Abstract
Antifolate drugs such as methotrexate are commonly used in cancer chemotherapy. It may be possible to increase the antitumor activity of antifolates by the coadministration of drugs that inhibit nucleoside transport, thereby blocking the capacity of tumor cells to salvage nucleotide precursors. An important limitation of this approach is severe myelosuppression caused by many of these drug combinations. For this reason, we have developed a gene therapy strategy to protect bone marrow cells against combined treatment with antifolates and nitrobenzylmercaptopurine riboside (NBMPR), a potent inhibitor of thees nucleoside transporter. A retroviral vector (MeiIRG) was constructed that expressed the NBMPR-insensitive eitransporter, hypothesizing that transduced bone marrow cells would survive drug treatment because of the preservation of nucleoside salvage pathways. In vitro clonogenic assays confirmed that the MeiIRG vector did protect myeloid progenitors against the toxic effects of 3 different antifolates when each was combined with NBMPR. On testing this system in vivo, decreased myelosuppression was observed in mice transplanted with MeiIRG-transduced bone marrow cells and subsequently treated with trimetrexate and NBMPR-P. In these mice, significant increases were noted in absolute neutrophil count nadirs, reticulocyte indices, and the numbers of myeloid progenitors in the bone marrow. Furthermore, a survival advantage was associated with transfer of the MeiIRG vector, indicating that significant dose intensification was possible with this approach. In summary, the MeiIRG vector can decrease the toxicity associated with the combined use of antifolates and NBMPR-P and thereby may provide a strategy for simultaneously sensitizing tumor cells while protecting hematopoietic cells.
Antifolate drugs have been used for many years for the treatment of neoplastic and viral disorders. In addition to the well-established agents methotrexate (MTX) and 5-fluorouracil (5-FU), many novel antifolates are under evaluation for clinical efficacy.1 These compounds exert their cytotoxic effects by targeting key enzymes such as dihydrofolate reductase (DHFR), thymidilate synthetase (TS), and glycinamide ribonucleotide formyltransferases (GARFT) in the de novo biosynthesis of purine and pyrimidine nucleotides.2
There are many forms of acquired and inherent resistance to antifolate drug therapies that are well characterized, such as decreased expression of the reduced folate carrier, mutation or amplification of the DHFR gene, and increased expression of the TS gene.2-4We and others3-8 have postulated that another possible mechanism of resistance to antifolate drugs results from the ability of tumor cells to salvage extracellular nucleosides. Many malignant cells are able to use membrane-spanning nucleoside transporter proteins to salvage extracellular nucleosides and thereby circumvent antifolate toxicity by bypassing the de novo nucleotide synthesis pathway. To date, 5 such transport proteins have been characterized in mammalian cells.5,6 Three of these are sodium-dependent transporters and are predominantly expressed in normal tissues.6,7 The other 2 are equilibrative and are subdivided into 2 classes, esand ei (equilibrative sensitive and equilibrative insensitive, respectively), based on their relative sensitivity to the nucleoside analog NBMPR. Low levels of ei are seen in most normal tissues and some tumor cell lines, whereas es is the major nucleoside transport activity expressed in both normal and malignant tissues.6,7 The high levels of es transporter expression in tumor cells suggests that NBMPR could be used to increase the antitumor effect of antimetabolite drugs by blocking salvage activity. Accordingly, cultured cell lines have been shown to be greatly sensitized to the cytotoxic effects of MTX, 5-FU, and PALA (N-phosphonoacetyl-L-aspartate) in vitro by the addition of nucleoside transport inhibitors such as NBMPR or dipyridamole.2,9-12NBMPR-P, a 5′-monophosphate pro-drug of NBMPR, has been demonstrated to block nucleoside transport in vivo in mice treated with lethal doses of tubercidin, a cytotoxic adenosine analog.13,14 However, it is not clear whether tumor cells can be sensitized to antifolates in vivo using nucleoside transport inhibitors. In clinical trials in which patients were treated with dipyridamole and 1 or more antifolate drugs, any potential clinical benefit was masked by severe myelosuppression and, in some instances, mild to moderate gastrointestinal toxicities.15-21 The gastrointestinal toxicities noted are unlikely to result from the addition of NBMPR or dipyridamole because the major nucleoside transporters in gastrointestinal epithelial cells are known to be sodium dependent6,7 and therefore are not inhibited by either of these compounds. However, the severe myelosuppression resulting from the drug combination can be explained by the fact that immature bone marrow (BM) cells predominantly express the estransporter7 and are very sensitive to the combined effects of antifolate drugs and NBMPR.22
A wide body of evidence has accumulated that suggests that the transient myelosuppression associated with antifolate chemotherapy, in the absence of a nucleoside transport inhibitor, results from toxicity at relatively late stages of hematopoietic development.22-26 The severe myelotoxicity seen with a combination of antifolate and nucleoside transport inhibitors results from the sensitization of primitive hematopoietic precursor cells that express the es transporter.22 The resultant myelosuppression is more pronounced because the combination therapy effectively depletes the more primitive progenitor and stem cells.22,26 27 Therefore, protection of hematopoietic cells will be necessary to treat patients effectively with a combination of antifolates and nucleoside transport inhibitors.
Transfer of drug-resistant genes to hematopoietic stem cells has shown success as an experimental strategy for protecting hematopoiesis against drug-induced myelosuppression.28 This approach has been validated in preclinical model systems using a variety of drug-resistant genes and cytotoxic drug combinations.29With regard to antifolates, drug-resistant variants of DHFR gene have been shown to protect mice from MTX and TMTX-induced myelosuppression.30-32 One disadvantage with DHFR vectors is that the protection is limited to antifolate drugs that only target the DHFR enzyme, not drugs such as 5-FU and Tomudex, which are TS inhibitors, or LY231514, a multi-targeting drug that inhibits DHFR, TS, and GARFT. We hypothesized that transfer of a nucleoside transporter gene would confer protection against a broad spectrum of antifolate drugs by allowing transduced cells to salvage preformed nucleotide precursors from the serum, thereby bypassing the antifolate-induced block in de novo synthesis. Because we intend to use NBMPR to sensitize tumor cells to antifolate toxicity, an NBMPR-insensitive nucleoside transporter gene (ei)33 was selected for hematopoietic protection. In the current study, we show that retroviral-mediated transfer of the ei gene protects bone marrow cells from antifolates when used together with NBMPR, both in vitro and in transplanted mice in vivo.
Materials and methods
Materials
Female C57BL/6J and B6.C-H1b/ByJ mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were used for experiments between 12 and 24 weeks of age. Nucleosides and NBMPR were obtained from Sigma (St. Louis, MO). Dipyridamole was generously provided by Boehringer-Ingelheim (Ridgefield, CT). [6-3H]-Thymidine (15 Ci/mmol), was purchased from DuPont NEN (Wilmington, DE). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Bio Whittaker (Walkersville, MD). Dialyzed FBS was purchased from Hyclone (Logan, UT). Murine interleukin-3 (IL-3), human IL-6 and rat stem cell factor were kindly provided by Amgen (Thousand Oaks, CA), and recombinant human erythropoietin was purchased from Amgen. MethoCult M3530 semisolid media were purchased from Stem Cell Technologies (Vancouver, BC, Canada). Trimetrexate (TMTX) base was kindly provided by the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment, NCI (Bethesda, MD) and converted to the glucuronate salt as previously described.30 Neutrexin was purchased from US Bioscience (West Conshohocken, PA). Tomudex was kindly provided by Zeneca Pharmaceuticals (Macclesfield, UK), and LY231514 was a gift from Dr Peter J. Houghton (SJCRH).
MeiIRG retroviral vector and ecotropic producer cells
The open reading frame of the ei gene was polymerase chain reaction (PCR) amplified from a pDR2/N1-71 construct, containing a full-length hENT2 cDNA isolated by expression cloning from a HeLa S3 cDNA library.33 Gene-specific primers 5′ CAGGTGCAGGTGGCGGCTTCT and 5′ TCCGGAAGGAGACGTCGAGA were designed within the 5′ and 3′ untranslated regions of the hENT2 cDNA (GenBank/EBI accession number AF034102), respectively, and used to amplify the ei open reading frame. PCR conditions were optimized to minimize misincorporations by using a commercially available mixture of Taq and Vent DNA polymerases (Clontech Laboratories, Palo Alto, CA) and reducing the PCR cycles to 15, the minimal number required for product amplification. The amplified product was cloned to a PCR2.1 TA cloning vector (Invitrogen, San Diego, CA) and sequenced to confirm homology with the published hENT2 sequence.33 A MeiIRG bicistronic retroviral vector was constructed by subcloning a 1486 bp hENT2 fragment containing the open reading frame into a murine stem cell virus (MSCV) vector (kindly provided by Dr Robert G. Hawley, Toronto),34 upstream of an encephalomyocarditis virus internal ribosome entry site (ires).35 The vector contains as a reporter gene the enhanced green fluorescent protein (GFP; Clontech Laboratories) cDNA downstream of the ires.
A GP+E86 ecotropic retroviral producer cell line36 was generated as previously described.37 Briefly, HEK 293T cells were transiently co-transfected with the retroviral construct MeiIRG and pEQ-PAM3 amphotropic helper plasmid. Supernatants were collected 72 hours after co-transfection and used to infect GP+E86 ecotropic producer cells. A polyclonal population of MeiIRG-transduced GP+E86 cells was used for subsequent experiments. The full-length MeiIRG vector genome was stably transmitted to target cells, and the supernatant was free of replication-competent retrovirus, as determined by previously described assays.37
MGirDHFR(L22Y/F31R) and irGFP producer lines, which express a TMTX-resistant variant of DHFR38 and only the GFP reporter, respectively, were used as positive and negative controls for the in vitro studies. Titers of the polyclonal producer cell lines used for these experiments were in the range of 1 to 10 × 105 particles per milliliter.
Functional characterization of MeiIRG vector
2.0 × 105 BALB/3T3 clone A31 cells were transduced with supernatant from MeiIRG producer cell line for 48 hours until 100% of cells were GFP positive, as determined by FACS analysis.3H thymidine (Thd) uptake was measured in wild-type or MeiIRG-transduced 3T3 cells, in the presence or absence of nucleoside transport inhibitors NBMPR (100 nmol/L) or dipyridamole (10 μmol/L). 3H Thd (1 μmol/L at 4 μCi/mL) influx was measured over 10 seconds in sodium-free buffer containing 120 mmol/L N-methyl-D-glucamine (MGA), 20 mmol/L Tris-HCl, 3 mmol/L K2HPO4.3H2O, 10 mmol/L glucose, 1 mmol/L MgCl2, and 1 mmol/L CaCl2. A sodium-free buffer was used to eliminate nucleoside uptake by any endogenous sodium-dependent transporters in A31 3T3 cells.
Retroviral-mediated bone marrow cell transduction
Bone marrow cells were harvested from tibia and femurs of untreated female C57BL/6J mice (for in vitro myeloid progenitor cells assays) or for transplant studies 48 hours after treatment with 150 mg/kg 5FU (Sigma) given intraperitoneally. Retroviral-mediated gene transfer was carried out by coculture of harvested bone marrow cells on producer cells as previously described.30,37 Briefly, bone marrow cells (1 × 106/mL) were prestimulated for 48 hours in DMEM medium supplemented with 20 ng/mL murine IL-3, 50 ng/mL human IL-6, 50 ng/mL rat stem cell factor, and 20% FBS (Hyclone). Prestimulated BM cells were subsequently cocultured for 48 hours with either MeiIRG, MGirDHFR (L22Y/F31R) or irGFP ecotropic retroviral producer cell lines, rendered replication defective by irradiation (11 Gy), with the above cytokines and 6 μg/mL polybrene. After transduction, nonadherent cells were harvested and resuspended in either DMEM plus 15% dialyzed FBS (Hyclone) for in vitro myeloid progenitor cell assays or PBS containing 2% FBS plus 20 U/mL heparin for transplantation. Dialyzed FBS was purchased as a single lot that contained undetectable thymidine and hypoxanthine as determined previously.22
In vitro drug sensitivity assays
Bone marrow cells were harvested from tibia and femurs of untreated C57Bl/6J mice and transduced with the MeiIRG, MGirDHFR(L22Y/F31R) or irGFP vectors as described above. Nonadherent, transduced BM cells were the collected and cultured in 6-well plates (Corning, Corning, NY) at 1.0 × 106 cell/mL in growth media (DMEM) containing 15% dialyzed FBS, cytokine cocktail (as described above), 1 μmol/L or 100 nmol/L Thd, and 100 μmol/L hypoxanthine. Transduced BM cells were treated in suspension cultures for 4 days at varying concentrations of TMTX, Tomudex, or LY231514 in the presence or absence of NBMPR and dipyridamole. After treatment, nonadherent and adherent BM cells were extensively washed to remove residual drugs and were resuspended in equal volumes of PBS. The volume equivalent of 3.0 × 104 BM cells from drug-free cultures were resuspended in 3 mL methylcellulose semisolid culture media (MethoCult M3530) supplemented with 3 U/mL recombinant human erythropoietin. The progenitor colony numbers were determined 7 days after plating by scoring all colonies consisting of more than 50 cells. A stock solution of NBMPR (10 mmol/L) was prepared in dimethyl sulfoxide before the addition to culture medium, TMTX was dissolved in sterile water and Tomudex and LY231514 were dissolved in 0.4 mol/L sodium bicarbonate, and all 3 were diluted in DMEM before use.
Transplantation
Transduced or untransduced control bone marrow cells (2 to 4 × 106) were transplanted through lateral tail veins into lethally irradiated (11 Gy at 1.24 Gy/minute) B6.C-H1b/ByJ (also referred to as HW80) recipients. Mice were placed on chlorinated drinking water and filter top cages 2 days before and for 4 weeks after transplantation.
GFP flow cytometric analysis
Whole blood obtained from the retro-orbital sinus 10 weeks after transplantation was diluted 100-fold in PBS and used to determine GFP expression in RBCs and platelets, which were gated based on forward and side light scatter as previously described.37 Peripheral blood leukocytes were depleted of RBCs by incubation for 10 minutes on ice in RBC lysis buffer (0.9% ammonium chloride), and then viable (based on propidium iodine exclusion) cells were analyzed for GFP expression.
Drug treatment of mice
Control and ei transplanted mice were treated with a combination of TMTX and NBMPR-P for 5 consecutive days 10 (cohort 1) and 25 (cohort 2) weeks after transplantation. TMTX glucuronate, provided by the Drug Synthesis and Chemistry Branch at the NCI, was used to treat mice in cohort 1 at a dose of 100 mg/kg. Neutrexin (trimetrexate glucuronate), purchased from US Bioscience, was found to be more active than the NCI TMTX and was given to cohort 2 at a dose of 80 mg/kg. Both the TMTX salt and Neutrexin were dissolved in sterile water and delivered as subcutaneous injections. NBMPR-P was synthesized as previously described39 and dissolved in sterile water, and 20 mg/kg was administered as intraperitoneal injections.
Hematology
Peripheral blood was obtained from the retro-orbital sinus with microcapillary tubes after Metofane anesthesia and was used to determine standard hematologic parameters. White blood cell counts were performed on erythrocyte-depleted blood diluted 1000-fold in Isoton using a Coulter (Hialeah, FL) counter analyzer. The hematocrit was determined by reading the height of packed red cells. Differentials were performed manually on Wright Giemsa-stained whole blood films.
Progenitor cell counts
Bone marrow was harvested from femurs of mice transplanted with untransduced or ei transduced BM cells 24 hours after final drug treatment and resuspended in 1 mL PBS per femur. Immature progenitor cell colonies were assessed as described above and expressed as clonogenic colonies per volume of femur relative to untreated control animals.
Statistical methods
The probability of statistical differences between experimental groups was determined by a 2-tailed Student t test using Instat 2.03 software for Macintosh (Apple, Cupertino, CA).
Results
MeiIRG vector
The MeiIRG vector was constructed by cloning the open reading frame of the ei gene33 into the MSCV retroviral backbone.34 An IRES-GFP fragment was inserted downstream of the ei gene to facilitate detection of transduced cells.37 Stable ecotropic GP+E86 producer cells36 were made as previously described.37 A polyclonal population with a titer of 5 × 105 on NIH 3T3 cells was used for all subsequent experiments. Southern blot analysis of transduced 3T3 and murine bone marrow cells showed that the proviral genome was transmitted unrearranged to these target cells.
Thymidine uptake in MeiIRG-transduced BALB/3T3 clone A31 cells
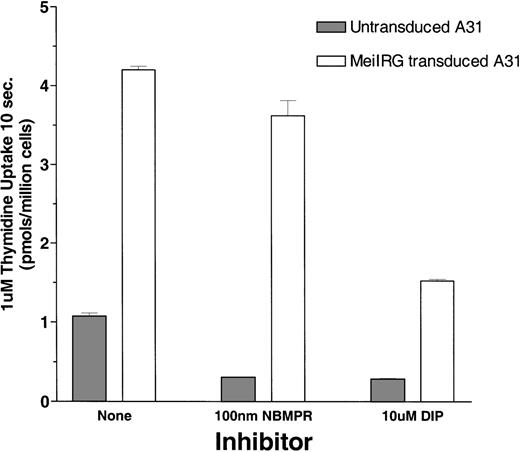
Functional activity of the MeiIRG retroviral vector was determined by comparing the influx of 3H thymidine (Thd) in untransduced cells and MeiIRG-transduced BALB/3T3 (A31) cells. A31 cells were transduced by incubation with supernatant from the MeiIRG GP+E86 producer cells until more than 98% of the cells were GFP positive. Influx of 3H Thd was measured at 24°C over a 10-second interval in sodium-free MGA buffer in the presence or absence of 100 nmol/L NBMPR or 10 μmol/L dipyridamole (Figure1). The major nucleoside transporter in WT A31 cells is es (J.A.B., unpublished data) consistent with our observation that the addition of 100 nmol/L NBMPR diminishes Thd influx in the untransduced cells down to background levels. In A31 cells transduced with MeiIRG construct, influx of Thd was approximately 3-fold greater than in untransduced cells. Furthermore, this activity was not inhibited by NBMPR, consistent with the known resistance of theei transporter to NBMPR. Both the endogenous es and the retroviral transduced ei activities were significantly inhibited in the presence of 10 μmol/L DIP, a nucleoside transport inhibitor known to inhibit both es and ei transport activities.5 6
Thymidine uptake in cells transduced with the MeiIRG retroviral vector.
BALB/3T3 A31 cells were transduced with MeiIRG retroviral vector, expressing the human nitrobenzylmercaptopurine riboside (NBMPR)-insensitive equilibrative nucleoside transporter eigene and a green fluorescent protein (GFP) reporter gene. Uptake of3H thymidine (Thd; 1.0 μmol/L at 4 μCi/mL) was measured in untransduced and MeiIRG-transduced A31 cells in the presence or absence of 100 nmol/L NBMPR or 10 μmol/L dipyridamole (DIP) in sodium-free MGA transport buffer. Cells were preincubated for 5 minutes at 22°C with either MGA buffer alone or MGA containing 100 nmol/L NBMPR or 10 mmol/L DIP before the addition of 3H Thd. Nucleoside uptake was determined at a constant time interval of 10 seconds. The values presented are from assays conducted in triplicate.
Thymidine uptake in cells transduced with the MeiIRG retroviral vector.
BALB/3T3 A31 cells were transduced with MeiIRG retroviral vector, expressing the human nitrobenzylmercaptopurine riboside (NBMPR)-insensitive equilibrative nucleoside transporter eigene and a green fluorescent protein (GFP) reporter gene. Uptake of3H thymidine (Thd; 1.0 μmol/L at 4 μCi/mL) was measured in untransduced and MeiIRG-transduced A31 cells in the presence or absence of 100 nmol/L NBMPR or 10 μmol/L dipyridamole (DIP) in sodium-free MGA transport buffer. Cells were preincubated for 5 minutes at 22°C with either MGA buffer alone or MGA containing 100 nmol/L NBMPR or 10 mmol/L DIP before the addition of 3H Thd. Nucleoside uptake was determined at a constant time interval of 10 seconds. The values presented are from assays conducted in triplicate.
NBMPR potentiates cytotoxicity of Tomudex and LY231514 in control murine progenitor cells in vitro
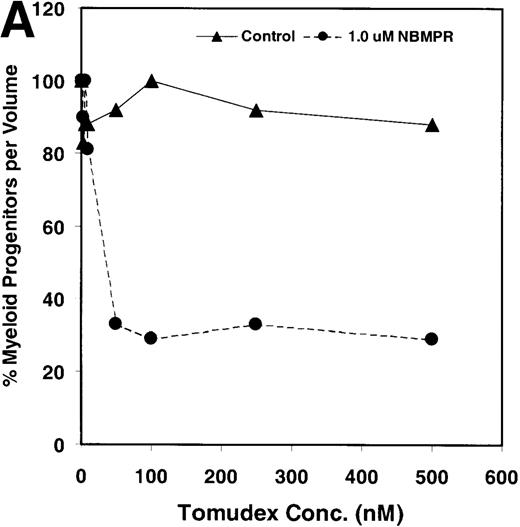
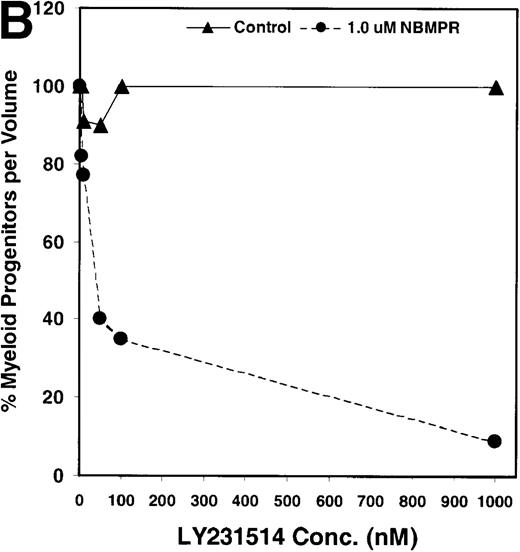
Salvage of extracellular nucleotide precursors by nucleoside transporter proteins has been postulated as a mechanism of resistance to antimetabolite drugs in certain tumor cell lines and in undifferentiated BM progenitor cells.4,7,15,22 These studies predict that NBMPR should sensitize both tumor cells and bone marrow cells to a variety of antifolate drugs. To test whether this was true for bone marrow cells, the cytotoxic effects of Tomudex, a thymidylate synthase (TS) inhibitor,1,40 and LY231514, a multi-targeting antifolate that inhibits DHFR, TS, and GARFT1 41 were evaluated in vitro in the presence or absence of 1 μmol/L NBMPR. Murine bone marrow cells were transduced with the control irGFP vector and treated with varying concentrations of Tomudex (0 to 500 nmol/L) or LY231514 (0 to 1000 nmol/L) in suspension cultures containing 1 μmol/L Thd and 100 μmol/L hypoxanthine for 4 days. Figure 2A shows that in the absence of NBMPR, no significant reduction in progenitor colony number was observed at the highest concentration of Tomudex (500 nmol/L) tested. However, when the BM cells were treated with a combination of Tomudex and 1.0 μmol/L NBMPR, the number of progenitor colonies fell below 50% at Tomudex concentration of less than 40 nmol/L (IC50 = 36 nmol/L). Similar observations were noted with LY231514, a multi-targeting antifolate drug. When BM cells were treated with LY231514 alone, the progenitor colony number remained unaffected even at 1000 nmol/L. However, when the antifolate was tested in combination with 1.0 μmol/L NBMPR, the number of progenitor cell colonies again dropped to below 50% at LY231514 concentrations less than 35 nmol/L (IC50 = 31 nmol/L). The findings from these in vitro studies show that NBMPR markedly sensitizes murine progenitor cells to the cytotoxic effects of multi-targeting antifolate drugs.
NBMPR-induced potentiation of Tomudex and LY231514 cytotoxicity in control murine myeloid progenitor cells.
Murine bone marrow cells were transduced with a control retroviral vector expressing only GFP. Transduced cells were treated in growth medium supplemented with 15% dialyzed FBS, myeloid cytokines, 1 μmol/L thymidine, and 100 μmol/L hypoxanthine. Varying concentrations of Tomudex (0 to 500 nmol/L) (A) or LY231514 (0 to 1000 nmol/L) (B) were added to culture media in the presence or absence of 1 μmol/L NBMPR. After 4 days of drug treatment, the volume equivalent 1 × 105 BM cells from control untreated cultures were washed and plated in 3 mL drug-free methylcellulose semisolid media. Myeloid progenitor colony counts were determined 7 days later by scoring colonies with more than 50 cells. Progenitor contents of the drug-treated cultures are expressed as a percentage of the colony number observed in untreated cultures.
NBMPR-induced potentiation of Tomudex and LY231514 cytotoxicity in control murine myeloid progenitor cells.
Murine bone marrow cells were transduced with a control retroviral vector expressing only GFP. Transduced cells were treated in growth medium supplemented with 15% dialyzed FBS, myeloid cytokines, 1 μmol/L thymidine, and 100 μmol/L hypoxanthine. Varying concentrations of Tomudex (0 to 500 nmol/L) (A) or LY231514 (0 to 1000 nmol/L) (B) were added to culture media in the presence or absence of 1 μmol/L NBMPR. After 4 days of drug treatment, the volume equivalent 1 × 105 BM cells from control untreated cultures were washed and plated in 3 mL drug-free methylcellulose semisolid media. Myeloid progenitor colony counts were determined 7 days later by scoring colonies with more than 50 cells. Progenitor contents of the drug-treated cultures are expressed as a percentage of the colony number observed in untreated cultures.
MeiIRG vector confers antifolate resistance in murine progenitor cells in vitro
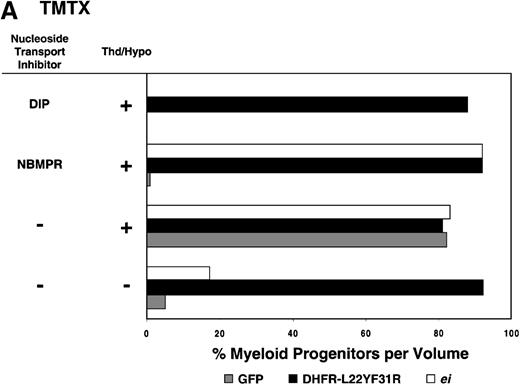
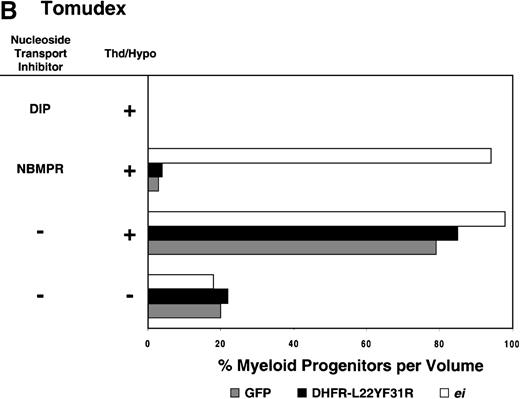
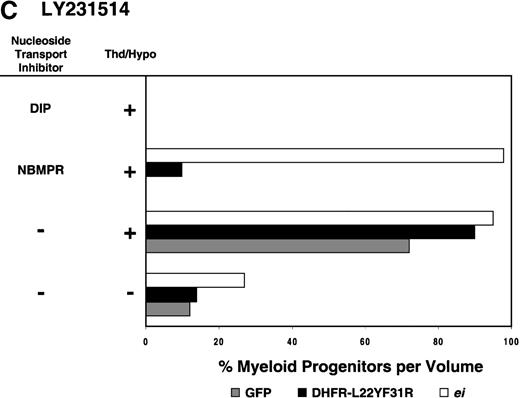
Having shown that NBMPR sensitizes murine progenitor cells to the cytotoxic effects of antifolate drugs, we examined whether the MeiIRG vector could protect murine BM cells against antifolate/NBMPR drug combinations. Murine BM cells were transduced with retroviral vectors irGFP, MGirDHFR(L22Y/F31R), or MeiIRG, expressing either GFP alone, a TMTX-resistant DHFR variant, or the ei gene, respectively. Transduced cells were treated for 4 days with TMTX, Tomudex, or LY231514 in suspension cultures in the presence or absence of NBMPR or DIP. All drug treatments were carried out in dialyzed FBS supplemented with 100 nmol/L Thd, which approximates levels measured in human serum,42 and 100 μmol/L hypoxanthine as indicated. The irGFP and MeiIRG-transduced progenitor cells were highly sensitive to 150 nmol/L TMTX when treated in the absence of exogenous Thd and hypoxanthine, whereas the addition of 100 nmol/L Thd and 100 μmol/L hypoxanthine resulted in complete rescue (Figure3A). When NBMPR was added to the latter culture conditions, myeloid progenitor colonies in irGFP-transduced cells were reduced to 1% of that seen in TMTX-free cultures, whereas BM cells transduced with the MeiIRG vector or the TMTX resistant MGirDHFR(L22Y/F31R) vector were completely protected. As expected, when dipyridamole was added, a drug that inhibits bothes and ei transporters, BM cells transduced with the MeiIRG vector were entirely sensitized to TMTX toxicity whereas the MGirDHFR(L22Y/F31R)-transduced BM cells continued to be protected.
Protection of murine progenitor cells in vitro from the cytotoxic effects of Trimetrexate, Tomudex, or LY231 514 together with NBMPR.
Murine BM cells were transduced with retroviral vectors expressing only the GFP gene, an L22YF31R trimetrexate (TMTX)-insensitive variant of the DHFR gene, or the NBMPR-insensitive nucleoside transporterei gene. Cells were then treated in suspension culture for 4 days in dialyzed FBS supplemented with myeloid cytokines in the presence or absence of 100 nmol/L Thd and 100 μmol/L hypoxanthine. 150 nmol/L TMTX (A), 100 nmol/L Tomudex (B), and 1 μmol/L LY231514 (C) were added to the suspension cultures in the presence or absence of 100 nmol/L NBMPR or 10 μmol/L DIP as indicated. After 4 days of drug treatment, cells were washed and plated in drug-free semisolid media, and the myeloid progenitor counts were determined. The progenitor cell counts in drug-treated cultures are expressed as a percentage of the colony number observed in untreated cultures.
Protection of murine progenitor cells in vitro from the cytotoxic effects of Trimetrexate, Tomudex, or LY231 514 together with NBMPR.
Murine BM cells were transduced with retroviral vectors expressing only the GFP gene, an L22YF31R trimetrexate (TMTX)-insensitive variant of the DHFR gene, or the NBMPR-insensitive nucleoside transporterei gene. Cells were then treated in suspension culture for 4 days in dialyzed FBS supplemented with myeloid cytokines in the presence or absence of 100 nmol/L Thd and 100 μmol/L hypoxanthine. 150 nmol/L TMTX (A), 100 nmol/L Tomudex (B), and 1 μmol/L LY231514 (C) were added to the suspension cultures in the presence or absence of 100 nmol/L NBMPR or 10 μmol/L DIP as indicated. After 4 days of drug treatment, cells were washed and plated in drug-free semisolid media, and the myeloid progenitor counts were determined. The progenitor cell counts in drug-treated cultures are expressed as a percentage of the colony number observed in untreated cultures.
Similar experiments using Tomudex showed that myeloid progenitors modified with the ei gene were protected against the cytotoxic effects of Tomudex combined with NBMPR (Figure 3B). Using concentrations of Tomudex and NBMPR that resulted in more than 95% killing of cells transduced with either GFP or DHFR variant gene, the MeiIRG-transduced progenitors were completely protected. Again, as with the TMTX, protection of the murine progenitors was exclusively through the salvage of extracellular nucleotide precursors by the transducedei gene, as indicated by the addition of dipyridamole, which completely sensitizes the MeiIRG-transduced cells to Tomudex. Dipyridamole, in the absence of antifolate drugs, was not toxic to BM progenitor cells at the concentration (10 μmol/L) used (data not shown). Similar observations were noted when transduced BM cells were treated with the multi-targeting antifolate LY23151441 43(Figure 3C). Under conditions that resulted in high levels of killing of GFP and DHFR-transduced BM cells, the ei-transduced progenitor cells were completely protected. These findings show that the ei gene protects murine BM cells, in the presence of NBMPR, from the cytotoxic effects of multiple antifolate drugs that collectively target at least 3 different enzymes in the nucleotide biosynthetic pathways.
TMTX- and NBMPR-P-induced myelosuppression is abated in MeiIRG-transplanted mice
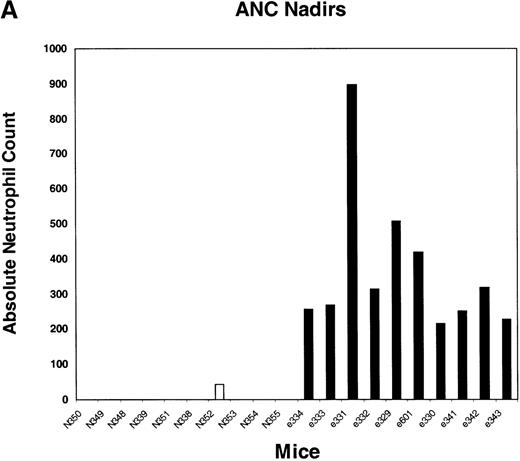
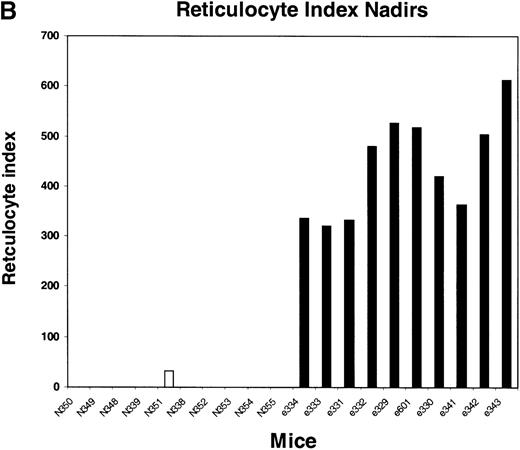
We next tested the protective properties of the ei gene in vivo using a mouse model for stem cell gene transfer. Briefly, primary BM cells from C57BL/6J mice were transduced with the MeiIRG vector and transplanted into lethally irradiated recipient mice as previously described.44 Control mice were transplanted with fresh untransduced BM cells from C57BL/6J mice. The BM transduction efficiency of the MeiIRG vector ranged from 62% to 91%, as determined by GFP expression in peripheral blood leukocytes, red blood cells, and platelets in 15 mice evaluated 10 to 25 weeks after transplantation (Table 1). Transplanted mice were treated with 100 mg/kg TMTX daily for 5 days, together with 20 mg/kg NBMPR-P. Peripheral blood was collected from the retro-orbital sinus 24 hours after the fifth day of treatment and the absolute neutrophil count and reticulocyte index were determined. Figures4A and 4B showed that both the absolute neutrophil count and the reticulocyte index fell to zero in all but 1 of the control mice (N). In comparison, the absolute neutrophil count and the reticulocyte indices were significantly higher in allei-transplanted mice. These findings were reproduced with a second cohort of control and ei-transplanted mice treated for 5 consecutive days with 80 mg/kg trimetrexate glucuronate (Neutrexin; US Bioscience) and 20 mg/kg NBMPR-P 25 weeks after transplantation (data not shown). In the second experiment, the Neutrexin and NBMPR-P doses used resulted in a 50% (5 of 10) loss of mice in the control group on day 6 after the start of treatment, whereas all 12 of theei-transplanted mice survived the treatment. Indeed, other than the 5 mice used for BM progenitor cell counts, the remaining 7 were still alive 14 weeks after treatment. BM cells from 5ei-transplanted and the 5 surviving control mice were harvested 24 hours after the fifth treatment, and the progenitor CFU-C was determined as clonogenic cells per volume of femur relative to untreated animals. Figure 5 shows that the myeloid progenitor CFU-C was conserved in 4 of 5 of theei-transplanted mice, whereas only 1 of the control transplanted mice showed any surviving progenitors (4%) after 5 consecutive days of drug treatment. The protection of BM progenitors inei-transplanted mice was statistically significant when compared to control transplanted mice (P < .02).
Peripheral blood absolute neutrophil count and reticulocyte index nadirs in transplanted mice treated with TMTX and NBMPR-P.
Mice transplanted with untransduced (N) or MeiIRG (e)-transduced BM cells were treated with 100 mg/kg TMTX together with 20 mg/kg NBMPR daily for 5 days, 10 weeks after transplantation. The absolute neutrophil count (ANC; A) and the reticulocyte index (B) were determined 24 hours after the final treatment. Each bar represents the value for an individual mouse.
Peripheral blood absolute neutrophil count and reticulocyte index nadirs in transplanted mice treated with TMTX and NBMPR-P.
Mice transplanted with untransduced (N) or MeiIRG (e)-transduced BM cells were treated with 100 mg/kg TMTX together with 20 mg/kg NBMPR daily for 5 days, 10 weeks after transplantation. The absolute neutrophil count (ANC; A) and the reticulocyte index (B) were determined 24 hours after the final treatment. Each bar represents the value for an individual mouse.
In vivo protection of bone marrow progenitor cells from TMTX and NBMPR-P treatment.
Mice transplanted with untransduced (N) or MeiIRG (e)-transduced BM cells were treated daily for 5 days with 80 mg/kg Neutrexin and 20 mg/kg NBMPR 25 weeks after transplantation. BM cells were harvested from femurs of treated mice 24 hours after final drug treatment, and the volume equivalent of 1 × 105 cells, from untreated animals, were plated in 3 mL methylcellulose. Colonies containing more than 50 cells were scored 7 days later and are expressed as a percentage of the colony number observed from untreated control mice.
In vivo protection of bone marrow progenitor cells from TMTX and NBMPR-P treatment.
Mice transplanted with untransduced (N) or MeiIRG (e)-transduced BM cells were treated daily for 5 days with 80 mg/kg Neutrexin and 20 mg/kg NBMPR 25 weeks after transplantation. BM cells were harvested from femurs of treated mice 24 hours after final drug treatment, and the volume equivalent of 1 × 105 cells, from untreated animals, were plated in 3 mL methylcellulose. Colonies containing more than 50 cells were scored 7 days later and are expressed as a percentage of the colony number observed from untreated control mice.
Discussion
The inherent or acquired resistance to cytotoxic drugs in certain tumor cells is a major concern in the treatment of neoplastic disorders.2-4 The conventional mechanisms of resistance, such as p53 status, gene amplification, mutation of target genes, and altered drug transport, have been well characterized.4 More recently some of these resistance mechanisms, initially considered to be an impediment, have been used in gene therapy approaches for the protection of normal tissues from the cytotoxic effects of chemotherapeutic agents. A number of chemoresistance genes have been identified for potential bone marrow protection, including P-glycoprotein (MDR-1),45,46 aldehyde dehydrogenase (ADH),47 DHFR,30-32 TS,48,49O6-methylguanine DNA methyltransferase (MGMT),46,50,51 glutathione-S transferase (GST).52 Some of these enzymes, either wild-type or mutated forms, have already been demonstrated to protect murine and human BM cells in vitro and in vivo. In the current study we report the use of a nucleoside transporter gene for the protection of murine BM cells against the cytotoxic effects of a broad spectrum of antifolate drugs and in doing so are introducing a new class of drug-resistant genes for hematopoietic protection.
Nucleoside transporter proteins may to play an important role in determining the antitumor activity of antifolate drugs. These transporters may circumvent antifolate cytotoxicity by allowing tumor cells to salvage exogenous nucleosides, thereby bypassing the antifolate-induced block in de novo nucleotide synthesis. Accordingly, designing new treatment modalities that combine antifolate chemotherapy with nucleoside transport inhibitors may result in increased chemosensitization of tumor cells. Although this possibility has been demonstrated in multiple tumor cell line models in vitro,2,9,10,12 in vivo trials have been less successful.15-21 One drawback to this form of combination therapy is that normal bone marrow progenitor cells predominantly express the es transporter5,7,40,53 and as such are also sensitized.22 We have therefore explored a novel gene transfer strategy for protecting myeloid progenitors from such combination therapies and believe that this approach may allow simultaneous sensitization of tumor cells and bone marrow protection.
We have shown that the salvage of extracellular nucleosides protects BM progenitor cells from a variety of antifolate drugs and that blocking salvage with NBMPR dramatically potentiates antifolate-induced killing of BM progenitor cells. This is consistent with our previous observation22 that the NBMPR-sensitive esnucleoside transport protein plays a critical role in determining TMTX toxicity in immature hematopoietic cells. Furthermore, we have shown that BM progenitor cells were protected from antifolate-induced cytotoxicity, in the presence of NBMPR, by retroviral transfer of the MeiIRG vector. This vector provided BM protection against a broad spectrum of antifolate drugs, TMTX, Tomudex, or LY231514, that target multiple enzymes in the de novo nucleotide biosynthetic pathway. The observation that progenitor protection was entirely dependent on the presence of extracellular thymidine and hypoxanthine and that protection was lost with the addition of dipyridamole, an inhibitor of both es and ei,5,6,33 demonstrates that the protection was mediated by nucleoside influx. Interestingly, complete progenitor protection was achieved at extracellular thymidine concentrations that are comparable to those found in human serum.42 This suggests that salvage-induced protection, by transfer of the ei gene, may be a feasible clinical approach for protecting hematopoiesis during antitumor chemotherapy.
One question regarding this system is the degree to which it will allow for dose intensification and improved anti-tumor responses. Our current transplant data prove that mice can be protected from the myelosuppression associated with TMTX and NBMPR-P; however, it is not yet clear whether protection with the L22Y DHFR variant30would be more effective. The potential advantage of the hENT2 gene is that protection can be afforded using antifolates other than DHFR inhibitors. A second question is whether toxicities other than hematopoietic suppression will quickly become dose limiting and prevent the potential advantage of hematopoietic protection. Although gastrointestinal toxicity was not examined in this study and it is known that antifolates exert significant toxicity in the gastrointestinal tract, we predict that the benefits of hematopoietic protection will not be abolished by gut toxicity. NBMPR-P does not increase antifolate toxicity in gastrointestinal epithelial cells, which express concentrative nucleoside transporters that are unaffected by NBMPR-P, so that this drug combination actually increases the specificity of toxicity to the bone marrow. We have been able to achieve significant dose escalation of TMTX and NBMPR-P without dose-limiting gut toxicity using a DHFR vector for hematopoietic protection (J.A.A., manuscript in preparation). It has been demonstrated that hematopoietic protection can result in reduced severity of gut toxicity through as yet unknown mechanisms.54 Proof of the benefits of hematopoietic protection against antifolate toxicity has been obtained using other gene therapy protection systems.55
In conclusion, we have shown that the ei gene confers broad-spectrum resistance in BM progenitor cells against antifolate drugs that target multiple enzymes in the nucleotide biosynthetic pathway including DHFR, TS, and GARFT when they are combined with NBMPR. With regard to safety, the ei gene product is naturally expressed in multiple human tissues and therefore not susceptible to immune responses. It is also an equilibrative transporter with affinity for all 4 nucleosides; therefore, the overexpression of eiwould not result in an accumulation of toxic levels of nucleosides. Because the ei gene offers protection against a broad range of antifolate compounds, multiple agents could be used during chemotherapy without the need for BM transduction with multiple chemoresistant genes.
Supported in part by the ASSISI Foundation of Memphis grant 94-00, National Heart, Lung and Blood Institute Program Project grant P01 HL 53749, Cancer Center Support grant P30 CA 21765, National Cancer Institute research grant RO1-CA55056, and the American Lebanese Syrian Associated Charities.
Reprints:Divyen H. Patel, Division of Experimental Hematology, St. Jude Children's Research Hospital, 332 N. Lauderdale, Memphis, TN 38105; e-mail: divyen.patel@stjude.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.