiveness cannot be ascertained. In this study we showed that both CD4+ and CD8+ cells contain G-CSF receptor mRNA, suggesting a mechanism by which G-CSF directly interacts with T cells.
Clinically, the use of unmanipulated PBSC transplants derived from donors treated with G-CSF is associated with an unexpected low rate of GVHD2,3 given the relatively large number of T cells infused. If G-CSF can mediate a shift in the balance between TH1 and TH2 cells, G-CSF administration to the PBSC donors might influence the course of GVHD. Acute GVHD appears to be mediated by TH1 cells and their proinflammatory cytokines IL-2, TNF, and IFN-γ.21 Although IFN-γ is a marker for TH1 cells, IFN-γ secreted by CD4+ and CD8+ donor T cells is one of the earliest events occurring in acute GVHD.22 IFN-γ is thought responsible for the delayed hematopoietic recovery and development of cytopenias.22,23IFN-γ is also present in the intestinal lesions of acute GVHD, and anti–IFN-γ antibodies prevented bowel damage in a murine GVHD model. In contrast, TH2 cells appeared to favorably modify acute GVHD. IL-4, a product of TH2 cells, decreases the expression of other proinflammatory cytokines, which may lead to immunologic tolerance.24 TH2 cells, however, may mediate chronic GVHD.
G-CSF also has been used to treat aplastic anemia. Especially in combination with SCF25 or with antithymocyte globulin and cyclosporine,26 G-CSF may have therapeutic activity apart from its stimulatory effects on hematopoiesis. Both GVHD and aplastic anemia have been reported to be related to the overexpression of IFN-γ.27 28 For both, the beneficial effects of G-CSF may be secondary to changes in IFN-γ production and the balance in the TH1 and TH2 lymphocyte subsets. The effects of G-CSF on the cytokine secretion pattern of lymphocytes derived from G-CSF–treated normal subjects were comparable to those observed in aplastic anemia. In studies to be reported elsewhere, we found that when patients with aplastic anemia and abnormally decreased TH1/TH2 ratios were treated with G-CSF, significant changes in the TH1/TH2 subsets were observed (unpublished data).
In summary, our results suggest that G-CSF, in addition to its stimulatory effects on hematopoiesis, can exert immunomodulatory effects on T lymphocytes, and these may be therapeutically exploited in conditions associated with TH1/TH2 imbalance such as immune-mediated bone marrow failure syndromes and GVHD.
Reprints:Elaine M. Sloand, NIH, National Heart, Lung, and Blood Institute, 31 Center Dr, MSC 2490, Bldg 31, Room 4A11, Bethesda, MD 20892-2490; e-mail: sloande@nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.