The influence of human T-cell leukemia/lymphoma virus type II (HTLV-II) in individuals also infected with HIV-1 is poorly understood. To evaluate the reciprocal influence of HTLV-II and HIV-1 infection, primary peripheral blood mononuclear cell (PBMC) cultures from coinfected individuals were established in the presence of interleukin 2 (IL-2). In these cultures, the kinetics of HTLV-II replication always preceded those of HIV-1. Noteworthy, the kinetics of HIV-1 production were inversely correlated to the HTLV-II proviral load in vivo and its replication ex vivo. These observations suggested a potential interaction between the 2 retroviruses. In this regard, the levels of IL-2, IL-6, and tumor necrosis factor- (TNF-) were measured in the same coinfected PBMC cultures. Endogenous IL-2 was not produced, whereas IL-6 and TNF- were secreted at levels compatible with their known ability to up-regulate HIV-1 expression. The HIV-suppressive CC-chemokines RANTES, macrophage inflammatory protein-1 (MIP-1), and MIP-1β were also determined in IL-2–stimulated PBMC cultures. Of interest, their kinetics and concentrations were inversely related to those of HIV-1 replication. Experiments were performed in which CD8+ T cells or PBMCs from HTLV-II monoinfected individuals were cocultivated with CD4+ T cells from HIV-1 monoinfected individuals separated by a semipermeable membrane in the presence or absence of antichemokine neutralizing antibodies. The results indicate that HTLV-II can interfere with the replicative potential of HIV-1 by up-regulating viral suppressive CC-chemokines and, in particular, MIP-1. This study is the first report indicating that HTLV-II can influence HIV replication, at least in vitro, via up-regulation of HIV-suppressive chemokines.
Human T-cell leukemia/lymphoma types I and II (HTLV-I and -II) are T-lymphotropic retroviruses. HTLV-I mostly infects CD4+ T cells, whereas HTLV-II shows a preferential tropism for CD8+ T lymphocytes.1 A strong association of HTLV-I infection with the subsequent development of adult T-cell leukemia,2 as well as of HTLV-I–associated myelopathy-tropical spastic paraparesis (HAM/TSP)3 has been described. In contrast, no definitive relationship between HTLV-II and human diseases has been yet established.4
Both HTLV-I and HTLV-II are potential viral cofactors in individuals also infected by HIV-1. In Europe, HTLV-II is predominant among intravenous drug users (IVDUs),5 and it is frequently found in individuals coinfected with HIV-1.6 Some studies in homosexual men concomitantly infected by HTLV-I and HIV-1 have suggested that they have a significantly accelerated HIV disease progression toward AIDS, compared with individuals infected with HIV-1 alone.7 Similarly, it has been suggested that a concomitant HTLV-I/HIV-1 infection causes a higher mortality in IVDUs with AIDS.8 This potentiation of HIV pathogenicity is supported by in vitro studies on HTLV-I tax gene products, showing that the production of infectious HIV-1 is enhanced in cell lines transformed by HTLV-I.9 This hypothesis has not been confirmed thus far in individuals with concomitant HIV-1 and HTLV-II infections, likely as a result of the different cellular tropism of these 2 viruses.10 Unlike HTLV-I, HTLV-II coinfection was not statistically associated with progression to AIDS in subjects from some cohorts of IVDUs.11 12
Some Italian individuals coinfected with HIV-1 and HTLV-II have shown a less steep decline of CD4+ T cells and a very long interval before the development of AIDS, in association with a high HTLV-II proviral load, even in the presence of high levels of HIV-1 replication, as measured by plasma viremia.13 14 These findings suggest that HTLV-II may play a protective rather than potentiating role on HIV disease progression.
Infection by HTLVs leads to several immune dysfunctions, including spontaneous proliferation of T cells, which have been attributed to the effect of the viral regulatory protein Tax, encoded by the pxgene.15 In addition to acting on the long terminal repeat (LTR) region to enhance viral RNA transcription, this protein is also capable of transactivating both eukaryotic promoters involved in T-cell activation and proliferation16 and the promoter of HIV-1 via activation of NF-κB.4 In this regard, in vitro experiments have shown that reciprocal virus activation occurs in cell lines coinfected with members of these virus families.17 18
Recently, it has been defined that expression of some chemokine receptors is required for fusion and entry of HIV-1 into CD4+ cells. In particular, CCR5, a cell surface receptor for the CC-chemokines RANTES, MIP-1α, and MIP-1β, acts as a coreceptor for nonsyncytium-inducing (NSI) macrophage-tropic strains of HIV-1, which dominate after transmission and during early disease. In addition, CXCR4, which binds stromal cell-derived factor-1 (SDF-1), is the coreceptor of SI viruses19 emerging with disease progression in approximately 50% of the individuals.20 The acquisition of CXCR4 usage as coreceptor corresponds to the switch from an NSI to an SI phenotype, the loss of sensitivity to the suppressive effects of CC-chemokines, and a steeper decrease in CD4+ T-cell counts.20 21
Of interest, the 3 CCR5 binding chemokines, RANTES, MIP-1α, and MIP-1β, were characterized as the major HIV-1–suppressive factor released by HTLV-I immortalized T cells, as well as by primary CD8+ T cells.22 In this regard, constitutive expression of various chemokines has been described in HTLV-I positive T-cell lines as a consequence of the effect of the viral transactivator Tax.23 In addition, HTLV-I–specific CD8+cytotoxic T lymphocyte (CTL) clones derived from patients with HAM/TSP are an important source of MIP-1α and MIP-1β,24 suggesting the possibility that HTLV-I may profoundly influence HIV replication via chemokine expression and release. In contrast, little information is available on whether HTLV-II can modulate the expression of CC chemokines.22
To address this issue and to investigate the potential relationship between HIV and HTLV-II replication, we have established peripheral blood mononuclear cell (PBMC) cultures from 6 coinfected individuals in whom the proviral load of both retroviruses was previously determined. We observed that both retroviruses were able to replicate in PBMC cultures in the presence of exogenous IL-2.
Materials and methods
Patients
The patient population included 12 IVDUs (6 men and 6 women, mean age 37.8 ± 7.4 years) belonging to the Parma cohort.14 Dual seropositivity for HTLV-I/II and HIV-1 was documented for patients PR-7, PR-8, PR-9, PR-10, PR-11, and PR-12 since 1986-87 (Table 1). Three individuals were infected exclusively with HTLV-II (PR-1, PR-2, and PR-3), and 3 additional patients exclusively with HIV-1 (PR-4, PR-5, and PR-6). The clinical history and immunologic features of all 12 patients were available through medical records, including the determination of CD4+ and CD8+ T-cell counts at entry and every 3 to 4 months thereafter. At the time of inclusion in the study, monoinfected and coinfected individuals presented the same pattern of seroprevalence for herpes simplex types 1 and 2; cytomegalovirus (CMV); hepatitis B, C, and D viruses; and Toxoplasma gondii. The stage of HIV-1 infection was classified according to Centers for Disease Control and Prevention criteria.25 The HTLV-II subtype was identified by sequencing the LTR region of the viral isolates.26 At regular intervals, each patient was tested for full hematology and chemistry panels and a complete physical examination was performed. The clinical and virologic features of the patients included in this study are summarized in Table 1. A competitive polymerase chain reaction (PCR) method was used to quantify HIV-1 and HTLV-II proviral DNA in PBMC obtained from infected subjects.13,27 Among the 6 coinfected patients, PR-11 (before therapy) and PR-12 presented with a high HTLV-II proviral load, compared with HIV-1 (ratio HIV-1/HTLV-II, < 0.1); PR-9 and PR-10 had equivalent proviral loads (ratio 1.1 and 1.3, respectively); and PR-7 and PR-8 had very high HIV-1 and very low HTLV-II proviral loads (ratio 40.6 and >800). PR-7 and PR-8 were under antiretroviral therapy at study entry, whereas PR-9, PR-10, and PR-12 were and remained untreated throughout the study. PR-11 underwent antiviral therapy after a sensory neuropathy concomitant developed with a drop in circulating CD4+ T cells below 400 cells/μL. Three HIV-1 monoinfected patients were chosen among antiretroviral naive individuals belonging to the same cohort of the coinfected patients. Coinfected individuals had an average followup of about 10 years. HIV-1 staging at entry before therapy was between A1 and B3 for those with an HIV-1/HTLV-II ratio ranging from 0.1 to <2. Patient PR-7 progressed to AIDS and an autonomic sensory neuropathy developed; he was treated with antiretroviral agents from 1993 until his death in 1996. The monthly rate of decline of CD4+ T lymphocytes in all coinfected individuals, expect PR-12, and in HIV-1 monoinfected patients was 1.3 up to 3.4 × 106 cells, compatible with the definition of slow progressors.28 PR-12 was characterized by a high HTLV-II load and an extremely low HIV-1 load, very high CD4+ T-cell number since 1987, and no sign of HIV disease progression. Nevertheless, he was able to transmit both retroviruses to his partner.
Patients mono-infected with HTLV-II maintained CD4 counts of more than or equal to 800 cells/μL since enrollment. Persistent HTLV-II antigen (Ag) expression was detected in the plasma of PR-1 (64 pg/mL) and PR-11 (40 pg/mL). No correlations between HTLV-II Ag expression and proviral load were observed. The HTLV-II strain of PR-7 and PR-11 belonged to the subtype IIa, whereas the others were of the subtype IIb.
IL-2 stimulated PBMC cultures
PBMCs were cultured in 96-well plates (Costar Corning, Cambridge, MA) at the concentration of 1 × 106 cells/mL in RPMI 1640 (Gibco BRL, Life Technologies Italia, Milano) medium, supplemented with 20% fetal calf serum (FCS) (Hyclone Europe, Cramlington, UK) in the presence or absence of 20 U/mL of rIL-2 (Genzyme, Cambridge, MA). Half (50%) of the culture medium was changed every 8 days with fresh medium containing 20% FCS. Supernatants were harvested for analysis of HIV-1 or HTLV-II p24 Ag, cytokine, and chemokine contents. Exogenous rIL-2 was added every 3 to 4 days. p24gag HIV-1 and HTLV-II Ag levels were tested on collected culture supernatants. HIV-1 expression was determined using an HIV-1 p24 Ag capture enzyme-immunoassay (EIA) (Abbott Laboratories, North Chicago, IL), whereas HTLV-II production was determined by using a p24gag Ag capture assay (Retrovirology Coulter Corp, Hialeah, FL), according to the manufacturers' instructions. At various time points, aliquots of cells were removed for proliferation assays, phenotypic analyses, and detection of HIV-1 and HTLV-II proviral DNA. Cell viability was determined by trypan blue dye exclusion. For calculation of spontaneous proliferation, triplicate samples of 1 × 106 cells/mL were pulsed with 0.037 MBq (1 μCi) [3H]-thymidine, cultured for 6 hours, and acid-insoluble precipitates were counted in a liquid scintillation β-counter. For phenotype determination, cells were analyzed by flow cytometry after staining with the mAbs directed to the following Ags: CD3, CD4, CD8, CD14, CD20, CD25, and HLA-DR (Becton Dickinson, San Jose, CA) as previously described.10
Cultures of cells separated by a semipermeable membrane
CD4+ or CD8+ T cells from PBMC were negatively selected by column exclusion (CD4+ or CD8+ Subset Enrichment Columns; R & D Systems, Minneapolis, MN). Purity of CD4+ or CD8+ T cells was 96% or more, determined by flow cytometric analysis. CD4+ T cells, 1.5 × 106, and CD8+ T cells, 1.5 × 106, (autologous or allogeneic) were cultured in the same well, but separated by a semipermeable polyester membrane (0.4-μm Costar Transwell; Costar Europe, Badhoevedorp, The Netherlands), as described for PBMC primary cultures. Supernatants from both the upper and the lower compartments of the well were removed separately once a week, frozen, and subsequently tested for the presence of HIV-1 p24 Ag. In some experiments, a mixture of neutralizing anti-RANTES, anti–MIP-1α, and anti–MIP-1β polyclonal Ab (R & D Systems) was added at 2 μg/mL each to the cultures, both at the beginning of the experiments and at every medium change.
Characterization of HIV-1 isolates
Primary viral stocks of mono–HIV-1 or coinfected patients were serially 4-fold diluted in sestuplicates, added to wells containing 3-day-old PHA-stimulated T-cell blasts from seronegative donors, and maintained in RPMI 1640, 10% FCS (Hyclone Europe) plus rIL-2 (20 U/mL). The culture supernatants were tested every 48 to 72 hours by a Mg++-dependent reverse transcriptase (RT) activity assay.29
To assess the coreceptor usage of the HIV-1 isolates, U87 cells stably expressing either CD4 or CD4 plus 1 of the following chemokine R, CCR2b, CCR3, CCR5, and CXCR-4,30 were inoculated with the different primary isolates (1/100 vol/vol of the viral stock). Viral stocks of 100 μL were diluted in 1 mL of D-MEM (Bio Whittaker, Verviers, Belgium), containing 15% FCS (Sigma Chemical Corp, St Louis, MO), were added to 1 × 105/mL cells in a 48-well plate (Falcon; Becton Dickinson Labware, Lincoln Park, NJ). Standard laboratory HIV-1 strains, such as HIV-1 IIIB and BaL, using CXCR4 (X4 virus) and CCR5 (R5 virus), respectively, were used as positive controls.19 Twenty-four hours later, the medium was replaced with fresh medium. Cultures were examined daily for syncytia formation with an inverted microscope, whereas culture supernatants were collected every 2 to 3 days over a 3-week period and stored at −80°C until tested for Mg++-dependent RT activity contents.29
Cytokine and chemokine determinations
Levels of cytokines present in the PBMC culture supernatants were determined by commercially available enzyme-linking immunosorbent assay (ELISA) kits, recognizing only bioactive cytokines: IL-2, IL-3, IL-6, and TNF-α (Biosource International, Camarillo, CA). Chemokine (RANTES, MIP-1α, MIP-1β) concentrations were measured by ELISA according to the manufacturer's instructions (R & D Systems).
Results
Establishment of PBMC cultures from HTLV-II infected individuals
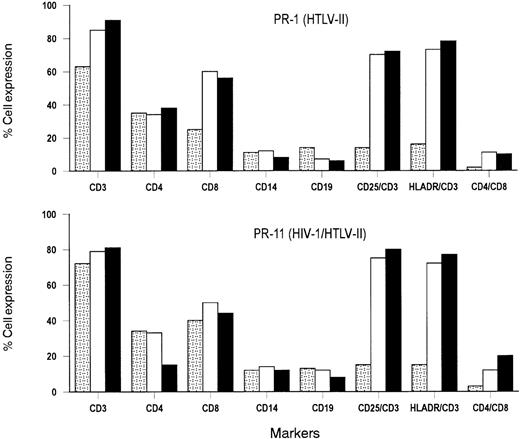
After PBMC isolation, the majority of PBMCs of HTLV-II monoinfected PR-1 and coinfected PR-11 cultures were T lymphocytes, as shown by the expression of CD3 Ag (Figure 1). Similar levels of CD4+ T cells were present in these cultures, whereas the number of CD8+ T cells was lower in PR-1 (25%) than in PR-11 (40%). Approximately, 14% to 16% of CD3+cells expressed the IL-2R α-chain (CD25) and class II HLA-DR Ag on their cell surface. The proportion of B lymphocytes (CD19+) and monocytes (CD14+) was similar to those of uninfected donors. The level of spontaneous cell proliferation in PBMC primary cultures varied from patient to patient (range, 3050 to 4654 cpm) but it was 2- to 3-fold greater than that seen in the cultured cells from HTLV-seronegative individuals (mean 1452 cpm), with the exception of the PBMC culture obtained from patient PR-7. This individual was characterized by a very low HTLV-II proviral load (< 240 copies per 105 cells). All primary PBMC cultures remained viable for at least 45 days, as determined by trypan blue dye exclusion, and expressed CD25 and HLA-DR activation markers on their cell surface that increased slightly over time, with respect to starting conditions (with 18%-22% vs 14%-16% positive cells at day 7 vs day 0, respectively). In the absence of IL-2, these primary cultures did not support HTLV-II or, in the case of dual infections, HIV-1 p24 Ag production (data not shown).
Analysis of cell surface markers in primary cultures established from a mono–HTLV-II–infected patient (PR-1) and a patient (PR-11) coinfected with HIV-1 and HTLV-II.
Results are representative of 3 phenotypic analyses performed at the indicated time, and are expressed as percentages of positive cells. , day 0; □, day 30; ▪, day 60.
, day 0; □, day 30; ▪, day 60.
Analysis of cell surface markers in primary cultures established from a mono–HTLV-II–infected patient (PR-1) and a patient (PR-11) coinfected with HIV-1 and HTLV-II.
Results are representative of 3 phenotypic analyses performed at the indicated time, and are expressed as percentages of positive cells. , day 0; □, day 30; ▪, day 60.
, day 0; □, day 30; ▪, day 60.
In the presence of exogenous IL-2 (20 U/mL), the majority of CD3+ T cells expressed CD25 and HLA-DR cell surface activation markers after 30 days of culture, at which time HTLV-II expression was observed. Approximately 50% to 60% CD8+ T cells and 30% to 35% CD4+ T cells were more than 80% of the cells in culture. B cells were either stable or diminished overtime. A substantial fraction of cells (ranging from 10% to 20% of the total cell numbers) coexpressing a double CD4+/CD8+ phenotype emerged in cultures of HTLV-II–infected individuals (Figure 1).
After 30 days of culture, HIV-1–associated CD4+ T-cell depletion was observed in primary cultures in coinfected patients, and the percentage of CD4+ cells among viable CD3+T cells decreased rapidly to 10% to 15%. A moderate decrease (5%-10%) of CD8+ T cells was also observed that was, however, compensated by a rise in T cells coexpressing CD4+and CD8+.
HIV-1 and HTLV-II expression in PBMC cultures from coinfected patients
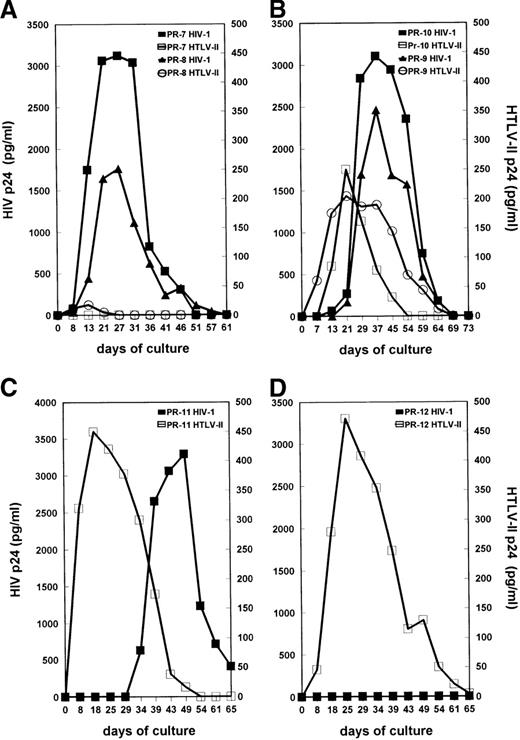
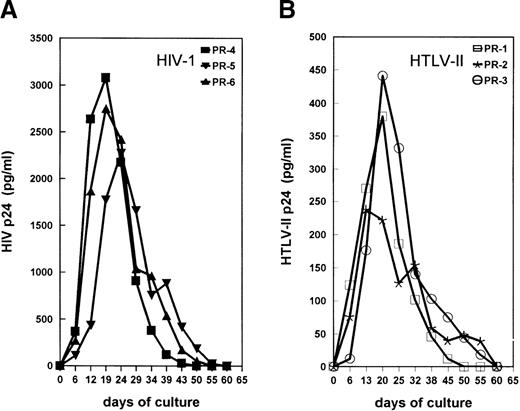
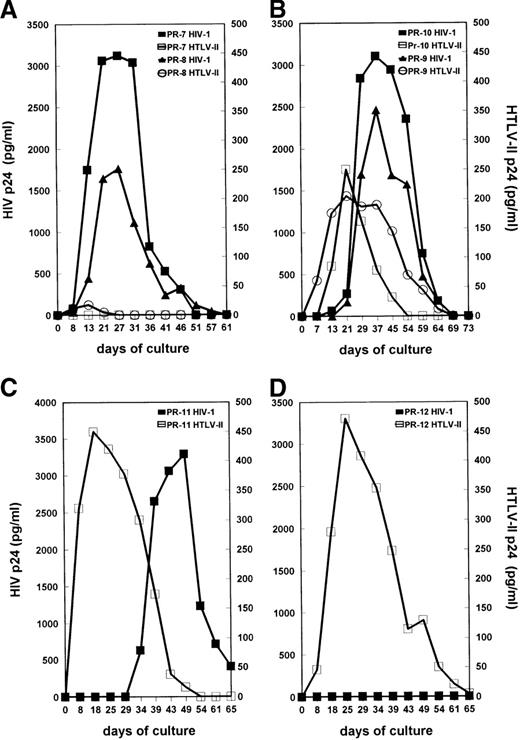
Primary cultures were established from the PBMC of all 12 subjects in the presence of IL-2 without additional mitogenic stimulation. This condition has been described as an optimal system to allow replication of both SI and NSI strains of HIV-1.31 HIV-1 and HTLV-II replication were measured by testing the levels of supernatant-associated p24 Gag proteins at different time points of PBMC cultures. The kinetics of viral Ag production in mono–HIV-1–infected PR-4, PR-5, and PR-6 and mono–HTLV-II–infected PR-1, PR-2, and PR-3 are shown in Figure 2A and 2B, respectively. Consistent with their low HTLV-II proviral load, HTLV-II expression was not detected in cultures of cells from coinfected individuals PR-7 and PR-8 (Figure3A). In PBMC cultures of coinfected individuals PR-9, PR-10, and PR-11, characterized by an HIV-1/HTLV-II ratio of less than or equal to 1, the kinetics of HTLV-II production always preceded those of HIV-1 (Figure 3B and 3C). HIV replication in these PBMC cultures was considerably delayed (3 to 4 weeks) in comparison with that observed in cultures established from mono-HIV–infected individuals (Figure 2A). Strikingly, the absence of HIV expression was observed up to 5 weeks of culture in PBMC of patient PR-11 (Figure 3C), although his HIV-1 proviral load in PBMC was similar to that of the mono–HIV-1–infected individual PR-4 (Table 1). Finally, in PBMC cultures of PR-12, characterized by a very low HIV-1/HTLV-II ratio, no replication of HIV-1 was observed (Figure 3D).
Kinetics of virus production in IL-2–stimulated PBMCs obtained from mono–HIV-1–infected (A), or mono–HTLV-II–infected (B) individuals.
Supernatants from PBMC cultures were harvested every 3 to 5 days. Supernatants were tested for concentrations of HIV-1 and HTLV-II p24 Gag Ags; the results are representative of 3 independent experiments performed at 6-month intervals.
Kinetics of virus production in IL-2–stimulated PBMCs obtained from mono–HIV-1–infected (A), or mono–HTLV-II–infected (B) individuals.
Supernatants from PBMC cultures were harvested every 3 to 5 days. Supernatants were tested for concentrations of HIV-1 and HTLV-II p24 Gag Ags; the results are representative of 3 independent experiments performed at 6-month intervals.
Kinetics of virus production by primary cultures obtained from HIV-1/HTLV-II–coinfected patients. (A) Cultures from PR-7 and PR-8, characterized by a high HIV-I/HTLV-II proviral load ratio. (B) Cultures from PR-9 and PR-10, with an HIV-I/HTLV-II ratio equal to 1. (C) Culture from PR-II before therapy, with a low HIV-I/HTLV-II ratio. (D) Culture from PR-12 with a very low HIV-I/HTLV-II ratio. See Figure2 for procedures.
Kinetics of virus production by primary cultures obtained from HIV-1/HTLV-II–coinfected patients. (A) Cultures from PR-7 and PR-8, characterized by a high HIV-I/HTLV-II proviral load ratio. (B) Cultures from PR-9 and PR-10, with an HIV-I/HTLV-II ratio equal to 1. (C) Culture from PR-II before therapy, with a low HIV-I/HTLV-II ratio. (D) Culture from PR-12 with a very low HIV-I/HTLV-II ratio. See Figure2 for procedures.
In these PBMC cultures, HTLV-II expression was associated with cellular proliferation and moderate (approximately 10%) cell death, whereas a dramatic cytopathic effect was observed during the peak of HIV-1 replication (data not shown).
HIV-1 coreceptor usage
All HIV-1 primary isolates were NSI by the MT-2 cells32and used CCR5 as entry coreceptor (Table 1). The primary PR-11–derived HIV did not score positive in a coreceptor usage assay and was further passaged onto allogeneic PHA-activated PBMC of a seronegative donor. This second passage isolate showed usage of both CCR5 and CXCR4 in the U87 assay coreceptors assay (Table 1). Finally, in the face of several attempts, the PR-10–derived HIV-1 could not be characterized for coreceptor usage.
Cytokine secretion in primary cultures of PBMCs from coinfected patients
It has been reported that T-cell lines infected by HTLVs constitutively express high levels of different cytokines, including IL-2, IL-6, and TNF-α,33,34 which in turn can profoundly influence the state of activation of T lymphocytes and monocytes. Expression of cytokines in IL-2–stimulated PBMC infected in vitro with HIV-1 has been previously shown to significantly sustain virus replication.31 35 Therefore, the cytokine content in the supernatants of primary PBMC cultures from mono- and coinfected patients with high HTLV-II proviral loads was investigated both in the presence or absence of exogenous IL-2.
Unstimulated PBMC cultures obtained from HTLV-II monoinfected PR-1 and from coinfected PR-11 initially secreted very low levels of IL-6 and TNF-α, whereas production progressively increased after 15 days and peaked after approximately 40 days of culture (Figure4A). A modest (up to 2-fold) increase of cytokine production was noted in PBMC cultures from the coinfected patients in comparison with those of monoinfected patients. Of interest is the fact that in both cases the cytokine levels that were reached during cultivation were in the range of concentrations capable of sustaining HIV replication after in vitro infection.31Secretion of endogenous IL-2 was not detected during the entire period of both in monoinfected PR-1 and coinfected PR-11 cultures (Figure 4A).
Cytokine production in primary cultures obtained from mono-HTLV-II–infected patient PR-1 and from HIV-1/HTLV-II–coinfected patient PR-11.
Results are representative of 3 separate experiments. Patients' PBMCs were cultivated in the absence (A) or presence (B) of IL-2. Supernatants were harvested at various times and tested for cytokine content.
Cytokine production in primary cultures obtained from mono-HTLV-II–infected patient PR-1 and from HIV-1/HTLV-II–coinfected patient PR-11.
Results are representative of 3 separate experiments. Patients' PBMCs were cultivated in the absence (A) or presence (B) of IL-2. Supernatants were harvested at various times and tested for cytokine content.
No significant differences in the levels or kinetics of cytokine production in monoinfected or coinfected cell cultures were observed between unstimulated and IL-2–stimulated cultures (Figure 4B). Worthy of note, cytokine production peaked simultaneously or close to maximal HIV-1 replication, suggesting their positive role on HIV production, as reported in vitro– infected PBMC.31
Chemokine secretion and HIV-1 replication in IL-2–stimulated PBMC cultures
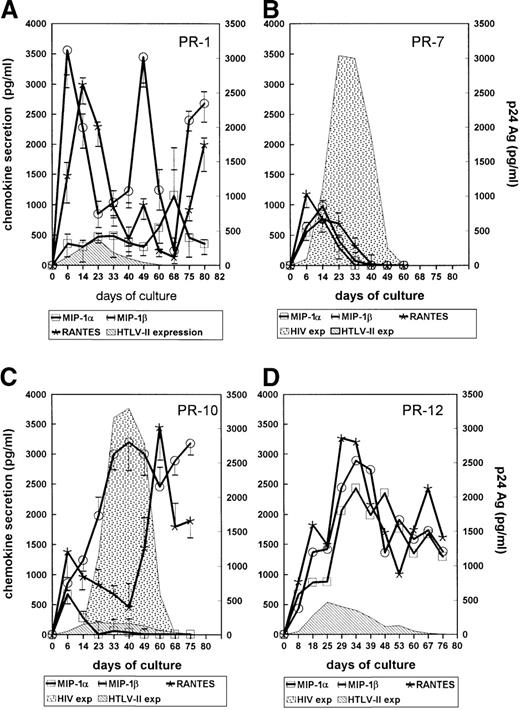
The ability of HTLV-I/-II to up-regulate expression of chemokines has been reported.22,23 Because some CC-chemokines, such as MIP-1α, MIP-1β, and RANTES, have shown inhibitory effects on NSI HIV-1 replication,19 22 their concentrations were tested in the supernatants of primary cultures established from HTLV-II–monoinfected PR-1, PR-2, and PR-3 and HIV-1/HTLV-II–coinfected PR-7, PR-8, PR-10, PR-9, PR-11, and PR-12. All chemokines were indeed secreted on a 6-day time course by primary cultures from PR-1 (Figure 5A) in the presence of IL-2; similar levels were found in PBMC cultures established from PR-2 and PR-3. Substantial variability in terms of chemokine secretion was observed in cultures from coinfected individuals. The PBMC cultures of PR-7 showed early peaks of MIP-1α, MIP-1β, and RANTES secretion within 6 to 14 days of culture, which then decreased to undetectable levels (Figure 5B); a similar pattern was observed in PBMC cultures of PR-8, who, like PR-7, was characterized by a very low HTLV-II load (Table 1). MIP-1α was produced early at low levels in the cultures of PR-10 (Figure 5C) and PR-9, whereas MIP-1β and RANTES peaked at higher levels than MIP-1α later during the culture (Figure 5C). In the case of PR-12, characterized by a particularly low HIV-1/HTLV-II ratio, a substantial production of all 3 chemokines occurs and was maintained during the whole culture period (Figure 5D). Of interest, no evidence of HIV replication was obtained in PBMC cultures of PR-12 (Figure 5D). These findings suggested a potential linkage between HTLV-II load, chemokine secretion, and HIV replication in IL-2–stimulated PBMC cultures of these individuals. This hypothesis was further reinforced with the cell cultures of PR-11, which showed considerable levels of chemokine secretion and delayed kinetics of HIV replication compared with the PBMC culture of PR-7 (Figure 6A). Furthermore, chemokine secretion and HTLV-II expression occurred simultaneously and preceded HIV replication in PR-11's cultures (Figure 6A). Viral replication as well as chemokine secretion in PR-11's cell cultures were also investigated after 3 months of zidovudine monotherapy; HTLV-II proviral load decreased from 16 239 to 500 copies per 105 cells, whereas no substantial changes in HIV-1 DNA load (1060 vs 1844 copies per 105 cells) occurred before and after therapy, respectively. Quite strikingly, increased HIV RNA plasma levels (from 4810 to 36 530 copies per milliliter, before and after therapy, respectively) likely indicated the presence of zidovudine-resistant strains in this individual. A more rapid kinetic of HIV replication ex vivo in the absence of HTLV-II production and in reduced levels of MIP-1α, MIP-1β, and RANTES was noted in cultures established 90 days after initiation of zidovudine monotherapy (Figure6B).
Chemokine secretion in culture supernatants of IL-2–stimulated PBMCs.
Cultures were established from a mono–HTLV-II–infected patient (PR-1), whereas the bars indicate the range of chemokine concentrations of PR-2 and PR-3 (A). Similar cultures were performed with PBMCs of the HIV-1/HTLV-II–coinfected patient PR-7, with the bars indicating the range of PR-8, (B); of HIV-1/HTLV-II–coinfected patients PR-10, with bars indicating the chemokine levels observed with cells of PR-9 (C); and of HIV-1/HTLV-II–coinfected patients PR-12 (D). Cells were seeded at 1 × 105 cells per well and culture supernatants were harvested at regular intervals and then tested for chemokine concentrations and HIV-1 or HTLV-II p24 Gag Ags. Results are representative of 3 independent experiments.
Chemokine secretion in culture supernatants of IL-2–stimulated PBMCs.
Cultures were established from a mono–HTLV-II–infected patient (PR-1), whereas the bars indicate the range of chemokine concentrations of PR-2 and PR-3 (A). Similar cultures were performed with PBMCs of the HIV-1/HTLV-II–coinfected patient PR-7, with the bars indicating the range of PR-8, (B); of HIV-1/HTLV-II–coinfected patients PR-10, with bars indicating the chemokine levels observed with cells of PR-9 (C); and of HIV-1/HTLV-II–coinfected patients PR-12 (D). Cells were seeded at 1 × 105 cells per well and culture supernatants were harvested at regular intervals and then tested for chemokine concentrations and HIV-1 or HTLV-II p24 Gag Ags. Results are representative of 3 independent experiments.
Chemokine secretion in culture supernatants of IL-2–stimulated PBMCs from mono–HTLV-II–infected patient PR-11 before (A) and after (B) 3 months of zidovudine monotherapy.
Chemokine secretion in culture supernatants of IL-2–stimulated PBMCs from mono–HTLV-II–infected patient PR-11 before (A) and after (B) 3 months of zidovudine monotherapy.
These findings support a strong correlation between HTLV-II load and expression, CC-chemokine secretion, and HIV-1 replication ex vivo, which was further investigated.
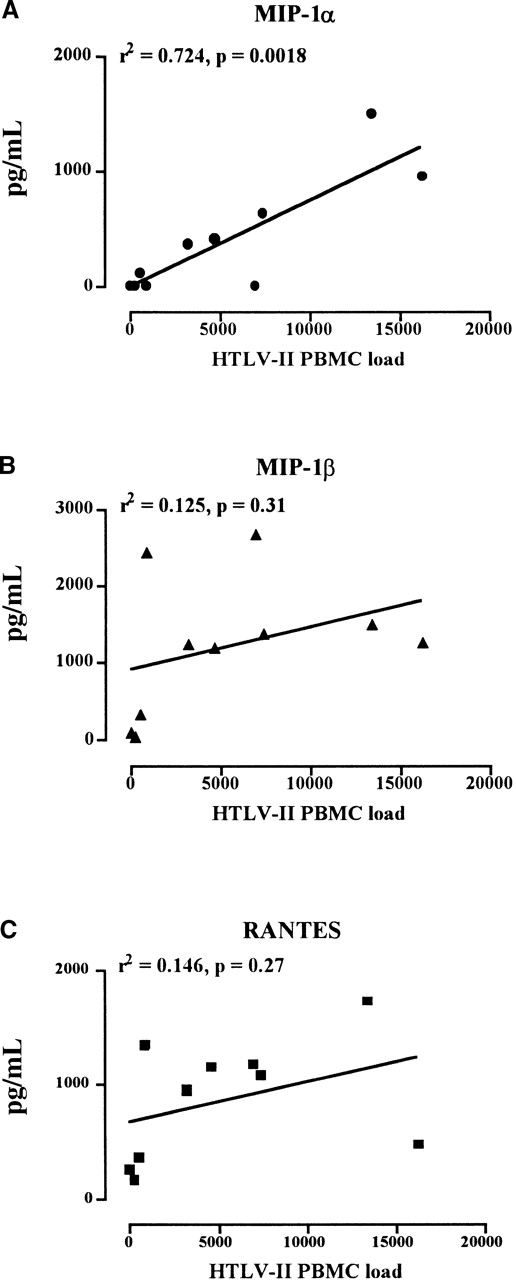
When the levels of the 3 HIV-suppressive chemokines secreted in IL-2–stimulated PBMC cultures were expressed as median values and then plotted against HTLV-II DNA load, MIP-1α, but not MIP-1β or RANTES, was found significantly associated with the HTLV-II copy number (Figure 7). This observation suggested that MIP-1α could play a crucial role in controlling HIV replication in autologous PBMC cultures of coinfected individuals.
Correlation between the HTLV-II proviral load in PBMCs and the MIP-1 secretion in ex vivo cultures.
HTLV-II copy number was evaluated by competitive PCR on 1 × 105 cells (horizontal axis). Median levels of MIP-1α (A), MIP-1β (B), and RANTES (C) secreted by IL-2–stimulated PBMCs from mono–HTLV-II–infected and HIV-1/HTLV-II–coinfected patients are shown. Median values represents the chemokine concentrations (vertical axis) observed by multiple determinations.
Correlation between the HTLV-II proviral load in PBMCs and the MIP-1 secretion in ex vivo cultures.
HTLV-II copy number was evaluated by competitive PCR on 1 × 105 cells (horizontal axis). Median levels of MIP-1α (A), MIP-1β (B), and RANTES (C) secreted by IL-2–stimulated PBMCs from mono–HTLV-II–infected and HIV-1/HTLV-II–coinfected patients are shown. Median values represents the chemokine concentrations (vertical axis) observed by multiple determinations.
Enhanced inhibitory effect of CD8+ T cells from HTLV-II–monoinfected patients on HIV-1 replication in CD4+ T cells from HIV-1–monoinfected individuals: dominant role of MIP-1
A critical role for a noncytolytic anti-HIV activity of CD8+ T cells in controlling HIV replication has been reported by several authors36; the 3 CC-chemokines investigated here have previously been implicated as major players for this antiviral activity.37 Of interest, HTLV-II is known to express a preferential tropism for CD8+ T lymphocytes in vivo.38 Therefore, CD8+ T cells from HTLV-II monoinfected PR-1 and PR-3 individuals (characterized by a high proviral load) were cocultivated with CD4+ cells of PR-5 and PR-6 HIV-1 monoinfected individuals (characterized by high HIV proviral DNA and viremia), separated by a semipermeable membrane. The ability of different numbers of allogeneic CD8+ T cells inhibiting HIV replication in this system was compared with that of autologous CD8+ T cells. Results of a typical experiment (representative of 3 independently performed experiments) are shown in Figure 8. As expected, HIV-1 p24 Gag production occurred in CD8-depleted CD4+ cells of PR-5 and PR-6, and peaked at day 24 of cultures (Figure 8). In contrast, CD4+ T cells from PR-5 or PR-6, incubated with equal numbers (1:1 ratio) of either purified autologous (PR-5/PR-5 and PR-6/PR-6) or allogeneic (PR-1/PR-5 and PR-3/PR-6) CD8+ T cells, showed a drastic reduction of viral expression. Interestingly, autologous CD8+ T cells from both HIV-1–monoinfected individuals lost their inhibitory capacity at a 1:5 ratio with CD4+ T cells, whereas CD8+ T cells of HTLV-II–monoinfected individuals maintained a potent suppressive effect up to a 1:20 ratio with CD4+ cells (Figure 8). A clear, although only partial, HIV inhibitory activity in mixed CD8/CD4 cocultures was present even at 1:50 (Figure 8). Of note is the fact that a cocktail of antihuman MIP-1α, MIP-1β, and RANTES polyclonal antibodies completely reverted the suppressive effects exhibited by CD8+ T cells of HTLV-II–monoinfected individuals on HIV-1 replication (Figure 8).
CD8+/CD4+ cell-dependent suppressive effect of CD8+ T cells from HIV-1 (PR-5 and PR-6, respectively) or HTLV-II (PR-1 and PR-3, respectively) monoinfected patients on HIV-1 p24 Ag production in cultures of CD4+ cells purified from a mono–HIV-1–infected individual.
From PR-1 and PR-5 (A), and from PR-6 and PR-3 (B), respectively. CD8+ cells were separated from HIV-infected CD4+ cells by a semipermeable membrane in a Transwell system. Cultures were performed in the absence or presence of anti-RANTES, anti-MIP-1α, and anti-MIP-1β polyclonal Abs (2 μg/mL each).
CD8+/CD4+ cell-dependent suppressive effect of CD8+ T cells from HIV-1 (PR-5 and PR-6, respectively) or HTLV-II (PR-1 and PR-3, respectively) monoinfected patients on HIV-1 p24 Ag production in cultures of CD4+ cells purified from a mono–HIV-1–infected individual.
From PR-1 and PR-5 (A), and from PR-6 and PR-3 (B), respectively. CD8+ cells were separated from HIV-infected CD4+ cells by a semipermeable membrane in a Transwell system. Cultures were performed in the absence or presence of anti-RANTES, anti-MIP-1α, and anti-MIP-1β polyclonal Abs (2 μg/mL each).
Similar results were obtained when unfractionated PBMCs, instead of purified CD4+ and CD8+ cells, were used in the same cocultivation system. PBMCs from PR-4 or PR-5 HIV-1 monoinfected and from PR-1 or PR-2 HTLV-II monoinfected were cocultivated in the same wells but separated by a semipermeable membrane up to 30 days in the presence of IL-2. The results of a typical experiment are shown in Figure 9. HIV-1 p24 Ag production was promptly observed in PBMC cultures of PR-5, which was fully suppressed in the presence of PR-2 PBMCs on the other side of the chamber (Figure9), as previously observed with purified CD4+ and CD8+ cells (Figure 8). Antichemokine neutralizing Abs (Nabs) were added either individually or as cocktails in the attempt to identify whether some chemokines were playing a dominant role in terms of HIV suppression in this system. Reversion of HTLV-II PBMC suppressive effect was indeed observed when either a cocktail of all 3 Nabs or anti–MIP-1α Nab alone was added to the cocultures (Figure9). In contrast, and quite surprisingly, no reversion of PBMC-mediated anti-HIV effect was observed when anti-RANTES or anti–MIP-1β Nabs were added alone or in combination to the cultures (Figure 9). As previously observed in terms of correlation between chemokine secretion in culture and HTLV-II proviral load in vivo, MIP-1α seems to play a crucial role in mediating HIV suppression by HTLV-II–infected cells.
Neutralizing activity of antichemokine Abs in cocultures of unfractionated PBMCs from HIV-1 (PR-5) and HTLV-II (PR-2) monoinfected patients.
PBMCs from the 2 individuals were cocultivated in the same chamber but separated by a semipermeable membrane up to 30 days in the presence or absence of antichemokine Abs (2 μg/mL each) added either individually or in combination.
Neutralizing activity of antichemokine Abs in cocultures of unfractionated PBMCs from HIV-1 (PR-5) and HTLV-II (PR-2) monoinfected patients.
PBMCs from the 2 individuals were cocultivated in the same chamber but separated by a semipermeable membrane up to 30 days in the presence or absence of antichemokine Abs (2 μg/mL each) added either individually or in combination.
Recombinant (r) MIP-1 inhibits HIV replication in CD8-depleted PBMC cultures of HIV-1–monoinfected individuals
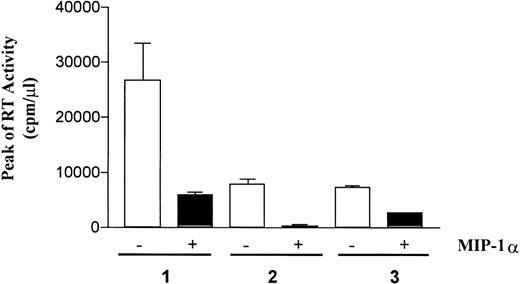
To further demonstrate the capacity of MIP-1α to suppress HIV-1 replication, PBMC cultures from HIV-1–monoinfected individuals were depleted of CD8+ T cells, stimulated with phytohemagglutinin (PHA-P, Sigma Chemical) and IL-2 (20 U/mL; Boehringer-Mannheim) in the presence or absence of rMIP-1α (100 ng/mL; R & D Systems). The excess of PHA was removed by cell centrifugation after 72 hours and cells were resuspended in medium enriched with IL-2 as described above. As shown in Figure10, rMIP-1α exerted profound inhibitory effects in these PBMC cultures established from different HIV-1–monoinfected individuals, thus confirming and extending our findings with cell cultures of HTLV-II/HIV-1–infected persons.
Recombinant MIP-1 inhibits HIV-1 replication in CD8-depleted PBMC cultures of monoinfected individuals.
PBMCs were isolated from 3 individuals with progressive HIV-1 disease, and CD8+ T cells were removed by a single round of immunomagnetic beads (Dynal, Oslo, Norway). rMIP-1α (100 ng/mL) that was added twice (time 0 and 72 hours from the establishment of the culture) inhibited virus replication in these autologous cell cultures, although with variable potency.
Recombinant MIP-1 inhibits HIV-1 replication in CD8-depleted PBMC cultures of monoinfected individuals.
PBMCs were isolated from 3 individuals with progressive HIV-1 disease, and CD8+ T cells were removed by a single round of immunomagnetic beads (Dynal, Oslo, Norway). rMIP-1α (100 ng/mL) that was added twice (time 0 and 72 hours from the establishment of the culture) inhibited virus replication in these autologous cell cultures, although with variable potency.
Discussion
In this work we describe that simultaneous replication of both HIV-1 and HTLV-II can be investigated in cultures of PBMCs obtained from coinfected individuals and maintained in IL-2–enriched medium. HTLV-II expression occurred earlier than that of HIV-1, and its kinetics were directly correlated to the number of HTLV-II copies present in patient PBMCs (viral load). Furthermore, evidence of HTLV-II interference on HIV-1 replication was obtained both in terms of kinetics of virus production and, more importantly, of cytokine and chemokine secretion, which were consistent with their respective roles of positive and negative regulators of HIV replication. CD8+ T cells from HTLV-II–monoinfected individuals released soluble factors that suppressed HIV-1 replication more potently than autologous CD8+ T lymphocytes in the cultures of both unfractionated PBMCs and purified CD4+ cells of mono HIV-1–infected individuals. Antichemokine Nabs reverted the suppressive effects of CD8+ cells of HTLV-II–monoinfected individuals. Among others, MIP-1α appeared to play a major role in these culture systems, also supported by the ability of rMIP-1α to inhibit HIV replication in the cultures of HIV-1–monoinfected individuals. Of note, MIP-1α secretion, but not that of either RANTES or MIP-1β, was significantly correlated to the HTLV-II load in vivo.
A major determinant of HIV-1 infection is the efficiency of virus entry. In contrast to what was observed for HTLV-I,39 in our ex vivo system of PBMCs of HIV-1/HTLV-II–coinfected individuals, we have not observed any down-regulation of the CD4 surface molecules after HTLV-II infection. In fact, expression of CD3 and CD8 Ag was unchanged, whereas CD4 was down-regulated only in concomitance with HIV-1 replication in the cocultures of both HTLV-II–monoinfected and HTLV-II/HIV-1–coinfected individuals.
Recently, it has been shown that both CCR5 and CXCR4 are up-regulated in response to IL-2.40 In this regard, we have previously reported that PBMC stimulation with IL-2 without prior mitogen (PHA) activation represented an in vitro system more physiologic in comparison with standard mitogen-activated T-cell blasts, in that both NSI and SI viruses, including primary HIV isolates, could efficiently replicate.31 Furthermore, under an IL-2–dependent condition, both monocytes and endogenous proinflammatory cytokines were shown to play an important role in virus spreading,31likely resembling in vivo events. Thus, it can be inferred that the expression levels of HIV-1 coreceptors in our culture systems were not the limiting factor for HIV-1 replication. This hypothesis was confirmed by the observation that the HIV primary isolates obtained from the IL-2–stimulated PBMCs of coinfected PR-7, PR-8, and PR-9 individuals were all defined as R5, with the sole exception of a second passage virus from PR-11, which was dualtropic (R5/X4).19
Unlike HTLV-I and HIV-1 coinfection,39 41 the analysis of viral expression and cellular phenotypes in the primary cultures of HIV-1– and HTLV-II–coinfected patients suggests that the 2 viruses were not coinfecting the same cell type because no evidence of reciprocal activation was observed.
The role of IL-2 in the expression of HIV-1 and HTLV-II is correlated to lymphocyte proliferation and activation, resulting in cytokine production.31,42 Resting T cells can be infected by HIV-1, but production of high levels of virus in T cells requires further cellular activation/proliferation.43-46 Autocrine and paracrine regulation of viral expression by endogenous cytokines has been demonstrated in cell lines either acutely or chronically infected by HIV-1,47,48 and in primary cells, both infected in vitro, as well as during viral isolation from patient PBMCs.49-51 T-cell lines infected by HTLVs are known to constitutively express various cytokines, including IL-2, IL-6, and TNF-α,33,34 which together induce activation of resting CD4+ T cells.52 Proinflammatory cytokines such as TNF-α, IL-1β, IFN-γ, and IL-6 were shown to profoundly affect the capacity of HIV to replicate because they act as potent up-regulators of viral expression in both T-lymphocytic and monocytic cells.47,51 53-55 Of interest, in our unstimulated primary cultures from coinfected patients, substantial levels of proinflammatory cytokines were observed. However, the lack of both IL-2 production and of CD25 expression indicated that TNF-α was not sufficient to activate either the IL-2Rα–chain or HIV-1 gene expression.
IL-2–stimulation of PBMCs induces autocrine/paracrine release of proinflammatory cytokines as well as HIV replication.31,56HIV-1 expression occurred in the IL-2–stimulated PBMCs of coinfected individuals after HTLV-II replication and when cytokine production was at its peak, confirming and extending previous observation,31 but also suggesting that proinflammatory cytokines may contribute to, but are not the only determinants of, HIV replication, at least in the presence of HTLV-II–infected cells.
HTLV-I–infected T cells expressed a wide spectrum of chemokines and that the virally encoded transcriptional activator Tax is capable of inducing a number of chemokine genes.23 The results reported here extend these findings to HTLV-II–infected cells. The levels of the CC-chemokines, RANTES, MIP-1α, and MIP-1β, earlier identified as components of the CD8+ T-cell–derived nonlytic suppressor factor for HIV-1 replication,19 22 in supernatants of the IL-2–stimulated PBMC cultures, were indeed correlated to the patterns of HIV-1 replication. HIV-1 expression was not detected until chemokine secretion was maximal, whereas it emerged when a decrease of their secretion occurred. This negative correlation was confirmed in the peculiar case of cultures established from cells obtained from coinfected PR-11 before and after zidovudine monotherapy, which was associated with a remarkable decrease of HTLV-II proviral load, but also with an increase of HIV-1 viral load. Ex vivo, a substantial loss of chemokine production in the primary culture established after therapy was reflected by earlier HIV replication kinetics and no HTLV-II expression.
In view of the fact that HIV-1 replication in our cellular system could be critically influenced by CC-chemokines, particularly in consideration that all viruses with the possible exception of 1 were R5, the effect of chemokine neutralization on HIV-1 expression was further investigated. Our results show unambiguously that soluble factors from HTLV-II–infected CD8+ T cells from HTLV-II–monoinfected patients were able to modulate HIV-1 expression in naturally infected CD4+ T cells in a negative fashion. Strikingly, either unfractionated PBMCs or CD8+ T cells from HTLV-II–monoinfected individuals were more potent than autologous CD8+ T cells in suppressing HIV replication. The major component of these factors was represented by CC-chemokines, because direct addition of antichemokine NAbs in cultures strongly decreased the soluble anti-HIV activity of HTLV-II–infected cells. These findings confirm and extend the previous observation that CC-chemokines released from HTLV-I–infected MT-2 cells are able to suppress HIV-1 replication in CD4+ T cells.57
Quite surprisingly, MIP-1α alone seemed to account for most the anti-HIV activity, and its levels of secretion in culture, unlike those of RANTES and MIP-1β, were correlated significantly with HTLV-II load in vivo. Of interest, CD8+ T lymphocytes have recently been shown to represent a biologically relevant source of MIP-1α in vivo,58 whereas spontaneous CC-chemokine production by a CD8+ lymphocyte subpopulation from HTLV-II–infected individuals, but not from individuals infected with HIV-1 alone or unstimulated normal donors, has been demonstrated.59 In addition, evidence that CD8+ cytotoxic T lymphocytes produce chemokines after engagement of viral Ags and that MIP-1α is required for an inflammatory response to virus challenge suggests that these molecules are key elements in the generation of effective antiviral immunity.60 Human MIP-1α is encoded by 2 highly related nonallelic genes producing 2 different isoforms designed LD78α and LD78β.61 The MIP-1α isoform differs only in 3 amino acids (a.a.): the penultimate NH2-terminal residue and a.a. 39 and 47. Important is the NH2-terminal regions for their biologic activity, because the difference in 1 residue may be important for receptor binding.62 LD78α signals predominantly via CCR1; in contrast, LD78β activates CCR5 more efficiently than LD78α or RANTES.63,64 On PBMCs, LD78β isoforms show 10- to 50-fold higher antiviral activity against M-tropic HIV-1 strains compared with RANTES and LD78α.63 64 It is conceivable that different isoforms of MIP-1α could account for its biologic relevance in our culture system.
Taken together, these results provide evidence that HTLV-II may exert a potent control over HIV replication via secretion of MIP-1α. The high HTLV-II proviral load might favor HIV-1 latency in coinfected patients through a chemokine-dependent mechanism.
Several questions remain unanswered in terms of the in vivo interaction between human pathogenic retroviruses. The optimization of an appropriate in vitro system of primary cells directly established from coinfected patients represents an important step for the understanding of these interactions and pathogenesis in vivo.
Supported in part by grants of the National AIDS Research Program Against AIDS of the Istituto Superiore di Sanità, and by the AIRC program 1998. The Universities of Parma and of Verona participated in the HERN concerted action, supported by the BIOMED Program of the European Commission. A.C. is currently at the Columbia University of New York, NY.
Reprints:Claudio Casoli, Istituto di Patologia Medica, Facoltà di Medicina, Università di Parma, v. Gramsci, 14, I-43100 Parma, Italy; e-mail: claucaso@ipruniv.cce.unipr.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.









 , day 0; □, day 30; ▪, day 60.
, day 0; □, day 30; ▪, day 60.