Abstract
Vascular endothelial growth factor (VEGF), a multifunctional cytokine, potently stimulates angiogenesis including tumor neovascularization. Although well established in solid tumors, the role of VEGF in bone marrow neoangiogenesis and paracrine tumor-stromal cell interactions in lymphohematopoietic malignancies has not been fully elucidated. In multiple myeloma (MM), marrow neovascularization parallels disease progression. This parallel prompted us to investigate the expression and secretion of VEGF by myeloma cells and its potential effects in myeloma-marrow stroma interactions. The biologically active splice variants VEGF165 and VEGF121 were expressed and secreted by myeloma cell lines and plasma cells isolated from the marrow of patients with MM. As shown by immunocytochemistry or RT-PCR, myeloma cells did not express or weakly expressed the VEGF receptors FLT-1 and FLK-1/KDR, indicating that autocrine stimulation is unlikely. In contrast, FLK-1/KDR was abundantly expressed by marrow stromal cells. Therefore, we studied the effects of VEGF on marrow stroma, focusing on the secretion of interleukin-6 (IL-6), a potent growth factor for myeloma cells and an inhibitor of plasma cell apoptosis. Exposure of stromal and microvascular endothelial cells to recombinant human (rh) VEGF165 or VEGF121 induced a time- and dose-dependent increase in IL-6 secretion (14- to 27-fold at 50 ng/mL after 24 hours, P < .001). Conversely, rhIL-6 stimulated VEGF expression and secretion in myeloma cell lines (40%-60%; P < .05) and to a variable degree (up to 5.3-fold; P < .005) in plasma cells purified from the marrow of patients with MM. This mutual stimulation suggests paracrine interactions between myeloma and marrow stromal cells triggered by VEGF and IL-6.
Multiple myeloma (MM) is a human B-cell neoplasm characterized by the clonal expansion of malignant plasma cells in the bone marrow. Interleukin-6 (IL-6) has been identified as a major cytokine involved in the emergence of the tumor clone and in tumor-associated toxicities in patients with MM.1-6 Indeed, high levels of IL-6 have been found in the serum of patients with active MM,7-9 and a reduction of the tumor cell mass has been reported by inhibition of myeloma cell proliferation with anti-IL-6 monoclonal antibodies.6,10 In addition, IL-6 is known to inhibit fas- and dexamethasone-induced plasma cell apoptosis in vitro.11-14 Thus, IL-6 is considered a most important cytokine involved in myeloma cell growth, both in vitro and in vivo.
Interactions between myeloma cells and the bone marrow stroma, a heterogeneous compartment of various cell types and the main producer of IL-6, are well described.15-21 Although various cytokines have been shown to mediate IL-6 secretion by myeloma-derived marrow stromal cells,21-24 the involvement of angiogenic factors has not been investigated. Vascular endothelial growth factor (VEGF) is considered a potent stimulator of angiogenesis in vivo.25 In solid tumors, VEGF expression is closely associated with the induction of neovascularization and correlates with tumor growth and metastatic potential.26-32 In MM, marrow neoangiogenesis parallels tumor progression and correlates with poor prognosis, suggesting an angiogenesis-dependent regulation of disease activity.33-35 In addition, VEGF expression36,37 and angiogenic activity38 of myeloma cells have recently been described.
In the present study, we have demonstrated that VEGF165 and VEGF121 are expressed and secreted by myeloma cells and that both isoforms stimulate the expression of IL-6 by microvascular endothelial cells (MVECs) and bone marrow stromal cells (BMSCs). In turn, IL-6 stimulated the expression of both VEGF splice variants by myeloma cells, suggesting a paracrine role for VEGF in tumor-stroma interactions in MM.
Materials and methods
Patients and control subjects
Bone marrow samples were obtained from 17 patients aged 38 to 69 years with stage III MM.39 Monoclonal isotype was immunoglobulin G (IgG) in 9 patients, IgA in 6, and light chains only in 2 patients. Marrow sampling was performed at diagnosis (n = 3), at relapse off therapy (n = 4), in refractory progressive disease (n = 5), or in chemotherapy responsive or stable disease (n = 5). In addition, mononuclear cells (MNCs) were isolated from nonaffected bone marrow obtained from patients with solid tumors (n = 1) or various skin diseases (n = 4) who served as control subjects. B lymphocytes were isolated from the peripheral blood of healthy volunteers. Marrow aspirates from three patients with active MM and three patients with non-Hodgkin's lymphoma or solid tumors without bone marrow involvement were used for the preparation of bone marrow stromal cell cultures (MM-BMSC and BMSC, respectively). Patients and volunteers gave informed written consent prior to the sampling procedure.
Immunofluorescence and cell sorting
Anti-CD38-phycoerythrin/cyanin5 and anti-CD19-fluorescein isothiocyanate were purchased from Coulter-Immunotech (Hamburg, Germany). Anti-CD56 and anti-CD14 conjugated to phycoerythrin were obtained from Becton Dickinson (Heidelberg, Germany), and anti-CD138-fluorescein isothiocyanate was obtained from Serotec (Oxford, UK).
Bone marrow MNCs were separated by density gradient centrifugation using Ficoll-Paque (Pharmacia, Upsala, Sweden). CD38high/CD138+ or CD38high/CD56+ plasma cells were isolated from the marrow MNC fraction of patients with MM by fluorescence-activated cell sorting using a FacsVantage (Becton Dickinson). The remaining cells that were negative for the myeloma phenotype were defined as the nontumor cell fraction. CD19+/CD14- B lymphocytes were sorted from peripheral blood MNCs of healthy volunteers. On reanalysis, sorted populations had a purity of at least 96% for the defining phenotype.
Cell cultures
The human myeloma-derived cell lines RPMI-8226, U-266, OPM-2, and IM-9 were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). Cell lines, marrow MNC, and sorted cells from individual patients were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; GIBCO-BRL, Eggenstein, Germany) and 2 mmol/L l-glutamine (GIBCO-BRL) at 37°C and 5% CO2. Human MVECs isolated from skin biopsies (pool: 20 samples; Technoclone, Austria) were cultured at 37°C and 5% CO2 in MCDB 131 medium (GIBCO-BRL) supplemented with 20% FCS. BMSC and MM-BMSC cultures were established from the MNC fraction of nonaffected or MM bone marrow, respectively, according to the method of Lagneaux et al40 with minor modifications. In brief, 5 × 105 cells/mL were cultured in α-minimal essential medium (GIBCO-BRL) supplemented with 10% FCS at 37°C and 5% CO2 in 75-cm2flasks. The culture medium was replaced at weekly intervals until a confluent monolayer had developed (usually after 4-8 weeks). All culture media were supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin. For stimulation experiments, MVECs and BMSCs in passages 7 and 3, respectively, were grown in 24-well plates. Prior to stimulation, confluent cells were starved for 24 hours (MVEC: 5% FCS, BMSC: 1% FCS).
Cell extracts
Extracts from myeloma cell lines were generated by lysis of cells at 4°C, using a Triton-based extraction buffer (100 mmol/L Tris-HCl, pH 8.0, 0.5% Triton X-100, 10 mmol/L EDTA). Supernatants were collected and centrifuged for 5 minutes at 12 000 rpm at 4°C. Extracts were frozen in liquid nitrogen and stored at -80°C until analysis. The protein content in cell extracts was determined by the bicinchoninic acid method using a commercial assay (Pierce Chemical, Rockford, IL).
Stimulation of cell cultures and cytokine measurements
MVECs and BMSCs were stimulated with different concentrations of recombinant human VEGF165 or VEGF121 (R&D Systems, Wiesbaden, Germany) for 6, 24, and 48 hours in their respective starvation medium. In addition, MM-BMSCs were stimulated with different concentrations of both VEGF isoforms for 48 hours. Thereafter, cells were pelleted and the supernatants were analyzed for IL-6. From BMSC pellets, total RNA was isolated for analysis of IL-6 transcripts. IL-6 concentrations in culture supernatants were determined by a commercial enzyme-linked immunosorbent assay (ELISA; Genzyme, Cambridge, MA) with a lower detection limit of 2 pg/mL. Concentrations of IL-6 are presented as pg/mL corrected for 105 cells. Inhibition experiments were performed, using a polyclonal goat anti-human VEGF antibody (R&D Systems).
Sorted marrow myeloma cells from patients were cultured for 24 hours prior to stimulation. Myeloma cells at a concentration of 1 × 106 cells/mL were stimulated with recombinant human IL-6 (PeproTech, Rocky Hill, NJ) at concentrations up to 10 ng/mL. After 72 hours of stimulation, cells were harvested and centrifuged, and the supernatants were analyzed for VEGF. From pelleted cells, either total RNA was isolated or proteins were extracted and subjected to quantitative analyses. VEGF levels in culture supernatants and in cell extracts were determined by a commercial ELISA (Cytimmune Science, MD) that detects both isoforms, VEGF121 and VEGF165. The lower detection limit of the assay is 12.5 pg/mL. Calibration curves were prepared by dilution of the VEGF standard provided by the manufacturer either in culture medium or extraction buffer as required. VEGF concentrations in supernatants are presented as pg/mL corrected for 106 cells. For inhibition experiments, a polyclonal goat anti-human IL-6 neutralizing antibody was used (R&D Systems).
RT-PCR analyses
Total RNA was prepared, using the guanidine isothiocyanate/phenol method.41 Complementary DNA (cDNA) was synthesized for 2 hours at 37°C, using 1 μg total RNA, random hexamers, and M-MLV reverse transcriptase (Promega, Heidelberg, Germany). VEGF transcripts were amplified, using Taq polymerase (Promega) on a Hybaid thermocycler (MWG-Biotech, Ebersberg, Germany) for 35 cycles as follows: at 94°C for 1.5 minutes, at 60°C for 3 minutes, and at 72°C for 4 minutes. VEGF primers 5′-TCGGGCCTCCGAAACCATGA-3′ (sense) and 5′-CCTGGTGAGAGATCTGGTTC-3′ (antisense) corresponding to sequences in the 3′ and 5′ untranslated regions were used, allowing the amplification of the known splice variants (516 base pair [bp], 648 bp, 720 bp, and 771 bp).42 PCR products were separated on a 1.2% low-melting agarose gel, and DNA fragments of the expected size were cloned in the pCR II-TOPO vector using the TOPO TA cloning kit (Invitrogen, San Diego, CA). Specificity of the PCR was verified by sequencing the cloned fragments, using an automated DNA sequencer (ABI PRISM 310; Perkin Elmer, Weiterstadt, Germany). Detection of IL-6 receptor (IL-6R) transcripts was performed by amplification for 35 cycles at 94°C for 1 minute, at 65°C for 30 seconds, and at 72°C for 30 seconds, using a human IL-6R amplimer set (Clontech Laboratories, Heidelberg, Germany). Expression of the VEGF receptors FLK-1/KDR and FLT-1 by myeloma cells isolated from patients was analyzed, using specific primer pairs as described previously.43 Amplification was performed for 35 cycles composed of 45 seconds at 94°C, 45 seconds at 58°C, 1 minute at 72°C, and, finally, 5 minutes at 72°C.
For semiquantitative VEGF messenger RNA (mRNA) analyses in patient samples, 300 ng total RNA was reversely transcribed, and cDNA was amplified as described above with a reduced cycle number (28-32 cycles). IL-6 mRNA expression was determined by the amplification of cDNA generated from 300 ng total RNA for 28 cycles at 94°C for 45 seconds, 60°C for 45 seconds, and 72°C for 2 minutes, followed by a 7-minute final extension step, using a human IL-6 amplimer set (Clontech). In all PCR amplifications, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was co-amplified as an internal control for RNA integrity and quantification, using the GAPDH primers 5′-CCCTCCAAAATCAAGTGGGG-3′ (sense) and 5′-CGCCACAGTTTCCCGGAGGG-3′ (antisense). All PCR-amplified products were separated on 6% polyacrylamide gels and stained with ethidium bromide. For the estimation of VEGF and IL-6 expression, the corresponding signals were normalized against GAPDH, using a fluorometric analysis system (Gel Doc 1000; Bio-Rad Laboratories, München, Germany).
Ribonuclease protection assay
VEGF mRNA expression in myeloma cell lines was quantitated by ribonuclease protection assays. Radioactive full-length antisense cRNA probes were generated in vitro from a 648 bp VEGF165 cDNA cloned in the PCR II-TOPO vector and from a 316 bp GAPDH cDNA (pTRI-GAPDH; Ambion, Austin, TX) in the presence of α-[32P]CTP or [35S]CTPαS (Amersham Buchler, Freiburg, Germany), using the Riboprobe Transcription System (Promega, Madison, WI). Probes and sample RNAs were denatured in hybridization buffer (80% formamide; 0.5 mol/L NaCl; 40 mmol/L Pipes, pH 6.4; 0.2 mmol/L EDTA, pH 8.0) and hybridized at 53°C for at least 16 hours. Samples were co-assayed for GAPDH mRNA as an internal quantification standard. Protected RNA fragments were electrophoresed through a 5% denaturating polyacrylamide gel. After exposure of the dried gel to an x-ray film (Hyperfilm; Amersham, Heidelberg, Germany), VEGF signals were quantitated by densitometric scanning (GelScan XL; Pharmacia LKB, Freiburg, Germany) and normalized against GAPDH.
Immunocytochemistry
BMSC cultures grown to semiconfluence on 8-well chamber slides and cytospin preparations of the above myeloma cell lines were processed for immunocytochemical detection of the VEGF receptors FLT-1 and FLK-1/KDR. The cells were dried and fixed for 4 minutes with cold acetone (-20°C). Unspecific binding of antibodies was blocked by TBS/0.5% BSA for 30 minutes at 4°C. Immunocytochemical analyses were performed, using polyclonal rabbit anti-human FLT-1 and FLK-1/KDR antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; working dilution 1:200) for 30 minutes at 4°C. Bound receptor antibodies were detected by the alkaline phosphatase/anti-alkaline phosphatase method according to the manufacturer's instructions, using Fast Red as a substrate (DAKO Diagnostica, Hamburg, Germany). After counterstaining with hematoxylin, cells were evaluated for receptor expression by light microscopy.
Murine monoclonal antibodies against human CD54, CD68, CD31, and thrombomodulin were purchased from DAKO and used for phenotypic characterization of BMSCs.
Statistics
Data are presented as individual data plots or as medians and interquartile ranges (IQRs). Statistical significance of overall differences between multiple groups was analyzed by the Kruskal-Wallis one-way analysis of variance. If the test was significant, pairwise comparisons were done by the multiple-comparisons' criterion. Differences between two independent groups were analyzed by the Mann-Whitney rank sum test. The Wilcoxon matched-pair signed rank test was used for comparison of differences within pairs.44 AP value of .05 or less was considered significant.
Results
Expression and secretion of VEGF by myeloma cells
The expression of VEGF transcripts was determined by RT-PCR in myeloma-derived cell lines (RPMI-8226, OPM-2, U-266, and IM-9) and in purified marrow myeloma cells from 12 patients. Peripheral blood B lymphocytes from healthy individuals served as controls. Myeloma cells consistently expressed VEGF transcripts of the two lower molecular weight isoforms, VEGF165 and VEGF121. In contrast, VEGF mRNA was not detected in normal peripheral blood B lymphocytes (Figure1).
Vascular endothelial growth factor (VEGF) expression in myeloma cells.
VEGF transcripts in human myeloma cell lines (lanes 2-5) and in CD38high/CD138+ or CD38high/CD56+ sorted myeloma cells from the bone marrow of patients with multiple myeloma (MM 1-4) were analyzed by RT-PCR. The primers used allowed the amplification of the known splice variants of VEGF. Only the two lower molecular weight isoforms VEGF165 (648 base pairs [bp]) and VEGF121 (516 bp) were detected. Peripheral blood B lymphocytes from healthy volunteers served as controls (PBB-L 1-4). Glyceraldehyde-3-phosphate dehydrogenase (347 bp) was used as a control for RNA integrity. M = size marker; C = PCR control.
Vascular endothelial growth factor (VEGF) expression in myeloma cells.
VEGF transcripts in human myeloma cell lines (lanes 2-5) and in CD38high/CD138+ or CD38high/CD56+ sorted myeloma cells from the bone marrow of patients with multiple myeloma (MM 1-4) were analyzed by RT-PCR. The primers used allowed the amplification of the known splice variants of VEGF. Only the two lower molecular weight isoforms VEGF165 (648 base pairs [bp]) and VEGF121 (516 bp) were detected. Peripheral blood B lymphocytes from healthy volunteers served as controls (PBB-L 1-4). Glyceraldehyde-3-phosphate dehydrogenase (347 bp) was used as a control for RNA integrity. M = size marker; C = PCR control.
Basal secretion of VEGF protein was similar in the myeloma cell lines RPMI-8226, OPM-2, and U266. Median concentrations in culture supernatants after 72 hours were 239.8 pg/mL (IQR, 235.8-260.6 pg/mL), 264.5 pg/mL (IQR, 247.1-291.5 pg/mL), and 368.3 pg/mL (IQR, 342.9-406.0 pg/mL) per 106 cells, respectively. By comparison, VEGF levels in IM-9 cells were significantly lower (median 19.8 pg/mL; IQR, 18.8-20.5 pg/mL; P < .001, Mann-Whitney test). After 72 hours of culture, VEGF concentrations in the media of sorted marrow myeloma cells from patients ranged from 16.0 to 321.5 pg/mL (median 115.3 pg/mL; IQR, 34.3-172.7 pg/mL) (Figure2).
Vascular endothelial growth factor (VEGF) secretion by myeloma cells.
Basal VEGF concentrations were determined in supernatants of myeloma cell lines (n = 5 independent experiments per cell line performed in triplicates) and of sorted marrow myeloma cells from patients with multiple myeloma (MM; n = 12) after 72 hours of culture. Data are presented as medians and interquartile ranges.
Vascular endothelial growth factor (VEGF) secretion by myeloma cells.
Basal VEGF concentrations were determined in supernatants of myeloma cell lines (n = 5 independent experiments per cell line performed in triplicates) and of sorted marrow myeloma cells from patients with multiple myeloma (MM; n = 12) after 72 hours of culture. Data are presented as medians and interquartile ranges.
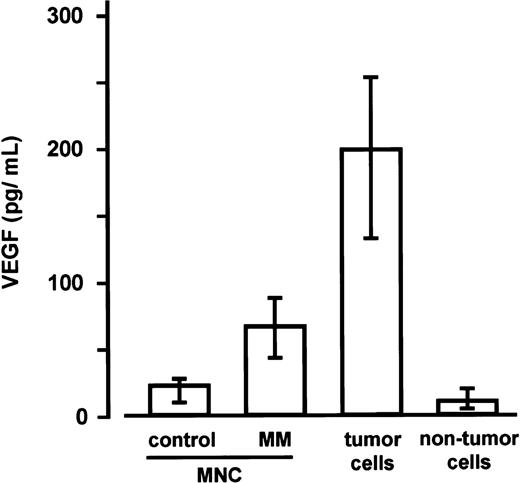
As determined in a subsequent series of experiments, VEGF levels in the culture media of marrow MNCs from patients with MM (n = 5; median 67.1 pg/mL; IQR, 43.6-88.2 pg/mL) were found to be significantly higher than in the media of marrow MNCs from control subjects (n = 5, see “Materials and methods” section; median 22.9 pg/mL; IQR, 10.3-28.1 pg/mL; P < .0025, Mann-Whitney test). In addition, a highly significant difference between sorted marrow myeloma cells (median 199.2 pg/mL; IQR, 132.6-253.4 pg/mL) and the corresponding nontumor cell fractions (median 10.9 pg/mL; IQR, 5.0-20.1 pg/mL) was observed (P < .001, Mann-Whitney)(Figure3).
Secretion of vascular endothelial growth factor (VEGF) by marrow mononuclear cells (MNCs) as well as tumor and nontumor cells.
VEGF concentrations were determined in supernatants of marrow MNCs from control subjects (n = 5), marrow MNCs from patients with multiple myeloma (MM; n = 5), and of the corresponding sorted tumor and nontumor cells of the MM marrows after 72 hours of culture. Data are presented as medians and interquartile ranges. The Mann-Whitney test was employed to identify differences between control MNCs and MM-MNCs (P < .0025) and between tumor and nontumor cells (P < .001).
Secretion of vascular endothelial growth factor (VEGF) by marrow mononuclear cells (MNCs) as well as tumor and nontumor cells.
VEGF concentrations were determined in supernatants of marrow MNCs from control subjects (n = 5), marrow MNCs from patients with multiple myeloma (MM; n = 5), and of the corresponding sorted tumor and nontumor cells of the MM marrows after 72 hours of culture. Data are presented as medians and interquartile ranges. The Mann-Whitney test was employed to identify differences between control MNCs and MM-MNCs (P < .0025) and between tumor and nontumor cells (P < .001).
VEGF receptors in myeloma cells and BMSCs
To examine whether VEGF might have an autocrine regulatory effect on myeloma cells, expression of the VEGF receptors FLT-1 and FLK-1/KDR was analyzed by immunocytochemistry. None of the four myeloma cell lines showed positive signals for either receptor. In contrast, BMSCs stained strongly positive for FLK-1/KDR, whereas expression of the FLT-1 receptor was consistently low (data not shown).
In addition, expression of the two VEGF receptors by sorted myeloma cells from patients was analyzed by RT-PCR. Ten of 12 patients expressed neither the FLK-1/KDR nor the FLT-1 receptor. One patient expressed exclusively transcripts for FLK-1/KDR, whereas another patient showed a weak signal for FLT-1 (data not shown).
Effects of VEGF on IL-6 secretion by BMSCs, MM-BMSCs, and MVECs
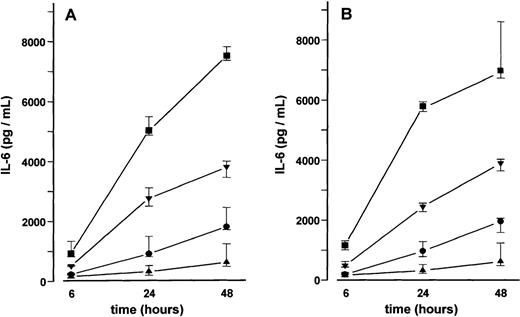
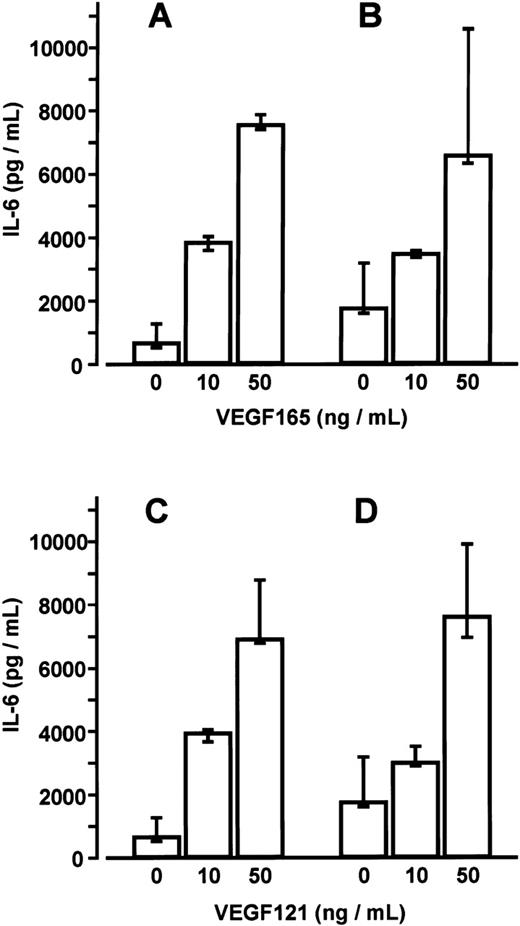
BMSC cultures were established from bone marrow aspirates of three patients with malignant disorders who had no evidence of marrow involvement. Adherent cells in third passage showed mainly CD54+ fibroblast-like cells with few CD68+macrophages. No endothelial cells were detected by CD31 and thrombomodulin immunoreactivity. Stimulation of BMSCs with VEGF165 or VEGF121 for up to 48 hours induced a time- and dose-dependent increase in IL-6 secretion (17- and 19-fold, respectively, at 50 ng/mL after 24 hours; P < .001 for overall group differences) (Figure4). This increase in IL-6 secretion was inhibited in the presence of an anti-human VEGF antibody (working concentration 100 μg/mL) (data not shown). Stimulation of MM-BMSC cultures for 48 hours with VEGF165 or VEGF121 revealed a similar dose-dependent increase in IL-6 levels as observed in BMSC cultures. Notably, basal IL-6 concentrations in MM-BMSCs tended to be higher than in BMSCs (median 1729.2 pg/mL; IQR, 1580.4-3165.2 pg/ml vs 624.7 pg/mL; IQR, 488.1-1244.1 pg/mL; P < .05, Mann-Whitney test) (Figure 5).
Effect of vascular endothelial growth factor (VEGF) on interleukin-6 (IL-6) secretion by bone marrow stromal cells (BMSCs).
IL-6 concentrations were determined in culture supernatants of BMSCs after stimulation with 2 (•), 10 (▾), or 50 (▪) ng/mL of VEGF165 (A) or VEGF121 (B) for 6, 24, and 48 hours, respectively; (▴), unstimulated controls. Data represent median values and interquartile ranges of stimulation experiments performed in triplicates in three different BMSC cultures. Analysis of significance for overall group differences was performed by the Kruskal-Wallis test (P < .001). The multiple comparisons' criterion was employed to identify differences between groups (10 ng/mL vs controls,P < .01; 50 ng/mL vs controls, P < .001; 50 ng/mL vs 2 ng/mL, P < .001 for all time periods).
Effect of vascular endothelial growth factor (VEGF) on interleukin-6 (IL-6) secretion by bone marrow stromal cells (BMSCs).
IL-6 concentrations were determined in culture supernatants of BMSCs after stimulation with 2 (•), 10 (▾), or 50 (▪) ng/mL of VEGF165 (A) or VEGF121 (B) for 6, 24, and 48 hours, respectively; (▴), unstimulated controls. Data represent median values and interquartile ranges of stimulation experiments performed in triplicates in three different BMSC cultures. Analysis of significance for overall group differences was performed by the Kruskal-Wallis test (P < .001). The multiple comparisons' criterion was employed to identify differences between groups (10 ng/mL vs controls,P < .01; 50 ng/mL vs controls, P < .001; 50 ng/mL vs 2 ng/mL, P < .001 for all time periods).
Comparison of interleukin-6 (IL-6) secretion by bone marrow stromal cells (BMSCs) and multiple myeloma (MM)-BMSCs.
IL-6 levels in culture supernatants of BMSCs (A, C) and MM-BMSCs (B, D) were measured after stimulation with 0, 10, or 50 ng/mL of VEGF165 or VEGF121 for 48 hours, respectively. Data represent median values and interquartile ranges of stimulation experiments performed in triplicates using three different BMSC and MM-BMSC cultures.P < .05 for differences between basal IL-6 secretion of BMSCs and MM-BMSCs (Mann-Whitney test).
Comparison of interleukin-6 (IL-6) secretion by bone marrow stromal cells (BMSCs) and multiple myeloma (MM)-BMSCs.
IL-6 levels in culture supernatants of BMSCs (A, C) and MM-BMSCs (B, D) were measured after stimulation with 0, 10, or 50 ng/mL of VEGF165 or VEGF121 for 48 hours, respectively. Data represent median values and interquartile ranges of stimulation experiments performed in triplicates using three different BMSC and MM-BMSC cultures.P < .05 for differences between basal IL-6 secretion of BMSCs and MM-BMSCs (Mann-Whitney test).
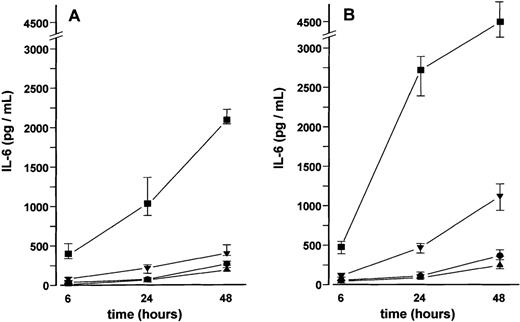
A time- and dose-dependent increase in IL-6 secretion was also observed in MVEC cultures stimulated with VEGF165 and VEGF121, respectively, although IL-6 levels in supernatants were consistently lower than in BMSCs. At higher concentrations, VEGF121 tended to be more potent than VEGF165 in stimulating IL-6 secretion by MVECs (median fold increase over controls, × 27 vs × 14 at 50 ng/mL after 24 hours;P < .05, Mann-Whitney test) (Figure6).
Effect of vascular endothelial growth factor (VEGF) on interleukin-6 (IL-6) secretion by microvascular endothelial cells (MVECs).
IL-6 concentrations were determined in culture supernatants of MVECs after stimulation with 2 (•), 10 (▾), or 50 (▪) ng/mL of VEGF165 (A) or VEGF121 (B) for 6, 24, and 48 hours, respectively; (▴), unstimulated controls. Data represent median values and interquartile ranges of three independent experiments performed in triplicates. Analysis of significance for overall group differences was performed by the Kruskal-Wallis test (P < .001). The multiple comparisons' criterion was employed to identify differences between groups (10 ng/mL vs controls, P < .05; 50 ng/mL vs controls, P < .001; 50 ng/mL vs 2 ng/mL,P < .001 for all time periods).
Effect of vascular endothelial growth factor (VEGF) on interleukin-6 (IL-6) secretion by microvascular endothelial cells (MVECs).
IL-6 concentrations were determined in culture supernatants of MVECs after stimulation with 2 (•), 10 (▾), or 50 (▪) ng/mL of VEGF165 (A) or VEGF121 (B) for 6, 24, and 48 hours, respectively; (▴), unstimulated controls. Data represent median values and interquartile ranges of three independent experiments performed in triplicates. Analysis of significance for overall group differences was performed by the Kruskal-Wallis test (P < .001). The multiple comparisons' criterion was employed to identify differences between groups (10 ng/mL vs controls, P < .05; 50 ng/mL vs controls, P < .001; 50 ng/mL vs 2 ng/mL,P < .001 for all time periods).
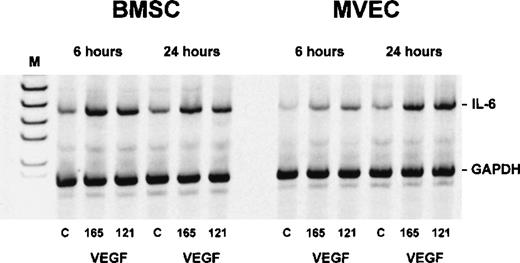
Semiquantitative RT-PCR showed an increase in IL-6 transcripts in both BMSCs and MVECs after exposure to 50 ng/mL VEGF165 or VEGF121 for 6 and 24 hours, respectively, indicating that VEGF-induced stimulation of IL-6 occurred at the mRNA level (Figure 7).
Interleukin-6 (IL-6) transcripts in bone marrow stromal cells (BMSCs) and microvascular endothelial cells (MVECs) after exposure to vascular endothelial growth factor (VEGF).
Adherent cells were incubated with 50 ng/mL of VEGF165 or VEGF121 for 6 and 24 hours, respectively, and analyzed for IL-6 expression by RT-PCR. Levels of IL-6 messenger RNA (628 base pairs) were estimated by normalization against co-amplified glyceraldehyde-3-phosphate dehydrogenase. C = unstimulated controls; M = size marker.
Interleukin-6 (IL-6) transcripts in bone marrow stromal cells (BMSCs) and microvascular endothelial cells (MVECs) after exposure to vascular endothelial growth factor (VEGF).
Adherent cells were incubated with 50 ng/mL of VEGF165 or VEGF121 for 6 and 24 hours, respectively, and analyzed for IL-6 expression by RT-PCR. Levels of IL-6 messenger RNA (628 base pairs) were estimated by normalization against co-amplified glyceraldehyde-3-phosphate dehydrogenase. C = unstimulated controls; M = size marker.
IL-6 stimulates VEGF expression in myeloma cells
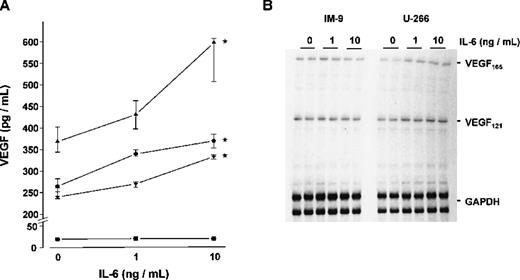
To study whether IL-6 would in turn stimulate the expression and secretion of VEGF by myeloma cells, the four myeloma cell lines were cultured for 72 hours with and without the addition of exogenous IL-6. Except for IM-9 cells, a dose-dependent increase in VEGF concentrations was observed in culture supernatants on stimulation with IL-6 (Figure8A). In the presence of 10 ng/mL IL-6, median VEGF concentrations increased by 62% over controls in U-266 (P < .005), by 39% in OPM-2 (P < .025), and by 38% in RPMI-8226 (P < .005). The effect of IL-6 on VEGF secretion was completely abrogated in the presence of an IL-6-neutralizing antibody (data not shown). Consistent with these results, RNase protection assays revealed an IL-6-induced increase of VEGF mRNA transcripts in the IL-6-sensitive cell lines, whereas no effect of IL-6 on VEGF mRNA levels was evident in IM-9 cells. Representative experiments in U-266 and IM-9 are shown in Figure 8B.
Effect of interleukin-6 (IL-6) on vascular endothelial growth factor (VEGF) expression in myeloma cell lines.
(A) VEGF concentrations in culture supernatants. The myeloma cell lines U-266 (▴), OPM-2 (•), RPMI-8226 (▾), and IM-9 (▪) were exposed to 0, 1, and 10 ng/mL IL-6, respectively, for 72 hours. Data represent median values and interquartile ranges of five independent experiments performed in triplicates. Stars denote significant differences versus unstimulated controls (U-266, P < .005; OPM-2,P < .025; RPMI-8226, P < .005; multiple-comparisons' criterion). (B) RNase protection assays showing VEGF messenger RNA (mRNA) levels in U-266 and IM-9 cells after exposure to 0, 1, and 10 ng/mL IL-6, respectively, for 72 hours. Total RNA was hybridized against radioactively labeled complementary RNA probes for VEGF and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Sizes of protected fragments were 648 nucleotides for VEGF165, 438 nt for VEGF121, and 316/258 nt for GAPDH. Signals were analyzed by densitometric scanning and normalized against GAPDH. In contrast to U-266, VEGF mRNA levels were not upregulated in IM-9 cells lacking IL-6 receptor.
Effect of interleukin-6 (IL-6) on vascular endothelial growth factor (VEGF) expression in myeloma cell lines.
(A) VEGF concentrations in culture supernatants. The myeloma cell lines U-266 (▴), OPM-2 (•), RPMI-8226 (▾), and IM-9 (▪) were exposed to 0, 1, and 10 ng/mL IL-6, respectively, for 72 hours. Data represent median values and interquartile ranges of five independent experiments performed in triplicates. Stars denote significant differences versus unstimulated controls (U-266, P < .005; OPM-2,P < .025; RPMI-8226, P < .005; multiple-comparisons' criterion). (B) RNase protection assays showing VEGF messenger RNA (mRNA) levels in U-266 and IM-9 cells after exposure to 0, 1, and 10 ng/mL IL-6, respectively, for 72 hours. Total RNA was hybridized against radioactively labeled complementary RNA probes for VEGF and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Sizes of protected fragments were 648 nucleotides for VEGF165, 438 nt for VEGF121, and 316/258 nt for GAPDH. Signals were analyzed by densitometric scanning and normalized against GAPDH. In contrast to U-266, VEGF mRNA levels were not upregulated in IM-9 cells lacking IL-6 receptor.
Determinations of intracellular VEGF levels in protein extracts of U-266, OPM-2, and RPMI-8226 cells demonstrated an increase on stimulation with 10 ng/mL IL-6 for 72 hours (median fold increase over controls × 1.54; range: × 1.21 to × 1.73; n = 3 independent experiments per cell line; P < .01 for IL-6-stimulated vs unstimulated myeloma cells, Wilcoxon test). In contrast, VEGF protein concentrations in IM-9 extracts remained below the detection limit of the ELISA, even when the cells were stimulated with IL-6. The IL-6-sensitive cell lines U-266, OPM-2, and RPMI-8226 expressed IL-6R mRNA as revealed by RT-PCR, whereas no IL-6R transcripts were detected in IM-9 cells (not shown).
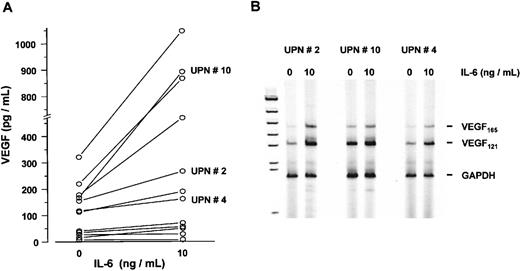
In a subsequent series of experiments, myeloma cells sorted from bone marrow aspirates of 12 patients were cultured in the presence or absence of IL-6 (10 ng/mL) for 72 hours. Although to a variable degree, IL-6 stimulated VEGF secretion into culture supernatants up to 5.3-fold (P < .005, Wilcoxon test) (Figure9A). In line with these results, an increase in VEGF mRNA levels was observed in IL-6-stimulated marrow myeloma cells using semiquantitative RT-PCR analysis (Figure 9B).
Effect of interleukin-6 (IL-6) on vascular endothelial growth factor (VEGF) expression in sorted marrow myeloma cells from patients (n = 12).
(A) VEGF concentrations in supernatants of myeloma cell cultures after exposure to 0 and 10 ng/mL IL-6 for 72 hours, presented as pg/mL corrected for 106 cells. P < .005 for difference between unstimulated and stimulated myeloma cells (Wilcoxon test). (B) VEGF transcription in IL-6-stimulated myeloma cells from patients. RT-PCR analysis of VEGF transcripts after exposure to 0 or 10 ng/mL IL-6 for 72 hours. Representative examples of three patients with increased VEGF messenger RNA levels after IL-6 stimulation are shown. Corresponding patient numbers (UPN) are indicated in panels A and B.
Effect of interleukin-6 (IL-6) on vascular endothelial growth factor (VEGF) expression in sorted marrow myeloma cells from patients (n = 12).
(A) VEGF concentrations in supernatants of myeloma cell cultures after exposure to 0 and 10 ng/mL IL-6 for 72 hours, presented as pg/mL corrected for 106 cells. P < .005 for difference between unstimulated and stimulated myeloma cells (Wilcoxon test). (B) VEGF transcription in IL-6-stimulated myeloma cells from patients. RT-PCR analysis of VEGF transcripts after exposure to 0 or 10 ng/mL IL-6 for 72 hours. Representative examples of three patients with increased VEGF messenger RNA levels after IL-6 stimulation are shown. Corresponding patient numbers (UPN) are indicated in panels A and B.
Discussion
In MM, three lines of evidence suggest a role for angiogenic factors, such as VEGF, in the regulation of tumor cell growth and disease activity. First, bone marrow neoangiogenesis parallels disease progression and predicts poor outcome.33,35,38 Second, the marrow microenvironment supports both the growth of myeloma cells and the neovascularization of areas with myeloma cell infiltration.45 Third, myeloma cells express both angiogenic activity and angiogenic cytokines.36-38 46 On the basis of these premises, we studied the role of VEGF in myeloma-marrow stroma interactions.
In accordance with a recent report,37 we found consistent expression of the splice variants VEGF165 and VEGF121 in purified myeloma cells from patients and in human myeloma-derived cell lines. Basal VEGF secretion was variable in cultured marrow myeloma cells and particularly low in IM-9 cells. Purified myeloma cell fractions of MM marrow were found to secrete significantly more VEGF than the corresponding nontumor fractions. This finding suggests that the presence of myeloma cells indeed accounts for the higher amounts of VEGF secretion by marrow MNCs from patients with MM as compared with marrow MNCs from control subjects.
In general, basal VEGF secretion rates appeared to be low in comparison with other types of tumor cells.37,47 However, VEGF concentrations in culture supernatants were within its range of biological activity. Native VEGF at concentrations of 150 to 300 pg/mL has been reported to stimulate endothelial cells in vitro.48 49 Thus, VEGF concentrations within the tumor microenvironment should also suffice to exert potential biological effects on marrow stromal cells. Because of the limited number of samples from patients with MM that were available for the study, we were unable to relate VEGF secretion by cultured myeloma cells to the actual stage and activity of the disease.
The activity of VEGF is mediated by the tyrosine kinase receptors FLT1 (VEGFR-1) and FLK1/KDR (VEGFR-2).50-52 However, none of the cell lines studied (U-266, RPMI-8226, OPM-2, and IM-9) expressed VEGFR-1 or VEGFR-2. In addition, myeloma cells of only 1 of 12 patients expressed VEGFR-2, and, in another patient, weak expression of VEGFR-1 by myeloma cells was observed. This finding indicates that relevant autocrine stimulation of myeloma cells by VEGF is unlikely. In contrast, FLK1/KDR (VEGFR-2) was abundantly expressed by BMSCs, suggesting a potential paracrine role for VEGF in MM.
Paracrine interactions between myeloma cells and the stromal cell compartment of the bone marrow have been extensively studied.15-24 IL-6, a key cytokine of myeloma cell growth,2,3,6,10 is expressed in BMSC cultures and its release is stimulated by adhesion of myeloma cells.17-20 On the one hand, bone marrow cultures mainly consisted of fibroblast-like cells, which is in agreement with previously reported data.53 On the other hand, MVECs, another functional component of the bone marrow stroma in vivo, have been shown to release cytokines relevant to the regulation of hematopoiesis.54Likewise, stimulation of human umbilical vein endothelial cells with VEGF leads to increased secretion of granulocyte-macrophage colony-stimulating factor and an up-regulation of several cytokine and growth factor transcripts.37,42 Thus, it is conceivable that the stromal cell compartment may, in response to angiogenic factors, release cytokines capable of sustaining tumor growth. Our results demonstrate that the VEGF isoforms 165 and 121 induce a time- and dose-dependent increase in IL-6 secretion by BMSCs and MVECs. Furthermore, a similar increase in IL-6 levels was found in MM-BMSCs and BMSCs from nonaffected marrow. The findings suggest that VEGF is a paracrine mediator supporting myeloma cell growth through the induction of IL-6 release by bone marrow stroma. In MM, stimulation of IL-6 secretion by marrow stromal cells was previously shown for tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and transforming growth factor-β (TGF-β)21-24 but had not been described for angiogenic cytokines. Because plasma cells can produce various cytokines able to induce the expression of IL-6 (eg, IL-1β, TNF-α, TGF-β, and VEGF),21,22 24 it is likely that the close proximity of myeloma and stromal cells creates favorable conditions for myeloma cell growth in vivo.
We also demonstrated that IL-6 in turn stimulates VEGF secretion by IL-6R-expressing myeloma cell lines and sorted marrow myeloma cells from patients. This observation is in line with recent reports on VEGF induction by both cellular and viral IL-6 in epidermoid carcinoma and NIH3T3 cells, respectively, further supporting the notion that IL-6 can indirectly promote angiogenesis.55,56 However, in 5 of 12 cases studied, we found no or only minor effects of IL-6 on VEGF secretion by myeloma cells. This finding may reflect an insensitivity to IL-6 caused by altered expression of or impaired signaling through the IL-6R. Thus, IL-6-induced VEGF secretion may only occur in a subset of myeloma cells, eg, more mature myeloma cells. This notion would be in line with reports on a variable response pattern of myeloma cells to exogenous IL-6, particularly in freshly isolated tumor cells.5 57 In this context, it is again tempting to speculate that the IL-6 sensitivity of VEGF secretion by myeloma cells may be related to the actual activity of the disease, a hypothesis that we were unable to confirm or refute in the present study.
Along with IL-6, other cytokines and growth factors, such as IL-1β, platelet-derived growth factor, insulin-like growth factor, TGF-β, fibroblast growth factor, TNF-α, and keratinocyte growth factor, have been reported to stimulate VEGF expression, whereas IL-10 and IL-13 inhibit the release of VEGF.58 This plethora of regulating factors suggests that cytokines other than IL-6 may also be involved in the control of VEGF production by myeloma cells.
In conclusion, our data provide strong evidence for a mutual stimulation of myeloma and marrow stromal cells triggered by VEGF and IL-6. What appears to represent a perpetual paracrine cooperation between VEGF and IL-6 may be part of a complex regulatory mechanism sustaining tumor growth and protecting myeloma cells from treatment-induced apoptosis. In addition, directly or indirectly, both cytokines are likely to be involved in the neovascularization of bone marrow infiltrated by myeloma cells. The data add to current evidence providing a rationale for anti-angiogenic therapy in MM.
Supported in part by a grant (ZE 378/1NW) from the Stifterverband für die Deutsche Wissenschaft, Essen, Germany.
Preliminary results presented as oral communication at the 40th (1998) Annual Meeting of the American Society of Hematology, Miami Beach, FL.
Reprints:Joachim Kienast, Department of Medicine/Hematology and Oncology, University of Muenster, Albert-Schweitzer-Str 33, D-48129 Muenster, Germany; e-mail: kienast@uni-muenster.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Vascular endothelial growth factor (VEGF) expression in myeloma cells. / VEGF transcripts in human myeloma cell lines (lanes 2-5) and in CD38high/CD138+ or CD38high/CD56+ sorted myeloma cells from the bone marrow of patients with multiple myeloma (MM 1-4) were analyzed by RT-PCR. The primers used allowed the amplification of the known splice variants of VEGF. Only the two lower molecular weight isoforms VEGF165 (648 base pairs [bp]) and VEGF121 (516 bp) were detected. Peripheral blood B lymphocytes from healthy volunteers served as controls (PBB-L 1-4). Glyceraldehyde-3-phosphate dehydrogenase (347 bp) was used as a control for RNA integrity. M = size marker; C = PCR control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2630/4/m_bloo00805001w.jpeg?Expires=1769241381&Signature=QOCk7aFmHuTY8TJq-xAXBag~68abhv14lGWN9hdBbrPq48ubkFy65~tr7Whe34vq~jbyDXM9YJADdYUeJxNSIK5ByM7u8rCzDmJzbFkV~TLDNZ78tWTtxgz8mYO9VMl47UWqjwtuWaCttJ9-3a4qFLP6PAUyAx4OBgAvWhcwRLjkkihzkGxWuP~zIJsTKGHZk4sgBAn1eNUX6zpPMSB2eYVU7Fwk0P5eFCt5wNopV7vHiPBCoW4zW-GcRjRMo9mLW2OGktMWN~1NUk~KP9FcB9NDIQBY235YkAGMTK9ivi8nEBNHxs2DM75VR-DFMWge~h5kqOWroQ9HOZqFe401-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)