Abstract
Adenoviral gene transfer to primitive hematopoietic progenitor cells (HPCs) would be useful in gene therapy applications where transient, high-level transgene expression is required. In the present investigations, we have used an adenoviral vector expressing the green fluorescent protein (AdGFP) to quantify transduction of primitive HPCs and assess adenoviral-associated toxicity in long-term culture. Here we show that a cytokine cocktail protects mass populations of CD34+ cells and primary colony forming unit–cultures (CFU-Cs) from toxicity, enabling transduction of up to 79% of CD34+ cells. Transduction of CFU-Cs and more primitive HPCs was quantified following fluorescence activated cell sorting for green flourescence protein expression. Our results demonstrate transduction of 45% of primary CFU-Cs, 33% of week-5 cobblestone area forming cells (CAFCs), and 18% of week-5 CFU-Cs. However, AdGFP infection inhibited proliferation of more primitive cells. Although there was no apparent quantitative change in week-5 CAFCs, the clonogenic capacity of week-5 AdGFP-infected cells was reduced by 40% (P < .01) when compared with mock-infected cells. Adenoviral toxicity specifically affected the transduced subset of primitive HPCs. Transduction of primitive cells is therefore probably underestimated by week-5 CFU-Cs and more accurately indicated by week-5 CAFCs. These studies provide the first functional and quantitative evidence of adenoviral transduction of primitive HPCs. However, further investigations will be necessary to overcome adenoviral toxicity toward primitive HPCs before adenoviral vectors can be considered a safe option for gene therapy.
Genetic modification of hematopoietic stem cells (HSCs) is a much pursued goal that has been met with limited success. To date, most attempts to transduce HSCs have used murine leukemia virus (MLV)–based retroviral vectors. Although MLV-based vectors transduce CD34+ progenitor cells with reasonable efficiency, analysis of progenitor subsets and results from gene therapy trials indicate that the most primitive progenitor cells are more resistant to retroviral infection.1-5 This problem may be attributed to HSC quiescence6,7 and to the necessity for mitosis for integration of MLV-based vectors.8,9 Efficient retroviral gene transfer to hematopoietic progenitor cells can be achieved following cytokine prestimulation of CD34+ cells. However, the standard cytokine cocktails employed during prestimulation may be detrimental to primitive hematopoietic repopulating cells.10,11 In addition, retroviral transduction of human hematopoietic cells is restricted by limited availability of retroviral receptors on target cells.12
Considering the obstacles surrounding retroviral infection of HSCs, recombinant adenoviral vectors are promising alternatives for gene delivery to HSCs. In contrast to retroviral vectors, adenoviral vectors efficiently infect nondividing cells. Hence cytokine prestimulation is not required, and quiescent hematopoietic cells are feasible targets for adenovirus-mediated gene transfer. Also, the cell surface molecules involved in adenovirus attachment and entry are expressed by CD34+ cells. For the majority of susceptible cell types, adenovirus attachment is mediated via a high-affinity association of the viral fiber protein with the coxsackie and adenovirus receptor (CAR).13,14 We have recently shown CAR messenger RNA (mRNA) expression in CD34+ cells.15 Alternative receptors and viral proteins may also play a role in adenoviral attachment, particularly at high multiplicities of infection (MOI) or when CAR expression is limiting. For instance, adenovirus serotype 2 binds to mature monocytes via a fiber-independent mechanism that is mediated by interaction of β2 integrins with the adenoviral penton base.16 Regardless of the mode of initial attachment, adenoviral entry is promoted by association of integrins with the penton base.16,17 Specifically, the αv and α1 (very late antigen [VLA]) integrins appear to have an important role in internalization.16-18 It may be significant that VLA4 and VLA5 are expressed on CD34+cells, including granulocyte/macrophage colony forming cells (CFU-GM),19 20 whereas the αv integrins are not expressed at significant levels on hematopoietic progenitors.
Adenoviral transduction of HSCs is a desirable goal for certain applications, such as the transient expression cytokines, cytokine receptors, or other growth regulators that stimulate HSC self-renewal or homing. Recently, several groups, including our own, have demonstrated transduction of CD34+ progenitor cells using recombinant adenoviral vectors.15,21,22 However, to date, there is still no convincing evidence that adenoviral vectors transduce subsets of hematopoietic progenitor cells (HPCs) that are functionally more primitive. Toxicity at high MOIs apparently limits the efficacy of adenoviral transduction of CD34+ cells.15,21,22Similarly, various nonhematopoietic cells were reported to be adversely affected by exposure to high concentrations of adenoviral vectors.23,24 For example, exposure of lung epithelial cells to high concentrations of an adenoviral vector was shown to restrict recruitment into the S-phase of the cell cycle and induce apoptosis.24 Low-level expression of viral proteins, such as the protein encoded by adenovirus E4 open reading frame 4, may contribute to apoptosis or growth arrest of vector-transduced cells.25-27 The consequences of toxic effects during adenoviral transduction are clearly an important consideration in gene transfer protocols targeting HSCs.
In the present investigations, we have used an adenoviral vector expressing the green fluorescent protein (AdGFP) to assess cytotoxic effects and quantify transduction of primitive HPCs. First, we demonstrate that toxicity toward mass populations of CD34+cells and primary colony forming unit–cultures (CFU-Cs) can be minimized by performing adenoviral infections in the presence of a cytokine cocktail. Second, we quantify transduction of CFU-Cs and primitive HPCs, read out as week-5 cobblestone area forming cells (CAFCs) and secondary (week-5) CFU-Cs, using fluorescence activated cell sorted (FACS) populations of transduced cells. Our results demonstrate that primitive HPCs can be transduced with relative efficiency. However, adenoviral infection was found to be detrimental to the proliferation of more primitive HPCs.
Materials and methods
Isolation of CD34+ and CD38+/- cells
Frozen units of granulocyte colony stimulating factor (G-CSF) plus cyclophosphamide mobilized peripheral blood (mPB) were used as a source of CD34+ cells. The units were specimens from deceased patients who were previously treated with chemotherapeutic agents for various malignancies. Leukapharesis products were thawed quickly at 37°C, then mixed with 1/10 volume of anticoagulant citrate dextrose solution (Baxter Health Care, Deerfield, IL), and diluted 1:1 with 1% fetal calf serum (FCS)/Hanks' balanced salt solution (HBSS). Mononuclear cells (MNCs) were harvested by Ficoll-Paque (Pharmacia-Uppsala, Sweden) density gradient separation and resuspended in 10 mL of 0.1% bovine serum albumin (BSA) fraction V (Sigma) in HBSS. CD34+ cells were selected from MNCs by means of the CellPro Ceparate System (kindly provided by CellPro, Bothell, WA) and immunomagnetic beads (Dynal, AS, Oslo, Norway) according to the manufacturer's instructions. Briefly, MNCs were incubated with 300 μg of anti-CD34 monoclonal antibody 11.1.6 (developed by M. A. S. Moore and available through Oncogene Science, Uniondale, NY) for 30 minutes at 4°C followed by a wash with 0.1% BSA/HBSS and 30 minutes' incubation with sheep antimouse immunoglobulin (Ig)–G1 (Fc) immunomagnetic beads. The CD34+ and CD34− cells were separated on a magnetic separator; then the CD34+ fraction was incubated overnight in Iscove's Modified Dulbecco's Medium (IMDM) with 20% FCS (Gibco BRL, Gaithersburg, MD) to allow the cells to detach from the beads. The purity of the CD34+ fraction was between 87% and 99% according to FACS analysis with the use of R-phycoerythrin (PE)–conjugated anti-CD34 monoclonal antibody (clone 581, Pharmingen, San Diego, CA). Dynal beads were also used for magnetic separation of CD38 ± subsets of CD34+ cells as described above. To obtain maximal purity, 2 rounds of selection were performed with the use of 50 μg anti-CD38 monoclonal antibody (clone H1T2, Pharmingen) for 2 × 107 CD34+ cells.
Adenoviral vectors and infections
Construction of AdGFP and AdNull were previously described.15,28 AdGFP is a replication-deficient, E1- and E3-deficient adenovector that includes a humanized green fluorescence protein (GFP) gene under transcriptional control of the cytomegalovirus promoter. AdNull is identical to AdGFP except AdNull contains no GFP gene. The titer of the adenoviral vector stocks was determined by plaque formation on 293 cells. We used 3 different stocks of AdGFP in these studies. Titers of AdGFP and AdNull stocks ranged between 1010 and 1012 plaque-forming units/mL.
For adenoviral transductions, freshly isolated CD34+ cells were suspended at 1.5 × 106 cells/mL in X-Vivo 15 (BioWhittaker, Walkersville, MD) supplemented with gentamycin and cytokines. Unless otherwise indicated, the cytokines used for infections were human Flt-3/Flk-2 ligand (FL, 300 ng/mL) (Imclone, New York, NY), human kit-ligand (KL, 20 ng/mL, Amgen, Thousand Oaks, CA), human interleukin (IL)–1 (IL-1, 100 U/mL) (Syntex, Palo Alto, CA), and human IL-3 (50 ng/mL) (Sandoz, Basel, Switzerland). Infections were performed by inoculating the cell suspensions with adenoviral vectors at the specified MOI, then incubating for 14 hours in a humidified incubator at 37°C with 5% CO2. At the end of the incubation period, the cells were washed twice in 20% FCS/IMDM, resuspended at 2 × 105 cells/mL in X-Vivo 15 with the same cytokines and incubated for a further 72 hours before analysis for transgene expression by FACS analysis. Viability of the cells was monitored throughout the transduction procedure by trypan blue exclusion.
Flow cytometry
Quantitation of GFP expression and phenotyping for CD34 and CD38 expression was performed by FACS analysis on a Becton Dickinson FACScan. For CD34 and CD38 determinations, 1 × 105cells were washed in 2% FCS/phosphate buffered saline (PBS), then stained with PE-conjugated anti-CD34 and/or fluoroscein isothiocyanate (FITC)–conjugated anti-CD38 monoclonal antibodies (Pharmingen) for 30 minutes at 4°C. Excess antibody was then washed away by resuspending the cells in 1 mL 2% FCS/PBS and centrifuging at 1000g for 6 minutes. Cells were finally resuspended in 400 μL 2% FCS/PBS for FACS analysis. Dead cells and debris were excluded from analysis by staining with propidium iodide (PI) or gating on forward and side scatter parameters. Cell sorting was performed on a Becton Dickinson FACS Star Plus.
Cell cycle analysis was performed by DNA content analysis of PI-stained cells 3 days after transduction with AdNull at an MOI of 2000. To prepare the cells for analysis, 2 × 105 cells were washed with PBS, resuspended in 5 mmol/L EDTA/PBS and fixed by dropwise addition of an equal volume of cold 100% ethanol. After 30 minutes at room temperature, the cells were harvested by centrifugation at 3000 rpm for 8 minutes. Following resuspension in 1 mL of 5 mmol/L EDTA/PBS, RNA was digested with 10 μg DNAse-free RNAse (Boehringer Mannheim, Indianapolis, IN) for 30 minutes at room temperature. Finally, the cells were stained for cell cycle analysis by addition of PI to a final concentration of 100 μg/mL in 5 mmol/L EDTA/PBS for 45 minutes at room temperature.
Clonogenic assay
For quantitation of clonogenic progenitor cells, cells were plated at 1 × 103 cells/mL in 30% FCS/IMDM and 0.8% methlycellulose (Dow Chemicals, Saddlebrook, NJ) supplemented with KL, IL-3, IL-6, erythropoietin (EPO, 6 U/mL) (Amgen), human G-CSF (100 ng/mL) (Amgen), 50 μmol/L β-mercaptoethanol, 100 nm hemin, and 20 mmol/L glutamine. The cultures were incubated for 14 days with humidity and 5% CO2. Colonies that consisted of more than 50 cells were scored using a 4x objective on an inverted microscope.
Delta assay
Following adenoviral infection and sorting, cells were cultured at 4 × 104 cells/mL in 20% IMDM supplemented with KL, IL-3, IL-6, EPO, and G-CSF for 21 days. Every 7 days, the cells were harvested, counted, and resuspended at 4 × 104cells/mL.
Assays for primitive progenitors in long-term culture
CD34+ cells were cocultured over the MS5 murine bone marrow stromal cell line29 in 12.5% FCS/12.5% horse serum/IMDM supplemented with 7.9 μg/mL monothioglycerol, 10−6 mol/L hydrocortisone, 20 mmol/L glutamine and 750 μg/mL gentamycin. The cultures were incubated at 37°C with 5% CO2 and demi-depopulated each week. After 5 weeks in culture, CAFCs were quantified by counting cobblestone areas consisting of at least 8 phase dark cells. Mean CAFC frequencies and the SEM were calculated from triplicate or quadruplicate flasks. Progenitor cells within the nonadherent and adherent layers were quantified at week 5 in clonogenic assays by plating cells at 5 × 104 cells/mL as described above. Secondary CFU-Cs were quantified as the sum of the progenitor cells in nonadherent and adherent layers.
Results
Effect of cytokines on viability of adenoviral transduced hematopoietic cells assessed in short-term assays
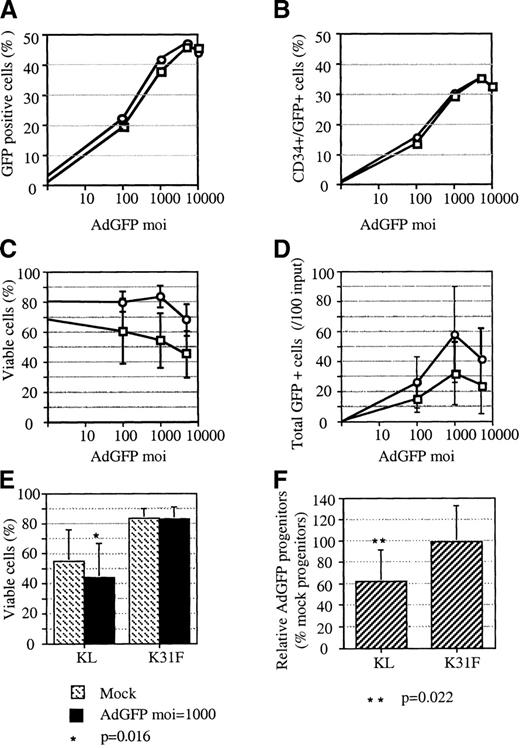
In order to study and optimize adenoviral transduction of HSCs, we used AdGFP to mark transduced progenitor cells. Our laboratory and others have shown that efficient adenoviral transduction of CD34+ cells requires infection at a high MOI.15,21 22 However, each of those studies also revealed that higher rates of transduction were limited by cytotoxicity at MOIs of 500 and greater. To address the problem of toxicity, we explored the potential for cytokines to promote cell survival and proliferation during adenoviral infection. In a series of experiments, CD34+ cells were maintained in either KL alone or in KL, FL, IL-1, and IL-3 during, and for 72 hours following, AdGFP infection. The cells were not prestimulated with cytokines prior to infection. Transduction was quantified by FACS analysis 72 hours after infection. Our results show no significant difference in transduction efficiency over a range of MOIs (100 to 100 000) when the cells were cultured in KL alone versus KL, FL, IL-1, and IL-3 (Figure1A). Moreover, the frequency of double-positive (GFP+/CD34+) cells was also identical for the 2 groups of cells (Figure 1B).
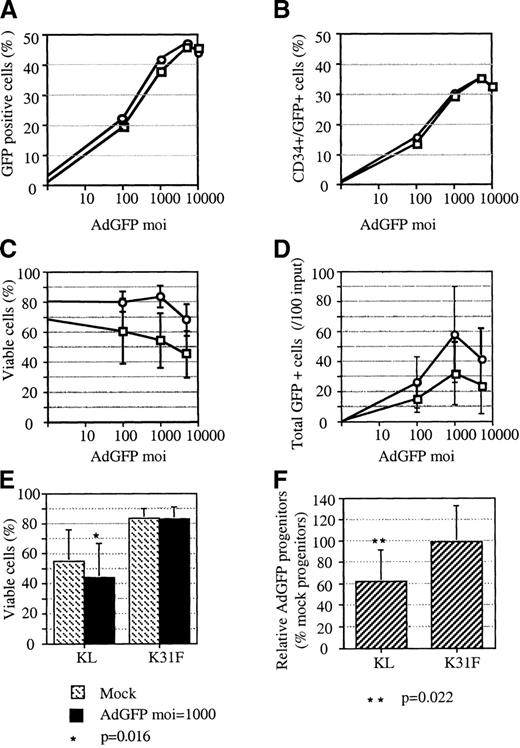
The effect of cytokines on adenoviral vector–transduced progenitors.
In a series of experiments, progenitor cells were cultured either in KL alone □ or in KL, FL, IL-1, and IL-3 ○ during infection and for 72 hours posttransduction with AdGFP or AdNull. At 3 days after initiation of the infection, FACS analysis, cell counts, and colonogenic assays were performed. Viability was assessed using trypan blue. (A) Total transduced cells in 1 representative experiment. (B) Transduced CD34+ cells in the same experiment shown in panel A. (C) Average cell viability evaluated from 3 experiments. (D) Average number of total transduced cells enumerated by multiplication of the percentage of transduced cells and the total number of viable cells in 3 experiments. (E) Average cell viability following either mock infection or adenoviral infection at MOI = 1000 enumerated from 7 independent experiments. The P value, derived from Studentt test, indicates a significant difference between mock-infected cells and infected cells when cultured in KL alone. (F) Clonogenicity of progenitor cells following adenoviral infection at MOI = 1000. Values, expressed as a percentage of colonies derived from mock-infected cells, are the averages of 7 independent tests. TheP value, derived from Student t test, indicates a significant difference in the cloning efficiency of infected cells compared with mock-infected cells when cultured in KL alone. Error bars represent the SEM.
The effect of cytokines on adenoviral vector–transduced progenitors.
In a series of experiments, progenitor cells were cultured either in KL alone □ or in KL, FL, IL-1, and IL-3 ○ during infection and for 72 hours posttransduction with AdGFP or AdNull. At 3 days after initiation of the infection, FACS analysis, cell counts, and colonogenic assays were performed. Viability was assessed using trypan blue. (A) Total transduced cells in 1 representative experiment. (B) Transduced CD34+ cells in the same experiment shown in panel A. (C) Average cell viability evaluated from 3 experiments. (D) Average number of total transduced cells enumerated by multiplication of the percentage of transduced cells and the total number of viable cells in 3 experiments. (E) Average cell viability following either mock infection or adenoviral infection at MOI = 1000 enumerated from 7 independent experiments. The P value, derived from Studentt test, indicates a significant difference between mock-infected cells and infected cells when cultured in KL alone. (F) Clonogenicity of progenitor cells following adenoviral infection at MOI = 1000. Values, expressed as a percentage of colonies derived from mock-infected cells, are the averages of 7 independent tests. TheP value, derived from Student t test, indicates a significant difference in the cloning efficiency of infected cells compared with mock-infected cells when cultured in KL alone. Error bars represent the SEM.
At all MOIs tested, in 3 independent experiments, the viability of cells was higher for cells cultured in the combination of 4 cytokines than in KL alone (Figure 1C). The disparity between the 2 groups increased with increasing MOIs and reached an average difference greater than 20% at an MOI of 1000. Maximal absolute transduction was achieved under the 4-cytokine condition (Figure 1D). At an MOI of 1000, there were approximately twice as many GFP+ cells in the 4-cytokine culture as in the culture with KL alone. The slight toxicity observed in the culture with 4 cytokines at an MOI of 5000 may have been due to the addition of excess glycerol (more than 10% of the culture media) from the viral stock rather than to the AdGFP transduction per se. Additional experiments (n = 7) demonstrated that the reduced viability of cells transduced in KL alone was statistically significant (P = .016, Student ttest [Figure 1E]). To investigate the potential for cytokines to protect CFU-C from cytotoxicity, CD34+ cells were either mock-infected or infected with AdGFP or a control vector (AdNull) at an MOI of 1000 to 2000 before plating in methylcellulose. Results from 7 independent experiments demonstrated adenoviral toxicity toward CFU-C when CD34+ cells were cultured with KL alone (Figure 1F). In contrast, no toxicity toward CFU-C was observed when the infections were performed in the presence of FL, K, IL-1, and IL-3. To further ensure that adenoviral transduction has no adverse effects under the 4-cytokine condition, cell cycle compartments of AdNull-transduced progenitors were analyzed. Cell cycle parameters were resolved by FACS analysis of PI-stained cells. Almost identical FACS profiles were obtained for progenitor cells that were either mock-infected or infected at an MOI of 2000 in the presence of the 4-cytokine cocktail (data not shown). Together, these results indicate that culture in FL, KL, IL-1, and IL-3 during adenoviral infection minimizes toxicity toward progenitor cells, including primary CFU-C.
AdGFP transduction of mPB progenitor cells
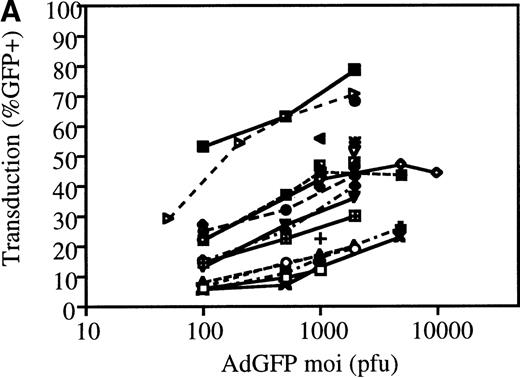
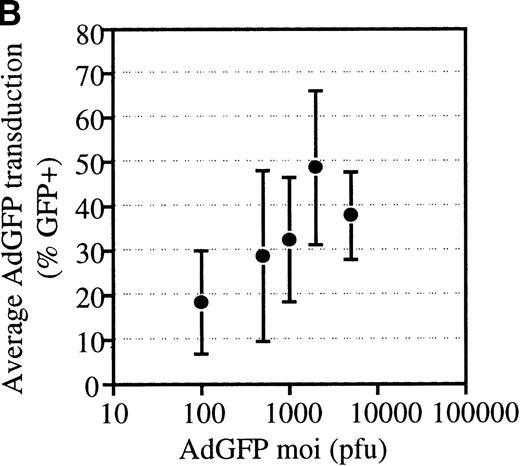
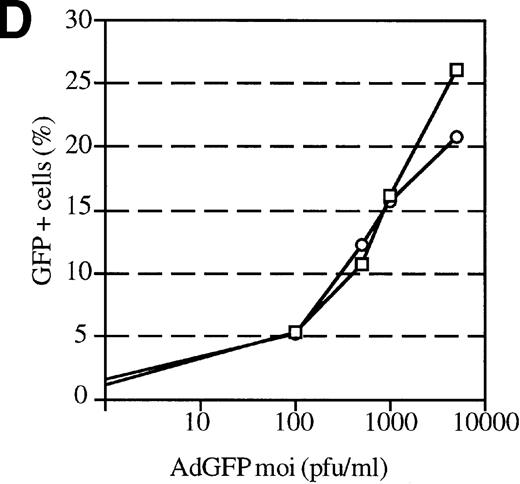
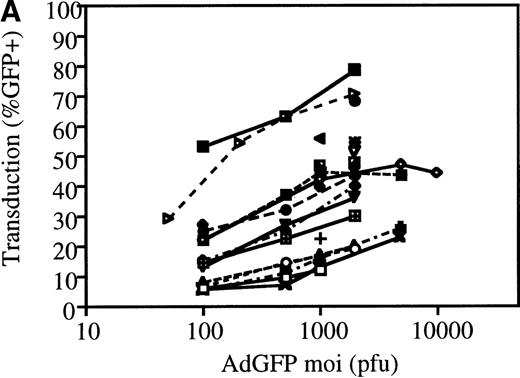
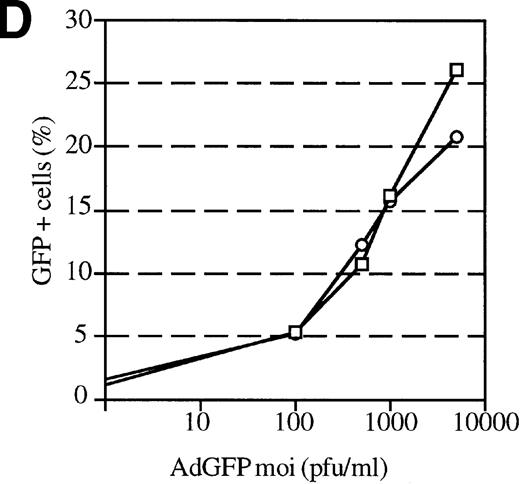
The efficiency of AdGFP transduction of mPB-derived CD34+ cells was assessed in a series of 23 experiments. CD34+ cells were transduced at various MOIs in the presence of KL, IL-1, IL-3, and FL. The percentage of transduced cells at specific MOIs is shown in Figure 2A. These results show considerable variation in the transduction efficiency for different samples at given MOIs. This variability appeared to be due to differences in the patient samples, since consecutive leukapharesis samples from the same patient were transduced with fairly reproducible efficiency (data not shown). The average transduction efficiency and SEM at each MOI tested is shown in Figure 2B.
Transduction efficiencies for mPB CD34+cells.
(A) Transduction efficiency as determined by FACs analysis of mPB CD34+ cells infected with AdGFP at various MOIs in 23 independent experiments. Different symbols indicate values obtained in distinct experiments. (B) Average transduction efficiency at given MOIs. Each point was derived from 3 to 11 independent transductions. Error bars represent the SEM.
Transduction efficiencies for mPB CD34+cells.
(A) Transduction efficiency as determined by FACs analysis of mPB CD34+ cells infected with AdGFP at various MOIs in 23 independent experiments. Different symbols indicate values obtained in distinct experiments. (B) Average transduction efficiency at given MOIs. Each point was derived from 3 to 11 independent transductions. Error bars represent the SEM.
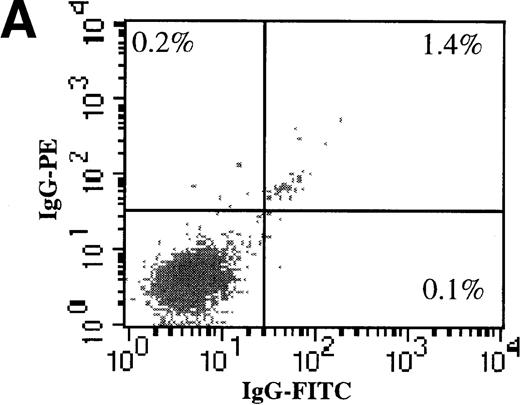
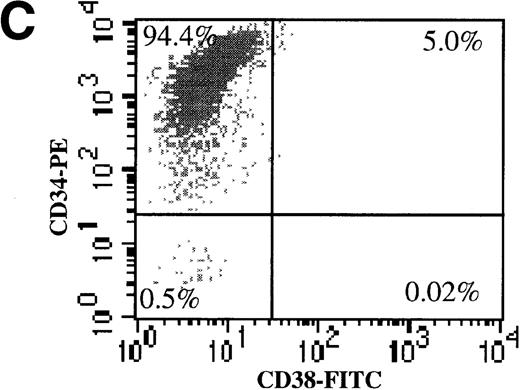
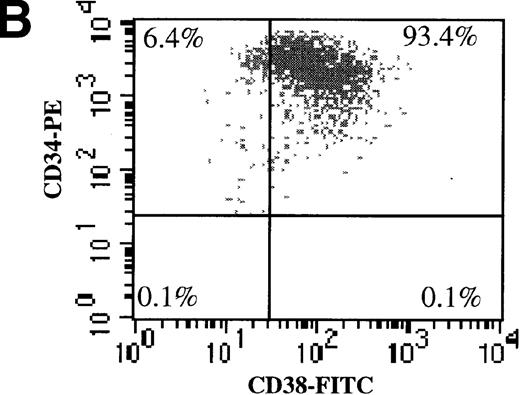
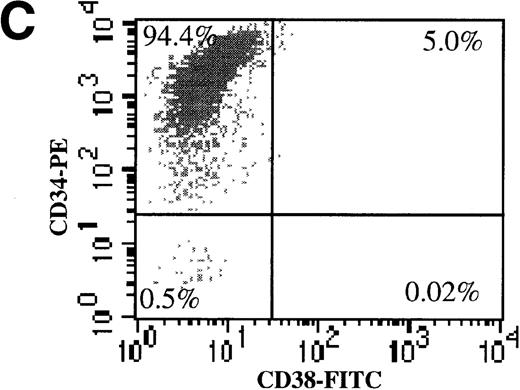
We also evaluated the efficiency of AdGFP transduction of CD34+/CD38− cells. To make this determination, freshly isolated CD34+ cells were subject to 2 rounds of negative selection for CD38 expression with the use of magnetic beads. Following this procedure, 3.4% of the input cells remained in the negative fraction. To confirm the efficiency of the separation procedure, dual-color FACS analysis for CD34 and CD38 was performed (Figure 3A-C). In the starting population, 6.4% of CD34+ cells were CD38−. After the bead selection, 94% of the negative fraction were CD34+/CD38−. Bead-separated CD34+/CD38− and CD34+/CD38+ cells were infected with AdGFP at various MOIs (up to 5000) in the presence of KL, FL, IL-1, and IL-3. FACS analysis for GFP expression showed that the CD38− fraction was transduced with a very similar efficiency to the CD38+ fraction (Figure 3D). However, there did appear to be some divergence in transduction at the highest MOI tested. At an MOI of 5000, fewer CD38− cells than CD38+ cells were transduced.
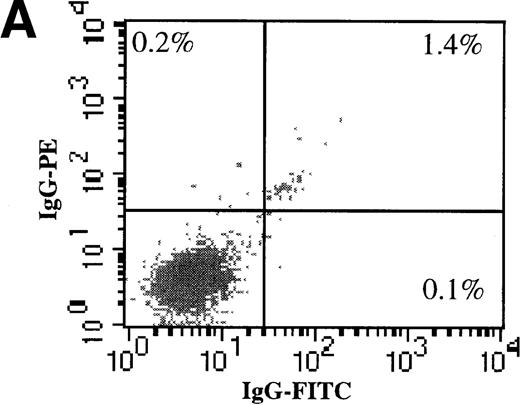
AdGFP transduction of CD38 ± subsets of mPB CD34+ cells.
(A) The purity of CD34+/CD38+ and CD34+/CD38− selected cells was analyzed by dual color FACs immediately after separation with the use of magnetic beads. (A) Total cells stained with FITC- and PE-labeled isotype control antibodies. (B) Total cells stained with PE-conjugated anti-CD34 and FITC-conjugated anti-CD38 antibodies. (C) CD38− subset following negative bead selection, stained with anti-CD34 and FITC-conjugated anti-CD38 antibodies. (D) Transduction of CD34+/CD38+ (□) and CD34+/CD38−(○) cells as assessed by FACs analysis 3 days after infection with AdGFP.
AdGFP transduction of CD38 ± subsets of mPB CD34+ cells.
(A) The purity of CD34+/CD38+ and CD34+/CD38− selected cells was analyzed by dual color FACs immediately after separation with the use of magnetic beads. (A) Total cells stained with FITC- and PE-labeled isotype control antibodies. (B) Total cells stained with PE-conjugated anti-CD34 and FITC-conjugated anti-CD38 antibodies. (C) CD38− subset following negative bead selection, stained with anti-CD34 and FITC-conjugated anti-CD38 antibodies. (D) Transduction of CD34+/CD38+ (□) and CD34+/CD38−(○) cells as assessed by FACs analysis 3 days after infection with AdGFP.
Functional analysis of AdGFP-transduced progenitor cells
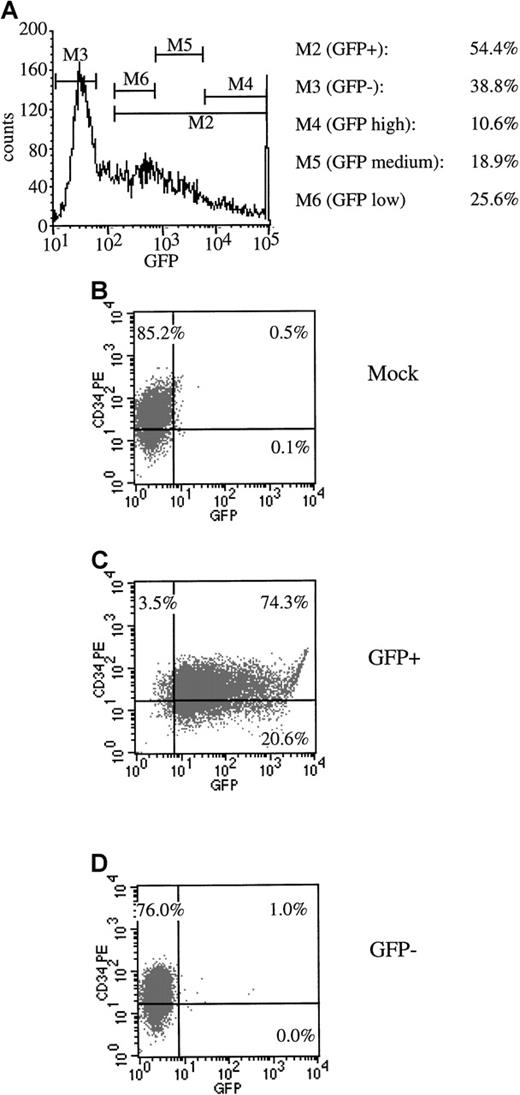
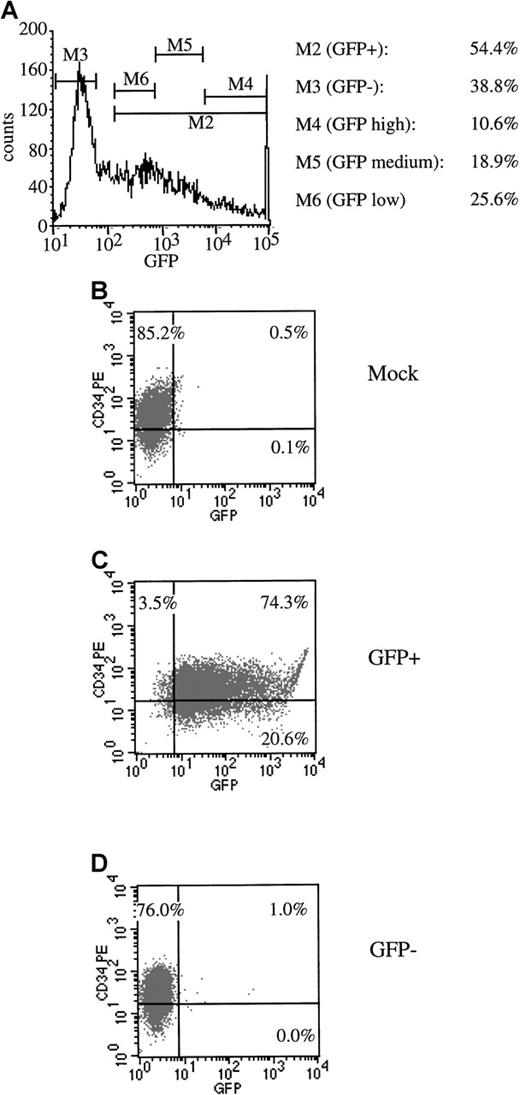
We next sought to functionally define the subsets of progenitors that could be transduced with AdGFP at a high MOI. For these determinations, CD34+ cells were infected with AdGFP at an MOI of 2000 in the presence of the 4-cytokine cocktail. At 72 hours postinfection, the bulk of the cells were sorted by flow cytometry into transduced (GFP+) and nontransduced (GFP−) fractions. An aliquot of nonsorted infected cells (AdGFP) was used as a control. In one experiment (experiment 1) GFP+ cells were sorted into 3 subsets (low, medium, and high) according to levels of GFP expression. FACS profiles demonstrating the sorting gates are shown in Figure4A. Sort purities were greater than 90% for the GFP+ fraction, and fewer than 1% of GFP+ cells contaminated the GFP− fraction (Figure 4B-D).
Flow cytometric sorting of transduced progenitor cells.
CD34+ mPB cells were transduced with AdGFP at an MOI of 2000. (A) An example of sorting gates set to collect GFP− and total GFP+ cells as well as GFP+ cells that exhibited high, medium, and low fluorescence. Post-sort FACS analysis was performed to check the purity of the sort. (B) Mock-infected cells. (C) GFP+sorted cells. (D) GFP− sorted cells.
Flow cytometric sorting of transduced progenitor cells.
CD34+ mPB cells were transduced with AdGFP at an MOI of 2000. (A) An example of sorting gates set to collect GFP− and total GFP+ cells as well as GFP+ cells that exhibited high, medium, and low fluorescence. Post-sort FACS analysis was performed to check the purity of the sort. (B) Mock-infected cells. (C) GFP+sorted cells. (D) GFP− sorted cells.
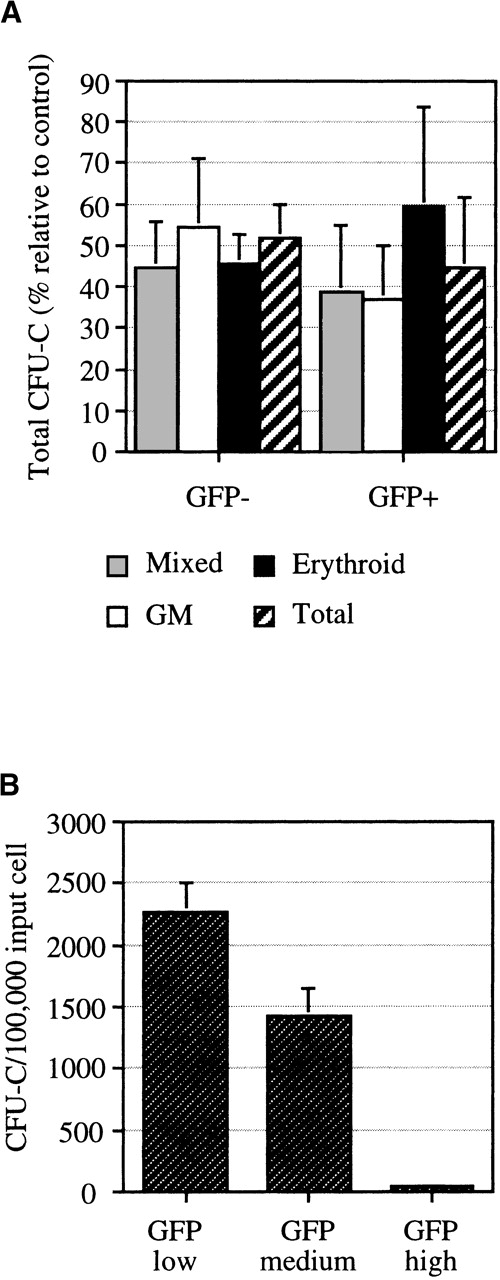
AdGFP transduction efficiencies and CFU-C content of sorted populations are shown in Table 1. In these experiments, the average transduction efficiency for the total population was 54% ± 7%. The average number of CFU-Cs in the GFP+and GFP− populations, calculated from 7 experiments, are shown in Figure 5. These data show that 45 ± 17% of the total primary CFU-Cs were transduced. The GFP+ fraction was enriched for erythroid progenitors, indicating that erythroid progenitors were more readily transduced. CFU-GM and CFU-granulocyte/erythroid/megakaryocyte/macrophage (GEMM) appeared to be more resistant to transduction. Assessment of the CFU-C content of sort fractions expressing low, medium, and high levels of GFP indicated that the majority of transduced CFU-Cs expressed low to medium levels of GFP (Figure 5A). Cells expressing high levels of GFP had a low cloning efficiency, which suggests this fraction of cells is a more differentiated subset.
CFU-Cs in sorted AdGFP-infected cells.
After sorting for GFP expression, colony assays were performed to assess CFU-C content of the fractionated cells. (A) Values represent the total number of CFU-Cs (calculated as frequency of CFU-Cs × the percentage of cells in each fraction) in GFP+ and GFP− fractions relative to the total CFU-Cs in the control (AdGFP) population. Error bars indicate SEM from 6 independent experiments. (B) Total CFU-Cs in GFP+ fractions exhibiting low, medium, and high fluorescence in experiment 1. Values were calculated as the CFU-C frequency × the percentage of cells in each fraction. CFU-C frequency is an average calculated from triplicate plates. Error bars represent the SEM.
CFU-Cs in sorted AdGFP-infected cells.
After sorting for GFP expression, colony assays were performed to assess CFU-C content of the fractionated cells. (A) Values represent the total number of CFU-Cs (calculated as frequency of CFU-Cs × the percentage of cells in each fraction) in GFP+ and GFP− fractions relative to the total CFU-Cs in the control (AdGFP) population. Error bars indicate SEM from 6 independent experiments. (B) Total CFU-Cs in GFP+ fractions exhibiting low, medium, and high fluorescence in experiment 1. Values were calculated as the CFU-C frequency × the percentage of cells in each fraction. CFU-C frequency is an average calculated from triplicate plates. Error bars represent the SEM.
Proliferation of sorted populations was also evaluated in cytokine-driven liquid media (delta culture, Figure6). Delta assay demonstrated that the proliferative ability of AdGFP cells was not different from the proliferative ability of mock-infected or GFP− cells, confirming that AdGFP transduction was not toxic to the CD34+ cells under the conditions employed. The more limited expansion of GFP+ cells in liquid culture is consistent with a lower CFU-C content in this fraction (Figure 5A).
Expansion of GFP fractionated cells in delta culture.
AdGFP-transduced and sorted cells from experiment 3 were cultured in KL, IL-3, IL-6, EPO, and G-CSF for 21 days. Each week, cells were harvested from triplicate wells and counted. [□] = Mock, [⋄] = AdGFP, [○] = GFP-, and [▵] = GFP+.
Expansion of GFP fractionated cells in delta culture.
AdGFP-transduced and sorted cells from experiment 3 were cultured in KL, IL-3, IL-6, EPO, and G-CSF for 21 days. Each week, cells were harvested from triplicate wells and counted. [□] = Mock, [⋄] = AdGFP, [○] = GFP-, and [▵] = GFP+.
We have demonstrated efficient transduction of CD34+/CD38− cells (Figure 3D). However, only a fraction of CD34+/CD38− exhibit properties of HSCs in functional assays.6,30,31 Moreover, both the CD38+ and the CD38− fraction were previously shown to contain similar numbers of long-term culture–initiating cells(LTC-ICs).32 We determined that 6.4% of CD34+ cells in an mPB sample were CD38− (Figure 3), whereas week-5 CFU-Cs within this sample and our other mPB samples were detected at frequencies of less than 1% (data not shown). To more accurately investigate transduction of primitive HPCs, sorted populations were assessed in LTC. The week-5 CFU-Cs (secondary CFU-Cs) and CAFCs contained in sorted fractions were quantified in 5 independent experiments (Table2). Week-5 CFU-Cs and CAFCs are primitive HPCs that are essentially the same as LTC-ICs. However, we do not use the term LTC-ICs since quantitations were not performed by limiting dilution analysis, as in the LTC-IC assay. The average efficiency of transduction of primitive subsets of cells are represented in Figure7A. These results demonstrate transduction of a significant number of primitive cells: 33 ± 13% of week-5 CAFCs and 18 ± 3% of secondary CFU-Cs.
Week-5 CFU-Cs and CAFCs in sorted AdGFP-infected cells.
(A) After sorting for GFP expression, long-term cultures were performed to assess primitive progenitors in the fractionated cells. Relative number of secondary CFU-Cs and CAFCs in sorted fractions, calculated as the frequency of secondary CFU-Cs or CAFCs × the percentage of cells in GFP+ [•] and GFP− [ ] fractions divided by the total secondary CFU-Cs and CAFCs in the control (AdGFP) population. (B) Average number of week-5 CAFCs (n = 6) and CFU-Cs (n = 4) for AdGFP (nonsorted) [▨] and mock-infected [□] cells. Values were calculated from data shown in Table 2 and additional data not shown. (C) The average ratio of week-5 CFU-Cs to week-5 CAFCs calculated from values shown in Table 2. Error bars indicate SEM.
] fractions divided by the total secondary CFU-Cs and CAFCs in the control (AdGFP) population. (B) Average number of week-5 CAFCs (n = 6) and CFU-Cs (n = 4) for AdGFP (nonsorted) [▨] and mock-infected [□] cells. Values were calculated from data shown in Table 2 and additional data not shown. (C) The average ratio of week-5 CFU-Cs to week-5 CAFCs calculated from values shown in Table 2. Error bars indicate SEM.
Week-5 CFU-Cs and CAFCs in sorted AdGFP-infected cells.
(A) After sorting for GFP expression, long-term cultures were performed to assess primitive progenitors in the fractionated cells. Relative number of secondary CFU-Cs and CAFCs in sorted fractions, calculated as the frequency of secondary CFU-Cs or CAFCs × the percentage of cells in GFP+ [•] and GFP− [ ] fractions divided by the total secondary CFU-Cs and CAFCs in the control (AdGFP) population. (B) Average number of week-5 CAFCs (n = 6) and CFU-Cs (n = 4) for AdGFP (nonsorted) [▨] and mock-infected [□] cells. Values were calculated from data shown in Table 2 and additional data not shown. (C) The average ratio of week-5 CFU-Cs to week-5 CAFCs calculated from values shown in Table 2. Error bars indicate SEM.
] fractions divided by the total secondary CFU-Cs and CAFCs in the control (AdGFP) population. (B) Average number of week-5 CAFCs (n = 6) and CFU-Cs (n = 4) for AdGFP (nonsorted) [▨] and mock-infected [□] cells. Values were calculated from data shown in Table 2 and additional data not shown. (C) The average ratio of week-5 CFU-Cs to week-5 CAFCs calculated from values shown in Table 2. Error bars indicate SEM.
To assess toxicity toward primitive progenitor cells, average values for week-5 CFU-Cs and CAFCs were calculated for AdGFP and mock-infected cells with the use of data shown in Table 2 and additional data (not shown). Week-5 CAFCs were quantitatively similar in mock and AdGFP cultures. However, AdGFP cultures generated fewer week-5 CFU-Cs (Figure7B). On average, there was a 40% reduction in the week-5 CFU-C content of AdGFP cultures (P < .01, Student t test). These results clearly indicate that the adenoviral infections were detrimental to primitive HPCs. The ratio of week-5 CFU-Cs to CAFCs is indicative of the proliferative potential of primitive cells. The week-5 CFU-Cs/CAFCs ratio for the AdGFP, mock, GFP+, and GFP− populations are represented in Figure 7C. In comparison with mock-infected cells, the week-5 CFU-Cs/CAFCs ratio was significantly reduced for AdGFP and GFP+ cells (P < .05, Student t test), but not significantly different for the GFP− group. Thus, adenoviral toxicity toward primitive progenitors appears to be due to reduced proliferative ability of the transduced subset.
Discussion
Toward the goal of efficient gene transfer to HSCs, we have investigated adenoviral gene transfer to CD34+ cells with emphasis on functional evaluation of transduction and toxicity. According to previous reports, cytotoxicity, resulting from exposure to high concentrations of adenoviral vectors, presents a limitation to the extent of gene transfer to CD34+ cells.15,21,22In the present report, we confirm that freshly isolated CD34+ cells exposed to adenoviral vectors at MOIs of 500 or greater have a reduced cloning efficiency and undergo cell death. These toxic effects were observed when progenitor cells were cultured in the absence of cytokines (data not shown) or in the presence of KL alone during adenoviral vector transduction. The adverse effects of adenoviral infection observed in short-term assays were minimized by culturing progenitor cells in a combination of 4 cytokines: KL, FL, IL-1, and IL-3. Culture in the 4-cytokine combination did not affect the vector-transduction efficiency, but rescued CD34+ cells from cell death and thereby enabled infection at high MOIs, resulting in maximal absolute transduction. When CD34+ cells were transduced at an MOI of 2000 with either AdGFP or AdNull in the presence of the 4-cytokine combination, there was no reduction in cloning efficiency or expansion ability in liquid culture and no change in cell cycle status. The exact cytokine or cytokines responsible for this protective effect warrant further investigation. In preliminary experiments, we found that only the 4-cytokine combination—not a 2- or 3-cytokine cocktail—rescued the cells from adenovirus-associated toxicity (data not shown). Previous reports indicate that in other cell types, adenovirus infection induces cell cycle arrest.24,25 It is plausible that the 4-cytokine combination, which very effectively induces cell cycling, overrides a growth-inhibitory effect in HPCs. In contrast to multiple-cytokine cocktails, KL alone protects progenitors from apoptosis and does not induce cell cycling.33 34
Unfortunately, the 4-cytokine combination failed to protect more primitive progenitors from adenoviral toxicity. Our investigations revealed that primitive progenitors, quantified as week-5 CAFCs, had a diminished cloning efficiency following exposure to adenovirus at an MOI of 2000. This was reflected as a 40% reduction in secondary CFU-Cs and a lower ratio of week-5 CFU/CAFCs for AdGFP compared with mock-infected cells. Furthermore, these antiproliferative effects were apparent only in the transduced subset (GFP+) of the infected cells, indicating that toxicity was a direct consequence of adenoviral transduction. Perhaps alternative cytokine combinations will be required to protect primitive HPCs from the adverse effects of adenovirus infection. For example, inclusion of thrombopoietin, which is a very potent early-acting cytokine,35 may be useful toward this goal. In relation to toxicity toward primitive HPCs, it may also be useful to investigate the effects of further modified adenoviruses, such as vectors with E4 deletion. The E4 region was recently shown to be responsible for the growth-inhibitory effect of adenovirus in endothelial cells.25
Although adenoviral vectors can efficiently transduce nonproliferating cells, it is conceivable that proliferating cells express higher levels of the cell surface receptors and integrins that facilitate adenoviral attachment and entry. In an attempt to increase adenoviral transduction of CD34+ cells, other investigators prestimulated CD34+ cells with cytokine combinations that included IL-3, granulocyte–macrophage colony stimulating factor and macrophage–colony stimulating factor.36Those investigators reported up-regulation of αvβ3 and αvβ5 integrins, but no significant increase in transduction efficiency was noted for the prestimulated cells. Similarly, we found that 72 hours' prestimulation with KL, FL, IL-1, and IL-3 had no significant effect on transduction efficiency (data not shown). Thus, it appears that the singular role of cytokines in adenoviral transduction of CD34+ cells may be to protect the cells from toxicity.
At an MOI of 2000 and in the presence of KL, FL, IL-1, and IL-3, up to 79% of the total CD34+ cells from mPB were transduced. However, there was great variation in the percentage of cells that were transduced under these conditions. The average transduction efficiency at an MOI of 2000 was 49 ± 17%. The variability in transduction efficiency appeared to be due to differences in the patient samples since consecutive leukapharesis samples from the same patient were transduced with fairly reproducible efficiency (data not shown). It would be of interest to further explore variations in susceptibility to adenovirus infection in search of a correlation with sample or patient characteristics.
Adenoviral transduction of HPCs is a controversial issue.37On the basis of an observation that bone marrow cells were seemingly resistant to adenoviral infection at MOIs that efficiently transduce tumor cells, adenoviral vectors were proposed as being useful for purging bone marrow of tumor cells.38-40 Those studies are in apparent contrast to the present investigations and previous reports that demonstrated transduction of CD34+cells.15,21,22 This discrepancy is probably a reflection of the relatively high MOIs required for efficient transduction of CD34+ cells. Thus far, attempts to demonstrate adenoviral transduction of more primitive subsets of progenitor cells are limited to phenotypic analyses.21,22 In those studies, infected CD34+ cells were cultured for a few days before tri-color FACS analysis was performed to simultaneously resolve CD34, CD38, and transgene expression. Results from those investigations suggest that transduction of CD38− cells parallels transduction of the total CD34+ cells. However, those results may overestimate transduction of HSCs since (1) cultured cells with the CD34+CD38− are not necessarily stem cells because the CD38 antigen is modulated in culture7 and (2) multicolor FACS analysis poses considerable technical challenges and false-positive results may ensue. Thus, to demonstrate transduction of the CD34+/CD38−subset of mPB cells, we took a slightly different approach, in which the transduction was performed on a freshly purified CD34+/CD38− subset. The results shown herein support the conclusion that transduction of CD34+/CD38− population is similar to transduction of total CD34+ cells at MOIs of 100 to 1000. At an MOI of 5000, transduction was slightly higher for the total CD34+ cells compared with the CD34+/CD38− fraction, suggesting that there may be a limit to the percentage of CD38−progenitors that can be transduced. Nonetheless, these results demonstrate relatively efficient transduction of CD34+/CD38− cells and contrast with data from retroviral transductions whereby transduction of CD38− subsets is approximately fivefold less than transduction of the overall CD34+ population.41
Although substantial evidence indicates that CD34+/CD38− cells are enriched for primitive progenitors,6,30,31 a recent report suggested that LTC-ICs and repopulating cells of nonobese diabetic mice with severe combined immunodeficiency disease are similarly distributed between CD38+ and CD38−fractions of CD34+ cells.32 Therefore, in order to more accurately quantify adenoviral transduction of primitive hematopoietic cells, we functionally analyzed primary CFU-Cs as well as week-5 (secondary) CFU-Cs and CAFC content of FACS-sorted transduced cells. In sorting experiments, where the overall CD34+ cell transduction frequency was around 54%, approximately 45 ± 17% of the input primary CFU-Cs, 33 ± 13% of week-5 CAFCs, and 18 ± 3% of secondary CFU-Cs were detected in GFP+fractions following FACS sorting. However, since AdGFP infection inhibited proliferation of primitive cells, we consider our secondary CFU-C results an underestimate of transduction. The percentage of week-5 CFU-Cs that were transduced is probably closer to the transduction efficiency enumerated for CAFCs.
The frequency of transduction of CAFCs indicated that the more primitive progenitor cells were not as efficiently transduced as the total progenitor pool. These results are not entirely consistent with the results from the CD34+/CD38−experiment and suggest that the CD34+/CD38− analysis may overestimate the efficiency of transduction of primitive hematopoietic progenitor cells. The frequency of adenoviral vector transduction of CAFCs shown herein compares favorably with reports demonstrating inefficient retroviral transduction of primitive hematopoietic cells.1-4 This may be due partially to the ability of adenoviral vectors to transduce nondividing cells. In contrast to adenoviruses, transduction by MLV-type retroviral vectors (which are to date the most commonly employed retroviral vectors) is strictly dependent upon timely cell division.8,9 Also, expression of adenoviral receptors may be less restricted than retroviral receptors on primitive hematopoietic cells.12
Adenoviral vectors would be most useful for HSC transductions where transient, high-level transgene expression is required. For example, adenoviral vectors could be employed to express cytokines or cytokine receptors to manipulate HSC self-renewal or differentiation during ex-vivo expansion or bone marrow transplantation. Adenoviral vectors could also be used to express retroviral receptors on CD34+ cells.42 Retroviral transduction of CD34+ cells via an adenovirally expressed receptor would exploit the advantages of both retroviral and adenoviral transductions; primitive cells would be efficiently transduced by the adenoviral vector, and the retroviral transgene would be integrated into the host cell genome and therefore passed on to all daughter cells. The present investigations provide important information for the application of adenoviral vectors in these applications. We have described herein means for minimizing some of the toxicity associated with adenoviral infection of HPCs and provide the first functional and quantitative evidence of adenoviral transduction of primitive HPCs. However, in order for adenoviral vectors to become a safe option for HSC gene therapy, future investigations should focus on protecting primitive progenitors from the antiproliferative effects of adenoviral transduction.
Acknowledgments
We thank Ms Dianna Ngok for invaluable technical assistance and Drs Shaheen Rafii and Beat Fry for helpful discussion and advice.
Supported by the Gar Reichman Fund of the Cancer Research Institute, National Institutes of Health Cancer Center Support grant CA-08748, and Memorial Sloan-Kettering Cancer Center (M.A.S.M.).
Reprints:Malcolm A. S. Moore, James Ewing Laboratory of Developmental Hematopoiesis, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Box 101, New York, NY 10021; e-mail:m-moore@ski.mskcc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Fig. 6. Expansion of GFP fractionated cells in delta culture. / AdGFP-transduced and sorted cells from experiment 3 were cultured in KL, IL-3, IL-6, EPO, and G-CSF for 21 days. Each week, cells were harvested from triplicate wells and counted. [□] = Mock, [⋄] = AdGFP, [○] = GFP-, and [▵] = GFP+.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.100/4/m_bloo01341006x.jpeg?Expires=1767836557&Signature=EqAvzyISluJI8QrImCwCbUj9oPqTMkuEeYVZw~tY7Z7-Ig0cUBNBImgoS-nlY4SzTt75JhP3mAsnOARcn8RPDL7t~yn~NCvyBNTNqzEZlJzFhvnBVkpAeKjn2l48KUNUI6kmNGK33BoAXlYMJd9LLX61A-wGCkAU15gkR1Lc5eAq5s7OIcK5SzsJHWu0DQZpuCivUbURvaB24fQYp1Gzu287icF3slqum2tRBIZatKYMUPro~miHUTACfZyW2LwYL6Xnl86f3QsJ0nl~XkA1mAfgNtP46vMd9fx~LsKeaEOlFgyqK67xf6-g-otUJYLW8ZkuBiUtKRiAtVmaOoU7lg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Week-5 CFU-Cs and CAFCs in sorted AdGFP-infected cells. / (A) After sorting for GFP expression, long-term cultures were performed to assess primitive progenitors in the fractionated cells. Relative number of secondary CFU-Cs and CAFCs in sorted fractions, calculated as the frequency of secondary CFU-Cs or CAFCs × the percentage of cells in GFP+ [•] and GFP− [] fractions divided by the total secondary CFU-Cs and CAFCs in the control (AdGFP) population. (B) Average number of week-5 CAFCs (n = 6) and CFU-Cs (n = 4) for AdGFP (nonsorted) [▨] and mock-infected [□] cells. Values were calculated from data shown in Table 2 and additional data not shown. (C) The average ratio of week-5 CFU-Cs to week-5 CAFCs calculated from values shown in Table 2. Error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.100/4/m_bloo01341007x.jpeg?Expires=1767836557&Signature=mbpLJDNpArZeBgRfu2leIiSBWMEaPC7Je9DWzU7ftLb6c2Gu1aLR9zXZvsD4xYCdxgrHzHxpIoGTIi4Nf3y6pvxfXxvki5hPj31MBUBWK3mh5Vqcj62nepz7zZvzpLZ5mORWttVQIqCq1OPf~b7Y8Jz~6OdmEtErYgJNAwjVJ2OvHkLjO8vJOKcyoniNG~hgci-KzFV1cQi8y-IOznIOLfXhBVl2xZLQrwl1PLArBYmEzIPrTEZ1chm4lbWdbGN5tCE5fjjXkgteFW49kZw9hW~Y6KQ1mdz-cT5ZLEcleD4GxwuoeHx2yj-2uvsGspC3IX-3uFupS-bZFnrF8~I1lg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 6. Expansion of GFP fractionated cells in delta culture. / AdGFP-transduced and sorted cells from experiment 3 were cultured in KL, IL-3, IL-6, EPO, and G-CSF for 21 days. Each week, cells were harvested from triplicate wells and counted. [□] = Mock, [⋄] = AdGFP, [○] = GFP-, and [▵] = GFP+.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.100/4/m_bloo01341006x.jpeg?Expires=1768204202&Signature=GYGrpEoxVynRIx1c5RfHL9YC8FyNwS35H2jxsMIqQd12olPPCRQaI9i6ZlRDyNGJsyy8END4dUvID~vL2dl4XIChIcUVOZXuuG-hl3bero-GhPZi5-MoajKRmQ~Zmw6pudvXcRR6z187gRpHucs8P5C2w9jkmZbdTdAjzeqRqORcFiOLA7rszK9pTtvfEuJPmZMVVGLRtWuZhpWNVFOZr9Cho50gRsY2IgA8cLFXkRNcFlFgq5TjDOWW39~BMsCzFKw3iooUotiYohYgjKhfqf-V0G0DHp4VhDhOtIrtEcnx0vYKz8LC-1ww2V1PQ5M8Y1KynYKrlUlaQdwW4LNItw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Week-5 CFU-Cs and CAFCs in sorted AdGFP-infected cells. / (A) After sorting for GFP expression, long-term cultures were performed to assess primitive progenitors in the fractionated cells. Relative number of secondary CFU-Cs and CAFCs in sorted fractions, calculated as the frequency of secondary CFU-Cs or CAFCs × the percentage of cells in GFP+ [•] and GFP− [] fractions divided by the total secondary CFU-Cs and CAFCs in the control (AdGFP) population. (B) Average number of week-5 CAFCs (n = 6) and CFU-Cs (n = 4) for AdGFP (nonsorted) [▨] and mock-infected [□] cells. Values were calculated from data shown in Table 2 and additional data not shown. (C) The average ratio of week-5 CFU-Cs to week-5 CAFCs calculated from values shown in Table 2. Error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.100/4/m_bloo01341007x.jpeg?Expires=1768204202&Signature=tXx9QFpwkAD2zEU17AlTpflVqnrgwJ2YnYEtdVK4XXa35O6dO~y16WHG7kfZE43IsUrpW1yhj1VJUSNkgbk4GjL06Kjg-24QVn4Bqq-VSY~QPkdFjZmEF7-WZ1XzI73RUV0Yl79rcZEMWecQSm5fwoH2CbOIoSfSTIh0MFAb5z7ITc45M0agA0RsU8tdCfpuypW7eM0FnYIhpdhWw2jZY7Kbd03giUsvwm1y98mWW2kfmxOw6ZzAWInAXz~k4t4RKtPURTuhMIkfHN7aOA5gpGKNHFS1oKNnUOG1HMJdJKoZrxpmT-ngUTPAXSbWt3aaQ8ZlT9RWqFLTMST-bXEHFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
 ] fractions divided by the total secondary CFU-Cs and CAFCs in the control (AdGFP) population. (B) Average number of week-5 CAFCs (n = 6) and CFU-Cs (n = 4) for AdGFP (nonsorted) [▨] and mock-infected [□] cells. Values were calculated from data shown in Table
] fractions divided by the total secondary CFU-Cs and CAFCs in the control (AdGFP) population. (B) Average number of week-5 CAFCs (n = 6) and CFU-Cs (n = 4) for AdGFP (nonsorted) [▨] and mock-infected [□] cells. Values were calculated from data shown in Table