Abstract
The serum-borne lysophospholipid mediators sphingosine 1-phosphate (Sph-1-P) and lysophosphatidic acid (LPA) have been shown to be released from activated platelets and to act on endothelial cells. In this study, we employed the repeated lipid extraction (under alkaline and acidic conditions), capable of detecting Sph-1-P, LPA, and possibly structurally similar lysophospholipids, whereby a marked formation of [32P]Sph-1-P, but not [32P]LPA, was observed in [32P]orthophosphate-labeled platelets. Platelet Sph-1-P release, possibly mediated by protein kinase C, was greatly enhanced in the presence of albumin, which formed a complex with Sph-1-P. This finding suggests that platelet Sph-1-P may become accessible to depletion by albumin when its transbilayer movement (flipping) across the plasma membrane is enhanced by protein kinase C. Although human umbilical vein endothelial cells expressed receptors for both Sph-1-P and LPA, Sph-1-P acted much more potently than LPA on the cells in terms of intracellular Ca++ mobilization, cytoskeletal reorganization, and migration. The results suggest that Sph-1-P, rather than LPA, is a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells, under the conditions in which critical platelet-endothelial interactions (including thrombosis, angiogenesis, and atherosclerosis) occur. Furthermore, albumin-bound Sph-1-P may account for at least some of the serum biological activities on endothelial cells, which have been ascribed to the effects of albumin-bound LPA, based on the similarities between LPA and serum effects.
Introduction
Lysophosphatidic acid (LPA)1-3 and sphingosine 1-phosphate (Sph-1-P)4-6 are now regarded as serum-borne lipid mediators that exert potent and pleiotropic biological effects. Although LPA and Sph-1-P are glycerolipid and sphingolipid metabolite, respectively, they resemble each other in structure as lysophospholipids.7 Furthermore, striking similarities between LPA and Sph-1-P actions have been reported, examples of which include platelet aggregation,8-10mitogenesis,1,5,11,12 and cytoskeletal remodeling.1,13-15 Such extracellular mediator activities of LPA and Sph-1-P are mainly mediated by subfamilies of G protein-coupled receptors, of which the most completely characterized are those encoded by the endothelial differentiation genes (edgs).16-18
Previously, we showed that in platelets Sph-1-P is rapidly formed from sphingosine (Sph) by the action of Sph kinase, abundantly stored intracellularly, and released into the extracellular environment on stimulation.8,9 Furthermore, we subsequently showed that Sph-1-P acts as an endothelial cell survival factor; this bioactive lipid prevents apoptosis induced by withdrawal of growth factors and stimulates DNA synthesis in human umbilical vein endothelial cells (HUVECs).19 Recently, Sph-1-P was defined as a novel regulator of angiogenesis.20 Accordingly, Sph-1-P should be added to the list of platelet-derived mediators interacting with endothelial cells. In addition, LPA is reported to be a bioactive lipid released from activated platelets1-3,21 and to interact with endothelial cells.22-24 Therefore, we, in this study, examined the relative involvement of these bioactive lipids in platelet-endothelial cell interactions.
Materials and methods
Materials
[32P]Sph-1-P and [3-3H]Sph-1-P were prepared by [γ-32P]ATP- and ATP-dependent phosphorylation of Sph and [3-3H]Sph, respectively, which were both catalyzed by Sph kinase obtained from human platelets.25
The following materials were obtained from the indicated suppliers: Sph-1-P and C6-ceramide (Cer) (Biomol, Plymouth Meeting, PA); LPA, phosphatidic acid (PA), Sph, 12-O-tetradecanoylphorbol 13-acetate (TPA), sphingomyelin, bovine serum albumin (BSA; essentially fatty acid-free), trypan blue, and FITC-conjugated phalloidin (Sigma, St Louis, MO); thrombin (Mochida Pharmaceutical, Tokyo, Japan); collagen (Hormon-Chemie, Munich, Germany); staurosporine (Kyowa Medex, Tokyo, Japan); recombinant human basic fibroblast growth factor (bFGF) (Becton Dickinson Labware, Lincoln Park, NJ); fetal calf serum (FCS) (ICN Biomedicals, Aurora, OH); fura2-am (Dojindo Laboratories, Kumamoto, Japan); anti-paxillin monoclonal antibody (MoAb) (Transduction Laboratories, Lexington, KY); the reconstituted basement membrane matrix Matrigel (Becton Dickinson Labware, Bedford, MA); [γ-32P]ATP (3000 Ci/mmol), [3H]LPA (51 Ci/mmol), [3-3H]Sph (22 Ci/mmol), [3H]C6-Cer (22.3 Ci/mmol), [14C]sphingomyelin (40-60 mCi/mmol), and [32P]orthophosphate (8810 Ci/mmol) (NEN Life Science Products, Boston, MA).
Cell preparation
The platelets were isolated from the blood of healthy adult volunteers. The blood was anticoagulated with 3.8% sodium citrate (9 vol of blood to 1 vol of sodium citrate) and centrifuged at 120g for 10 minutes to obtain platelet-rich plasma. Then, the washed platelets suspended in the HEPES buffer (containing 138 mmol/L NaCl, 3.3 mmol/L NaH2PO4, 2.9 mmol/L KCl, 1.0 mmol/L MgCl2, 1 mg/mL of glucose, and 20 mmol/L HEPES [pH 7.4]) were prepared and handled as described previously.8 NaH2PO4 was omitted when indicated.
HUVECs were isolated from human umbilical cords with trypsin treatment, plated onto 0.2% gelatin-coated dishes, and cultured in Dulbecco modified Eagle medium with 20% FCS, 10 ng/mL of bFGF, penicillin G (100 U/mL), and streptomycin sulfate (100 μg/mL) at 37°C under an atmosphere of 5% CO2 and 95% air. HUVECs were used between the third and the sixth passages.
Platelet labeling with [32P]orthophosphate
Platelets suspended in the HEPES buffer without added NaH2PO4 at a cell density of 1 × 109/mL were incubated with 5 mCi/mL of carrier-free [32P]orthophosphate for 2 hours at 37°C. Then, the platelets were diluted, washed twice, and finally adjusted to 3 × 108/mL. The [32P]orthophosphate-labeled platelets were stimulated, as indicated, under continuous shaking at 80 bpm.
Under these conditions, platelet phosphatidylinositol, phosphatidylinositol 4-phosphate, phosphatidylinositol 4,5-bisphosphate, and phosphatidylinositol 3,4,5-trisphosphate were heavily labeled with [32P] (data not shown), as we previously described.26
Lipid extraction and analysis
Lipids were extracted from 0.5-mL aliquots of the HEPES buffer, including the indicated radiolabeled lipid or [32P]orthophosphate-labeled platelets. Three milliliters of ice-cold chloroform/methanol (1:2) was added to the samples, followed by thorough mixing and sonication. Phases were separated by adding 2 mL of chloroform and 2 mL of 1 mol/L KCl, without or with further addition of 80 μL of 7N NH4OH or 40 μL of concentrated HCl. The lower chloroform phases were evaporated under N2 and assayed for radiolabeled lipids. When indicated, the alkaline upper phases (after the addition of NH4OH) were transferred to new tubes, to which 3 mL of chloroform and 160 μL of concentrated HCl were added. The final lower chloroform phases, formed under the new acidic conditions, were then evaporated and assayed for radiolabeled lipids. All the lower phase samples were subject to liquid scintillation counting or to thin-layer chromatography (TLC) autoradiography as previously described.8 The TLC plates (silica gel 60 HPTLC plates; Merck, Darmstadt, Germany) were developed in butanol/acetic acid/water (3:1:1), and autoradiography was performed with Konica Medical Film (Konica, Tokyo, Japan) at −80°C. When [3H]- or [14C]-labeled lipids were analyzed, enhancer (EN3HANCE Spray, NEN Life Science Products) treatment of TLC plates were performed.
[3H]Sph-1-P release from platelets labeled with [3H]Sph
Platelet suspensions (0.5 mL), without or with inclusion of 1% BSA, were incubated with 1 μmol/L [3H]Sph (0.2 μCi) and stimulated at 37°C with the indicated agent. At the times indicated, the platelets were centrifuged for 15 seconds at 12 000g. Lipids were then extracted from the resultant medium supernatant and cell pellet and analyzed for [3H]Sph metabolism by TLC autoradiography, as described above. For quantitative analysis, silica gel areas that contained radiolabeled Sph-1-P were scraped off and counted by liquid scintillation counting. The percentage of Sph-1-P release into the medium was calculated as 100 × ([3H]Sph-1-P in medium) / (total [3H]Sph-1-P in medium plus cell pellets), and stimulus-dependent Sph-1-P release as (percentage of Sph-1-P release into the medium on stimulation) − (percentage of Sph-1-P release into the medium without stimulation).
Gel-filtration chromatography of [32P]Sph-1-P/BSA mixtures
Sph-1-P, containing trace amounts of [3H]Sph-1-P, was incubated for 30 minutes with equimolar amounts of intact BSA or BSA denatured by boiling. The [3H]Sph-1-P/BSA mixtures were fractionated in a Sephadex G-25M (Pharmacia LKB Biotechnology AB, Uppsala, Sweden) gel-filtration column, which was equilibrated and run in a phosphate-buffered saline (PBS). Fractions (1 mL) were collected and assayed for their [3H]Sph-1-P content and albumin concentration, which were determined by liquid-scintillation counting and dye binding,27 respectively.
RNA isolation and reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was prepared from HUVECs with the total RNA isolation system (Isogen, Wako Pure Chemical Industries, Osaka, Japan), and the isolation of messenger RNA (mRNA) was performed with Oligotex-dT30 < Super > (Takara Biomedicals, Tokyo, Japan), according to the manufacturer's instructions. The isolated mRNA was reverse transcribed with the use of SuperScript Preamplification System (Gibco BRL, Life Technologies, Rockville, MD). Reverse transcribed complementary DNA was amplified in a Perkin-Elmer 9600R thermal cycler (The Perkin-Elmer, Norwalk, CT), using Gene Taq (rTaq DNA polymerase; Wako Pure Chemical Industries).
Oligonucleotide primer pairs used foredg-1, -2, -3, and -4 were 5′-CCAGAAAGACGAAGGGGACAAC-3′ (sense) and 5′-CAGCAGTCGAAGCCCAGAGATT-3′ (antisense) foredg-128; 5′-CAGGACCCAATACTCGGAGA-3′ (sense) and 5′-CTGTAGAGGGGTGCCATGTT-3′ (antisense) for edg-2 (GenBank accession no. Y09 479); 5′-TGGTCATCTGCAGCTTCATC-3′ (sense) and 5′-CGTCTTCTTGCCAGACATCA-3′ (antisense) for edg-3(GenBank accession no. X83 864); 5′-CCCAACCAACAGGACTGACT-3′ (sense) and 5′-GAGCCCTTATCTCTCCCCAC-3′ (antisense) foredg-4 (GenBank accession no. AF011 466).
Amplification was conducted with 35 cycles of 1 minute at 94°C, 1 minute at 60°C, and 1 minute at 72°C. Absence of contaminating DNA was confirmed by control reactions with RNA that had not been reverse transcribed. No amplification products were detected without addition of the reverse transcriptase (data not shown). PCR products (5 μL) were resolved by electrophoresis in a 2% agarose gel in TBE buffer (90 mmol/L Tris-Borate, 2 mmol/L EDTA, pH 8.3) and stained with ethidium bromide. The PCR products were cut from gels, solubilized, and sequenced with Dye Terminator Cycle Sequencing FS Ready Reaction Kits (The Perkin-Elmer) and analyzed with the use of an ABI PRISM 310 Genetic Analyzer (The Perkin-Elmer), according to the supplied protocols.
Measurement of the intracellular Ca++ concentration ([Ca ++]i)
The [Ca++]i measurement was performed with the use of the Ca++-sensitive fluorophore fura2. Confluent HUVECs were harvested by trypsinization, and the cell pellet was suspended in the HEPES buffer, containing 3 μmol/L fura2-am. After 1 hour at 37°C, cells were washed twice, resuspended in fresh buffer, and adjusted to 2 × 106/mL. Fluorescence measurements were made with the use of a FS100 (Kowa, Tokyo, Japan). The [Ca++]i values were determined from the ratio of fura2 fluorescence intensity at 340 and 380 nm excitation.29
HUVEC cytoskeletal reorganization
Formation of actin filaments was studied by visualization of filamentous actin with FITC-labeled phalloidin, and focal adhesion site assembly was examined by immunostaining of paxillin. HUVECs were serum-starved for 2 hours and then stimulated as indicated in serum-free conditions. The cells were fixed in 3% paraformaldehyde for 40 minutes, permeabilized in 0.2% Triton X-100 for 8 minutes, incubated with 2.5 μg/mL of anti-paxillin MoAb for 2 hours, and washed with PBS. The cells were subsequently incubated with FluoroLink Cy3-labeled goat anti-mouse immunoglobulin G (Amersham Pharmacia Biotech, Buckinghamshire, UK) for 1 hour and then with 5 μg/mL of FITC-conjugated phalloidin for 40 minutes. After washing 3 times with PBS, the cells were viewed on a BH2 microscope (Olympus, Tokyo, Japan), and photographed on Fujicolor super G400 film (Fujifilm, Tokyo, Japan).
HUVEC migration
HUVEC migration was assessed by the modified Boyden chamber assay, ie, in Transwell cell culture chambers (Costar, Cambridge, MA) basically as previously described.30 Polycarbonate filters with 8-μm pores were used to separate the upper and lower chamber and were coated with 500 μg/mL of Matrigel. The coated filters were washed by serum-free medium and dried immediately. Then, HUVECs were added to the upper compartment of the chamber at a density of 1 × 105/100 μL of medium containing 0.1% BSA and incubated at 37°C. HUVECs were allowed to migrate toward Sph-1-P or LPA in the lower chamber. After the reaction, the filters were removed, fixed, stained with trypan blue, and mounted on glass slides. After removal of nonmigrated cells by wiping with cotton swabs, cells that had migrated through the filter to the lower surface were counted manually under a microscope in 5 predetermined fields. Each experiment was conducted in triplicate, and the mean was calculated.
Data presentation
All experiments were performed at least 3 times. The results shown are from a single representative experiment or the mean ± SD (n = 3).
Results
Efficient extraction of lysophospholipids by repeated phase separations
Lipids are usually extracted into the lower chloroform phase after the phase separation; Sph, C6-Cer, sphingomyelin (Figure1), and PA (data not shown) were recovered into the lower phase, irrespective of pH conditions. In contrast, the water-soluble lipids Sph-1-P and LPA were extracted into the upper aqueous phase, especially under alkaline conditions (Figure1). However, these lysophospholipids were recovered into the lower phase under acidic conditions (Figure 1), which is possibly due to the elimination of polarity of the phosphate moiety. When lipid extraction was first performed under the alkaline conditions and the resultant upper aqueous phases were re-extracted under acidic conditions, more than 40% and 60% of Sph-1-P and LPA, respectively, were recovered in the final lower phase (Figure 2A). Under these conditions, both Sph-1-P and LPA were confirmed to be stable, and no degradation products were detected in the TLC autoradiography (Figure 2B). This procedure is an easy and efficient method for specific extraction of Sph-1-P, LPA, and possibly structurally related lysophospholipids; other lipids examined, including Sph, C6-Cer, and sphingomyelin, were not present at all in the final lower phase (Figure 2A).
Recovery of various radiolabeled lipids into the lower chloroform phase after the phase separation.
The HEPES buffer, containing [32P]Sph-1-P, [3H]LPA, [3H]Sph, [3H]C6-Cer, or [14C]sphingomyelin (SM), and chloroform/methanol (1:2) were mixed without (▨) or with further addition of NH4OH (■) or HCl (▪). The phases were then separated, and radioactivities of the lower phase were counted by liquid scintillation counting. The percentage of recovery of each radiolabeled lipid into the lower chloroform phase was calculated as 100 × the radioactivity in the lower chloroform phase/the radioactivity added.
Recovery of various radiolabeled lipids into the lower chloroform phase after the phase separation.
The HEPES buffer, containing [32P]Sph-1-P, [3H]LPA, [3H]Sph, [3H]C6-Cer, or [14C]sphingomyelin (SM), and chloroform/methanol (1:2) were mixed without (▨) or with further addition of NH4OH (■) or HCl (▪). The phases were then separated, and radioactivities of the lower phase were counted by liquid scintillation counting. The percentage of recovery of each radiolabeled lipid into the lower chloroform phase was calculated as 100 × the radioactivity in the lower chloroform phase/the radioactivity added.
Efficient recovery of Sph-1-P and LPA after the repeated phase separations under alkaline and acid conditions.
(A) After the initial phase separation of [32P]Sph-1-P, [3H]Sph-1-P, [3H]LPA, [3H]Sph, [3H]C6-Cer, or [14C]sphingomyelin (SM) under alkaline conditions, the resultant aqueous upper phase was phase-separated again under the acidic conditions. The radioactivities of each radiolabeled lipid into the final lower chloroform phase were counted by liquid scintillation counting. The percentage of recovery of each radiolabeled lipid into the final lower chloroform phase was calculated as 100 × the radioactivity in the final lower chloroform phase/the radioactivity originally added. (B) [3H]Sph-1-P (left lane) or [3H]LPA (right lane), recovered in the final lower chloroform phases, was analyzed by TLC autoradiography. Note that no bands other than [3H]Sph-1-P and [3H]LPA were detected.
Efficient recovery of Sph-1-P and LPA after the repeated phase separations under alkaline and acid conditions.
(A) After the initial phase separation of [32P]Sph-1-P, [3H]Sph-1-P, [3H]LPA, [3H]Sph, [3H]C6-Cer, or [14C]sphingomyelin (SM) under alkaline conditions, the resultant aqueous upper phase was phase-separated again under the acidic conditions. The radioactivities of each radiolabeled lipid into the final lower chloroform phase were counted by liquid scintillation counting. The percentage of recovery of each radiolabeled lipid into the final lower chloroform phase was calculated as 100 × the radioactivity in the final lower chloroform phase/the radioactivity originally added. (B) [3H]Sph-1-P (left lane) or [3H]LPA (right lane), recovered in the final lower chloroform phases, was analyzed by TLC autoradiography. Note that no bands other than [3H]Sph-1-P and [3H]LPA were detected.
Detection of [32P]Sph-1-P in [32P]orthophosphate-labeled platelets
To detect Sph-1-P and LPA in intact platelets, the repeated extraction procedures described above were performed on [32P]orthophosphate-labeled platelets. When the lower chloroform phase was analyzed by TLC autoradiography after a single-phase separation (either in the alkaline or acidic condition), numerous [32P]-labeled lipids were detected (data not shown). However, when the final chloroform phases were analyzed after the repeated phase separations (under the alkaline and then acidic conditions), [32P]Sph-1-P was mainly detected with a clear resolution (Figure 3). This finding confirms that our extraction procedure is very useful for detection of Sph-1-P and possibly structurally related lysophospholipids. In agreement with our previous report that Sph-1-P is abundantly stored even in resting platelets,31,32 [32P]Sph-1-P was detected in unstimulated platelets (Figure 3). When Sph, the substrate of Sph kinase, was added to platelets, the radioactivity of [32P]Sph-1-P was greatly enhanced (Figure 3), as expected from the fact that platelets possess high activity of Sph kinase.8,9 Although Sph kinase activity has been reported to be stimulated with receptor-mediated agonists or protein kinase C activators in several cells,5,6,12 the Sph-1-P levels were not enhanced by treatment of platelets with the potent and physiological agonists thrombin and collagen (Figure3).33
Detection of [32P]lysophospholipids in [32P]orthophosphate-labeled platelets.
Human platelets labeled with [32P]orthophosphate were incubated without (−) or with 1 U/mL of thrombin (Thr), 20 μg/mL of collagen (Col), or 5, 10, or 20 μmol/L Sph for 5 minutes. Sph-1-P and LPA were first extracted from these platelets into the upper aqueous phase under alkaline conditions, followed by re-extraction into the lower chloroform phase under acidic conditions. The final chloroform phase was analyzed by TLC autoradiography.
Detection of [32P]lysophospholipids in [32P]orthophosphate-labeled platelets.
Human platelets labeled with [32P]orthophosphate were incubated without (−) or with 1 U/mL of thrombin (Thr), 20 μg/mL of collagen (Col), or 5, 10, or 20 μmol/L Sph for 5 minutes. Sph-1-P and LPA were first extracted from these platelets into the upper aqueous phase under alkaline conditions, followed by re-extraction into the lower chloroform phase under acidic conditions. The final chloroform phase was analyzed by TLC autoradiography.
In contrast to [32P]Sph-1-P, [32P]LPA was not detectable under these conditions (Figure 3); the production of [32P]LPA seemed much less than that of [32P]Sph-1-P. Under the conditions employed, marked synthesis of PA was detected in stimulated platelets (Figure4), confirming failure to detect any PA-specific phospholipase A activity in human platelet lysates but arguing against the existence of active PA-specific phospholipase A2 in platelets.3,21 34
Formation of [32P]PA in [32P]orthophosphate-labeled platelets stimulated with thrombin or collagen.
Platelets labeled with [32P]orthophosphate were incubated without (−) or with 1 U/mL of thrombin (Thr), 20 μg/mL of collagen (Col), or 5 μmol/L Sph for 5 minutes. The lipids were extracted from these platelets, and the lower chloroform phase under alkaline conditions was analyzed for [32P]PA formation.
Formation of [32P]PA in [32P]orthophosphate-labeled platelets stimulated with thrombin or collagen.
Platelets labeled with [32P]orthophosphate were incubated without (−) or with 1 U/mL of thrombin (Thr), 20 μg/mL of collagen (Col), or 5 μmol/L Sph for 5 minutes. The lipids were extracted from these platelets, and the lower chloroform phase under alkaline conditions was analyzed for [32P]PA formation.
Albumin-dependent release of Sph-1-P from activated platelets
Previously, we reported that platelet Sph-1-P can be released extracellularly on stimulation, which may be mediated by protein kinase C activation.9 In this study, we checked the Sph-1-P release from platelets in the absence and presence of albumin in the medium. In accordance with our previous results,9[3H]Sph-1-P, formed from [3H]Sph in platelets, was released extracellularly on stimulation with thrombin or TPA (Figure 5); thrombin activates protein kinase C as a result of phosphoinositide turnover, whereas TPA directly activates this kinase as a substitute for diacylglycerol.33 Furthermore, this stimulated release was blocked by staurosporine, an inhibitor of protein kinases, including protein kinase C.35 It should be noted that the extracellular release of Sph-1-P into the medium was much higher in the presence of extracellular BSA than in its absence (Figure 5). We assume that albumin extracts Sph-1-P stored in the platelets, when Sph-1-P location inside the cells is shifted on protein kinase C activation. In fact, we confirmed that Sph-1-P co-elutes with BSA (but not with denatured BSA) when a mixture of [3H]Sph-1-P and BSA is applied to a gel-filtration column (Figure6), as is the case with LPA.21
Albumin dependence of Sph-1-P release from activated platelets.
(A) Platelet suspensions, containing 1% BSA (lower lanes) or not (upper lanes), were incubated with [3H]Sph for 6 minutes and further stimulated without (a and d) or with 0.5 U/mL of thrombin (b and c) or 1 μmol/L TPA (e and f) for 5 minutes. In c and f, 1 μmol/L staurosporine was added 2 minutes before stimulation; this protein kinase inhibitor, by itself, failed to affect Sph-1-P release. After incubation, samples were centrifuged, and lipids were extracted from the resultant medium supernatant (M) and cell pellet (C) to detect [3H]Sph-1-P release from platelets. (B) Thrombin- or TPA-induced [3H]Sph-1-P release from platelets pretreated without or with staurosporine in the absence (■) or presence (▪) of extracellular 1% BSA was calculated.
Albumin dependence of Sph-1-P release from activated platelets.
(A) Platelet suspensions, containing 1% BSA (lower lanes) or not (upper lanes), were incubated with [3H]Sph for 6 minutes and further stimulated without (a and d) or with 0.5 U/mL of thrombin (b and c) or 1 μmol/L TPA (e and f) for 5 minutes. In c and f, 1 μmol/L staurosporine was added 2 minutes before stimulation; this protein kinase inhibitor, by itself, failed to affect Sph-1-P release. After incubation, samples were centrifuged, and lipids were extracted from the resultant medium supernatant (M) and cell pellet (C) to detect [3H]Sph-1-P release from platelets. (B) Thrombin- or TPA-induced [3H]Sph-1-P release from platelets pretreated without or with staurosporine in the absence (■) or presence (▪) of extracellular 1% BSA was calculated.
Co-fractionation of [3H]Sph-1-P with BSA.
Sph-1-P, containing trace amounts of [3H]Sph-1-P, was incubated with equimolar amounts of intact BSA (A) or denatured BSA (B). The mixtures were fractionated in a gel-filtration column, and fractions were collected and assayed for their [3H]Sph-1-P radioactivity and BSA concentration.
Co-fractionation of [3H]Sph-1-P with BSA.
Sph-1-P, containing trace amounts of [3H]Sph-1-P, was incubated with equimolar amounts of intact BSA (A) or denatured BSA (B). The mixtures were fractionated in a gel-filtration column, and fractions were collected and assayed for their [3H]Sph-1-P radioactivity and BSA concentration.
The above results indicate that Sph-1-P (as well as LPA) can bind to albumin, that Sph-1-P stored inside platelets become accessible to depletion by albumin on protein kinase C activation, and that Sph-1-P may be present in the plasma or serum in an albumin-bound form.
Expression of mRNA transcripts for Sph-1-P and LPA receptors in HUVECs
To examine the effects of bioactive substances released from platelets on the endothelial cells is important in terms of platelet-endothelial interactions. For this purpose, we first confirmed the expression of the lysophospholipid receptorsedgs16-18 by RT-PCR. Edg-1 and -3 have been identified as receptors for Sph-1-P, whereas Edg-1, -2, and -4 have been identified as receptors for LPA.17,18,20 36 RNA from HUVECs was prepared and reverse transcribed, followed by PCR amplification of specific transcripts. As demonstrated in Figure7, HUVECs were found to express mRNA transcripts for edg-1, -2, -3, and -4; the sequence of each PCR product was confirmed (data not shown).
Detection of mRNA expression by RT-PCR for the Sph-1-P and LPA receptors in HUVECs.
Amplified products for edg-1 (lane 1), -2 (lane 2), -3 (lane 3), and -4 (lane 4) were resolved on 2% agarose gel. The far right lane contained the size standards with positions (base pairs [bp]) indicated. The specific amplified products for edg-1, -2, -3, and -4 are 158, 283, 170, and 289 bp, respectively.
Detection of mRNA expression by RT-PCR for the Sph-1-P and LPA receptors in HUVECs.
Amplified products for edg-1 (lane 1), -2 (lane 2), -3 (lane 3), and -4 (lane 4) were resolved on 2% agarose gel. The far right lane contained the size standards with positions (base pairs [bp]) indicated. The specific amplified products for edg-1, -2, -3, and -4 are 158, 283, 170, and 289 bp, respectively.
Sph-1-P, but not LPA, exhibits potent effects on HUVEC intracellular Ca++mobilization, cytoskeletal reorganization, and migration
Finally, we examined the effects of Sph-1-P and LPA on functional responses of HUVECs, which express receptors for both lysophospholipids. One of the most well-established short-term cellular responses to Sph-1-P5,6,8,37 and LPA1,2,21 is intracellular Ca++ mobilization. As reported,20 Sph-1-P rapidly and potently induced the increase in [Ca++]i in HUVECs (Figure8A) in a concentration-dependent manner (Figure 8B). Sph-1-P-induced Ca++ mobilization was not affected by the presence of BSA in the medium (data not shown). In contrast to Sph-1-P, the stimulatory effect of LPA on intracellular Ca++ mobilization was very weak (Figure 8).
Effects of Sph-1-P or LPA on HUVEC [Ca++]i.
(A) Fura2-loaded HUVECs were challenged with 1 μmol/L Sph-1-P or LPA. Changes in [Ca++]i were monitored by the ratio of fura2 fluorescence. Arrows indicate the addition of Sph-1-P and LPA. (B) Fura2-loaded HUVECs were stimulated with various concentrations of Sph-1-P (○) or LPA (●), and the increases in [Ca++]i (the ratio of fura2 fluorescence) were measured.
Effects of Sph-1-P or LPA on HUVEC [Ca++]i.
(A) Fura2-loaded HUVECs were challenged with 1 μmol/L Sph-1-P or LPA. Changes in [Ca++]i were monitored by the ratio of fura2 fluorescence. Arrows indicate the addition of Sph-1-P and LPA. (B) Fura2-loaded HUVECs were stimulated with various concentrations of Sph-1-P (○) or LPA (●), and the increases in [Ca++]i (the ratio of fura2 fluorescence) were measured.
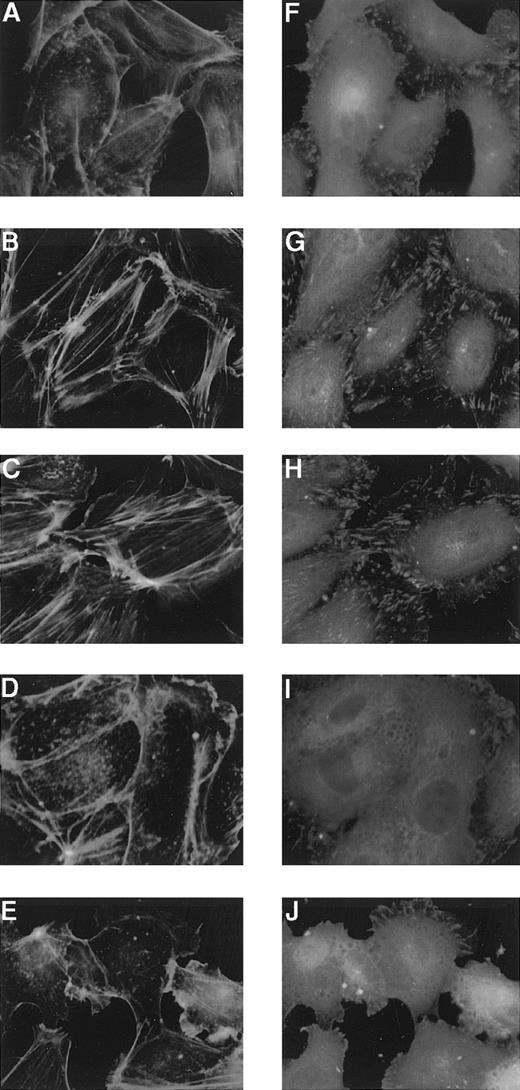
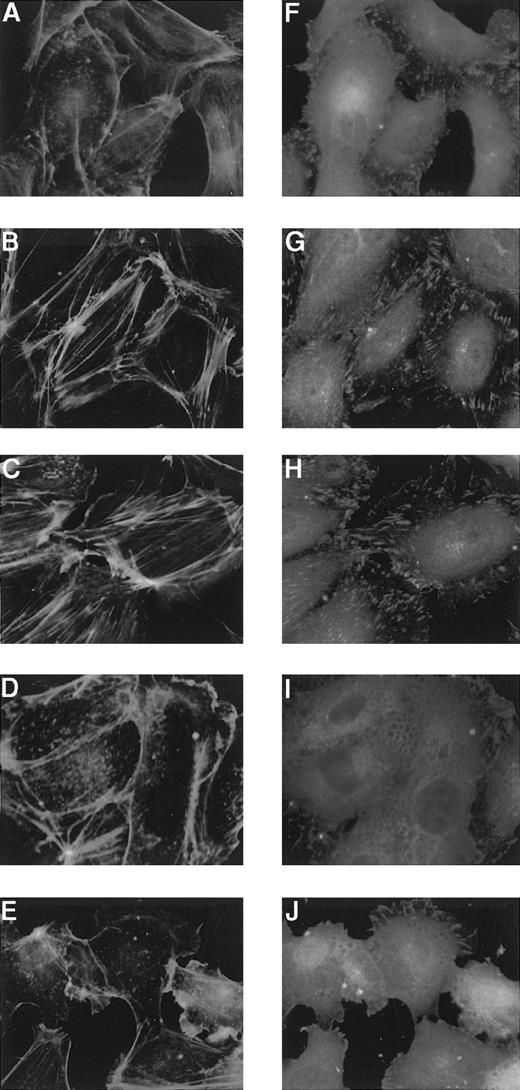
Both Sph-1-P and LPA have been shown to cause cytoskeletal remodeling in several cells.1,2,13-15 Formation of actin filaments was studied by visualization of filamentous actin with FITC-labeled phalloidin, and focal adhesion site assembly was examined by immunostaining of paxillin in HUVECs. Sph-1-P was found to markedly induce these cytoskeletal responses (Figure9). Stimulation of HUVECs with LPA reportedly leads to the formation of stress fibers,24 but the effect of LPA was apparently weaker than that of Sph-1-P (Figure9).
Effects of Sph-1-P or LPA on HUVEC cytoskeletal reorganization.
HUVECs were stimulated without (A and F) or with 100 nmol/L (B and G) or 1 μmol/L (C and H) Sph-1-P or 100 nmol/L (D and I) or 1 μmol/L (E and J) LPA for 30 minutes, fixed, and then incubated with anti-paxillin for detection of focal adhesions (F to J) and with FITC-conjugated phalloidin for actin stress-fiber staining (A to E).
Effects of Sph-1-P or LPA on HUVEC cytoskeletal reorganization.
HUVECs were stimulated without (A and F) or with 100 nmol/L (B and G) or 1 μmol/L (C and H) Sph-1-P or 100 nmol/L (D and I) or 1 μmol/L (E and J) LPA for 30 minutes, fixed, and then incubated with anti-paxillin for detection of focal adhesions (F to J) and with FITC-conjugated phalloidin for actin stress-fiber staining (A to E).
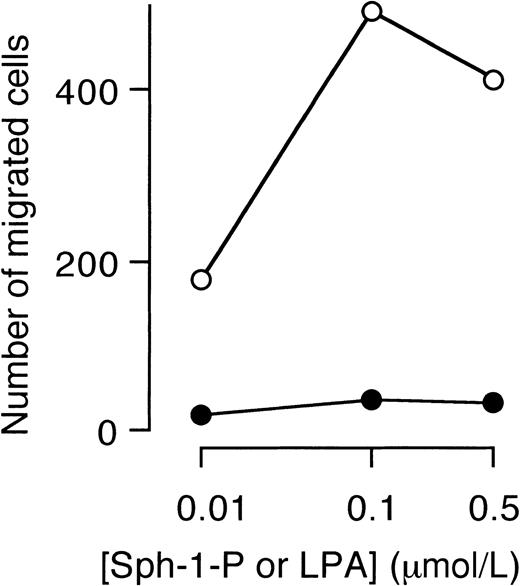
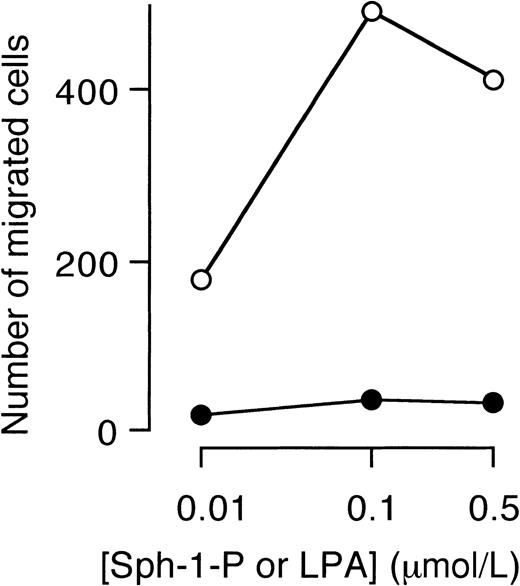
Finally, the effect of Sph-1-P or LPA on migration of HUVECs was examined. Sph-1-P, but not LPA, stimulated directional migration (chemotaxis) of HUVECs (Figure 10).
Stimulation of HUVEC migration by Sph-1-P, but not by LPA.
Indicated concentrations of Sph-1-P (○) or LPA (●) were placed in the lower chamber, and HUVECs were allowed to migrate for 4 hours.
Stimulation of HUVEC migration by Sph-1-P, but not by LPA.
Indicated concentrations of Sph-1-P (○) or LPA (●) were placed in the lower chamber, and HUVECs were allowed to migrate for 4 hours.
The results described above suggest that Sph-1-P, rather than LPA, functions as a potent HUVEC agonist.
Discussion
In the present study, we have demonstrated that Sph-1-P, abundantly stored in platelets, is released on activation and forms a high-affinity complex with albumin, while retaining its strong biological activities on HUVECs (possibly through Edg receptors). LPA is structurally related to Sph-1-P and has been shown to be released from activated platelets1-3,21 and to act on endothelial cells.22-24 However, both LPA release from platelets and its actions on HUVECs were minor in comparison with Sph-1-P.
[32P]Sph-1-P was mainly detected in [32P]orthophosphate-labeled platelets (both in resting and activated states) with the use of our detection system, which could possibly detect structurally similar lysophospholipids (other than Sph-1-P), if existing. Accordingly, Sph-1-P is by far the most important lysophospholipid produced in platelets, at least from a quantitative viewpoint. Furthermore, platelet Sph-1-P is released extracellularly on stimulation,8,9 although its mechanism remains to be solved. Because Sph-1-P stored in platelets becomes susceptible to depletion by BSA when platelet protein kinase C is activated, one possible explanation is that the route of Sph-1-P externalization is enhanced by transbilayer movement (flipping) across the plasma membrane, as a result of modifications in membrane physical properties (accompanying protein kinase C activation). Previously, it was demonstrated that the mdr2 P-glycoprotein may have an essential role in the secretion of phosphatidylcholine into bile38and hypothesized that the P-glycoproteins may transport other phospholipids.39,40 With previous reports in mind that such transport is ATP dependent and that protein kinase C phosphorylates P-glycoprotein,39,41 we examined the effect of verapamil, an inhibitor of P-glycoprotein.40 This inhibitor, however, did not affect Sph-1-P release under the conditions in which the release was completely inhibited by staurosporine (data not shown). Although the precise mechanism for extracellular release of Sph-1-P that follows platelet activation is still unclear, utilization of albumin to extract outer leaflet Sph-1-P would be a useful tool to detect transbilayer movement of Sph-1-P and hence to analyze Sph-1-P release.
The concentrations of LPA and Sph-1-P in the serum were estimated to be approximately 1 to 5 μmol/L21,42 and 0.5 μmol/L,32 respectively. Because they both have been shown to be released from activated platelets,8,9,21 much more formation of [32P]Sph-1-P than [32P]LPA in platelets labeled with [32P]orthophosphate and activated with potent agonists (such as thrombin and collagen) was unexpected. Although Sph-1-P is formed through the action of Sph kinase, which is abundantly expressed in platelets,8,9 the metabolic pathways as well as the enzymes responsible for LPA production are not well characterized. Although LPA has been postulated to be produced from platelets (possibly by hydrolysis of newly formed PA through the action of phospholipase A2),1,21,34 the production seems much less than that of Sph-1-P. Our present results support the previous idea that there may be another source(s) of LPA present in serum.3,34 One possible mechanism is LPA formation mediated through plasma lysophospholipase D, which selectively acts on lysophosphatidylcholine, the second most abundant phospholipid in the plasma.3,43,44 Blood cells other than platelets (eg, neutrophils45) may contribute to the LPA production. Another possibility is that activated platelets may release phospholipase(s), capable of acting on the substrate in plasma and producing LPA. In this context, worthy of notice is the report that LPA can be generated through secretory, cytokine-inducible phospholipase A2, acting on microvesicles shed from blood cells challenged with inflammatory stimuli.46 Recently, LPA was shown to be formed during mild oxidation of low-density lipoprotein (LDL) and to be the active compound in mildly oxidized LDL and minimally modified LDL.24 In a clinical study, elevated blood LPA levels were detected in gynecological cancer patients.47 These cancer cells are reportedly capable of producing LPA under certain conditions and are considered to be a potential source of elevated plasma LPA in vivo.48 Further studies are required to obtain a definite conclusion on LPA sources in the blood.
Although Edg-1, an inducible G protein-coupled receptor from vascular endothelial cells, was originally found to be a high-affinity receptor for Sph-1-P,17,20 LPA was found to stimulate this receptor, although as a low-affinity agonist.36 In addition, HUVECs were found to express Edg-3 (a Sph-1-P receptor) and Edg-2 and -4 (LPA receptors).18,20 Accordingly, it is possible that Sph-1-P and LPA equally act on endothelial cells. Contrary to our expectations, however, Sph-1-P was found to act as a much more potent agonist than LPA for HUVECs in terms of Ca++ mobilization, cytoskeletal remodeling, and migration. Consistent with our results, stimulation of HUVEC proliferation, chemotaxis, and FAK tyrosine phosphorylation by Sph-1-P, but not by LPA, has been reported recently.49 50 At present, the reason for much weaker effects of LPA on HUVECs is not clear.
It is established that LPA is an albumin-bound serum factor that elicits a variety of responses.51,52 Furthermore, many serum biological activities have been ascribed to the effects of albumin-bound LPA, based on the similarities between LPA and serum effects.1-3,11,13,15,21,22,53,54 Previously, it was reported that LPA as well as serum albumin does induce intracellular Ca++ mobilization in HUVECs and that the Ca++-releasing action of serum albumin may be attributed to LPA presence.22 However, because the concentrations of LPA used in this study were as high as 60 μmol/L22 and Sph-1-P is a much more potent Ca++ mobilizer than LPA, the reported action of serum albumin may be attributed to Sph-1-P (but not LPA). At least in some cases, albumin-bound Sph-1-P may also account for the biological activities of serum. To support this notion, invasion of T-lymphoma cells reportedly depends on the presence of serum, which can be replaced not only by LPA but also by Sph-1-P.15 Although LPA bound to albumin in FCS was considered to be a possible active principle that induced actin reorganization in serum-starved Swiss 3T3 fibroblasts,13this cytoskeletal response was later mimicked by Sph-1-P in the same cells.14 In HEK293 cells specifically transfected with Sph-1-P (but not LPA) receptors, rounded cell morphology was induced in the presence of serum.55 Furthermore, muscarinic K+ current in atrial myocytes was strongly activated by Sph-1-P, which caused desensitization of a response to the serum phospholipid challenge,56 suggesting that the serum effect may be ascribed to Sph-1-P.
In conclusion, Sph-1-P (but not LPA) seems to be a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells under the conditions in which critical platelet-endothelial interactions (such as thrombosis, angiogenesis, and atherosclerosis) occur. While this work was under consideration for publication, we found that, in the presence of extracellular Ca++, [32P]LPA can be clearly detected in [32P]orthophosphate-labeled platelets stimulated with thrombin; this had not been the case in the absence of added Ca++ (Figure 3). This result suggests that LPA may be formed extracellularly by the action of Ca++-requiring lipase(s). Even in the presence of extracellular Ca++, however, the radioactivities incorporated into [32P]LPA were less than 20% of those into [32P]Sph-1-P.
Acknowledgment
The authors thank Dr Nobuo Hisano for helpful discussions.
Supported in part by the Clinical Pathology Research Foundation of Japan, the Ryoichi Naito Foundation for Medical Research, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yutaka Yatomi, Department of Laboratory Medicine, Yamanashi Medical University, Nakakoma, Yamanashi 409-3898, Japan; e-mail: yatomiy@res.yamanashi-med.ac.jp.

![Fig. 1. Recovery of various radiolabeled lipids into the lower chloroform phase after the phase separation. / The HEPES buffer, containing [32P]Sph-1-P, [3H]LPA, [3H]Sph, [3H]C6-Cer, or [14C]sphingomyelin (SM), and chloroform/methanol (1:2) were mixed without (▨) or with further addition of NH4OH (■) or HCl (▪). The phases were then separated, and radioactivities of the lower phase were counted by liquid scintillation counting. The percentage of recovery of each radiolabeled lipid into the lower chloroform phase was calculated as 100 × the radioactivity in the lower chloroform phase/the radioactivity added.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364001.jpeg?Expires=1764972949&Signature=tr-9v5LJTqOh1~IQ-DoN6QAb~zoKdnvf3202gQDvotlVatt8g9tS5kJJAg-MtUYBYmfeFA3XpoP6IbnpRryjW2zK7xGoP2qZQUtdhUiSrOH~woLZ9Q3hSxqVOGIrxW8Mh7Df-2wtQjGM0cMVzqzm8pfGa4oOxUYzyBNsEO053P3~HbYsoQFo7TIoC~9nEhVwPlHZKgYqc3AJxCep4quXAg359Jgjy2dLnz2RHf4DsOt8OfIcgjsNTGdFJeWs6idjplfPtwAXE6TddFJQKC8auK-TKvP-9fuWJXJ5ICCkzX86tVzxlAI0C6tIRZ9~sfJt3PsHaFUTR0-Kv6A~M3~FuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Efficient recovery of Sph-1-P and LPA after the repeated phase separations under alkaline and acid conditions. / (A) After the initial phase separation of [32P]Sph-1-P, [3H]Sph-1-P, [3H]LPA, [3H]Sph, [3H]C6-Cer, or [14C]sphingomyelin (SM) under alkaline conditions, the resultant aqueous upper phase was phase-separated again under the acidic conditions. The radioactivities of each radiolabeled lipid into the final lower chloroform phase were counted by liquid scintillation counting. The percentage of recovery of each radiolabeled lipid into the final lower chloroform phase was calculated as 100 × the radioactivity in the final lower chloroform phase/the radioactivity originally added. (B) [3H]Sph-1-P (left lane) or [3H]LPA (right lane), recovered in the final lower chloroform phases, was analyzed by TLC autoradiography. Note that no bands other than [3H]Sph-1-P and [3H]LPA were detected.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364002.jpeg?Expires=1764972949&Signature=LQd9ZXiDq9J6q8mlXUdPqkAhwLc1JHBVbOsOUKfpZVIe3Fhm5UltpCshNlp53VOt2er4KTppv9Q2U60TMK29xE3oQCT9ACGB9NcM5ZSuqiTAr1TnFgYJtBVT1Vv1vruNmGRKekjNt8RaPoWs5EQA580WxjdBlD3aOe0nqemjWRiBiGwrvyW82L6FWktwdZTXki1PZLMzKlqdF4Gls7Yn15ovqBrMWGnJxJdxZoq6S9-0O04xBKZqEGur0qxMqeD87lKMOSfwfnVYkTzSWTncdjqkgppdUe4MgmLRzkQxCjCHXiLg6FJnP2sEEEt3Ce0ZoJuGC0aRaBT63bhTQztlLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Detection of [32P]lysophospholipids in [32P]orthophosphate-labeled platelets. / Human platelets labeled with [32P]orthophosphate were incubated without (−) or with 1 U/mL of thrombin (Thr), 20 μg/mL of collagen (Col), or 5, 10, or 20 μmol/L Sph for 5 minutes. Sph-1-P and LPA were first extracted from these platelets into the upper aqueous phase under alkaline conditions, followed by re-extraction into the lower chloroform phase under acidic conditions. The final chloroform phase was analyzed by TLC autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364003.jpeg?Expires=1764972949&Signature=OL9YZLqs0z1JVV2Ebx4d0HnRUOWbWCokME-3QfFhX~ARe0j6nxCr9aE~NwIs1lL67JXn8H~wzD~hFW9AlEjdLluJ8~57l7LhRgg5q1BYebdpSaH1ik9DT9RH5vYZSgYEoWRsYn5KtYDIOSiCOSPWW8jNKjvzyQC0v39aR~VhJaD0klzzOJQNGdt9bvtuYWDGMXjCTP~6hOVN4zZ6zA6Hfv~l4eS1C2Q2WqtS8ILtzi58JweXEBXSp7NooYPwg~UgDwgI2eXWlAZkYzilKIcMo11JPxaHNdtkerg39K8A5UsWxpNCeKiXe3sbLbZUb9RMaH01sPkNRQGkQpwUgD9vxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Formation of [32P]PA in [32P]orthophosphate-labeled platelets stimulated with thrombin or collagen. / Platelets labeled with [32P]orthophosphate were incubated without (−) or with 1 U/mL of thrombin (Thr), 20 μg/mL of collagen (Col), or 5 μmol/L Sph for 5 minutes. The lipids were extracted from these platelets, and the lower chloroform phase under alkaline conditions was analyzed for [32P]PA formation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364004.jpeg?Expires=1764972949&Signature=CR6YYcFN0oA1Ym28Go1mIci-ee5KDg30dp7CSvFknshixvHuRRZut7fgddnOKk-gybaIz5BtqoTEll8rdTBrZ2dGQyiEczW~lcOZgbh14owp2keY9zHXpaTX~QH4HWPH0SDnK4Rs3V4LuS-Bj41vWlN4TMplmLGGIHeQ-j75yKJ0boeQtNnAMvpAfMfyhjymm6wkH8yfj2rv15HAmyLFqEWpwy17NrWG161M8aISAIYwQ~dKJ59oFVvgc~~CMNsAR9Mh6o1O5Ajl-kW5n-BuQeO76RO6XngoacNs-poy4Ree2HdUjIGn9yM0JN0IHGyWOxH46WEp7hXEKD5x8A-JcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Albumin dependence of Sph-1-P release from activated platelets. / (A) Platelet suspensions, containing 1% BSA (lower lanes) or not (upper lanes), were incubated with [3H]Sph for 6 minutes and further stimulated without (a and d) or with 0.5 U/mL of thrombin (b and c) or 1 μmol/L TPA (e and f) for 5 minutes. In c and f, 1 μmol/L staurosporine was added 2 minutes before stimulation; this protein kinase inhibitor, by itself, failed to affect Sph-1-P release. After incubation, samples were centrifuged, and lipids were extracted from the resultant medium supernatant (M) and cell pellet (C) to detect [3H]Sph-1-P release from platelets. (B) Thrombin- or TPA-induced [3H]Sph-1-P release from platelets pretreated without or with staurosporine in the absence (■) or presence (▪) of extracellular 1% BSA was calculated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364005.jpeg?Expires=1764972949&Signature=2Qynu6JwebENfsEbpAq1~yJ8AkU9MrSLnNMsYlVhqGHW6FgZ2VNrh0g4VEGqUtBQ9iasDafr~sbtEzd03npo~qx5Z7GJSqbjjevIwj2~DZlX5dYFR1ygAiXzfv4syqLbDjjDSebL1YgNRc6tVMo9Ph5VvCeNno8imdqZlT~X1i0yCku4K9Esf1fEr2IqR3~wCUcfjYaOFkdOCiptTOWvCtKnD~WMGgSX6ToR6DzJ2iKntPce~6yr7FBkeBFAWK2f7Lbz7nOn3EWFKSJy~~kKHMNtgCvM7NMh5i3qHnqjUZMPI7K-HrP-SBhkD46N7QvL86OuzEnsQOxPbB6~4BgmVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Co-fractionation of [3H]Sph-1-P with BSA. / Sph-1-P, containing trace amounts of [3H]Sph-1-P, was incubated with equimolar amounts of intact BSA (A) or denatured BSA (B). The mixtures were fractionated in a gel-filtration column, and fractions were collected and assayed for their [3H]Sph-1-P radioactivity and BSA concentration.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364006.jpeg?Expires=1764972949&Signature=ArR7Totw~xSGmWeMHcOC4S0a75~R-FJBFtcS4GxBqoBCJDQ52G6kM-ukm3TufB82X0G4MVjBjc~-fGzeTklKWYCx4m2~GX0kmZQGKG0eUrOErMzrVWuiz8tjqCnQe4tOx1pxt1J~MfO9Je86ULp1aFFCL~j2L5fUL-lqZOZ37UObyPIygzXj155yt-UMya7oAzFDTQGBfBIaxuv5OC~C-4dudYjwdEH6aZxdY7HjtC0J15xedbYl9Y6yBeeM20PY6Mvkclfc23LckMpyr7byED8AKmZ4qo715mGDt1aIDKSK3Y5hglBj68gqrB3gA3E7OthpwcL-4t9f1pSM-RfHiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Detection of mRNA expression by RT-PCR for the Sph-1-P and LPA receptors in HUVECs. / Amplified products for edg-1 (lane 1), -2 (lane 2), -3 (lane 3), and -4 (lane 4) were resolved on 2% agarose gel. The far right lane contained the size standards with positions (base pairs [bp]) indicated. The specific amplified products for edg-1, -2, -3, and -4 are 158, 283, 170, and 289 bp, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364007.jpeg?Expires=1764972949&Signature=zROFW9xmXzfQdC2XKFXAsNXTvFHaZwZ4grHfDUujCieE35~3VTyqYCLFGn-dplOQVhu6Y2Ljq7LXolWOqhOEo0hS3ogswogc6WEzY~yT-tGxWB1Uq73qbdHd~txAj8VBOP7FhkyOPl53jyb2eEe2qE~fReRVp1EIDvAEC6n0Lrvv7Rzm6I12jNHSY-GQQrK7OpfXkdBxxOCkY3XGaurbU4LXfaPSe04yihCJ7K0CLT7dbaUiMS7PXzpx~YdRf8yrxNFBgG7xJ4Vi503vtUZXStUa2WsHfaz3dD73girSl3iVCZzh0CsY9PigXo4xcgUWja9ZQ5NEPjNOThqU80idYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effects of Sph-1-P or LPA on HUVEC [Ca++]i. / (A) Fura2-loaded HUVECs were challenged with 1 μmol/L Sph-1-P or LPA. Changes in [Ca++]i were monitored by the ratio of fura2 fluorescence. Arrows indicate the addition of Sph-1-P and LPA. (B) Fura2-loaded HUVECs were stimulated with various concentrations of Sph-1-P (○) or LPA (●), and the increases in [Ca++]i (the ratio of fura2 fluorescence) were measured.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364008.jpeg?Expires=1764972949&Signature=aIQ2S~2gnN9yJaS4SIWGGf3n99KaNNe2c~aESmnUx4VU0jQ2~j9oZ4eeUJMqENdlhY4ARZpu4zPQNBZvpATELxD9KAHYuhEXqXA2hBqS7hDs1VYbxj38YBXN-qzZMg7FLGhMQqYLIkwantvvONu1gRTPZooxApFM8i7Z8G56Hnsbz82oXwKS0UVVMWfHIwbH~4JTLOHp9u4yF-U1BV6EMAPpVw9xckt8puPkJ56LucP0KPeJrcGPEqJEsslt~9AUEVMvDoA58GOTQqGUGE0CAJ35REHUCDUozSbVdc-LBq2MyHMgN9~rnDvk-jW7FTr89U~IefxSwFBZcI35yb2b4w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Recovery of various radiolabeled lipids into the lower chloroform phase after the phase separation. / The HEPES buffer, containing [32P]Sph-1-P, [3H]LPA, [3H]Sph, [3H]C6-Cer, or [14C]sphingomyelin (SM), and chloroform/methanol (1:2) were mixed without (▨) or with further addition of NH4OH (■) or HCl (▪). The phases were then separated, and radioactivities of the lower phase were counted by liquid scintillation counting. The percentage of recovery of each radiolabeled lipid into the lower chloroform phase was calculated as 100 × the radioactivity in the lower chloroform phase/the radioactivity added.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364001.jpeg?Expires=1765247534&Signature=35nF2N-uq92~~2YNLeBCMxg8BbHV8EYhRYbwdC6onqGSwTh9~0eNl-PDwA9CwNogPjW1Nr7QAzaA1T-G9KW6qtekzxp~H6-EvnC-H6F5j9SczpgyRjf~VzRfhJGyhNTnftWNvoazgxlYKhnGHcvUqrFhZL3T0bkP9PWJFrh-jZyM4HYCjLl6OkbebeWcDriGpr6670WRDo8jxMEPFY4UaGlXUBjL2fqnAX96HERzha9d7hyufMVgUT3PBfrkj2k6JL~gLrlACaZgTTD49vtrdkIOqiWks6RIYXxPs6adyBIkT-nhO1PjY6tDS2VWGdk~oMUOzY9il3IVQ0ob92GhEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Efficient recovery of Sph-1-P and LPA after the repeated phase separations under alkaline and acid conditions. / (A) After the initial phase separation of [32P]Sph-1-P, [3H]Sph-1-P, [3H]LPA, [3H]Sph, [3H]C6-Cer, or [14C]sphingomyelin (SM) under alkaline conditions, the resultant aqueous upper phase was phase-separated again under the acidic conditions. The radioactivities of each radiolabeled lipid into the final lower chloroform phase were counted by liquid scintillation counting. The percentage of recovery of each radiolabeled lipid into the final lower chloroform phase was calculated as 100 × the radioactivity in the final lower chloroform phase/the radioactivity originally added. (B) [3H]Sph-1-P (left lane) or [3H]LPA (right lane), recovered in the final lower chloroform phases, was analyzed by TLC autoradiography. Note that no bands other than [3H]Sph-1-P and [3H]LPA were detected.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364002.jpeg?Expires=1765247534&Signature=g4o5MbgztYr1dWVMdBiUwLBVqtSsUB-XwdY60xQuwPH7vFEZPKDIlcwKVONajmox6lEoq2CSv4FtPsP7hYEsv~z1UWm95P4pW-BYDGqTsnyzWEMHh9yElKUrnZOH7LSJSs7hfPV3JmDfQut8HYUjqN9jIpcXwblrcoInItJ2Rc1AAqFHGAnbEcvXpmfua8xuaKqjMzywWpbkye0liUTdwvH94NHvSltvMvQGOAsMvmGCQrDlwZJ~1yzV0kBU0u870XNKvRDVS0jqy6Luepoc2yPS~mJV2UeWClf6WzlRq9aqYqxXF2SbdRpT-YhqJM7DeaZSxm~90EsKhJN~5Qv-cQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Detection of [32P]lysophospholipids in [32P]orthophosphate-labeled platelets. / Human platelets labeled with [32P]orthophosphate were incubated without (−) or with 1 U/mL of thrombin (Thr), 20 μg/mL of collagen (Col), or 5, 10, or 20 μmol/L Sph for 5 minutes. Sph-1-P and LPA were first extracted from these platelets into the upper aqueous phase under alkaline conditions, followed by re-extraction into the lower chloroform phase under acidic conditions. The final chloroform phase was analyzed by TLC autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364003.jpeg?Expires=1765247534&Signature=HP8PrycTgCfXsdacv8Z6GicYj9LvKs9t98rydVC-FUNdfQpWgPvKFxuCxCFFnj4tdfP3F5j2-FVpDPRG7tVR31hGF46OE7hDWFgS5DrygsysA0SevusM1reOpAKrXKMUnAsFMYOFCtrADPz72WzaFYmXHu53so985lcT8ynwz2kDhhAyzU7a1pyW3mL8XiO10KkwzDv6zZybwaWmN1DJwux8XX2Voz9pFwxpg-UHfxy~fdfdS9Wm8s2ufiNEve3vXWL7P2GSm9jAYyhSLM8OtCwBu4nLpmchYGiNwTOj06CWhDzVcrhoArXNYFWPLgf2Q-gOyQEwW-oA0OOyJGwfsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Formation of [32P]PA in [32P]orthophosphate-labeled platelets stimulated with thrombin or collagen. / Platelets labeled with [32P]orthophosphate were incubated without (−) or with 1 U/mL of thrombin (Thr), 20 μg/mL of collagen (Col), or 5 μmol/L Sph for 5 minutes. The lipids were extracted from these platelets, and the lower chloroform phase under alkaline conditions was analyzed for [32P]PA formation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364004.jpeg?Expires=1765247534&Signature=vPnhzGNxWr0BlkIwFX8p47hJu7xFe6j8sEcx3QQ4d1yzUERsQMTTh-TublGWPNYikLITIE4YetoJtKH1P4lpQo3e-O8rQBQAPyHXlZy6VUojyExkVMiJmHkt839ZN0hs~evjnfO-YQbDJjoBCRky5NbI6l0K-h~pDtDDikd~L8d3ETb3MaMbWdMXG~~-abF8YHPAExbUFGg8n65amkU6QSzmPbbjuXxZyxbg6IpVTfBBt2TkD9vufpNK2EHspbcdmrl9Lo60-iirMGX0l3XcXS-ok5gP4bcnx6XrLRIggXw2Tt1zYyhoncJ7jOuBr2znLK26cBhpoO03giyMIpTyPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Albumin dependence of Sph-1-P release from activated platelets. / (A) Platelet suspensions, containing 1% BSA (lower lanes) or not (upper lanes), were incubated with [3H]Sph for 6 minutes and further stimulated without (a and d) or with 0.5 U/mL of thrombin (b and c) or 1 μmol/L TPA (e and f) for 5 minutes. In c and f, 1 μmol/L staurosporine was added 2 minutes before stimulation; this protein kinase inhibitor, by itself, failed to affect Sph-1-P release. After incubation, samples were centrifuged, and lipids were extracted from the resultant medium supernatant (M) and cell pellet (C) to detect [3H]Sph-1-P release from platelets. (B) Thrombin- or TPA-induced [3H]Sph-1-P release from platelets pretreated without or with staurosporine in the absence (■) or presence (▪) of extracellular 1% BSA was calculated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364005.jpeg?Expires=1765247534&Signature=hjpzLs5O3F8t22QMV5zTtiSdPJZQSAvhhbQASSYBZ1UaoqiopoeloseCRM3Wuq-rPTX1Qj47osV9-XUM5pEa-8-qZE9H0MbxEfOjxqWJ9ioS5TWF7~zjOkIsf4fMjdnLu-BPQxmAdlLOHiuqoR7YxdnvehDSB460b1G0gQPzoBPHDij08Bem-TsOfG662Nqxho9Vqb94nRJSWNU3-ByGaCpxSdPhyuZueErK7-7Y7Mjb1rCWfnAbdxDHoocB36zmJJx~DEzcI5r-A4NSPjNNKBswvEtrEvnrKumCGVABF4mqFAUEKGJgkNhokxOljcql7EUoHKtsNajqS-LtD6YMJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Co-fractionation of [3H]Sph-1-P with BSA. / Sph-1-P, containing trace amounts of [3H]Sph-1-P, was incubated with equimolar amounts of intact BSA (A) or denatured BSA (B). The mixtures were fractionated in a gel-filtration column, and fractions were collected and assayed for their [3H]Sph-1-P radioactivity and BSA concentration.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364006.jpeg?Expires=1765247534&Signature=WpuwlLpagwHNGNNHEc7uDWuIltsOJKX4nP2lO5M2DclvG0gw7zdo-CfoYlMcFWrXOjz1k6RgzIMYqXmeVcGkIvSav1ZMgJ7X6atn4c8p8wsjuUbrlXcHFCns3m3QwhHPlFHbNl7TY9kwHFB25DIjfk4FyIi-sqUK-mKNGtGKUVB1JhDBoD8H0Hrz2J6vb~hOZoRNNswkuCvAAZHP-OJRtqVd0ZtzWm1vw4lgJ~WnsxUX71fklLrJpQ5mzJ0tEl8dYCxnKcFfgHgor6Z6OphV30576-PH2u7DCKTqjzbGPv2jPWtgJpY4WenlFDHOdcU5l8Tiuc5sna2fpD1DpmOjQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Detection of mRNA expression by RT-PCR for the Sph-1-P and LPA receptors in HUVECs. / Amplified products for edg-1 (lane 1), -2 (lane 2), -3 (lane 3), and -4 (lane 4) were resolved on 2% agarose gel. The far right lane contained the size standards with positions (base pairs [bp]) indicated. The specific amplified products for edg-1, -2, -3, and -4 are 158, 283, 170, and 289 bp, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364007.jpeg?Expires=1765247534&Signature=xBpqXwcHIbN0bHyU2Tfl6IkqxAuQkyfwaoU3cKc-hM2KV4HVLM8CEflbookmO9kSwphkp3wmYg2xjm1KFGEinMetX2BLfGv9Y8C0INYQylZxlBYXZqCp5xRM8o4CNG3dSFQvZWQuxd-KN4Fg~AWwAGqX5eKjkLu-9d20-eusmxm4uCUivw93V-yYkgm86DeEXdQU0eyHo~V6qwvRpOqbbPJp0hRfP-VedM1XcG3Ea2ucWEdwj0kRWj1RmQxRqGBAJe04BAJoPhmWOz3N1fikMIwi0eXG9A3de5lITXONrH0BcoJLkV9O87UH7RaelV3fGmvn29wqnqNix1pHZu1i8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effects of Sph-1-P or LPA on HUVEC [Ca++]i. / (A) Fura2-loaded HUVECs were challenged with 1 μmol/L Sph-1-P or LPA. Changes in [Ca++]i were monitored by the ratio of fura2 fluorescence. Arrows indicate the addition of Sph-1-P and LPA. (B) Fura2-loaded HUVECs were stimulated with various concentrations of Sph-1-P (○) or LPA (●), and the increases in [Ca++]i (the ratio of fura2 fluorescence) were measured.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3431/5/m_h82200364008.jpeg?Expires=1765247534&Signature=XN2QWcZ7mmlwUJeYPSquQDHF4EXnf78PL2bPd0JitMJx1IPk2IE0-fJbNtB7i8cjgpmK8qC4Dhu-F8pd17RWOQcnRHHGFMh4wIfgZsvxllZDXXrob8gAK27mXWP8PA~XI3mE1Z-WcBopXUVKukPKVldKpvBiSUlsq1b2GJIiYUYwcOxdqm2ZMHTXui5V8DQ7QaELZsKsdvYiAJEbGMlWEB7fVw67XnGl6ZRQ7JPd0K6T6CKgtaZnlu2iGzSn0MqyXsaWV7MyahnRlOvqsNfhkV2hdz0C65GnFZx4MgkXPrCJywPkucwnRaimS97-2DlEWnv~g8kff7C~Sy7ulvURCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)