Abstract
Dendritic cell (DC) precursors and immature DC reside in epithelium where they encounter pathogens and cytokines, which stimulate their differentiation. We hypothesized that type-I interferons (IFN- and -β), cytokines that are produced early in the innate immune response against viruses and some bacteria, may influence DC differentiation and function. To examine this possibility, we used an in vitro model of DC differentiation in which initial culture of human CD14+monocytes with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 generates immature DC, and subsequent culture with tumor necrosis factor (TNF)- drives the final development into mature DC. We found in this model that IFN-/β, added from the initiation of the culture on, significantly reduced the survival and altered the morphology and differentiation of DC. TNF-–dependent maturation of IFN-β–treated immature DC led to cells with reduced expression of CD1a, CD40, CD54, and CD80 when compared with mature DC controls. IFN-/β–treated DC further had a reduced capacity to induce naive Th-cell proliferation through allostimulation or anti-CD3 monoclonal antibody stimulation. In addition, IFN-/β–treated DC secreted less IL-12 upon stimulation with Staphylococcus aureus Cowan strain or with CD4+ T cells, and this decrease correlated directly with their inability to support CD4+ T-cell secretion of IFN-γ, even though T-cell lymphotoxin production was unaffected. These findings indicate that type-I IFNs can influence the generation of acquired immune responses by modifying T-helper cell differentiation through the regulation of DC differentiation and function.
Dendritic cells (DC) are highly specialized antigen-presenting cells (APC) required for primary T-cell activation and T-cell–dependent immunity.1 Immature DC reside within nonlymphoid tissues, where they actively capture and process antigen and where contact with proinflammatory cytokines and bacterial products induces their maturation. Upon activation, DC migrate via the afferent lymphatics from peripheral tissues, such as the skin and intestine, to secondary lymphoid tissues.2 Mature DC relocate to the T-cell zone of lymph node parenchyma where they reside as potent stimulators of naive T cells, presumably because of their high expression of costimulatory molecules and production of cytokines such as interleukin (IL)-12.2 3 CD4+ T cells that recognize their specific antigen in the context of major histocompatibility complex (MHC) class II presented on DC are retained in the lymph node, where they undergo expansion and differentiation. Unstimulated CD4+ T cells migrate through the medullary sinus and back into the blood.
In recent years, several cytokines have been identified that support in vitro maturation of DC from bone marrow or blood-derived progenitor cells. CD14+ mononuclear cells isolated from peripheral blood and cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 are efficient APC with morphology and cell surface molecule expression typical of immature DC.4 Alternatively, CD34+ progenitors cultured with IL-3 or stimulated through CD40 also differentiate into functional immature DC.5,6 In both instances, tumor necrosis factor (TNF)-α induces, in vitro and in vivo, further DC maturation and significantly enhances the capacity of DC to stimulate resting T cells by increasing the expression of costimulatory molecules and cytokines, promoting migration of DC to draining lymph nodes, and down-regulating DC antigen capture and processing.4,7 Other factors that can similarly drive DC maturation include lipopolysaccharides and IL-1.8 9 Thus, pathogen-derived components and cytokines produced at sites of infection by the innate immune response can contribute to DC maturation and, as a result, regulate the ability of DC to stimulate naive T cells in draining lymph nodes.
Our laboratory has been interested in how type-I IFNs (IFN-α and -β) may modulate DC development and, as a consequence, influence the generation of acquired immune responses by altering T-helper cell differentiation. Of particular relevance to our interest is that IFN-β is now a standard treatment for patients with multiple sclerosis,10,11 which leads to the question of what immunomodulatory effects a systemic increase in IFN-β has on the generation of DC. IFN-α/β are produced by many cell types, including macrophages, T cells, keratinocytes, and Langerhans cells, and have a broad range of immunomodulatory effects, including inhibition of viral replication and stimulation of natural killer cell activation.12 IFN-α/β production during early stages of the innate immune response against viral infection correlates with increased viral titers and natural killer cell function in both murine models and human disease13,14 and has been implicated in promoting transient immunosuppression.15-17 The role of IFN-α/β in shaping the acquired immune function is, however, not fully understood.12 Recently, we have shown that type-I IFNs influence T-helper cell differentiation and function both by acting directly on the T cell as well as by altering the function of mature DC.18-20 Specifically, we showed that type-I IFNs regulate homing receptor expression (E-selectin ligand and L-selectin) on human CD4+ T cells18 and inhibit T-cell–mediated CD40-induced secretion of functional IL-12 heterodimer by DC.19 20 In the present work, we have extended our studies to demonstrate that type-I IFNs influence the generation of monocyte-derived DC in vitro by reducing the number of cells that will differentiate into mature DC and altering the function of immunocompetent human DC.
Materials and methods
In vitro differentiation of DC
CD14+ monocytes were isolated from peripheral blood by counterflow centrifugal elutriation21 and frozen at 4 × 107 cells/mL. Cells were thawed as needed and cultured in 6-well tissue culture plates (Costar, Cambridge, MA) at approximately 5 × 106/mL in complete culture medium (RPMI-1640 [Gibco/BRL, Gaithersburg, MD] supplemented with 10% fetal bovine serum, 20 mmol/L L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin [BioWhittaker, Walkersville, MD]). IL-4 (biologic activity 108 IU/mg; Pharmingen, San Diego, CA) and GM-CSF (biologic activity 2 × 108 IU/mg; Pharmingen) were added to the cells at 30 ng/mL on day 1, day 4, and day 7 of the culture. Some wells also received recombinant human (rh)IFN-β-1a (biologic activity 2 × 108 IU/mg; provided by Biogen, Cambridge, MA) or IFN-αA (biologic activity 2 × 108 IU/mg; Biosource, Camarillo, CA) on days 1 and 4 of culture at concentrations indicated. On day 6 of culture, TNF-α (Pharmingen) was added at 100 U/mL. Cells were harvested on day 10 of culture with versene (EDTA) (Biowhittaker), washed twice with Ca/Mg-free phosphate-buffered saline (PBS), and used immediately in functional assays. Viable cell count was determined by trypan blue exclusion. Monocytes were cultured for 48 hours in complete medium without exogenous cytokines, washed, and used immediately in functional assays.
Isolation of CD4+, CD45RA+, CD45RO− T cells
Human peripheral blood mononuclear cells from buffy coats of anonymous healthy donors (Life Source, Glenview, IL) were isolated by Ficoll gradient centrifugation. Resting CD4+CD45RA+CD45RO− T cells were obtained by negative selection with antibodies and magnetic beads, as described.22 CD45RA+ cells were greater than 95% pure by flow cytometric analysis using mouse monoclonal antibody (mAb): CD45RA-phycoerythrin (PE) (clone B-C15; Biosource) and CD45RO-FITC (fluorescein isothiocyanate) (clone UCHL1; Caltag, South San Francisco, CA). Staining of cells with antibodies was performed according to standard procedures, as described previously,22 and cells were evaluated using a FACScan (Becton Dickinson, San Jose, CA).
CD4+ T-cell activation
Naive CD4+ T cells (5 × 105/well) were cultured with allogeneic irradiated DC (5 × 104/well) in a volume of 1 mL of complete culture medium for 48 hours in 48-well plates (Costar), which had been coated overnight at 4°C with 0.25 mL of 1 μg/mL humanized αCD3 mAb (h)OKT3 (CDR grafted on human IgG1 23) in PBS. At the end of the 48-hour stimulation period, the T cells were resuspended and transferred to 6-well plates (Costar), and 1 mL of fresh medium was added. Seven days after the initial stimulation, T cells were counted and restimulated under identical conditions as the primary stimulation, using fresh DC derived from the same donor used in the primary stimulation. Supernatants were harvested after the 48-hour secondary stimulation for analysis of cytokine production.
Enzyme-linked immunosorbent assays
Pairs of mAbs (Pharmingen) were used in sandwich enzyme-linked immunosorbent assays (ELISAs) to measure lymphotoxin (LT) (sensitivity 0.1 ng/mL), TNF-α (sensitivity 0.2 ng/mL), IFN-γ (sensitivity 0.2 ng/mL), p40 chain of IL-12 heterodimer (sensitivity 0.2 ng/mL), and p35/p40 (p70) heterodimer of IL-12 (sensitivity 0.1 ng/mL). MaxiSorp 96-well plates (Nunc Inc., Naperville, IL) were coated with capture mAbs (1-4 ng/mL) overnight at 4°C. The following day, plates were washed and blocked with 3% bovine serum albumin in PBS at room temperature for 2 hours. The plates were subsequently washed, and standards and samples were added to the wells and incubated overnight at 4°C. Biotinylated secondary mAb (1-3 μg/mL), avidin-peroxidase (Sigma, St. Louis, MO), and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS; Sigma) were used to quantify cytokine levels, as per the Pharmingen protocol.
CD4+ T-cell proliferation
Proliferation assays were performed using standard techniques, as described.24 Briefly, purified naive CD4+ T cells (5 × 104 cells/well) were cultured in complete culture medium in 96-well flat-bottom tissue culture plates (Costar) with irradiated DC or irradiated monocytes. Seventy-two hours later, cultures were pulsed with 25 μL/well of [3H]thymidine solution (5 mCi/mL, 2 mCi/mmol specific activity; New England Nuclear, Boston, MA) and harvested 24 hours later on glass fiber filters. For mixed lymphocyte cultures, purified naive CD4+ T cells (1 × 105 cells/well) were cultured in complete culture medium in 96-well round-bottom tissue culture plates (Costar) with allogeneic DC and were pulsed during the last 24 hours of a 6-day culture with 25 μL/well of [3H]thymidine solution. Incorporation of radioactive label was measured by a liquid scintillation counter. Results are expressed as the arithmetic mean counts per minute of triplicate cultures. Accessory cell–independent CD4+ T-cell proliferation was obtained by stimulation with the combination of phorbol myristate acetate (PMA) (1 ng/mL) and phytohemagglutinin (PHA)-M (Life Technologies, Gaithersburg, MD). Anti-CD80 (IF1) and anti-CD86 (3D1), both generously provided by Genetics Institute (Cambridge, MA), were used at a final concentration of 10 μg/mL.
Flow cytometry
The murine antihuman mAbs used to stain DC included: anti-CD1a clone B-B5 (Biosource), anti-CD14 clone B-A8 (FITC conjugated; Biosource), anti-CD40 clone EA-5 (a gift from Dr T. Lebien, University of Minnesota, Minneapolis, MN), anti-CD54 (intercellular adhesion molecule [ICAM]-1) clone 84H10 (a gift from Dr P. Mannoni, INSERM U 119, Marseille, France), anti-CD80 clone BB1 (FITC conjugated; Pharmingen), anti-CD86 clone IT2.2 (FITC conjugated; Pharmingen), anti-MHC class I clone 5H7 (a gift from Dr S. Woodle, University of Chicago, Chicago, IL), antiMHC class II clone IVA12 (a gift from Dr J. D. Capra, University of Texas Southwestern Medical Center, Dallas, TX), and anti-CD83–FITC (Immunotech, Marseille, France). Isotype controls were used either unconjugated (ICN, Costa Mesa, CA) or directly conjugated to FITC (Ancell, Bayport, MN). Staining of cells with antibodies was carried out according to standard procedure, as described previously,22 and the cells were evaluated using a FACScan. Propidium iodide (PI) was used to exclude dead cells from analysis.
Light microscopy photographs
Monocyte-derived DC were generated as described earlier. Cells were harvested on day 10 of culture and transferred with culture supernatant to fibronectin-coated (20 μg/mL; Life Technologies) glass chambers (Bioptech, Butler, PA) for overnight culture. The next day, differential interference contrast images were obtained with a Zeiss Axiovert TV 100 microscope (Thornwood, NY) using a × 63 NA 1.4 Plan-Apochromat oil objective and a 12-bit Micromax 1300 Y CCD camera (Princeton, Raleigh, NC). Digital images were subsequently converted into TIFF format and imported into Adobe Photoshop 4.0 (San Jose, CA) for editing and printing.
Induction of IL-12 secretion from DC
Naive CD4+ T cells (5 × 105) were cultured in a volume of 2 mL of complete culture medium with 5 × 104 irradiated allogeneic DC,DC–IFN-α, or DC–IFN-β for 48 hours in 24-well plates (Costar), which had been coated overnight at 4°C with 0.5 mL of 1 μg/mL of αCD3 mAb OKT323 in PBS. In some experiments, DC or DC–IFN-β (5 × 105) were stimulated for 48 hours with 0.1%Staphylococcus aureus Cowan strain (SAC) (Sigma).
Statistical analysis
Variations among culture conditions were examined by pairedt test.
Results
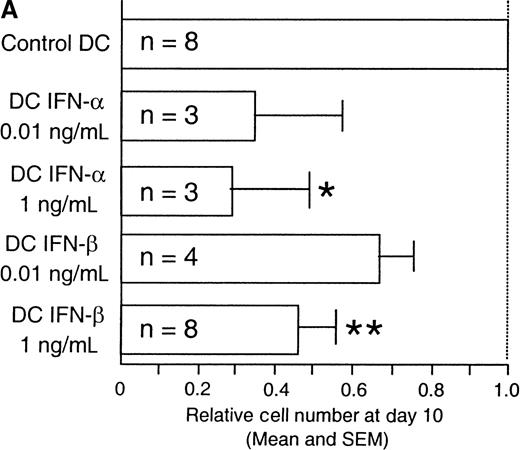
IFN- and -β reduce cell recovery of monocyte-derived DC
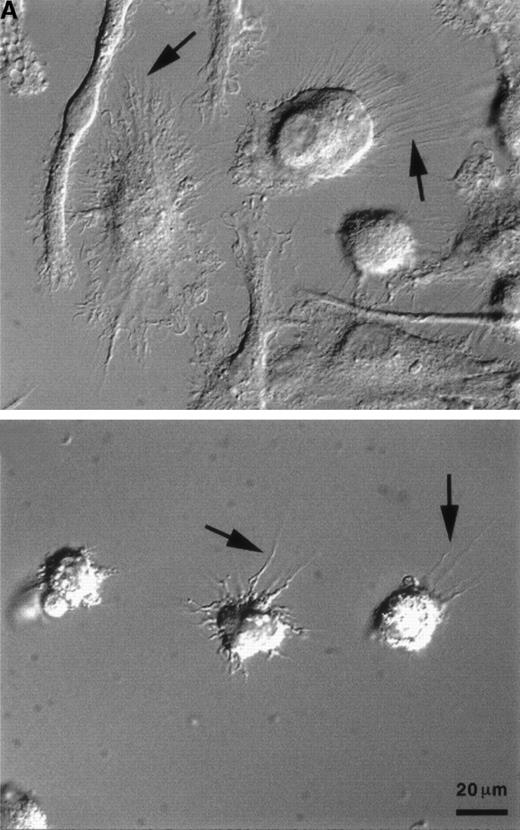
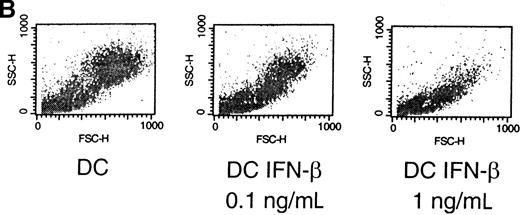
The cytokine environment at the time of primary T-cell activation has been shown to regulate Th-cell differentiation.25 We postulated that the cytokines generated during the innate immune response may similarly contribute to the environment that influences DC maturation, migration, and function. To test this hypothesis, we generated functional DC in vitro in the presence or absence of IFN-α or -β. Immature DC were generated by culturing CD14+-elutriated monocytes for 10 days in the presence of GM-CSF and IL-4. Addition of TNF-α on day 6 induced further DC maturation and resulted in large adherent cells with multiple dendritic processes making up the “veils” that are characteristic of mature DC (Figure 1A, top). The large majority of cells cultured with GM-CSF, IL-4, TNF-α, and 1 ng/mL IFN-α (referred to as DC–IFN-α) (data not shown) or IFN-β (referred to as DC–IFN-β) (Figure 1A, bottom) did not spread out as much as the control DC and had a much reduced or even absent veiled appearance. Furthermore, fluorescence-activated cell sorter analysis of both forward scatter and side scatter showed a pattern for DC–IFN-α and -β that was clearly distinct from control DC (Figure1B and data not shown). Most strikingly, there was a dose-dependent reduction in the number of viable cells recovered (as determined by trypan blue exclusion) at day 10 from DC generated with either IFN-α or IFN-β compared with control DC (Figure2A). The reduced cell number in IFN-α and -β cultures was thought most likely to reflect a decrease in DC survival and not inhibition of DC expansion because DC generated from monocytes do not undergo much cell division in our culture conditions. To test this possibility, we further analyzed the IFN-α/β–induced decrease in viable DC number by performing flow cytometric studies following PI staining of cells taken directly out of culture. This “no wash” analysis did not use any centrifugation step, and therefore it avoided the possible preferential loss of dead cells that may occur during a wash step. Using this method, we found that although the viability of IFN-β–treated DC (cultured at 1 ng/mL) was never less than 80% at day 10, it was always slightly and significantly decreased compared with control DC (Figure 2B). The mean percentages of PI-positive–staining cells at day 10 were 12.5% and 8.0%, respectively (n = 5, P < .05). Thus, it is possible that the large reduction in day-10 recovery in DC–IFN-β might have resulted from the cumulative effects of a decreased viability of IFN-α– and -β–treated DC.
Type-I IFNs alter the morphology of DC derived from CD14+ precursors.
Mature DC were produced in vitro by culture with GM-CSF, IL-4, and TNF-α, as described in Materials and methods. IFN-β was added to the wells at a final concentration of 1 ng/mL on days 1 and 4 of culture. (A) Photographs of DC were taken on day 10 of culture, as described in Materials and methods: control DC (A, top), IFN-β–cultured DC (A, bottom). Arrows indicate dendritic processes within veils, and the 20-μm reference bar is identical for both panels. (B) On day 10 of culture, cells were harvested and subjected to flow cytometric analysis to evaluate light-scattering properties by forward (x-axis) and side (y-axis) scatter. Nonviable cells were eliminated from analysis using propidium iodide. Results are representative of 5 independent experiments. IFN-β was added at days 1 and 4 of culture at the indicated concentrations.
Type-I IFNs alter the morphology of DC derived from CD14+ precursors.
Mature DC were produced in vitro by culture with GM-CSF, IL-4, and TNF-α, as described in Materials and methods. IFN-β was added to the wells at a final concentration of 1 ng/mL on days 1 and 4 of culture. (A) Photographs of DC were taken on day 10 of culture, as described in Materials and methods: control DC (A, top), IFN-β–cultured DC (A, bottom). Arrows indicate dendritic processes within veils, and the 20-μm reference bar is identical for both panels. (B) On day 10 of culture, cells were harvested and subjected to flow cytometric analysis to evaluate light-scattering properties by forward (x-axis) and side (y-axis) scatter. Nonviable cells were eliminated from analysis using propidium iodide. Results are representative of 5 independent experiments. IFN-β was added at days 1 and 4 of culture at the indicated concentrations.
Dose-dependent reduction in viable cells with type-I IFNs.
(A) Coculture with type-I IFNs reduced cell recovery of monocyte-derived DC. Relative viable cell recovery of DC at day 10 of culture is presented. Type-I IFNs were added at days 1 and 4 of culture at the indicated concentrations. n = number of independent experiments; *P < .05, **P << .01 as determined by pairedt test. (B) IFN-β induced a small but significant decrease in cell viability after 10 days of culture. On day 10 of culture, cells were harvested and subjected to flow cytometric analysis to evaluate the percentage of dead cells with propidium iodide (PI) staining. Numbers indicate the percentage of PI-positive cells. Results are 2 representatives of 5 independent experiments.
Dose-dependent reduction in viable cells with type-I IFNs.
(A) Coculture with type-I IFNs reduced cell recovery of monocyte-derived DC. Relative viable cell recovery of DC at day 10 of culture is presented. Type-I IFNs were added at days 1 and 4 of culture at the indicated concentrations. n = number of independent experiments; *P < .05, **P << .01 as determined by pairedt test. (B) IFN-β induced a small but significant decrease in cell viability after 10 days of culture. On day 10 of culture, cells were harvested and subjected to flow cytometric analysis to evaluate the percentage of dead cells with propidium iodide (PI) staining. Numbers indicate the percentage of PI-positive cells. Results are 2 representatives of 5 independent experiments.
IFN-β–treated monocyte-derived DC express less CD1a, CD40, CD54, and CD80
Although the finding that type-I IFNs can influence cell survival is of considerable interest, this study focused on defining the functional properties of the viable DC remaining in culture. The cell surface expression of viable DC recovered after 10 days in culture was analyzed to identify any phenotypic changes due to the presence of IFN-β. Elutriated monocytes (CD14+, CD1a−) expressed high levels of MHC class I and II, showed low levels of ICAM-1, and were negative for CD40, CD80, CD86, and CD83 (data not shown). As reported previously, monocyte-derived mature DC (CD14−, CD1a+) expressed high levels of ICAM-1, CD40, and CD86, and intermediate levels of CD80.4 Interestingly, although DC–IFN-β similarly lost CD14 expression, they failed to uniformly up-regulate CD1a (Figure3). Levels of ICAM-1 and CD40 were consistently lower on DC–IFN-β than on DC derived in the absence of IFN-β. Furthermore, IFN-β prevented optimal up-regulation of CD80 while inducing a slightly higher expression of CD86, a costimulatory molecule associated with mature DC (Figure 3).26 Expression of MHC class II and the mature DC marker CD8327 was not altered by culture with IFN-β (Figure 3). The above described changes in surface phenotype by coculture with IFN-β were observed only at the higher concentration of 1 ng/mL (Figure 3; compare upper and lower panels). Interestingly, although at the lower (0.1 ng/mL) concentration of IFN-β, no distinct cell surface phenotype was observed, some of the functional effects can still be seen (see Results).
Cell surface phenotype comparison of DC and DC–IFN-β generated with 0.1 ng/mL or 1 ng/mL IFN-β.
DC and DC–IFN-β were harvested on day 10 of culture, washed twice, and prepared for flow cytometry as described in Materials and methods. Light lines represent staining of DC generated in the absence of exogenous IFN-β, whereas bold lines indicate staining of DC–IFN-β generated with 0.1 ng/mL or 1 ng/mL IFN-β, as indicated. Unlabeled isotype controls are depicted in upper left corner panels, and direct FITC-conjugated isotype control for CD83 staining is depicted in the panels directly left of CD83. Specificity of the staining with specific mAbs is as indicated below the panels. Results are representative of 5 to 7 independent experiments.
Cell surface phenotype comparison of DC and DC–IFN-β generated with 0.1 ng/mL or 1 ng/mL IFN-β.
DC and DC–IFN-β were harvested on day 10 of culture, washed twice, and prepared for flow cytometry as described in Materials and methods. Light lines represent staining of DC generated in the absence of exogenous IFN-β, whereas bold lines indicate staining of DC–IFN-β generated with 0.1 ng/mL or 1 ng/mL IFN-β, as indicated. Unlabeled isotype controls are depicted in upper left corner panels, and direct FITC-conjugated isotype control for CD83 staining is depicted in the panels directly left of CD83. Specificity of the staining with specific mAbs is as indicated below the panels. Results are representative of 5 to 7 independent experiments.
In summary, DC that develop in the presence of IFN-β express to a varying degree lower levels of CD1a, CD40, CD54, and CD80, but otherwise exhibit a cell surface phenotype with low CD83 and high CD86 and MHC class II that is characteristic of mature DC.
DC–IFN- and DC–IFN-β have diminished capacity to support naive CD4+ T-cell proliferation
Competent APC provide the costimulatory signals and soluble factors necessary for T-cell differentiation and expansion. We used a naive Th-cell stimulation model to determine whether IFN-α or -β treatment of DC influenced the ability of these cells to provide T-cell costimulation. In this model, immobilized anti-CD3 mAb is used specifically to trigger the TCR/CD3 complex, while DC cells added to the culture provide costimulatory signals and soluble factors. This stimulation model allows analysis of the costimulatory capacity of the DC in isolation, as compared with a direct allogeneic stimulation model, which would measure both the antigen presentation and costimulatory capacity of the DC. (It should be noted that although allogeneic DC are used in this model, any possible allospecific T-cell response induced by the DC will provide only an extremely minor contribution to antigen receptor stimulation as compared with that induced by cross-linking with immobilized anti-CD3 mAb.) Naive CD4+ T cells proliferated well in response to immobilized hOKT3 and DC and required fewer DC to support optimal proliferation than did CD4+ T cells stimulated with DC–IFN-α or –IFN-β (Figure 4A,B). DC–IFN-α and -β often could stimulate only 50% to 80% of the proliferative response observed with normal DC. When blocking mAbs were used to disrupt specific costimulatory pathways, DC–IFN-β–induced T-cell proliferation was found to be largely dependent upon CD86 costimulation, whereas both CD80 and CD86 costimulation contributed to normal DC-induced T-cell proliferation (Figure 4C). These data suggest that DC–IFN-β depend largely on CD86 to support proliferation of naive T cells.
DC–IFN- and DC–IFN-β have a decreased capacity to support naive CD4+ T-cell proliferation.
Naive CD4+ T cells were stimulated with immobilized anti-CD3 and either DC, DC–IFN-α (A), or DC–IFN-β (B) at the indicated ratios. IFN-α and IFN-β were added at indicated concentrations at days 1 and 4 of the culture. APC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to the cultures for measurement of [3H]-thymidine incorporation. After 3 days in culture, wells were pulsed with 1 μCi of [3H]-thymidine per well. Twenty-four hours later, plates were harvested and incorporated [3H]-thymidine was measured. DC-induced T-cell proliferation was significantly different from DC–IFN-β–induced and DC–IFN-α–induced T-cell proliferation at a 1:0.1 ratio (n = 4; P < .05 as determined by a paired t test). Results depicted are representative of 4 independent experiments. (C) Naive CD4+ T-cell proliferation induced by DC–IFN-β (1 ng/mL) was not dependent on CD80 costimulation. Anti-CD80 and anti-CD86 were added at the beginning of the proliferative assays at a final concentration of 10 μg/mL. Data are expressed as the percentage of inhibition of proliferation in the presence of isotype-matched control Ig at a T-cell to DC ratio of 1:0.1. Results shown are the mean and SEM of 3 independent experiments.
DC–IFN- and DC–IFN-β have a decreased capacity to support naive CD4+ T-cell proliferation.
Naive CD4+ T cells were stimulated with immobilized anti-CD3 and either DC, DC–IFN-α (A), or DC–IFN-β (B) at the indicated ratios. IFN-α and IFN-β were added at indicated concentrations at days 1 and 4 of the culture. APC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to the cultures for measurement of [3H]-thymidine incorporation. After 3 days in culture, wells were pulsed with 1 μCi of [3H]-thymidine per well. Twenty-four hours later, plates were harvested and incorporated [3H]-thymidine was measured. DC-induced T-cell proliferation was significantly different from DC–IFN-β–induced and DC–IFN-α–induced T-cell proliferation at a 1:0.1 ratio (n = 4; P < .05 as determined by a paired t test). Results depicted are representative of 4 independent experiments. (C) Naive CD4+ T-cell proliferation induced by DC–IFN-β (1 ng/mL) was not dependent on CD80 costimulation. Anti-CD80 and anti-CD86 were added at the beginning of the proliferative assays at a final concentration of 10 μg/mL. Data are expressed as the percentage of inhibition of proliferation in the presence of isotype-matched control Ig at a T-cell to DC ratio of 1:0.1. Results shown are the mean and SEM of 3 independent experiments.
A characteristic function of mature DC is their capacity to generate a mixed lymphocyte reaction. The data provided in Figure5 show that DC–IFN-β also have a reduced capacity to induce a mixed lymphocyte reaction. The day-6 proliferative response of naive CD4+ Th cells to allogeneic DC–IFN-β (1.0 ng/mL) was significantly reduced when compared with control DC (P << .01, n = 4).
DC–IFN-β have reduced capacity to induce a mixed lymphocyte reaction.
Naive CD4+ T cells were stimulated with either allogeneic DC or DC–IFN-β (0.1 ng/mL or 1 ng/mL) at the indicated ratios. DC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to cultures for measurement of [3H]-thymidine incorporation. After 5 days in culture, wells were pulsed with 1 μCi of [3H]-thymidine per well. Twenty-four hours later, plates were harvested and incorporated [3H]-thymidine was measured. Means of triplicate measurements are shown. A representative of 4 independent experiments is shown.
DC–IFN-β have reduced capacity to induce a mixed lymphocyte reaction.
Naive CD4+ T cells were stimulated with either allogeneic DC or DC–IFN-β (0.1 ng/mL or 1 ng/mL) at the indicated ratios. DC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to cultures for measurement of [3H]-thymidine incorporation. After 5 days in culture, wells were pulsed with 1 μCi of [3H]-thymidine per well. Twenty-four hours later, plates were harvested and incorporated [3H]-thymidine was measured. Means of triplicate measurements are shown. A representative of 4 independent experiments is shown.
DC–IFN- and DC–IFN-β produce decreased levels of IL-12
IL-12 is a critical cytokine secreted by DC and influencing naive Th-cell differentiation. Previous studies using our naive Th-cell differentiation model demonstrated that anti-CD3 mAb–activated naive CD4+ T cells stimulated DC secretion of the p40 subunit of the IL-12 heterodimer.19 In these studies, the induction of DC secretion of biologically active p35/p40 (p70) IL-12 heterodimer was indicated by the finding that neutralizing anti-p70 IL-12 antibodies prevented the T-cell IFN-γ secretion normally observed in this model, even though p70 itself remained below the level of detection of our ELISA.19
We determined the influence of IFN-α and -β on the capacity of DC to secrete IL-12 during Th-cell activation. Mature DC produced significant levels of p40 IL-12 when stimulated with CD4+ T cells (Figure 6A), and we observed this secretion to be significantly inhibited by the addition of IFN-α and -β during the DC differentiation process. As expected from our previous work,17 both DC and DC–IFN-α and -β failed to secrete detectable levels of p70 IL-12 in this Th-cell stimulation model (data not shown). Monocytes also did not produce detectable levels of IL-12 in this assay (Figure 6A). To be able to determine the levels of secreted p35/p40 (p70) IL-12 by DC, we stimulated the DC with 0.1% SAC for 48 hours. The results in Figure 6B show that, consistent with their lower secretion of p40 IL-12, DC–IFN-β are significantly inhibited in their capacity to secrete p70 IL-12 in comparison with control DC.
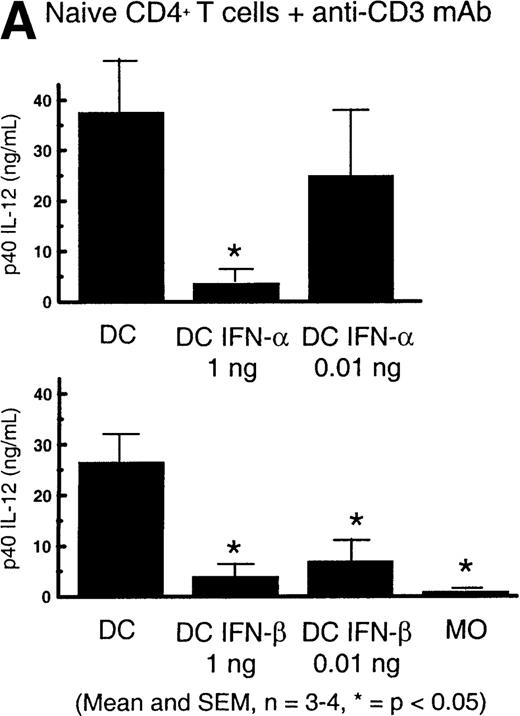
DC–IFN- and DC–IFN-β have reduced capacity to secrete IL-12.
(A) DC (5 × 104), DC–IFN-α (0.01 ng/mL or 1 ng/mL), or DC–IFN-β (0.01 ng/mL or 1 ng/mL) were cultured with 5 × 105 naive CD4+ T cells and immobilized anti-CD3. DC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to stimulation cultures. Supernatants were harvested after 48 hours of the primary stimulation. The results indicated with * show that p40 IL-12 production was significantly different from control DC p40 IL-12 production (n = 3-4, *P < .05 as determined by paired t test). Results shown are the mean and SEM. (B) DC–IFN-β had reduced capacity to produce p70 IL-12 in response to activation with 0.1% SAC. DC (5 × 104) or DC–IFN-β (1 ng/mL) were cultured with 0.1% SAC. DC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to stimulation cultures. Supernatants were harvested after 48 hours of stimulation. Results indicated with * show that p70 IL-12 production was significantly different from control DC p70 IL-12 production (n = 3; *P < .05 as determined by paired t test). Results shown are the mean and SEM.
DC–IFN- and DC–IFN-β have reduced capacity to secrete IL-12.
(A) DC (5 × 104), DC–IFN-α (0.01 ng/mL or 1 ng/mL), or DC–IFN-β (0.01 ng/mL or 1 ng/mL) were cultured with 5 × 105 naive CD4+ T cells and immobilized anti-CD3. DC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to stimulation cultures. Supernatants were harvested after 48 hours of the primary stimulation. The results indicated with * show that p40 IL-12 production was significantly different from control DC p40 IL-12 production (n = 3-4, *P < .05 as determined by paired t test). Results shown are the mean and SEM. (B) DC–IFN-β had reduced capacity to produce p70 IL-12 in response to activation with 0.1% SAC. DC (5 × 104) or DC–IFN-β (1 ng/mL) were cultured with 0.1% SAC. DC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to stimulation cultures. Supernatants were harvested after 48 hours of stimulation. Results indicated with * show that p70 IL-12 production was significantly different from control DC p70 IL-12 production (n = 3; *P < .05 as determined by paired t test). Results shown are the mean and SEM.
Thus, these and our previous results indicate that culture with IFN-α and -β during DC differentiation prevents the development of DC with full capacity to secrete functional IL-12.
DC–IFN-β have a greatly reduced capacity to provide naive Th-cell costimulation that leads to the generation of IFN-γ–secreting Th cells
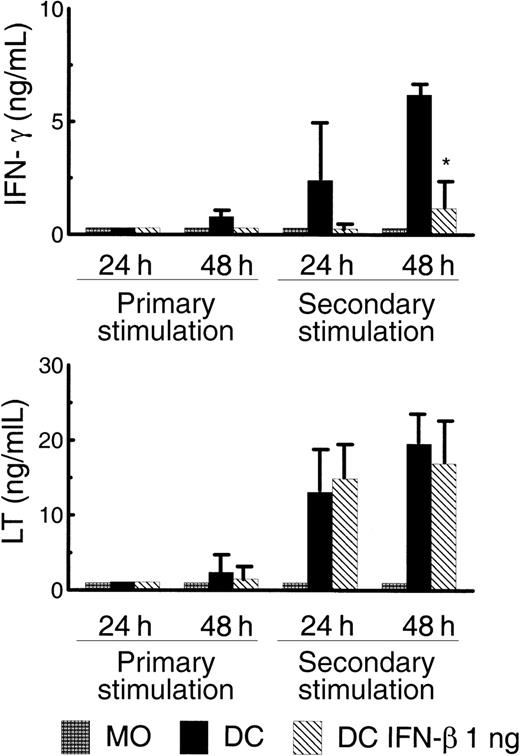
The ability of monocytes, DC, and DC–IFN-α (1 ng/mL) to support activation and differentiation of anti-CD3 mAb–stimulated naive CD4+ T cells was analyzed by measuring the T-cell cytokine secretion profile at 2 time points during primary and secondary stimulation. We previously reported that mature DC in this model generate Th cells that secrete only Th1 cytokines, such as IFN-γ and LT.19 In addition, the IFN-γ secretion observed in this model was shown to be IL-12 dependent, whereas LT secretion was found to be IL-12 independent. The results in Figure 7demonstrate that monocyte costimulation failed to generate any IFN-γ– or LT-secreting Th cells in either the primary or secondary stimulation. In contrast, mature DC costimulation supported IFN-γ production, which could be detected at low levels by 48 hours after the primary stimulation and at much higher levels after the second stimulation (Figure 7, top). DC–IFN-β could not induce IFN-γ production during primary T-cell activation, and induction of IFN-γ secretion was at significantly lower levels when compared with DC during the secondary stimulation. Of particular interest was the finding that both DC populations induced similar levels of T-cell LT secretion (Figure 7, bottom). Thus, although DC–IFN-β can induce normal levels of IL-12–independent T-cell LT secretion, they are deficient in stimulating IL-12–dependent IFN-γ secretion when compared with DC derived without IFN-β. This finding is consistent with the decreased secretion of IL-12 that was observed in this DC population (Figure 6A,B). The failure of DC–IFN-β to induce significant IFN-γ secretion did not lead to the induction of secretion of the Th2 cytokines IL-4, IL-5, and IL-13 (data not shown).
Costimulatory signals provided by DC–IFN-β are insufficient for optimal IFN-γ production by CD4+ T cells.
A total of 5 × 104 monocytes, DC, or DC–IFN-β were cultured with 5 × 105 naive CD4+ T cells and immobilized anti-CD3. APC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to stimulation cultures. Supernatants were harvested from the primary and secondary stimulations at the indicated time points. IFN-γ secretion (top) and lymphotoxin (LT) secretion (bottom) were measured by ELISA. Results indicate that DC–IFN-β induced IFN-γ production was significantly different from IFN-γ production induced by DC (n = 4; *P < .05 as determined by paired ttest). Results shown are the mean and SEM.
Costimulatory signals provided by DC–IFN-β are insufficient for optimal IFN-γ production by CD4+ T cells.
A total of 5 × 104 monocytes, DC, or DC–IFN-β were cultured with 5 × 105 naive CD4+ T cells and immobilized anti-CD3. APC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to stimulation cultures. Supernatants were harvested from the primary and secondary stimulations at the indicated time points. IFN-γ secretion (top) and lymphotoxin (LT) secretion (bottom) were measured by ELISA. Results indicate that DC–IFN-β induced IFN-γ production was significantly different from IFN-γ production induced by DC (n = 4; *P < .05 as determined by paired ttest). Results shown are the mean and SEM.
Discussion
We have found that type-I IFNs disrupt the developmental program of monocytes that is initiated by GM-CSF and IL-4, and which leads in a TNF-α–dependent manner to mature DC. This disruption appears to occur at least at 2 levels: (1) by decreasing viable cell recovery at the end of the culture, and (2) by altering the phenotypic and functional characteristics of the remaining viable cells. In regard to the first issue, a small but significantly higher (5% to 10%) level of cell death was observed at the end of culture in DC–IFN-β compared with control DC. We hypothesize that either the cumulative effects of such increased cell death over the 10-day culture period may account for the large (> 50%) decrease in viable cell recovery that occurred or that early during DC differentiation, a large reduction in viable cells occurred that is not adequately reflected in the viability at the end of the culture. The results from the phenotypic and functional studies were determined on viable cells and, therefore, the alterations induced by IFN-α and -β that were observed in these studies were likely to be caused by mechanisms independent from those resulting in decreased cell recovery. An important phenotypic alteration induced by IFN-β coculture was a change in the expression of costimulatory molecules, most notably a decrease in the up-regulation of CD1a, CD40, ICAM-1, and CD80 expression. Functionally, cells cultured under conditions normally leading to mature DC were altered by coculture with IFN-α and -β such that: (1) they provided less optimal costimulation for naive Th-cell proliferation, which for DC–IFN-β was more dependent on CD86 costimulation than for control DC; and (2) they secreted less IL-12, which led to a decrease in Th-cell IFN-γ secretion. Interestingly, both DC populations, generated with or without IFN-β, were comparable in their costimulatory capacity to induce Th-cell secretion of LT. The decrease in costimulatory capacity of DC–IFN-β to induce Th-cell proliferation was also observed in direct allogeneic responses.
The precise mechanism(s) involved in the observed IFN-α– and IFN-β–induced alterations have not been identified, but several possible pathways have been eliminated. In this model, type-I IFNs do not modulate expression of CD95 or CD95L, suggesting that Fas-mediated apoptosis is probably not responsible for the enhanced death of IFN-β–treated DC progenitors (data not shown). TNF-α potentiates DC development, at least in part through up-regulation of GM-CSF receptor.28 Consequently, we investigated the possibility that IFN-β might negatively regulate expression of this receptor, and found that IFN-β did not inhibit GM-CSF receptor levels (as determined by the CD116 staining) on the surface of DC progenitors (data not shown). We cannot, however, rule out the possibility that IFN-β may disrupt some other component of the GM-CSF signaling pathway. Similarly, we cannot exclude that the effect of coculture with type-I IFNs on DC differentiation is in part mediated through effects on other cell types present in the initial starting population of elutriated monocytes.
It has been difficult to classify definitively the cells derived in the presence of IFN-β. We have referred to these cells as dendritic because of their loss of the monocyte marker CD14 and their expression of CD83, despite the decreased expression of CD1a (Figure 3). Furthermore, DC–IFN-β expressed high levels of adhesion molecules associated with DC such as ICAM-3, CD11b, and CD11d (data not shown). Thus, the IFN-β phenotype does resemble that of direct blood-derived DC.29 Our results further emphasize the heterogeneity of monocyte-derived DC from the perspective of both the phenotypic markers and the functional properties.29
Our results demonstrating an inhibitory effect of IFN-β on DC differentiation from monocyte-derived DC progenitors are consistent with other reports on the inhibition of hematopoiesis by IFN-α/β. McNeill and Killen30 found that poly I–poly C, which stimulates type-I IFN production, inhibited colony formation in bone marrow cells. These results were confirmed and extended by other laboratories to show that type-I IFNs are cytotoxic for progenitor cells.31 More recently, transient suppression of hematopoiesis associated with viral infection in both humans and murine models has been attributed to high levels of IFN-α/β.16 17
Recent publications by Wang et al32 and Paquette et al33 confirm that IFN-α greatly reduces the number of DC that can be generated from monocytes, but no other detrimental effects of IFN-α on DC function were reported. However, the effects of IFN-α on DC function in those reports cannot be compared directly with our data because of significant differences in both the culture conditions used to generate DC and the purity and phenotype of the generated DC used in their function assays, as well as the T-cell populations used to analyze DC function. Specifically, although we used CD4+ naive T cells, Wang et al32 and Paquette et al33 both used unseparated T cells for their studies. Another recent report by Luft et al34 found that IFN-α and TNF-α synergize to enhance the transition from immature to mature DC under serum-free conditions. The differences between our results and this report may be due to the type of progenitor cell used (CD14+ versus CD34+, respectively) and the time point during the differentiation process at which the type-I IFN treatment was initiated (day 1 at DC progenitor stage versus day 4 at immature DC stage, respectively). Consistent with the data of Luft et al,34 we found that IFN-β had little effect on DC morphology when added on day 4 rather than day 1 of culture (data not shown). Thus, these contrasting results suggest that IFN-α/β can have both positive and negative regulatory effects on DC differentiation depending upon the progenitor cell treated and the stage during DC maturation at which IFN-β is introduced.
Another level of complexity in regard to the role of type-I IFNs in regulating T-cell immune responses is provided by data from Sprent et al, who have published a series of studies indicating that type-I IFNs promote CD8+ memory cell survival35 through the induction of IL-15 secretion.36,37 Interestingly, these growth- and survival-promoting effects did not extend to CD4+ Th cells. In fact, we found that type-I IFNs may lead to immune deviation by altering naive CD4+ Th-cell differentiation through the promotion of an IL-10–secreting Th-cell subset.19
In summary, several proinflammatory cytokines elaborated during the innate immune response (eg, IL-1 and TNF-α) are known to promote maturation and migration of DC, a step necessary for the eventual activation of naive CD4+ T cells in regional lymph nodes.8,38,39 In this way, the early cytokine response to various pathogens will determine the nature and efficacy of subsequent T-cell–dependent immunity.40,41 We now show that type-I IFNs, cytokines either elaborated during the early phase of the innate host defense or given as therapeutic treatment, can negatively regulate immune effector functions by influencing DC differentiation and function. Our data indicate that the presence of type-I IFNs from the initiation of DC differentiation can alter DC progenitor differentiation in such a way as to diminish IL-12–mediated inflammatory responses at sites of infection. These findings may explain why prolonged IFN-α/β production in peripheral tissues and draining lymph nodes, as occurs during some chronic viral infections, can lead to immunosuppression.15-17 They may also explain some of the beneficial effects observed in treatment of patients with multiple sclerosis with IFN-β.10 11
Acknowledgments
We thank Dr Mark P. Hayes and Valerie Calvert (Food and Drug Administration, Bethesda, MD) for providing elutriated monocytes, Drs Susan E. Goelz and Paula Hochman (Biogen Corp., Cambridge, MA) for providing human recombinant IFN-β, and Dr Mary Collins (Genetics Institute, Cambridge, MA) for the anti-CD80/86 antibodies. Beth Beilfuss provided expert technical help when needed. Dr Michael Model's help with the light microscopy is greatly appreciated.
Supported by grant AI34541 from the National Institutes of Health and a grant from Biogen, Inc. Also supported in part by a postdoctoral fellowship from the National Multiple Sclerosis Society (B.L.M.).
B.L.M. and T.N. contributed equally to the data presented in this manuscript.
Reprints:Gijs A. van Seventer, Department of Pathology, University of Chicago, 5841 S Maryland Ave, Room J541A, MC1089, Chicago, IL 60637; e-mail: gvsevent@flowcity.bsd.uchicago.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Fig. 4. DC–IFN- and DC–IFN-β have a decreased capacity to support naive CD4+ T-cell proliferation. / Naive CD4+ T cells were stimulated with immobilized anti-CD3 and either DC, DC–IFN-α (A), or DC–IFN-β (B) at the indicated ratios. IFN-α and IFN-β were added at indicated concentrations at days 1 and 4 of the culture. APC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to the cultures for measurement of [3H]-thymidine incorporation. After 3 days in culture, wells were pulsed with 1 μCi of [3H]-thymidine per well. Twenty-four hours later, plates were harvested and incorporated [3H]-thymidine was measured. DC-induced T-cell proliferation was significantly different from DC–IFN-β–induced and DC–IFN-α–induced T-cell proliferation at a 1:0.1 ratio (n = 4; P < .05 as determined by a paired t test). Results depicted are representative of 4 independent experiments. (C) Naive CD4+ T-cell proliferation induced by DC–IFN-β (1 ng/mL) was not dependent on CD80 costimulation. Anti-CD80 and anti-CD86 were added at the beginning of the proliferative assays at a final concentration of 10 μg/mL. Data are expressed as the percentage of inhibition of proliferation in the presence of isotype-matched control Ig at a T-cell to DC ratio of 1:0.1. Results shown are the mean and SEM of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.210/4/m_bloo01352004ax.jpeg?Expires=1766201881&Signature=cZ5VmVTyHOMfarEFdlCV4F3ew2V9WWysS9S3TWHwpPbUIzKnBB~QOlY2GihBf55hgHinuLqkb4j91uWObIJcme71m03A3AHv9urpxksrG5BSASLwVGlVZLT2Zuc4fpMUxtYu0zrNzXBtaR355r-DOoXtLjxsRKcsfTNXU1wE533EDHhlAU24yg1hJq~mzqdPLRxPkDuYhScEGgHgNp23~1OcT5~Q9VyQPcuC4Z-hAgBxsC3~OtG8J~JzWf9Y0RxYWLxRIzEm-pHiy-D2MysrgyMt3GTkaEDO5aJDeg4L~-c1R1DwRGa~THFC3O9win8V4GzBX2BfDNqLQ58JKg0YpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. DC–IFN- and DC–IFN-β have a decreased capacity to support naive CD4+ T-cell proliferation. / Naive CD4+ T cells were stimulated with immobilized anti-CD3 and either DC, DC–IFN-α (A), or DC–IFN-β (B) at the indicated ratios. IFN-α and IFN-β were added at indicated concentrations at days 1 and 4 of the culture. APC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to the cultures for measurement of [3H]-thymidine incorporation. After 3 days in culture, wells were pulsed with 1 μCi of [3H]-thymidine per well. Twenty-four hours later, plates were harvested and incorporated [3H]-thymidine was measured. DC-induced T-cell proliferation was significantly different from DC–IFN-β–induced and DC–IFN-α–induced T-cell proliferation at a 1:0.1 ratio (n = 4; P < .05 as determined by a paired t test). Results depicted are representative of 4 independent experiments. (C) Naive CD4+ T-cell proliferation induced by DC–IFN-β (1 ng/mL) was not dependent on CD80 costimulation. Anti-CD80 and anti-CD86 were added at the beginning of the proliferative assays at a final concentration of 10 μg/mL. Data are expressed as the percentage of inhibition of proliferation in the presence of isotype-matched control Ig at a T-cell to DC ratio of 1:0.1. Results shown are the mean and SEM of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.210/4/m_bloo01352004bx.jpeg?Expires=1766201881&Signature=AlnlWEMjyeo~6x9krhNbTs4XssFxEse4MOxkDPfZXbtJlq22HiP2Q44Xle3i0eiSwxXHIq9RZKYiSQ4Vz0y~arulfVW6eQlboWjGnz8uwvfeYAla0XbnMgyifbE7cft7OYEbzLIxF8OwawBl5T~qq1GKEo8AmO~1fYS1K6IoE6AeyOyYtYRmo07CVyG97vcneJ7MrtuetQkrS8yRvGzsckNwnxuXPDAGQxzLoz6UuIF308Gia5mCc6U~XsmrKpyn~SFz0n2eslkkvRyjLbOH6uc~dDdtykIYWHV~kPvmkhNsgLF2pSKpFyViFuk24gylKYUOcb7s-V~dNIDqKdbhiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. DC–IFN- and DC–IFN-β have a decreased capacity to support naive CD4+ T-cell proliferation. / Naive CD4+ T cells were stimulated with immobilized anti-CD3 and either DC, DC–IFN-α (A), or DC–IFN-β (B) at the indicated ratios. IFN-α and IFN-β were added at indicated concentrations at days 1 and 4 of the culture. APC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to the cultures for measurement of [3H]-thymidine incorporation. After 3 days in culture, wells were pulsed with 1 μCi of [3H]-thymidine per well. Twenty-four hours later, plates were harvested and incorporated [3H]-thymidine was measured. DC-induced T-cell proliferation was significantly different from DC–IFN-β–induced and DC–IFN-α–induced T-cell proliferation at a 1:0.1 ratio (n = 4; P < .05 as determined by a paired t test). Results depicted are representative of 4 independent experiments. (C) Naive CD4+ T-cell proliferation induced by DC–IFN-β (1 ng/mL) was not dependent on CD80 costimulation. Anti-CD80 and anti-CD86 were added at the beginning of the proliferative assays at a final concentration of 10 μg/mL. Data are expressed as the percentage of inhibition of proliferation in the presence of isotype-matched control Ig at a T-cell to DC ratio of 1:0.1. Results shown are the mean and SEM of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.210/4/m_bloo01352004cx.jpeg?Expires=1766201881&Signature=JgH4tmatGs8IaPm9dLW59AKUlMAefn9Q3y4I2IoAYEKB4LAn~n5oKU0HozquqAuE9y4SEoWbJocV0-IRM5qcsCvtcGaKs-pgqoohZCgUj4M-sxrcYktXoir6bH6TvCpXJSExcgmrAmUUkIlijPMGNxLV~8eX2zMsz9gMKr5AlQ6~31HHxKiAuQ-MOsVCbQpVquszxzDPx-3qT8HwQMDQEEbrdCnaii0Is3NbowEMG2pwQkwbZ7ESPPOPJ7mCOdtmeX0kTK7WRwvB7scOVRUCV9RvDLlogCo1zimM~o1vV2zDhXHZ6ti1FLh4ba3w4j3y1WnUo8s7HOpJ9n9fOu7s2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. DC–IFN-β have reduced capacity to induce a mixed lymphocyte reaction. / Naive CD4+ T cells were stimulated with either allogeneic DC or DC–IFN-β (0.1 ng/mL or 1 ng/mL) at the indicated ratios. DC were washed to remove cytokines used for in vitro differentiation. No exogenous cytokines were added to cultures for measurement of [3H]-thymidine incorporation. After 5 days in culture, wells were pulsed with 1 μCi of [3H]-thymidine per well. Twenty-four hours later, plates were harvested and incorporated [3H]-thymidine was measured. Means of triplicate measurements are shown. A representative of 4 independent experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/1/10.1182_blood.v96.1.210/4/m_bloo01352005x.jpeg?Expires=1766201881&Signature=OrziCanzKu~DTNINnoSfz6RlPoAwyWPbDxIswyIHuwNDm6-b18VBdFtHCwtBdPlDjr0ARaFoKRqFX7mJys4xQVpOu-1hkKDv7n-Z2-FIJBloBBu-e5QMIueO1zEg1fIV03qIxiy-7Qe7zRj91VWvWaFwiqJzMznUEw63hKb9OhJl8H~tTma1iDeeCjj19Wj3H6zQ5OYuI9OtWY4Pj9yhcz0~fYEfJRyibK2tkOzsugR-d3QNZ0o4UfZTEDvrgRJ54t-47OKEcYcr~Z28Nkadqezh9IRK~DSloeK05pVTFi8ddpY2bOzVa2O7APpi5gw9ObZ8WN9ITFJkAbY6gkl0rQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)