Abstract
FcγRIIA, the only Fcγ receptor present in platelets, is involved in heparin-associated thrombocytopenia (HIT). Recently, adenosine diphosphate (ADP) has been shown to play a major role in platelet activation and aggregation induced by FcγRIIA cross-linking or by sera from HIT patients. Herein, we investigated the mechanism of action of ADP as a cofactor in FcγRIIA-dependent platelet activation, which is classically known to involve tyrosine kinases. We first got pharmacologic evidence that the ADP receptor coupled to Gi was required for HIT sera or FcγRIIA clustering-induced platelet secretion and aggregation. Interestingly, the signaling from this ADP receptor could be replaced by triggering another Gi-coupled receptor, the α2A-adrenergic receptor. ADP scavengers did not significantly affect the tyrosine phosphorylation cascade initiated by FcγRIIA cross-linking. Conversely, the Gi-dependent signaling pathway, initiated either by ADP or epinephrine, was required for FcγRIIA-mediated phospholipase C activation and calcium mobilization. Indeed, concomitant signaling from Gi and FcγRIIA itself was necessary for an efficient synthesis of phosphatidylinositol 3,4,5-trisphosphate, a second messenger playing a critical role in the process of phospholipase Cγ2 activation. Altogether, our data demonstrate that converging signaling pathways from Gi and tyrosine kinases are required for platelet secretion and aggregation induced by FcγRIIA.
Introduction
There is now growing evidence that FcγRIIA, the subclass of Fc receptors predominantly expressed in platelets,1 is able to transduce an activating signal that contributes to the rapid destruction of these cells during immune thrombocytopenia. This is well documented in the case of important venous and arterial thrombotic complications occurring in heparin-induced thrombocytopenia (HIT) and in some autoimmune diseases.2-5 Moreover, a number of platelet-activating monoclonal antibodies directed against several major platelet membrane glycoproteins, such as CD9, CD36, GPIb, or the integrin αIIbβ3, require an intact immunoglobulin (Ig) G Fc domain for platelet activation, implying that binding to FcγRIIA, of the same or an adjacent platelet, is crucial in the activation process.6 The specific clustering of FcγRIIA is also sufficient per se to trigger platelet activation.7Addition of the nonactivating specific anti-FcγRIIA monoclonal antibody IV-3, followed by addition of F(ab′)2 fragments of a secondary antimouse antibody to immune cells or platelets, is now a classical model widely used to study the signaling pathways evoked by this receptor.8 The cytosolic tail of FcγRIIA bears 3 tyrosine residues. Two of them belong to a specific sequence closely related to the immunoreceptor-tyrosine based activation motif (ITAM) found in the cytoplasmic domains of several Ig gene family receptors, including B-cell receptor complex.7,9,10 Upon clustering, FcγRIIA becomes rapidly phosphorylated on tyrosines, potentially through a protein tyrosine kinase of the Src family.9 A role for Lyn in this phosphorylation has, for instance, been suggested in neutrophils.11 Subsequently, tyrosine phosphorylated FcγRIIA plays a role as a docking protein and specifically recruits SH2 domain-containing signaling proteins, including the tyrosine kinase Syk.7,9,12 The 2 SH2 domains of Syk interact with the 2 phosphotyrosines of the ITAM-like motif of FcγRIIA, leading, at least in vitro, to the stimulation of its tyrosine kinase activity.12 In platelets, this event is required for tyrosine phosphorylation and activation of phospholipase Cγ2 (PLCγ2, a key enzyme in the early activation process initiated by FcγRIIA.13 14
Recent data suggest that the early steps of platelet activation by FcγRIIA clustering may be modulated by an important cofactor secreted by platelets. Indeed, an interesting observation by Hérault et al,15 confirmed by Polgàr et al,16 indicates that secreted adenosine diphosphate (ADP) is required for platelet secretion and aggregation induced by HIT sera and FcγRIIA cross-linking. This major observation suggests that ADP receptor antagonists may be effective as therapeutic agents for prevention or treatment of HIT. At least 3 distinct ADP receptors17 are present on the platelet surface. The role of the ionotropic P2X1 purinergic receptor is not yet clearly understood in these cells. Conversely, both the P2Y1 purinergic receptor coupled to Gq and a not yet identified P2 receptor, negatively coupled to adenylyl cyclase, are required for ADP-induced aggregation.18 Evidence is now accumulating that the P2Y1 purinergic receptor coupled to Gq mediates Ca++mobilization and shape change, whereas the other P2 receptor activates Gi proteins.18 The Gi-coupled ADP receptor seems to be involved in potentiating the effects of other platelet agonists,19 including the thrombin receptor–activating peptide (TRAP).20 21
Here, we investigated the molecular mechanisms by which ADP plays its crucial role as coactivator following FcγRIIA cross-linking, a model classically known to involve tyrosine kinases.7 9 Our data indicate that both Gi-dependent and tyrosine kinase–dependent signaling pathways were required for platelet secretion and aggregation induced by FcγRIIA clustering or HIT sera. A key target of these converging signaling pathways appeared to be phosphatidylinositol 3,4,5-trisphosphate (PtdIns[3,4,5]P3), a lipid second messenger playing a critical role in the early steps of PLCγ2 activation and the subsequent calcium mobilization and phosphatidic acid (PtdOH) production.
Materials and methods
Reagents
The anti-FcγRII monoclonal antibody (moAb IV.3), the rabbit polyclonal antibody against the linker for activation of T cells (LAT), and the monoclonal antiphosphotyrosine 4G10 antibody were purchased from Upstate Biotechnology Inc. The specific F(ab′)2fragments were from Jackson Immunoresearch Laboratories, and the rabbit polyclonal anti-PLCγ2 antibody was from Santa Cruz Biotechnology Inc. [32P]orthophosphate, 5-hydroxy[14C]tryptamine (56.0 mCi/mmol), and enhanced chemiluminescence (ECL) Western blotting reagents were from Amersham International. AR-C69931MX was a generous gift from Dr J. Turner (ASTRA Charnwood, UK). Thin-layer chromatography (TLC) plates were from Merck (Nogent-sur-Marne, France), and all other reagents were purchased from Sigma (Saint Quentin-Fallavier, France) unless otherwise indicated.
HIT serum samples
HIT was identified in patients whose platelet count was below 100 × 109/L or who experienced a 50% decrease in platelet count for no apparent reason other than heparin administration. These sera were aliquoted and stored at −80°C until use. Prior to use in any HIT assay, the sera were heated at 56°C for 1 hour and centrifuged to remove any residual thrombin activity. Sera were designated as positive or negative based on their ability to promote platelet aggregation in an HIT aggregation system and with regard to the so-called “SRA” assay as described previously.22
Preparation and activation of platelets
Human blood platelet concentrates were obtained from the local blood bank (Etablissement de Transfusion Sanguine, Toulouse, France). Platelets were prepared essentially as described previously.23 Briefly, they were washed in a washing buffer (pH 6.5) containing 140-mmol/L NaCl, 5-mmol/L KCl, 5-mmol/L KH2PO4, 1-mmol/L MgSO4, 10-mmol/L HEPES, 5-mmol/L glucose, and 0.35% bovine serum albumin (wt/vol). The same buffer containing 1-mmol/L CaCl2 was added to the final suspension, and pH was adjusted to 7.4.
For inositol lipid analysis, platelets were labeled with 0.5-mCi/mL of [32P]orthophosphate during 60 minutes in a phosphate-free washing buffer (pH 6.5) at 37°C. [32P]-labeled platelets were then washed once in the same buffer and finally resuspended at a final concentration of platelets of 1000 × 109/L (pH 7.4). Cross-linking of the low-affinity receptor for IgG, FcγRIIA, was performed by preincubation of platelets for 1 minute with the monoclonal antibody IV.3 (2 μg/mL) followed by stimulation for different periods by addition of antimouse IgG F(ab′)2 (30 μg/mL) at 37°C under gentle shaking as described previously.8 For activation of platelets by HIT serum, 400 μL of platelet suspension was incubated with heparin (0.5 IU) and HIT sera (80 μL). When indicated, 1-IU/mL apyrase, 5-mmol/L creatine phosphate (CP), 40-IU/mL creatine phosphokinase (CPK), 500-μmol/L A3P5PS, 50-μmol/L adenosine triphosphate (ATP)γS, or 1-μmol/L epinephrine was added 1 minute before stimulation at 37°C.
Calcium flux measurements
Platelets were prepared as described above with slight modifications. Platelet-rich plasma was incubated for 30 minutes at 37°C with 1-μmol/L Fura 2-acetoxymethylester, washed, and the final platelet concentration adjusted to 300 × 109cells/L in the stimulation buffer. The fluorescence excitation wavelengths and the emission wavelength were 340 nm, 380 nm, and 510 nm, respectively. Platelets were preincubated and stirred for 1 minute at 37°C in the presence of 1-mmol/L EGTA and were stimulated by FcγRIIA cross-linking in the presence or absence of inhibitors and epinephrine as indicated. The changes in fluorescence were recorded using a PTI Deltascan spectrofluorometer.
Lipid extraction and analysis
Reactions were stopped by addition of chloroform/methanol (vol/vol), and lipids were extracted following a Bligh and Dyer modified procedure.24,25 Lipids were first resolved by TLC using chloroform/acetone/methanol/acetic acid/water (80/30/26/24/14, vol/vol). The spots corresponding to PtdIns(3,4,5)P3 were then scraped off, deacylated by 20% methylamine, and analyzed by high-performance liquid chromatography on a Whatman Partisphere 5 SAX column (Whatman International Ltd, United Kingdom) as described previously.25 For PtdOH quantification, lipids were resolved by TLC using CHCl3/CH3OH/HCl 10N (87/13/0.5, vol/vol) as described previously.26
Platelet aggregation and 5-hydroxytryptamine secretion studies
Aggregation was monitored by a turbidimetric method using a dual-channel Payton aggregometer (Payton Assoc, Scarborough, ON) with continuous stirring at 900 rev/min at 37°C (500 × 109platelets/L). Secretion of 5-hydroxytryptamine was performed as described previously.27 Briefly, platelets loaded with 5-hydroxy[14C]tryptamine were preincubated or not with different ADP inhibitors: A3P5PS (500 μM), CP (5 mmol/L), and CPK (40 IU/ML) for 1 minute and stimulated by FcγRIIA cross-linking during 3 minutes in the presence of 5-μmol/L imipramin. Incubations were stopped by addition of 3% formaldehyde, 0.1-mol/L ethylenediaminetetraacetic acid (EDTA), cooling on ice, and centrifugation. The 5-hydroxy[14C]tryptamine released from platelet-dense granules was determined by liquid-scintillation counting.25
Gel electrophoresis and immunoblotting
Proteins were resuspended in electrophoresis sample buffer containing 100-mmol/L Tris-HCl (pH 6.8), 15% (vol/vol) glycerol, 25-mmol/L dithiothreitol, and 3% sodium dodecyl sulfate (SDS), boiled for 5 minutes, separated on 7.5% SDS–polyacrylamide gel electrophoresis (PAGE), and transferred onto a nitrocellulose membrane (Gelman Sciences). The nitrocellulose was blocked for 60 minutes at room temperature with 1% (wt/vol) milk powder and 1% (wt/vol) bovine serum albumin in a TBST buffer containing 10-mmol/L Tris-HCl (pH 7.5), 150-mmol/L NaCl, and 0.05% (wt/vol) Tween 20 as reported previously.25 Immunodetection was achieved using the relevant antibody, peroxidase-conjugated secondary antibody, and the ECL system.
Immunoprecipitation
For PLCγ2 and LAT immunoprecipitations, reactions were stopped by addition of 1 volume of ice-cold 2 × lysis buffer containing 80-mmol/L Tris-HCl (pH 7.4), 200-mmol/L NaCl, 200-mmol/L NaF, 20-mmol/L EDTA, 80-mmol/L Na4P2O7, 4-mmol/L Na3VO4, 2% Triton X-100 (vol/vol), and 10 μg/mL each of aprotinin and leupeptin. After gentle shaking during 20 minutes at 4°C and centrifugation (12 000g for 10 minutes at 4°C), the soluble fraction was collected and precleared for 30 minutes at 4°C with protein A-Sepharose CL4B. The precleared suspensions were incubated overnight at 4°C with the anti-PLCγ2 antibody or anti-LAT antibody, and immune complexes were then precipitated by addition of 10% (wt/vol) protein A-Sepharose CL4B for 1 hour at 4°C and centrifugation (6000g for 5 minutes at 4°C). The immunoprecipitates were washed once in 1 × lysis buffer and twice in a washing buffer containing 10-mmol/L Tris-HCl (pH 7.4), 100-mmol/L NaCl, 100-μmol/L Na3VO4, and 1 μg/mL each of aprotinin and leupeptin. Immunoprecipitated proteins were resolved by 7.5% SDS-PAGE and analyzed by Western blotting. For FcγRIIA immunoprecipitation, reactions were stopped by addition of 1 volume of ice-cold 2 × RIPA buffer containing 2-mmol/L Na3VO4, 10-mmol/L EDTA, 20-mmol/L Tris (pH 7.4), 320-mmol/L NaCl, 0.2% SDS, 2% sodium deoxycholate, 2% NP-40, and 10 μg/mL each of aprotinin and leupeptin. After gentle shaking during 20 minutes at 4°C and centrifugation (12 000g for 10 minutes at 4°C), the soluble fraction was collected. The suspensions were then incubated for 1 hour at 4°C with the MoAb IV.3, and immune complexes were then precipitated by addition of 10% (wt/vol) pansorbin for 30 minutes at 4°C and centrifugation (6000g for 5 minutes at 4°C). The immunoprecipitates were washed 3 times in 1 × RIPA buffer. Immunoprecipitated proteins were resolved by 10% SDS-PAGE and analyzed by Western blotting.
Results
Requirement of a Gi-dependent pathway initiated either by ADP or epinephrine for FcγRIIA-mediated platelet secretion and aggregation
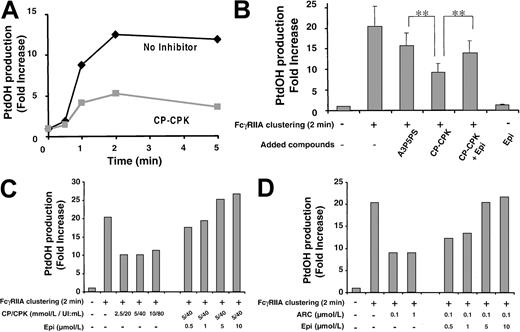
As previously shown,15,16 platelet aggregation induced by FcγRIIA clustering (Figure1A) or by addition of HIT sera (Figure1B) were both fully inhibited in the presence of the 2 unrelated ADP scavengers, apyrase or CP-CPK. In agreement with these results, ATPγS, an antagonist of ADP platelet receptors, strongly inhibited platelet aggregation. In the presence of A3P5PS, a specific platelet P2Y1 purinergic receptor antagonist,28-30 aggregation was not significantly affected. Conversely, the selective antagonist of the ADP receptor coupled to Gi, AR-C69931MX,31 strongly inhibited FcγRIIA-mediated platelet aggregation. Epinephrine, known to selectively activate Gi proteins via the α2-adrenergic receptor, could overcome the inhibitory effect of CP-CPK on FcγRIIA and HIT sera-induced platelet aggregation (Figure 1, right panels). It is noteworthy that epinephrine per se does not induce platelet shape change, calcium mobilization, inositol trisphosphate formation, fibrinogen binding, and aggregation,32,33although it potentiates platelet aggregation induced by other agonists.32 Altogether, these results strongly suggest that the not yet identified platelet ADP receptor coupled to Gi was essential for the coactivation effect of ADP. Moreover, serotonin secretion was also strongly inhibited by ADP scavengers (Figure2) but not affected by A3P5PS. Again, epinephrine did not induce secretion per se but was able to replace ADP as a coactivator of FcγRIIA to obtain secretion (Figure2).
The inhibitory effect of ADP scavengers on platelet aggregation induced by FcγRIIA cross-linking or by HIT sera is reversed by epinephrine.
Human platelet suspensions were preincubated (1 minute, 37°C) with 500-mol/L A3P5PS, 0.1 μmol/L AR-C69931MX, 1-IU/mL apyrase, 50-μmol/L ATPγS, 5-mmol/L CP, and 40-IU/mL CPK or CP-CPK plus 0.5-μmol/L or 1-mol/L epinephrine and then stimulated by FcγRIIA cross-linking (A) or by 80 μL of sera from HIT patients (B) as indicated in “Materials and methods.” Aggregation was assessed using a Chrono-Log dual-channel aggregometer with stirring at 900 rev/min (5 × 108 cells/mL).
The inhibitory effect of ADP scavengers on platelet aggregation induced by FcγRIIA cross-linking or by HIT sera is reversed by epinephrine.
Human platelet suspensions were preincubated (1 minute, 37°C) with 500-mol/L A3P5PS, 0.1 μmol/L AR-C69931MX, 1-IU/mL apyrase, 50-μmol/L ATPγS, 5-mmol/L CP, and 40-IU/mL CPK or CP-CPK plus 0.5-μmol/L or 1-mol/L epinephrine and then stimulated by FcγRIIA cross-linking (A) or by 80 μL of sera from HIT patients (B) as indicated in “Materials and methods.” Aggregation was assessed using a Chrono-Log dual-channel aggregometer with stirring at 900 rev/min (5 × 108 cells/mL).
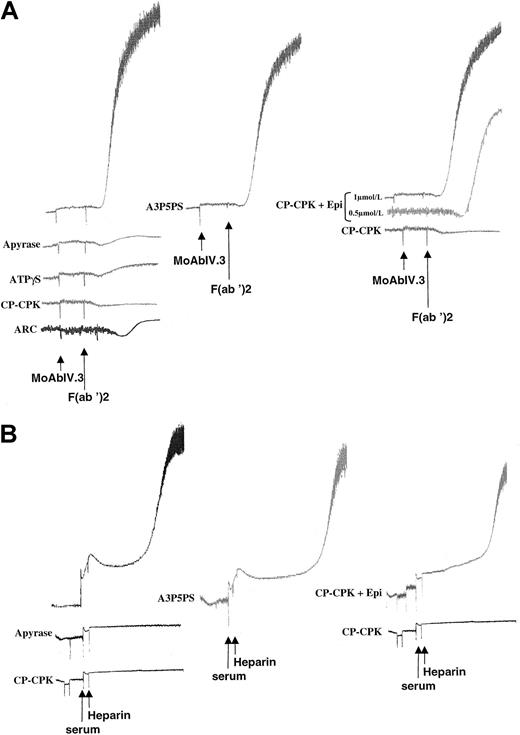
The inhibitory effects of ADP scavengers on serotonine secretion evoked by FcγRIIA is reversed by epinephrine.
Platelets were previously loaded with 5-hydroxy[14C]tryptamine, and secretion was determined as described in “Materials and methods.” A3P5PS, CP-CPK, and CP-CPK and 1-μmol/L epinephrine were added 1 minute before stimulation by FcγRIIA cross-linking. The effect of 1-μmol/L epinephrine alone was also assessed. Data are the mean of 2 independent experiments. (A) Stimulation by FcγRIIA cross-linking; (B) stimulation by HIT sera.
The inhibitory effects of ADP scavengers on serotonine secretion evoked by FcγRIIA is reversed by epinephrine.
Platelets were previously loaded with 5-hydroxy[14C]tryptamine, and secretion was determined as described in “Materials and methods.” A3P5PS, CP-CPK, and CP-CPK and 1-μmol/L epinephrine were added 1 minute before stimulation by FcγRIIA cross-linking. The effect of 1-μmol/L epinephrine alone was also assessed. Data are the mean of 2 independent experiments. (A) Stimulation by FcγRIIA cross-linking; (B) stimulation by HIT sera.
These results suggest that an early step of the signal transduction cascade initiated by FcγRIIA was regulated by concomitant signaling from Gi.
Optimal FcγRIIA-mediated PLC activation and Ca++signaling require a Gi-dependent pathway initiated either by ADP receptor or by α2-adrenergic receptor
PLC activation, one of the key biochemical events related to platelet secretion, was first investigated. In32P-labeled platelets, the production of32P-PtdOH is a good marker of PLC activation.34,35 As shown in Figure3A,C, FcγRIIA-mediated PtdOH synthesis was strongly inhibited in the presence of CP-CPK. A3P5PS had a weak inhibitory effect probably due, at least in part, to the inhibition of the Gq-dependent activation of PLC via the P2Y1 ADP receptor. Increasing concentrations of A3P5PS, up to 1.1 mmol/L, did not significantly amplify this effect (not shown). This is consistent with the fact that 10 μmol/L of ADP alone was able to induce a very modest production of PtdOH (not shown) as previously observed.36In agreement with these results, Ca++ mobilization (Figure4) was also strongly inhibited in the presence of CP-CPK (72% ± 8% of inhibition, n = 4) whereas A3P5PS had a weak but significant inhibitory effect (36% ± 13% of inhibition, n = 3). Nevertheless, the partial inhibition of PLC and Ca++ mobilization by the P2Y1 antagonist A3P5PS was not sufficient to lead to a detectable decrease in platelet secretion and aggregation (Figures 1 and 2).
CP-CPK inhibits FcγRIIA-mediated PtdOH production.
32P-labeled platelets were incubated or not with CP-CPK, A3P5PS, or CP-CPK and 1-μmol/L epinephrine (1 minute, 37°C) as described in “Materials and methods” and activated by FcγRIIA cross-linking during different periods of time (A) or during 2 minutes (B). Increasing concentrations of CP-CPK (C) or AR-C69931MX (D) were also tested. The inhibitory effects of these compounds were overcome in a dose-dependent manner by epinephrine (C,D). Lipids were immediately extracted, PtdOH was separated by TLC, and the radioactivity incorporated into PtdOH was quantified by PhosphorImager analysis. Data are representative of 2 independent experiments with very similar results (A), mean ± standard errors of 6 independent experiments (B), or representative of 3 independent experiments (C,D). **Significant difference (P < .01) according to Studentt test.
CP-CPK inhibits FcγRIIA-mediated PtdOH production.
32P-labeled platelets were incubated or not with CP-CPK, A3P5PS, or CP-CPK and 1-μmol/L epinephrine (1 minute, 37°C) as described in “Materials and methods” and activated by FcγRIIA cross-linking during different periods of time (A) or during 2 minutes (B). Increasing concentrations of CP-CPK (C) or AR-C69931MX (D) were also tested. The inhibitory effects of these compounds were overcome in a dose-dependent manner by epinephrine (C,D). Lipids were immediately extracted, PtdOH was separated by TLC, and the radioactivity incorporated into PtdOH was quantified by PhosphorImager analysis. Data are representative of 2 independent experiments with very similar results (A), mean ± standard errors of 6 independent experiments (B), or representative of 3 independent experiments (C,D). **Significant difference (P < .01) according to Studentt test.
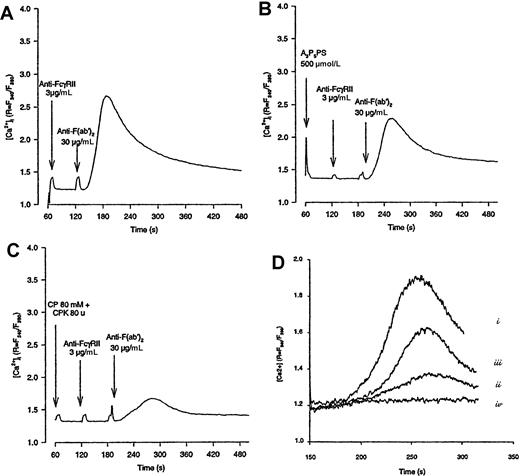
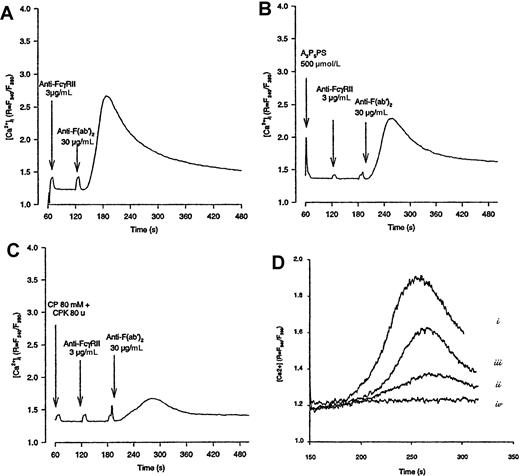
Epinephrine overcomes the inhibitory effect of CP-CPK on FcγRIIA-mediated Ca++ mobilization.
Platelets were loaded with 1-μmol/L Fura-2 as described in “Materials and methods” and stimulated by FcγRIIA cross-linking in the absence (A) or in the presence of A3P5PS (B) or CP-CPK (C). In (D), platelets were stimulated by FcγRIIA cross-linking in the absence (i) or in the presence of CP-CPK (ii) or CP-CPK and epinephrine (iii). In (iv), platelets were stimulated by 1-μmol/L epinephrine alone. The variations in fluorescence, reflecting changes in intracellular Ca++ concentration, were monitored using a PTI Deltascan spectrofluorometer. Data are representative of 2 to 4 independent experiments.
Epinephrine overcomes the inhibitory effect of CP-CPK on FcγRIIA-mediated Ca++ mobilization.
Platelets were loaded with 1-μmol/L Fura-2 as described in “Materials and methods” and stimulated by FcγRIIA cross-linking in the absence (A) or in the presence of A3P5PS (B) or CP-CPK (C). In (D), platelets were stimulated by FcγRIIA cross-linking in the absence (i) or in the presence of CP-CPK (ii) or CP-CPK and epinephrine (iii). In (iv), platelets were stimulated by 1-μmol/L epinephrine alone. The variations in fluorescence, reflecting changes in intracellular Ca++ concentration, were monitored using a PTI Deltascan spectrofluorometer. Data are representative of 2 to 4 independent experiments.
Interestingly, as with CP-CPK, AR-C69931MX was able to strongly inhibit the production of PtdOH. In agreement with the results shown in Figures 1 and 2, epinephrine was able to overcome, in a dose-dependent manner, the inhibitory effects of CP-CPK or AR-C69931MX on FcγRIIA-mediated PtdOH production (Figure 3B-D). The inhibitory effect of CP-CPK on calcium mobilization was also significantly overcome by epinephrine (Figure 4D).
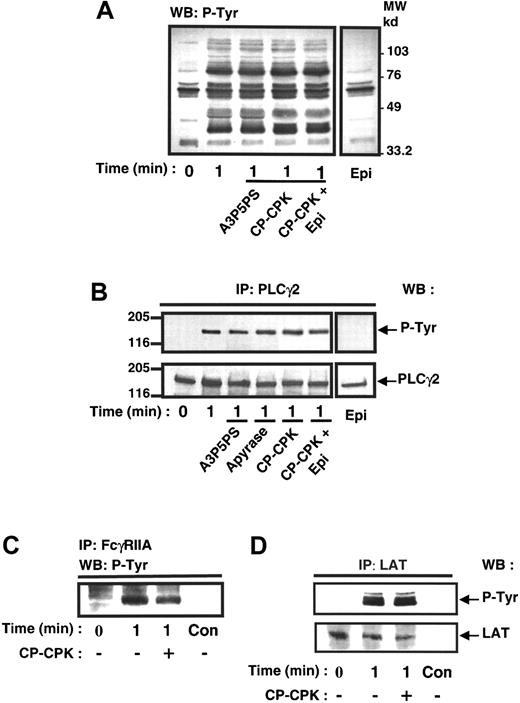
FcγRIIA stimulates the tyrosine phosphorylation of a set of proteins, including PLCγ2, independently of ADP and Gi pathway
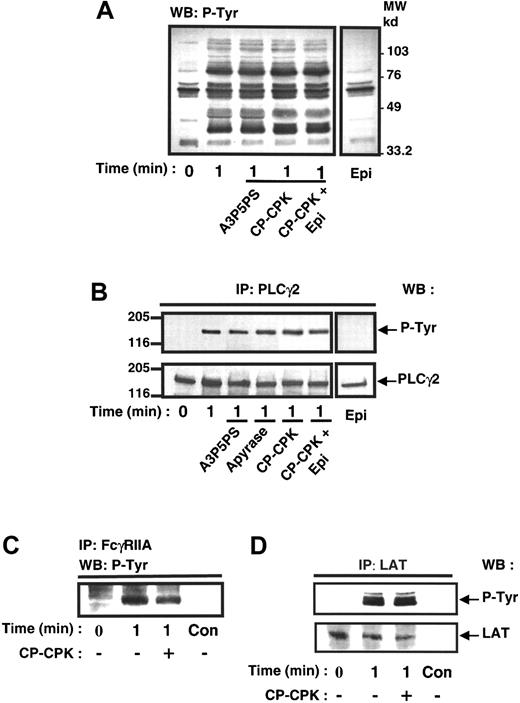
It is now well established that FcγRIIA cross-linking induces the activation of PLCγ2 through a mechanism involving its tyrosine phosphorylation.9,37 The contribution of the Gi pathway on tyrosine phosphorylation of PLCγ2 following FcγRIIA clustering was therefore evaluated. Figure 5A indicates that the whole pattern of phosphotyrosyl proteins in platelets stimulated by FcγRIIA cross-linking was not significantly affected by the ADP scavenger CP-CPK. Furthermore, the tyrosine phosphorylation of PLCγ2 was not impaired by addition of ADP scavengers (Figure 5B). In addition, Figure 5C,D shows that the rapid tyrosine phosphorylation of FcγRIIA itself as well as the tyrosine phosphorylation of LAT, a docking protein recently identified in platelets,38 were not significantly affected by the ADP scavenger.
ADP is not significantly involved in FcγRIIA-mediated protein tyrosine phosphorylation.
Platelets were preincubated in the absence or in the presence of A3P5PS, CP-CPK, and CP-CPK and 1 μmol/L epinephrine and stimulated by FcγRIIA cross-linking. The effect of 1-μmol/L epinephrine alone was also assessed. (A) Immunoblotting of platelet total protein extracts with the antiphosphotyrosine antibody 4G10. PLCγ2 (B), FcγRIIA (C), and LAT (D) were immunoprecipitated and submitted to immunoblotting with 4G10 antibody. As a loading control, the nitrocellulose membranes were stripped and reprobed with anti-PLCγ2 antibody and anti-LAT antibody (lower panels). (C) Immunoprecipitations performed with nonimmune serum as a control (C,D). Con, control. Data are representative of 3 to 4 independent experiments.
ADP is not significantly involved in FcγRIIA-mediated protein tyrosine phosphorylation.
Platelets were preincubated in the absence or in the presence of A3P5PS, CP-CPK, and CP-CPK and 1 μmol/L epinephrine and stimulated by FcγRIIA cross-linking. The effect of 1-μmol/L epinephrine alone was also assessed. (A) Immunoblotting of platelet total protein extracts with the antiphosphotyrosine antibody 4G10. PLCγ2 (B), FcγRIIA (C), and LAT (D) were immunoprecipitated and submitted to immunoblotting with 4G10 antibody. As a loading control, the nitrocellulose membranes were stripped and reprobed with anti-PLCγ2 antibody and anti-LAT antibody (lower panels). (C) Immunoprecipitations performed with nonimmune serum as a control (C,D). Con, control. Data are representative of 3 to 4 independent experiments.
These results indicate that the tyrosine kinase pathway initiated by FcγRIIA cross-linking is independent of ADP.
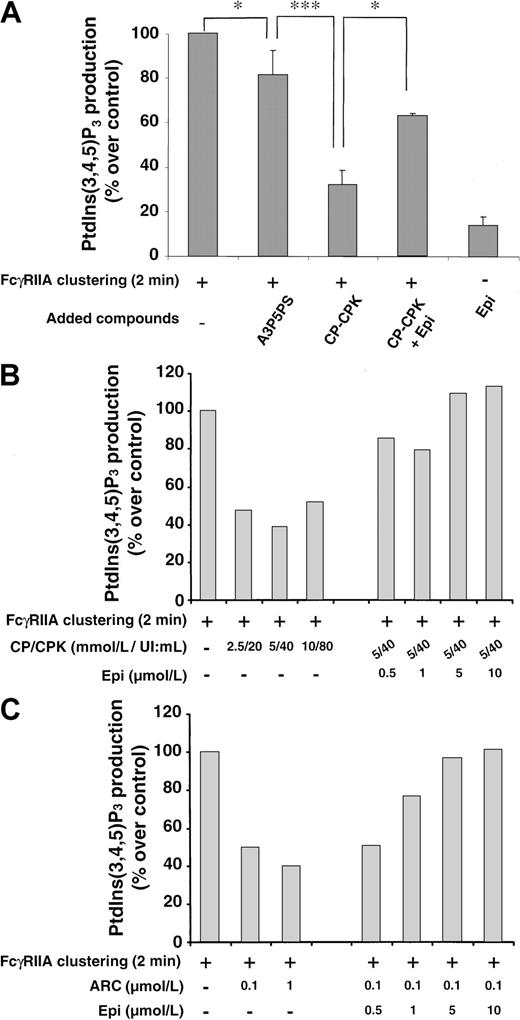
ADP is required for an optimal production of PtdIns(3,4,5)P3 in FcγRIIA-stimulated platelets and can be replaced by epinephrine
Recently, we have shown that the early synthesis of PtdIns(3,4,5)P3 is absolutely required for the activation of the tyrosine phosphorylated PLCγ2 upon FcγRIIA cross-linking.25 The role of ADP in the synthesis of this particular phosphoinositide was therefore investigated. Figure6A shows that PtdIns(3,4,5)P3production was strongly inhibited by the ADP scavenger CP-CPK (68.2% ± 12% of inhibition, n = 4). The P2Y1 antagonist A3P5PS was a weak inhibitor of this production (Figure 6A) even at high concentrations, up to 1.1 mmol/L (not shown). Conversely, AR-C69931MX was as efficient as CP-CPK to inhibit PtdIns(3,4,5)P3production (Figure 6B,C). As already described, ADP39 40 or epinephrine alone was only able to induce the production of trace amounts of PtdIns(3,4,5)P3. However, again, epinephrine could overcome, in a dose-dependent manner, the inhibitory effects of CP-CPK or AR-C69931MX (Figure 6B,C), indicating that this α2-adrenergic receptor agonist could replace ADP as a coactivator of FcγRIIA–mediated PtdIns(3,4,5)P3production.
The inhibitory effect of ADP scavengers on FcγRIIA-mediated PtdIns(3,4,5)P3 synthesis is reversed by epinephrine.
32P-labeled platelets were incubated with or without A3P5PS, CP-CPK, and CP-CPK and 1-μmol/L epinephrine (1 minute, 37°C) and activated by FcγRIIA cross-linking (A). The effect of 1-μmol/L epinephrine alone was assessed. Increasing concentrations of CP-CPK (B) and AR-C69931MX (C) were tested, and the inhibitory effects of these compounds were overcome in a dose-dependent manner by epinephrine (B,C). After 2 minutes of stimulation, lipids were extracted, and the radioactivity incorporated in PtdIns(3,4,5)P3 was determined as described in “Materials and methods.” Data are expressed as a percentage of PtdIns(3,4,5)P3 produced compared with control (100% being the production obtained by FcγRIIA clustering at 2 minutes) and are means ± standard errors of 3 independent experiments (A) or representative. *,***Significant difference (P < .05) and (P < .001), respectively, according to Student ttest.
The inhibitory effect of ADP scavengers on FcγRIIA-mediated PtdIns(3,4,5)P3 synthesis is reversed by epinephrine.
32P-labeled platelets were incubated with or without A3P5PS, CP-CPK, and CP-CPK and 1-μmol/L epinephrine (1 minute, 37°C) and activated by FcγRIIA cross-linking (A). The effect of 1-μmol/L epinephrine alone was assessed. Increasing concentrations of CP-CPK (B) and AR-C69931MX (C) were tested, and the inhibitory effects of these compounds were overcome in a dose-dependent manner by epinephrine (B,C). After 2 minutes of stimulation, lipids were extracted, and the radioactivity incorporated in PtdIns(3,4,5)P3 was determined as described in “Materials and methods.” Data are expressed as a percentage of PtdIns(3,4,5)P3 produced compared with control (100% being the production obtained by FcγRIIA clustering at 2 minutes) and are means ± standard errors of 3 independent experiments (A) or representative. *,***Significant difference (P < .05) and (P < .001), respectively, according to Student ttest.
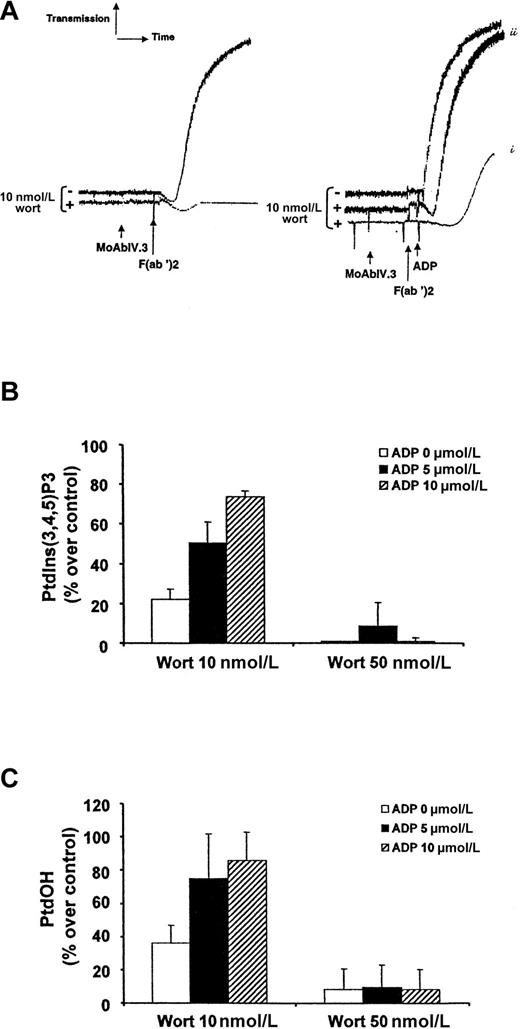
To further confirm the role of ADP as a cofactor in FcγRIIA-mediated phosphatidylinositol (PI) 3-kinase activation, we used the PI 3-kinase inhibitor wortmannin at a low concentration (10 nmol/L) to strongly, but not completely, inhibit the production of PtdIns(3,4,5)P3 (78% of inhibition) (Figure7). Under these conditions, platelet aggregation was fully inhibited (Figure 7A) and the production of PtdOH was inhibited by 63.8 ± 10% (Figure 7C). It is important to note that wortmannin does not affect tyrosine phosphorylation of PLCγ2 25 and that the remaining production of PtdIns(3,4,5)P3 did not induce sufficient activation of PLCγ2 to obtain platelet secretion (not shown) and aggregation (Figure 7A). Interestingly, addition of exogenous ADP to 10-nmol/L wortmannin-treated platelets could overcome the FcγRIIA-mediated PtdIns(3,4,5)P3 production (Figure 7B), PtdOH synthesis (Figure 7C), and platelet aggregation (Figure 7A). This effect of ADP was dose-dependent (Figure 7). The intracellular production of PtdIns(3,4,5)P3 and PtdOH is correlated with the intensity of platelet aggregation (Figure 7A-C). As expected, this effect of ADP was no longer observed with a dose of wortmannin (50 nmol/L) capable of irreversibly inhibiting most PI 3-kinase copies, subsequently blocking the production of PtdIns(3,4,5)P3 (Figure 7B). Although ADP by itself was a very weak activator of PtdIns(3,4,5)P3production (maximum, 1.4-fold increase upon 10 μmol/L ADP), these results indicate that it is a crucial FcγRIIA cofactor for the production of this lipid second messenger, subsequent PtdOH synthesis, calcium mobilization, and platelet aggregation. Moreover, epinephrine could again replace ADP to overcome the inhibitory effect of low doses of wortmannin (not shown), indicating that a Gi pathway is required for this mechanism. Finally, when exogenous ADP was added during FcγRIIA-mediated normal platelet activation, we observed a clear potentiation of PtdIns(3,4,5)P3 production in a dose-dependent manner (1.8- and 2.2-fold increase at 5- and 10-μmol/L ADP, respectively).
ADP overcomes the inhibitory effect of low doses of wortmannin on PtdIns(3,4,5)P3 production, PtdOH synthesis, and platelet aggregation.
Human platelet suspensions were preincubated with or without 10-nmol/L wortmannin (2 minutes, 37°C) and stimulated by FcγRIIA cross-linking in the absence (left panel) or in the presence (right panel) of 5-μmol/L (i) or 10-μmol/L (ii) ADP (A). Aggregation was assessed using a Chrono-Log dual-channel aggregometer with stirring at 900 rev/min. (B,C) 32P-labeled platelets were incubated with 10 or 50-nmol/L wortmannin (2 minutes, 37°C) and activated by FcγRIIA cross-linking in the presence or in the absence of 5-μmol/L or 10-μmol/L ADP as indicated. After 2 minutes of stimulation, lipids were extracted and the radioactivity incorporated in PtdIns(3,4,5)P3 (B) or PtdOH (C) was determined as described in “Materials and methods.” Data are expressed as a percentage of PtdIns(3,4,5)P3 or PtdOH produced compared with control (100% being the production obtained by FcγRIIA clustering at 2 minutes) and are means ± standard errors of 3 independent experiments.
ADP overcomes the inhibitory effect of low doses of wortmannin on PtdIns(3,4,5)P3 production, PtdOH synthesis, and platelet aggregation.
Human platelet suspensions were preincubated with or without 10-nmol/L wortmannin (2 minutes, 37°C) and stimulated by FcγRIIA cross-linking in the absence (left panel) or in the presence (right panel) of 5-μmol/L (i) or 10-μmol/L (ii) ADP (A). Aggregation was assessed using a Chrono-Log dual-channel aggregometer with stirring at 900 rev/min. (B,C) 32P-labeled platelets were incubated with 10 or 50-nmol/L wortmannin (2 minutes, 37°C) and activated by FcγRIIA cross-linking in the presence or in the absence of 5-μmol/L or 10-μmol/L ADP as indicated. After 2 minutes of stimulation, lipids were extracted and the radioactivity incorporated in PtdIns(3,4,5)P3 (B) or PtdOH (C) was determined as described in “Materials and methods.” Data are expressed as a percentage of PtdIns(3,4,5)P3 or PtdOH produced compared with control (100% being the production obtained by FcγRIIA clustering at 2 minutes) and are means ± standard errors of 3 independent experiments.
Discussion
ADP is required for platelet activation and aggregation induced by FcγRIIA cross-linking using either specific antibodies or sera from HIT patients.16 Besides the important therapeutic developments, these findings raise a number of crucial questions relating to the molecular mechanism of agonist-induced platelet activation. Several recent reports have also shown that, although ADP is a weak agonist per se, it plays an important role as coactivator of other platelet agonists such as collagen, TRAP, or thrombin.19-21,41,42 Moreover, according to the agonist used, ADP can play a role at different stages of platelet activation. It is involved in the stabilization of thrombin or TRAP-induced human platelet aggregation21 but also plays a critical role in the very early steps of FcγRIIA-dependent platelet activation.16 ADP can also potentiate platelet secretion independently of aggregation.43 These results strongly suggest that specific signaling pathways initiated by ADP receptors may modulate or amplify intracellular biochemical events initiated by other platelet agonists.
Here we show that A3P5PS, a P2Y1 ADP receptor antagonist, had no effect on FcγRIIA-mediated secretion and aggregation. Conversely, AR-C69931MX, a selective antagonist of the ADP receptor coupled to Gi,31 was a potent inhibitor of FcγRIIA-mediated platelet secretion (not shown) and aggregation. Interestingly, triggering of another receptor selectively coupled to Gi, the α2A-adrenergic receptor,18,32 33 could overcome the inhibitory effect of ADP scavengers. These observations indicated that a Gi-dependent pathway either initiated by ADP or epinephrine participated in FcγRIIA-induced platelet activation.
One of the first intracellular signaling events required for FcγRIIA-mediated platelet activation is tyrosine phosphorylation of the ITAM-like motif of this receptor. We found that this phosphorylation as well as the phosphorylation of other downstream targets, including the adaptor molecule LAT or PLCγ2, were not significantly affected by ADP scavengers. Another early major event of this activation cascade is the stimulation of enzymes of the phosphoinositide metabolism. Interestingly, the PtdOH production and the calcium mobilization, reflecting PLC activation, were both strongly inhibited by ADP scavengers. The selective block of the P2Y1 receptor had a weak effect on the production of PtdOH. This effect may correspond, at least partially, to the slight activation of PLC observed upon ADP addition by several groups.36Interestingly, AR-C69931MX inhibited the PtdOH production following FcγRIIA clustering as efficiently as CP-CPK, indicating that the major cofactor effect of ADP was actually due to its receptor negatively coupled to adenylyl cyclase. Moreover, addition of epinephrine could restore this inhibitory effect of CP-CPK on PtdOH production and calcium mobilization in a dose-dependent manner. PLCγ2 is activated downstream of tyrosine kinases and is an effector of Syk in FcγRIIA-mediated platelet activation.7,9,37 Recently, we demonstrated that the PI 3-kinase product PtdIns(3,4,5)P3 was absolutely required for activation of the tyrosine phosphorylated PLCγ2 following FcγRIIA clustering.25 It is interesting to note that, in contrast to the situation in B lymphocytes where BTK, a Tec family tyrosine kinase activated through PtdIns(3,4,5)P3production, is involved in the phosphorylation and the activation of PLCγ2,44 our results strongly suggest that PLCγ2 is not a major substrate for Tec family kinases in platelets stimulated through FcγRIIA, as also observed following activation of glycoprotein VI in mice platelets.45 These discrepancies may reflect a difference in the role of BTK between cell types or agonist-dependent activation and highlight a critical role of PtdIns(3,4,5)P3 as a direct cofactor of PLCγ2 activation possibly by allowing an adequate localization of this enzyme at the membrane-cytoskeleton interface. Thus, calcium mobilization and activation of protein kinase C, 2 events required for secretion, inside-out activation of the integrin αIIbβ3 and aggregation, are also indirectly regulated by PtdIns(3,4,5)P3 production. Interestingly, the production of PtdIns(3,4,5)P3 was strongly inhibited by ADP scavengers and AR-C69931MX, whereas the P2Y1 receptor antagonist, A3P5PS, had only a weak inhibitory effect. It is noteworthy that ADP or epinephrine alone are very weak activators of PtdIns(3,4,5)P3 production.39 40 Again, epinephrine could overcome the inhibitory effect of CP-CPK or AR-C69931MX in a dose-dependent manner. These results strongly suggest that concomitant signaling from tyrosine kinases, initiated by FcγRIIA cross-linking, and Gi-dependent pathway, initiated either by ADP or epinephrine, were required to reach a sufficient level of PtdIns(3,4,5)P3.
This is consistent with several reports that have placed a wortmannin-sensitive PI 3-kinase as a key signaling molecule in FcγRIIA-mediated platelet activation.12,25 46 To confirm the role of ADP in the regulation of the amount of PtdIns(3,4,5)P3, we have used low doses of wortmannin (10 nmol/L) capable of irreversibly inhibiting a large number of, but not all, PI 3-kinase copies. Under these conditions, 22% of the PtdIns(3,4,5)P3 produced upon FcγRIIA cross-linking remained, but this production was not sufficient to allow platelet aggregation. Interestingly, addition of ADP could restore, in a dose-dependent manner, the production of PtdIns(3,4,5)P3, allowing sufficient PLC activation for platelet aggregation. In contrast, ADP, at any concentration used, was unable to overcome the inhibitory effect of a higher dose of wortmannin (50 nmol/L), which blocked most PI 3-kinase copies and fully inhibited PtdIns(3,4,5)P3 production. These results clearly demonstrate that ADP can up-regulate the rapid FcγRIIA-mediated PtdIns(3,4,5)P3 production in human platelets. This effect of ADP could be replaced by the activation of the α2-adrenergic receptor, indicating that a Gi-dependent pathway was involved early on in the production of PtdIns(3,4,5)P3.
How can ADP signaling via Gi regulate the level of PtdIns(3,4,5)P3 production? Type IA PI 3-kinase has been shown to transiently associate with FcγRIIA,12possibly through the interaction of its adaptor subunit p85α with the docking protein Cbl,46 and may therefore account for the rapid production of PtdIns(3,4,5)P3. This isoform of PI 3-kinase is activated by tyrosine kinase–dependent mechanisms and specific protein-protein interactions.47 Interestingly, a synergistic effect involving βγ subunits of G proteins and phosphotyrosyl peptide has been recently described in the activation of the heterodimeric PI 3-kinase p85α-p110β but not of p85α-p110α.48 The βγ subunits may also activate p110β independently of the p85 subunits in vitro.49These results suggest that 2 different types of membrane receptors, one activating the tyrosine kinase pathway and the other activating GTP-binding proteins, may cooperate for the production of PtdIns(3,4,5)P3. Among the p85 subunits, p85α is preferentially expressed in human platelets. The p110α catalytic subunit is also present,39 and we recently observed, by immunoblotting experiments, the expression of p110β (not shown). Thus, a p85α-p110β form of PI 3-kinase may exist in platelets and could require synergistic activation via βγ of Gi and a tyrosine-phosphorylated docking protein.
The βγ subunits of Gi may also directly involve type IBPI 3-kinase (p110γ),49 which is less sensitive to wortmannin than type IA.39 However, because ADP or epinephrine alone are poor activators of PtdIns(3,4,5)P3 synthesis, this hypothesis becomes invalidated. Moreover, we cannot exclude the possibility that unidentified isoforms of PI 3-kinase, weakly sensitive to low concentrations of wortmaninn, might be involved in this mechanism.
At last, although we did not observe an accumulation of PtdIns(3,4)P2 in the presence of CP-CPK, ADP might also modulate the degradation of PtdIns(3,4,5)P3 through specific phosphatases such as the 5-phosphatase SHIP1.45 50
In conclusion, our results demonstrate that converging signaling pathways from Gi and tyrosine kinases are required for platelet activation and aggregation induced by FcγRIIA and suggest that antagonists of the ADP receptor coupled to Gi may be effective as therapeutic agents for prevention or treatment of HIT. Based on our results and recent reports, we propose that concomitant signaling through Gi and either Gq18 51 or tyrosine kinases, according to the primary agonist used, might be a general mechanism by which platelet activation and aggregation occurs.
Acknowledgments
The authors thank Drs P. Raynal, F. Gaits, J. Ragab, C. Trumel, G. Mauco, S. Giuriato, and K. Missy for stimulating discussions and C. Greenland for correcting the English.
Supported by grants from Association pour la Recherche sur le Cancer, European Union Biomed 2 Program BMH4-CT-97 2609, and Région Midi-Pyrénées.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bernard Payrastre, INSERM U326, Hôpital Purpan, 31059 Toulouse, France.

![Fig. 2. The inhibitory effects of ADP scavengers on serotonine secretion evoked by FcγRIIA is reversed by epinephrine. / Platelets were previously loaded with 5-hydroxy[14C]tryptamine, and secretion was determined as described in “Materials and methods.” A3P5PS, CP-CPK, and CP-CPK and 1-μmol/L epinephrine were added 1 minute before stimulation by FcγRIIA cross-linking. The effect of 1-μmol/L epinephrine alone was also assessed. Data are the mean of 2 independent experiments. (A) Stimulation by FcγRIIA cross-linking; (B) stimulation by HIT sera.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3439/5/m_h82200374002.jpeg?Expires=1770759931&Signature=DZQgXV6GEyHfcf9Y~lmYfQwc1oXpxOGlbAnjS4DxXZC5qBg1c9onVUCWTE4UdInaSaqGl4KYZHmpNT6vEuAg2dn5EHgrt1w88tncwOCAnx5~dRa4e5AgMhjnWHtPr16ILziF1BP0wDrsuqv6mRRVeq-tO7LzAK26Rl-nvpE8N-21unXOXmEprbQbMIg6GX2Bbuxe~vw6bCvthjm7SPXZLFVUvErRCRK8l2AGXGzWMXp2iwnMrmUIWx9n8fApUNw8m4mnGWiudN8xckUKO87nMJUVHbvSUnrUPqEQs0R5-iD4WbZMqm~tTcvEho8KdsbA~O2JSfcWcHjlx4nYuuhomg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. The inhibitory effects of ADP scavengers on serotonine secretion evoked by FcγRIIA is reversed by epinephrine. / Platelets were previously loaded with 5-hydroxy[14C]tryptamine, and secretion was determined as described in “Materials and methods.” A3P5PS, CP-CPK, and CP-CPK and 1-μmol/L epinephrine were added 1 minute before stimulation by FcγRIIA cross-linking. The effect of 1-μmol/L epinephrine alone was also assessed. Data are the mean of 2 independent experiments. (A) Stimulation by FcγRIIA cross-linking; (B) stimulation by HIT sera.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3439/5/m_h82200374002.jpeg?Expires=1770991635&Signature=zFOgWJynwNrTx~4N~qKI4DEbUrAE67JpKrELx6j2k2C9BA3UhRD0iC9AWQczvfpi~sSHQf2Eh0MDTy4V5VXFODHnxo0VW9~~ka-pBDHIgFhUcpHDrnDB9a3mPT2vMY6vVZZ4Y95TV~38lalmbzDs7KF3FS~~GfrlEhifm65jGOZtXaWu68NN8ft~zM8GVNN83XL-zxhRHaeN6gb7tNYR7WVhNviZMFKibJVkfmebDw0VI9ZaoV1boPNv9D7pkkUNAw2q9IPiB9YSr4RmMP1MUGVoUN6GGUM8kZDbdvaDR5uDxjSrjuIqxpGT60wnajdLTOxqApFMmXLdPeVJ6aQE6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)