Abstract
Dendritic cells (DCs) have the unique ability to initiate an immune response in vivo by capturing antigens (Ags) in peripheral tissues and migrating to secondary lymphoid organs, where they sensitize naive CD4+ T cells. To mimic this process in vitro, previous studies have shown that DCs directly isolated from peripheral blood can be used to elicit primary responses to neoantigens (neoAgs). In other studies, when monocyte-derived DCs have been utilized to sensitize total CD4+ T cells in vitro, only secondary proliferation to neoAgs could be elicited. In the present study, the relative abilities of CD40 ligation, protein kinase C activation, and culture in tumor necrosis factor α (TNF-α) to induce functional and phenotypic maturation of human DCs from monocyte precursors were compared. Optimal TNF-α–induced maturation of DCs required a prolonged 4-day culture. It was then found that loading immature DCs with the neoAgs keyhole limpet hemocyanin or human immunodeficiency virus-1 p24 gag prior to TNF-α–induced maturation, rather than after maturation, was crucial to sensitize CD4+ T cells to new Ags. This primary proliferation to neoAgs was initiated from the CD4+ CD45RA+naive T-cell population. Finally, it was found that monocyte-derived DCs acquired the ability to secrete interleukin-12 p70, after contact with Ag-specific T cells. The ability to prime and expand Ag-specific CD4+ T cells ex vivo to neoAgs in serum-free conditions has potential application for cellular vaccination and adoptive immunotherapy.
Introduction
Dendritic cells (DCs) are described as professional antigen-presenting cells (APCs) because they elicit strong proliferative responses to alloantigens1 and to recall antigens (Ags).2,3 Most importantly, DCs have the unique ability to initiate the immune response in vivo by capturing Ags in peripheral tissues and migrating to secondary lymphoid organs, where they sensitize naive CD4+ T cells to the Ag.4-7 DC migration is concomitant with maturation,8 during which the DCs lose their ability to acquire and process Ags. However, mature DCs express large amounts of peptide–major histocompatibility complex (MHC) class II and costimulatory molecules on their surface,9 thereby acquiring the ability to prime CD4+ T cells.
Mature, Ag-presenting DCs have typically been generated in vitro from peripheral blood monocytes by means of a 2-step culture analogous to the in vivo maturation process. First, in a 3- to 7-day culture in the presence of interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF), monocytes are differentiated into immature DCs.10 Then, maturation signals are provided by either monocyte-conditioned medium,11-15 tumor necrosis factor α (TNF-α),10,16,17 or prostaglandin E2.18,19 However, in these systems Ag was added for processing at the completion of the DC maturation process. The practical efficiency of these approaches may be limited since multiple rounds of T-cell stimulation have been required in vitro to generate Ag-specific proliferation from naive precursors. Furthermore, the induction of Ag-specific proliferation from naive precursors has to date been described with mature DCs isolated directly from peripheral blood.20-22
In the present studies, we have characterized the requirements for the generation of monocyte-derived DCs optimized for naive CD4+T-cell activation. CD40 ligation, protein kinase C activation, and culture in TNF-α were examined for their relative ability to induce differentiation of DCs. We found that TNF-α and CD40 ligation were superior to protein kinase C activation for induction of functional and phenotypic maturation of DCs. Further, we found that sensitization of CD4+ T cells by neoantigens (neoAgs) using monocyte-derived DCs pulsed with either keyhole limpet hemocyanin (KLH) or human immunodeficiency virus (HIV)–1SF2 p24 gag (p24) before their maturation with TNF-α was optimal; Ag loading after DC maturation was ineffective. We showed that loading immature DCs with Ag resulted in more potent APCs, as evidenced by their ability to stimulate specific proliferation of unprimed CD4+CD45RA+ naive T cells after a single cycle of presentation of neoAg. Our results therefore more closely mimic the physiologic maturation process by pulsing monocyte-derived DCs with Ag prior to initiating the maturation process.
Materials and methods
Reagents
Tetanus toxoid (TT) was purchased from Lederle Laboratories (Pearl River, NY); KLH (7.0 endotoxin unit/mg protein or less) from PerImmune (Rockville, MD); GM-CSF from Immunex (Seattle, WA); TNF-α from R&D Systems (Minneapolis, MN); IL-2 from Chiron (Emeryville, CA); IL-1-β from Endogen (Boston, MA); and phorbol 12-myristate 13-acetate (PMA) from Sigma Chemical Co (St. Louis, MO). Purified recombinant HIV-1SF2 p24 (p24) was obtained from the National Insitutes of Health AIDS Research and Reference Reagent Program (Rockville, MD). IL-4 was generously provided by Schering-Plough (Levallois-Perret, France), and human CD40 ligand trimer (CD40LT)23 from Immunex. All reagents and medium were endotoxin free as determined by a Quantitative Chromogenic Limulus Amebocyte Lysate Assay (BioWhittaker, Walkersville, MD).
Isolation of monocytes from peripheral blood
Peripheral blood monocytes were isolated by Percoll (Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation of leukopacks obtained by apheresis of healthy donors. The monocyte population was further enriched by gravity sedimentation. Briefly, cells were resuspended in 50 mL phosphate-buffered saline (PBS) without calcium and magnesium (BioWhittaker), mixed by inversion and allowed to settle for 1 hour. The cells still in suspension at the end of 1 hour were discarded, and the monocyte-enriched population in the formed pellet was washed and centrifuged at low speed (130g for 5 minutes) 3 times to eliminate platelets. The cell volume was analyzed on a Coulter Counter ZM and Coulter Channalyzer 256 (Coulter, Hialeah, FL) before and after gravity sedimentation to verify monocyte enrichment. T-cell contamination of the enriched monocytes was evaluated by flow cytometry and varied from 3% to 15%, depending on the donor. Monocytes were either frozen or cultured as described below.
Ag-loading and generation of DCs from monocytes
To induce DC differentiation, monocytes were cultured in 6-well plates (Costar; Cambridge, MA) at 5 × 106 cells per well in 3 mL of AIM V supplemented with 1% autologous serum or 3% heat-inactivated human AB serum (NorML Cera-Plus; NABI, Boca Raton, FL), 1000 IU/mL IL-4, and 1000 IU/mL GM-CSF at 37°C in 5% CO2 for 4 days. The culture medium and cytokines were renewed every other day. Unless specified otherwise, DCs were pulsed once with Ag on day 3 after culture initiation. The following Ag concentrations were used: TT at 0.01 Lf/mL, KLH at 25 μg/mL, and p24 at 10 μg/mL. Where indicated, Ag was removed by washing the cells and renewing the supplemented medium. The following maturation-inducing agents were used in their respective optimized conditions. TNF-α was added to the supplemented culture medium at day 4 (20 ng/mL) and at day 6 (10 ng/mL), whereas PMA (10 ng/mL) and CD40LT (3 μg/mL) were added to the supplemented culture medium at day 6, for 2 days. PMA, a phorbol ester that induces protein kinase C activation, has been shown to induce DC differentiation from CD34+ hematopoietic progenitors.24 After the experiments described in Figure1, DC maturation was induced for 4 days in the presence of TNF-α as above. Cells were collected at the end of the culture (day 8) by pipetting and by incubation at 4°C with PBS containing 0.2 mmol/L EDTA (Quality Biological Inc, Gaithersburg, MD) for 5 minutes.
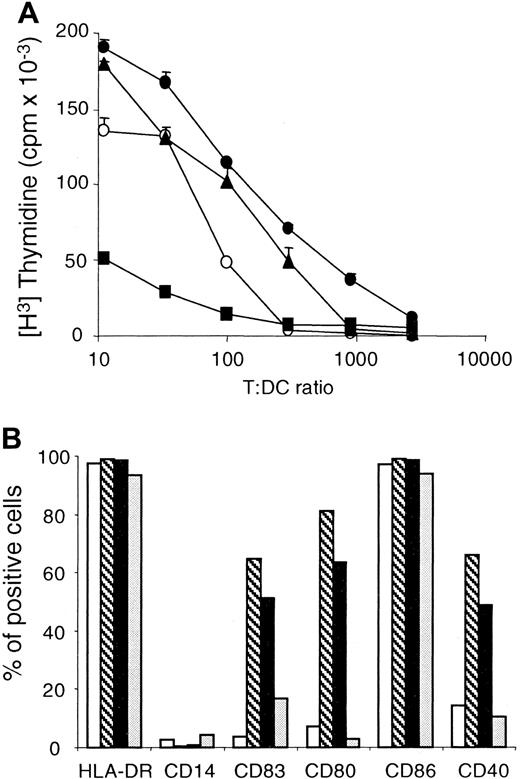
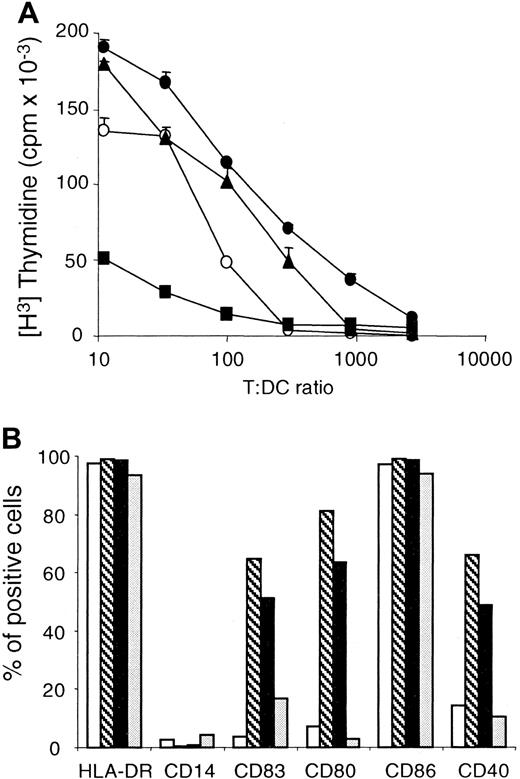
DCs obtained by TNF-α or CD40LT exposure express characteristics of mature DCs.
(A) Monocytes were cultured for 4 days in AIM V containing 3% human AB serum, IL-4, and GM-CSF, as described in “Materials and methods.” TNF-α (●) was added at day 4, whereas PMA (▪) and CD40LT (▴) were added at day 6 as maturation stimuli. As a control, we used DCs to which no maturation signals were added (○). Cells were harvested at day 8, irradiated, and tested for CD4+ T-cell stimulatory capacity in a primary allogeneic mixed lymphocyte reaction at the indicated T/DC ratio. These results are the mean of round-bottom triplicate wells and are representative of 3 different experiments with different donors. (B) Flow cytometric analysis of immature DCs (■) or DCs matured by TNF-α (▧), CD40LT (▪), and PMA (░), as described in panel A. DCs were double-stained with a panel of phycoerythrin (PE)–conjugated monoclonal antibodies (mAbs) and with fluorescein isothiocyanate (FITC)–conjugated mAb HLA-DR. Cells were also stained with isotype controls for each PE- and FITC-conjugated mAb. Results are expressed as percentage of positive cells for each marker, in comparison with its isotype control. These results are representative of 3 different experiments with different donors.
DCs obtained by TNF-α or CD40LT exposure express characteristics of mature DCs.
(A) Monocytes were cultured for 4 days in AIM V containing 3% human AB serum, IL-4, and GM-CSF, as described in “Materials and methods.” TNF-α (●) was added at day 4, whereas PMA (▪) and CD40LT (▴) were added at day 6 as maturation stimuli. As a control, we used DCs to which no maturation signals were added (○). Cells were harvested at day 8, irradiated, and tested for CD4+ T-cell stimulatory capacity in a primary allogeneic mixed lymphocyte reaction at the indicated T/DC ratio. These results are the mean of round-bottom triplicate wells and are representative of 3 different experiments with different donors. (B) Flow cytometric analysis of immature DCs (■) or DCs matured by TNF-α (▧), CD40LT (▪), and PMA (░), as described in panel A. DCs were double-stained with a panel of phycoerythrin (PE)–conjugated monoclonal antibodies (mAbs) and with fluorescein isothiocyanate (FITC)–conjugated mAb HLA-DR. Cells were also stained with isotype controls for each PE- and FITC-conjugated mAb. Results are expressed as percentage of positive cells for each marker, in comparison with its isotype control. These results are representative of 3 different experiments with different donors.
Flow cytometry
Cell staining was performed on 1 × 105 cells per sample with the following phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs): CD4 (Leu3a/SK3), CD14 (Leu-M3/MoP9), CD25, (2A3) CD45RO (Leu-45RO/UCHL-1), and CD80 (anti-BB1/L307.4) (Becton Dickinson, San Jose, CA), CD40 (EA-5) (Ancell, Bayport, MN), CD86 (B70/B7-2/IT2.2) (PharMingen, San Diego, CA), and CD83 (HB15a) (Immunotech, Westbrook, ME). Each sample was double-stained with the fluorescein isothiocyanate (FITC)–conjugated mAb HLA-DR (L243) (Becton Dickinson). Negative controls were irrelevant isotype-matched mAbs from Becton Dickinson, except for immunoglobulin (Ig)–G2b (Southern Biotechnology Associates; Birmingham, AL). Cells were stained for 20 minutes at 4°C in fluorescence-activated cell sorter (FACS) staining medium (PBS with 0.05% fetal bovine serum, 2 mmol/L EDTA, and 0.01% sodium azide), washed twice, and then fixed with 1% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in PBS. Usually, 10 000 viable cells were analyzed by means of a Coulter Epics Elite (Beckman-Coulter, Miami, FL), equipped with a 488-nm argon laser and Elite 4.2 software (Coulter). Cell-surface expression on DCs was determined by means of a forward versus side scatter gate, on the basis of their unique scatter properties. Proper laser alignment was confirmed with FITC-beads (Becton Dickinson). All isotype controls were set to be less than 2% positive for statistical analysis.
Measurement of endocytosis
Endocytic activity of dendritic cells was quantified by measuring dextran–fluorescein isothiocyanate (DX-FITC) (Molecular Probes; Eugene, OR) uptake, as described.25 Aliquots of 105 cells in 100 μL of AIM V with 1% autologous serum were incubated for 30 minutes with 0.5 mg/mL DX-FITC at either 37°C or 4°C. The cells were washed 3 times with cold FACS staining medium and kept at 4°C without fixation until analysis. The samples were analyzed the same day on a Coulter Epics Elite for DX-FITC expression. The endocytic activity of DCs incubated at 37°C was compared with that of the same cells incubated at 4°C.
Isolation of naive and memory CD4+peripheral blood T cells
Peripheral blood lymphocytes (PBLs) were isolated from leukopacks obtained by apheresis of healthy donors and Percoll gradient centrifugation. CD28+CD4+ T cells were isolated from the PBLs by negative magnetic immunoadherence as described previously.26 Purified CD4+CD28+ T cells (greater than 98% CD3+, greater than 98% CD28+, and less than 3% CD8+ as evaluated by flow cytometry) were separated into CD4+CD45RA+ (naive) and CD4+CD45RO+ (memory) subsets by negative magnetic immunoadherence as previously described.27 These preparations were routinely greater than 95% pure as determined by flow cytometry.
Allogeneic mixed lymphocyte reaction
Purified CD4+ T cells from an allogeneic donor were cultured at 105 cells per well in 96-well plates (Costar) in AIM V supplemented with 3% heat-inactivated human AB serum (NorML Cera-Plus) with increasing numbers of irradiated DCs (30 Gy from a 137Cs source). Thymidine incorporation was measured in triplicate on day 6 by an 18-hour pulse with [3H]-thymidine (1 μCi/well) (Dupont NEN, Boston, MA). Cells were harvested by means of a Mach II 96 cell harvester (TomTec, Hamden, CT), and [3H]-thymidine incorporation was measured by means of a 1205 Betaplate liquid scintillation counter (Wallac Inc, Gaithersburg, MD). When mixed lymphocyte reactions (MLRs) were performed after 2, 3, or 4 days of TNF-α–induced maturation, identical aliquots of frozen purified CD4+ T cells from the same allogeneic donor were used as the responder population.
Ag presentation assays
Freshly isolated CD4+ T cells were plated at 105 cells per well in flat-bottom 96-well plates in AIM V–3% heat-inactivated human serum. Autologous dendritic cells, prepared as described above and pulsed with Ag or not pulsed, were irradiated and added at 1 × 104, 5 × 103, and 2.5 × 103 cells per well to obtain T/DC ratios of 10:1, 20:1, and 40:1, respectively. Proliferation of Ag-specific CD4+ T cells was evaluated in triplicate after 6 days for TT, and after 7 days for KLH and p24, by measuring thymidine uptake during the last 18 hours of the assay. Subsequent stimulation of Ag-specific CD4+ T-cell lines was evaluated on day 3 after an 18-hour thymidine uptake.
Generation of Ag-specific CD4+T-cell lines
Fresh CD4+ T cells were stimulated at 106/mL/well in 24-well plates (Costar) with 105 Ag-pulsed autologous irradiated DCs in AIM V containing 3% human serum in the presence of IL-1 β (2 IU/mL), IL-2 (0.2 IU/mL), and IL-4 (50 IU/mL).21 The culture medium and cytokines were renewed on days 4 and 6. These Ag-specific CD4+ T-cell lines were further expanded by restimulation with Ag-pulsed DCs on day 9 of culture and every 2 weeks thereafter. The restimulations were performed at a T/DC ratio of 40:1, and IL-2 (100 IU/mL) was the only cytokine added to the culture medium used to propagate the T-cell culture.
IL-12 assays
IL-12 secretion by nonirradiated DCs was measured by enzyme-linked immunosorbent assay in supernatants collected at the end of DC culture (before adding T cells), or after 1, 2, or 3 days of coculture with a TT-specific CD4+ T-cell line (106 T cells per well). To evaluate Ag-specific IL-12 production, nonpulsed DCs or DCs pulsed with TT were added at 5 × 104 and 2.5 × 104 cells per well to obtain respective T/DC ratios of 20:1, and 40:1. The Predicta Total Interleukin-12 Kit (Genzyme Diagnostics, Cambridge, MA) was used to measure the IL-12 p40 subunit, whereas the Quantikine HS Human IL-12 Immunoassay (R&D Systems) was used to measure the heterodimer IL-12 p70. The sensitivity of these 2 kits was 10 pg/mL and 0.5 pg/mL, respectively.
Results
A comparison of TNF-α, PMA, and CD40 stimulation of monocyte-derived DCs for the ability to generate alloreactive CD4+ T cells
To optimize Ag presentation to CD4+ T cells by peripheral blood monocyte-derived DCs, we first evaluated several differentiation factors for their ability to induce DC maturation. All experiments were performed in a bovine-serum–free system, to avoid specific responses related to xenogeneic proteins.
Because DC maturation is best measured by functional assays rather than receptor surface expression, the relative ability of these stimuli to induce maturation of monocyte-derived DCs was compared in an allogeneic MLR. DCs matured in the presence of TNF-α, PMA, or CD40LT were used as APCs. Since the primary objective of this study was the development of Ag-specific CD4+ T cells, allogeneic CD4+ T cells were used in preference to CD8+ T cells as responder cells. Monocytes obtained from the peripheral blood of healthy donors were enriched by gravity sedimentation and plated in IL-4 and GM-CSF as described in “Materials and methods.” Optimized conditions for DC maturation were applied in using TNF-α for 4 days and PMA or CD40LT for 2 days in the DC culture. As shown in Figure1A, TNF-α and CD40LT were better stimuli for inducing mature monocyte-derived DCs, as judged by their ability to promote an alloreactive CD4+ T-cell proliferation. In Figure 1B, a higher surface expression of CD40, CD80, and CD83 correlated with the stronger induction of alloreactive CD4+ T-cell proliferation obtained with DCs matured with TNF-α or CD40LT compared with immature DCs. PMA did not promote CD40 and CD80 surface expression on DCs (Figure 1B). PMA even seemed to cause immature DCs to revert to a macrophagelike stage of differentiation, with a poor ability to induce allogeneic MLR (Figure1A). Because DCs generated with TNF-α were slightly superior to DCs generated with CD40LT in 2 donors and were equivalent in a third donor, we used TNF-α as the maturation signal for the subsequent experiments.
Prolonged culture is required for TNF-α–mediated DC differentiation
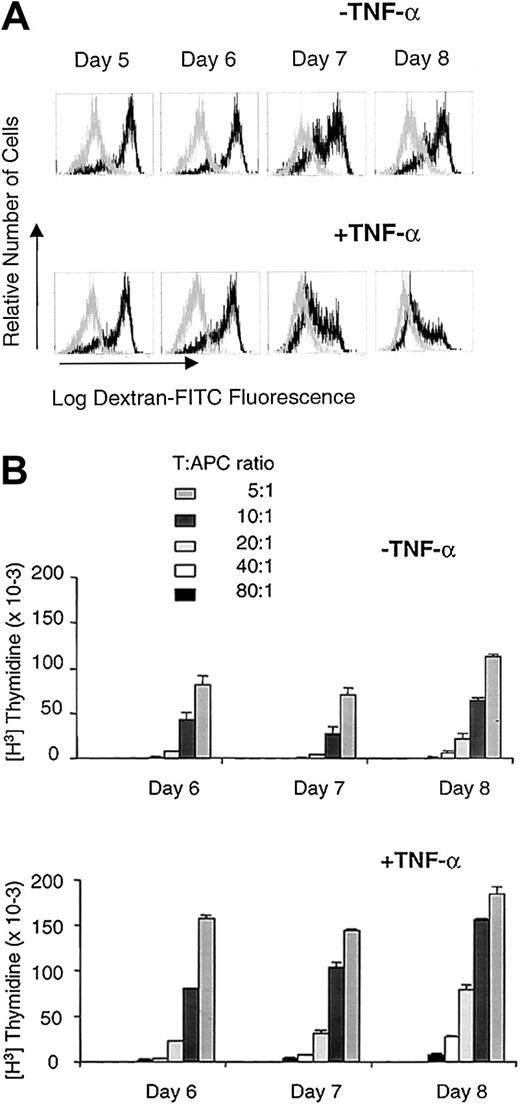
Immature DCs generated by culture with IL-4 and GM-CSF are able to endocytose soluble Ag via macropinocytosis and mannose receptor endocytosis, a process that can be measured with DX-FITC uptake.25 DC maturation induced by TNF-α results in a complete down-regulation of fluid phase pinocytosis, concomitant with an increase in T-cell stimulatory capacity.10 We therefore followed DX-FITC uptake after TNF-α exposure as an inverse correlate of DC maturation (Figure 2A). We found that DX-FITC uptake was significantly reduced at day 3 and virtually eliminated after a 4-day exposure of immature DCs to TNF-α, whereas immature DCs not exposed to TNF-α retained most of their endocytic ability (Figure 2A).
At least 4 days of exposure to TNF-α are required for full maturation of DCs.
(A) Accumulation of DX-FITC in DCs. Immature DCs (top panel) cultured for 8 days in IL-4 and GM-CSF, or mature DCs (bottom panel) cultured for 4 days in IL-4 and GM-CSF plus 4 days in IL-4 and GM-CSF and TNF-α, were evaluated for their ability to endocytose DX-FITC as a function of time of exposure to TNF-α. For mature DCs, day 5 represents 1 day of exposure to TNF-α; day 6, 2 days of exposure to TNF-α; day 7, 3 days of exposure to TNF-α; and day 8, 4 days of exposure to TNF-α. The dark line represents DX-FITC uptake by samples incubated at 37°C, whereas the light line depicts DX-FITC uptake by samples incubated at 4°C as a negative control. These results are representative of 3 separate experiments with 3 different donors. (B) CD4+ T-cell stimulatory capacity of irradiated immature DCs (top) or mature DCs (bottom) in an allogeneic mixed lymphocyte reaction, when harvested at days 6, 7, and 8 of their culture as described in panel A. Frozen aliquots of responder CD4+ T cells from the same allogeneic donor were used for each time point. These results are the mean of triplicate wells and are representative of 2 different experiments.
At least 4 days of exposure to TNF-α are required for full maturation of DCs.
(A) Accumulation of DX-FITC in DCs. Immature DCs (top panel) cultured for 8 days in IL-4 and GM-CSF, or mature DCs (bottom panel) cultured for 4 days in IL-4 and GM-CSF plus 4 days in IL-4 and GM-CSF and TNF-α, were evaluated for their ability to endocytose DX-FITC as a function of time of exposure to TNF-α. For mature DCs, day 5 represents 1 day of exposure to TNF-α; day 6, 2 days of exposure to TNF-α; day 7, 3 days of exposure to TNF-α; and day 8, 4 days of exposure to TNF-α. The dark line represents DX-FITC uptake by samples incubated at 37°C, whereas the light line depicts DX-FITC uptake by samples incubated at 4°C as a negative control. These results are representative of 3 separate experiments with 3 different donors. (B) CD4+ T-cell stimulatory capacity of irradiated immature DCs (top) or mature DCs (bottom) in an allogeneic mixed lymphocyte reaction, when harvested at days 6, 7, and 8 of their culture as described in panel A. Frozen aliquots of responder CD4+ T cells from the same allogeneic donor were used for each time point. These results are the mean of triplicate wells and are representative of 2 different experiments.
The loss of endocytic ability by TNF-α–treated DCs was concomitant with their increased ability to stimulate T cells (Figure 2B). Indeed, the ability of TNF-α–treated DCs to stimulate CD4+ T cells in an allogeneic MLR was consistently higher than that of immature DCs (Figure 2B).
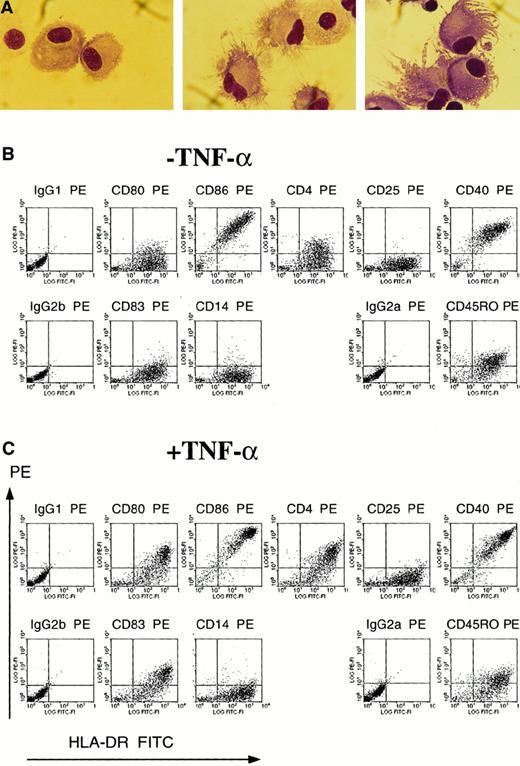
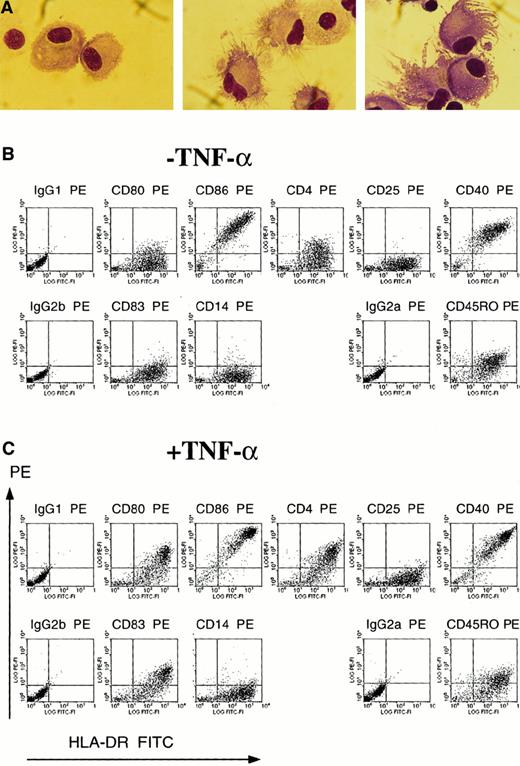
The efficacy of TNF-α as a maturation signal for monocyte-derived DCs was also apparent morphologically and phenotypically. TNF-α treatment induced the cellular morphologic processes characteristic of mature DCs (Figure 3A). After 4 days of maturation with TNF-α (Figure 3C), the cells exhibited phenotypic markers characteristic of mature DCs. In contrast to immature DCs cultured with only IL-4 and GM-CSF (Figure 3B), mature DCs expressed CD80 and CD83, a marker for mature DCs,16 as well as increased fluorescence for MHC class II, CD4, CD40, and CD86 (Figure3C).12,14,16,28 Both immature and mature DCs (Figure 3B-C) were negative for CD14, in contrast to monocytes (data not shown), negative for CD25, and positive for CD45RO, as previously described for TNF-α–matured DCs.12,14,16 28 Immature DCs at day 4 exhibited lower mean fluorescence intensity for CD86, CD40, and MHC class II than immature DCs at day 8 (data not shown).
Monocyte-derived DCs obtained after 4 days of maturation with TNF-α express the morphology and phenotype of mature DCs.
(A) Immature DCs cultured for 8 days in IL-4 and GM-CSF (left) or mature DCs cultured for 4 days in IL-4 and GM-CSF plus 4 days in IL-4 and GM-CSF and TNF-α (center and right) were cytospun onto glass slides and stained by Wright-Giemsa. Magnification × 100. (B) (C) Flow cytometric analysis of immature DCs cultured for 8 days in IL-4 and GM-CSF (B) and mature DCs cultured for 4 days in IL-4 and GM-CSF plus 4 days in IL-4 and GM-CSF and TNF-α (C). DCs were double-stained with a panel of PE-conjugated mAbs and with FITC-conjugated mAb HLA-DR. Cells were also stained with isotype controls for the PE-conjugated mAbs and were double-stained with FITC-IgG2a, the isotype control for FITC-conjugated HLA-DR.
Monocyte-derived DCs obtained after 4 days of maturation with TNF-α express the morphology and phenotype of mature DCs.
(A) Immature DCs cultured for 8 days in IL-4 and GM-CSF (left) or mature DCs cultured for 4 days in IL-4 and GM-CSF plus 4 days in IL-4 and GM-CSF and TNF-α (center and right) were cytospun onto glass slides and stained by Wright-Giemsa. Magnification × 100. (B) (C) Flow cytometric analysis of immature DCs cultured for 8 days in IL-4 and GM-CSF (B) and mature DCs cultured for 4 days in IL-4 and GM-CSF plus 4 days in IL-4 and GM-CSF and TNF-α (C). DCs were double-stained with a panel of PE-conjugated mAbs and with FITC-conjugated mAb HLA-DR. Cells were also stained with isotype controls for the PE-conjugated mAbs and were double-stained with FITC-IgG2a, the isotype control for FITC-conjugated HLA-DR.
Thus, of the conditions tested, 4 days of exposure to TNF-α were necessary for monocyte-derived DCs to express the characteristics of mature DCs: loss of Ag-uptake ability, enhanced capacity to induce an allogeneic CD4+ T-cell MLR, morphologic features, and increased costimulatory molecule surface expression.
Mature DCs induce a proliferative response of autologous CD4+ T cells to neoAg
Mature monocyte-derived DCs were analyzed for their ability to sensitize fresh autologous CD4+ T cells to the recall Ag TT or to the neoAg KLH. The donors used in this study had been vaccinated with TT, but had not been exposed to KLH. DCs were pulsed with Ag on day 3 of culture. Ag was neither removed nor supplemented during the remainder of the 8-day culture or during the T-cell proliferation assay. Purified CD4+ T cells were incubated with immature DCs or with mature DCs pulsed with TT or KLH for 6 days and 7 days, respectively, at various T/APC ratios.
With mature DCs, Ag-specific proliferation was observed for both Ags, in contrast to immature DCs. Importantly, only mature DCs were capable of sensitizing autologous CD4+ T cells to KLH upon a primary exposure (Figure 4). This proliferative response was observed with a T/APC ratio as low as 40:1. Immature DCs, cultured for 8 days in IL-4 and GM-CSF, induced autologous CD4+ T cells to proliferate to the TT recall Ag, but no response was observed to the KLH neoAg. As expected, mature DCs also induced proliferation of the CD4+ T cells to TT. The proliferation to the recall Ag induced by mature DCs was 2-fold higher than that induced by the immature DCs, and it was apparent at a lower T/APC ratio (Figure 4). KLH by itself did not induce maturation of the DCs, as immature DCs pulsed with KLH did not elicit a CD4+T-cell response (Figure 4). Ag-pulsed monocytes or peripheral blood mononuclear cells were relatively inefficient when used as APCs, as they could induce proliferation to TT only when one or more APCs per T cell were used in the assay (data not shown). Thus, only mature monocyte-derived DCs could sensitize sufficient CD4+T-cell precursors to KLH to elicit a response in a primary proliferation assay. The present results are consistent with data from DCs isolated directly from peripheral blood, indicating that initiation of primary responses by DCs is more stringently controlled than initiation of recall responses.4 21
Only mature monocyte-derived DCs can initiate a CD4+ T-cell response to a neoAg in a primary proliferation assay.
Immature DCs (top) or mature DCs (bottom) were pulsed on day 3 of culture with TT as a recall Ag or KLH as a neoAg, and were plated at day 8 with autologous CD4+ T cells at various T/APC ratios. T-cell Ag-specific proliferation was measured by [3H]-thymidine incorporation at day 6 after coculture initiation for TT, or at day 7 for KLH. Unpulsed DCs were used as negative controls for the 6- and 7-day assays, and these controls are shown separately. Each point was done in triplicate. These results are representative of 3 experiments with 3 different donors.
Only mature monocyte-derived DCs can initiate a CD4+ T-cell response to a neoAg in a primary proliferation assay.
Immature DCs (top) or mature DCs (bottom) were pulsed on day 3 of culture with TT as a recall Ag or KLH as a neoAg, and were plated at day 8 with autologous CD4+ T cells at various T/APC ratios. T-cell Ag-specific proliferation was measured by [3H]-thymidine incorporation at day 6 after coculture initiation for TT, or at day 7 for KLH. Unpulsed DCs were used as negative controls for the 6- and 7-day assays, and these controls are shown separately. Each point was done in triplicate. These results are representative of 3 experiments with 3 different donors.
Pulsing immature DCs with neoAg prior to maturation with TNF-α results in more effective presentation of neoAg
The enhanced endocytic ability of immature DCs (Figure 2) suggested that pulsing DCs on day 3 with KLH, prior to maturation with TNF-α, would allow for more effective Ag presentation than pulsing after maturation. We tested this hypothesis by pulsing DCs at various times during their maturation with either recall Ag (TT) or neoAg (KLH). As indicated in Figure 5A, DCs were cultured for 8 days in IL-4 and GM-CSF, and TNF-α was then added on day 4 to induce DC maturation. DCs were pulsed with Ag on day 3, 6, or 7. On day 8 of culture, the DCs were added to autologous CD4+ T cells at a T/APC ratio of 1:10, and thymidine uptake by the T cells was measured 7 days later.
Pulsing DCs with Ag prior to their maturation is required to prime CD4+ T cells but not to elicit a recall response.
(A) Experimental design. DCs were pulsed with Ag on day 3, 6, or 7 of their culture, either before or during TNF-α–induced maturation. DCs were then harvested on day 8, irradiated, and used as stimulator cells in a primary proliferation assay with autologous CD4+ T cells at a T/DC ratio of 1:10. (B) Proliferation to recall Ag. DCs loaded with TT at indicated times were used to stimulate autologous CD4+ T cells. When indicated, Ag was removed from the DC culture after 4 hours of uptake (rem. 4h). [3H]-thymidine incorporation was measured at day 6 of the assay. (C) Proliferation to neoAg. DCs were incubated with KLH at indicated times and used at day 8 to stimulate autologous CD4+ T cells in a primary assay. When indicated, Ag was removed from the DC culture after 4 hours, 1 day, or 2 days of Ag uptake (rem. 4h, rem. d4, rem. d5). [3H]-thymidine incorporation by CD4+ T cells was measured in triplicate at day 7 of the assay. These results are representative of 3 experiments with 3 different donors.
Pulsing DCs with Ag prior to their maturation is required to prime CD4+ T cells but not to elicit a recall response.
(A) Experimental design. DCs were pulsed with Ag on day 3, 6, or 7 of their culture, either before or during TNF-α–induced maturation. DCs were then harvested on day 8, irradiated, and used as stimulator cells in a primary proliferation assay with autologous CD4+ T cells at a T/DC ratio of 1:10. (B) Proliferation to recall Ag. DCs loaded with TT at indicated times were used to stimulate autologous CD4+ T cells. When indicated, Ag was removed from the DC culture after 4 hours of uptake (rem. 4h). [3H]-thymidine incorporation was measured at day 6 of the assay. (C) Proliferation to neoAg. DCs were incubated with KLH at indicated times and used at day 8 to stimulate autologous CD4+ T cells in a primary assay. When indicated, Ag was removed from the DC culture after 4 hours, 1 day, or 2 days of Ag uptake (rem. 4h, rem. d4, rem. d5). [3H]-thymidine incorporation by CD4+ T cells was measured in triplicate at day 7 of the assay. These results are representative of 3 experiments with 3 different donors.
As seen in Figure 5B, the time of pulsing was not critical for the TT recall response, as the T cells proliferated regardless of the time or duration of pulsing. In contrast, the time and duration of pulsing were critical to sensitizing autologous CD4+ T cells to the neoAg KLH (Figure 5C). T-cell proliferation was observed only when DCs were pulsed with KLH on day 3, prior to the addition of the maturation signal (TNF-α). No T-cell proliferation over background was observed when DCs were pulsed on day 6 or 7. When added to DCs on day 3, Ag was either removed from the culture after 4 hours, 1 day, or 2 days or left in for the duration of the culture. Surprisingly, the proliferative response was dramatically lower if KLH was removed from DC culture after 4 hours, in contrast to the results shown above for the TT response. Furthermore, the neoAg pulse was time-dependent, as removal of KLH at 1 or 2 days after addition resulted in progressively less diminution. Late addition of KLH on day 6 or 7 of culture was also ineffective. Thus, for a recall response, time of Ag loading on DCs was not crucial. In contrast, T-cell proliferation in response to KLH was dependent on the timing and, most critically, the duration of the neoAg pulse with DCs.
Primary response to neoAg is initiated from the CD45RA+ subpopulation of CD4+ T cells
To further characterize the requirements for a CD4+T-cell response to neoAg, subsequent experiments were conducted with KLH and another neoAg, HIV-1SF2 p24 gag (p24). In addition, CD4+ T cells were enriched into naive (CD4+CD45RA+) and memory (CD4+CD45RO+) fractions. Monocyte-derived DCs, primed with Ag (TT, KLH, or p24) on day 3, and matured with TNF-α, were harvested on day 8, irradiated, and added to CD4+CD45RA+ or CD4+CD45RO+ T-cell subpopulations. Thymidine uptake was measured 6 days later for TT and 7 days later for KLH or p24. As shown in Figure 6A, DCs pulsed with either KLH or p24 induced proliferation of the naive subpopulation that was greater than the proliferation induced by the nonpulsed day-7 control DCs. In CD45RO+ T cells, high background to unpulsed DCs was reproducibly observed, and no proliferation to DCs pulsed with either KLH or p24 was observed over background. In contrast, proliferative response of T cells to the recall Ag TT resided in the memory fraction, and not the naive fraction, of CD4+T cells (Figure 6A).
Peripheral blood monocyte-derived DCs can prime KLH-specific and HIV-1 p24–specific precursors from naive CD4+CD45RA+ T cells.
(A) Autologous naive CD4+CD45RA+ T cells (left) or memory CD4+CD45RO+ T cells (right) freshly isolated from peripheral blood were stimulated with unpulsed DCs (noAg d6 and noAg d7) or with DCs pulsed with KLH, p24, or TT, at various T/DC ratios. [3H]-thymidine incorporation was measured on day 6 for TT and on day 7 for KLH and p24. Each point was done in triplicate. A representative experiment out of 3 with 3 different donors is shown. (B) Secondary stimulation. After 9 days of culture for the first stimulation, T-cell lines RA/p24, RA/KLH, and RO/TT, derived from the same donor shown in panel A, were restimulated for 3 days with DCs pulsed with p24, KLH, or TT, respectively, or with DCs pulsed with an irrelevant Ag (control), ie, KLH for RA/p24, TT for RA/KLH, and KLH for RO/TT. [3H]-thymidine was added for the last 18 hours of the assay. Each point was done in triplicate.
Peripheral blood monocyte-derived DCs can prime KLH-specific and HIV-1 p24–specific precursors from naive CD4+CD45RA+ T cells.
(A) Autologous naive CD4+CD45RA+ T cells (left) or memory CD4+CD45RO+ T cells (right) freshly isolated from peripheral blood were stimulated with unpulsed DCs (noAg d6 and noAg d7) or with DCs pulsed with KLH, p24, or TT, at various T/DC ratios. [3H]-thymidine incorporation was measured on day 6 for TT and on day 7 for KLH and p24. Each point was done in triplicate. A representative experiment out of 3 with 3 different donors is shown. (B) Secondary stimulation. After 9 days of culture for the first stimulation, T-cell lines RA/p24, RA/KLH, and RO/TT, derived from the same donor shown in panel A, were restimulated for 3 days with DCs pulsed with p24, KLH, or TT, respectively, or with DCs pulsed with an irrelevant Ag (control), ie, KLH for RA/p24, TT for RA/KLH, and KLH for RO/TT. [3H]-thymidine was added for the last 18 hours of the assay. Each point was done in triplicate.
The previous results established that Ag-specific but modest proliferation of naive T cells could be detected after a single cycle of activation. To further this analysis, cells from the above experiments were used to derive CD4+ T-cell lines specific for KLH and p24 from CD4+CD45RA+ naive precursors, and CD4+ T-cell lines specific for TT were obtained from CD4+CD45RO+ memory T cells. CD4+ T cells were exposed for 9 days to Ag-pulsed DCs, and the resulting CD4+ T-cell lines were tested for specificity in a secondary proliferation assay (Figure 6B). Indeed, a single restimulation with mature DCs was sufficient to induce substantially more vigorous and specific proliferation to KLH and p24 (Figure 6B). For this donor, no T-cell lines specific to KLH or to p24 were obtained from the starting population of CD4+CD45RO+memory T cells. However, from 1 donor out of 3, a KLH-specific CD4+ T-cell line was obtained from memory CD4+T cells, suggesting the possibility of reactivation of Ag cross-reactive memory cells or incomplete removal of naive T cells; nonspecific activation of CD4+ T cells by KLH was excluded by use of endotoxin-free reagents.
Thus, monocyte-derived DCs pulsed with neoAg prior to their maturation can sensitize CD4+CD45RA+ naive T cells to neoAg, and this is detectable in a primary assay. Furthermore, selection and enrichment of neoAg-specific CD4+ T cells was efficiently achieved in only a single round of stimulation with neoAg-pulsed DCs.
DC production of biologically active IL-12 is induced only in the presence of activated T cells
We wanted to determine in our system whether mature monocyte-derived DCs, after 4 days of maturation with TNF-α, could produce biologically active IL-12 through activation by Ag-specific interaction with T cells.29,30 IL-12 is a heterodimeric cytokine composed of 2 subunits designated p35 and p40, which separately do not have any biologic activity.31 However, a p40 homodimer may function as an IL-12 antagonist.32
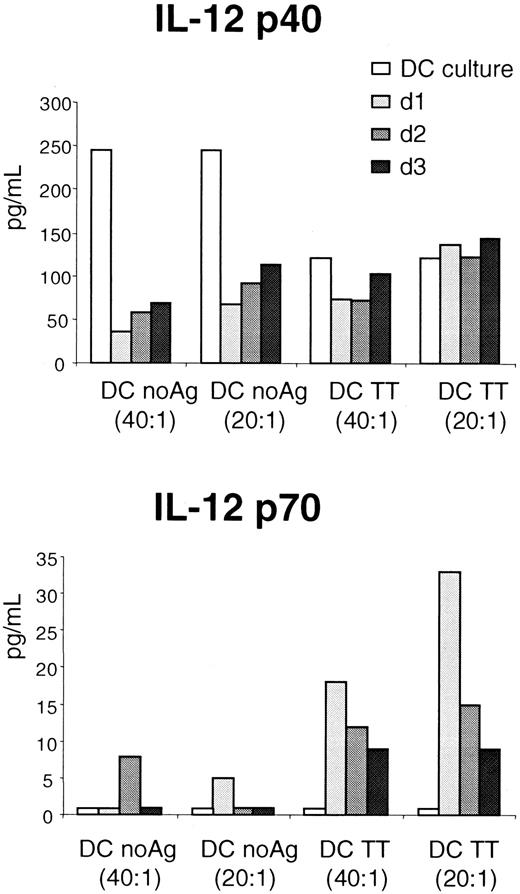
A TT-specific CD4+ T-cell line (106 cells) was stimulated with mature DCs pulsed with TT at a T/APC ratio of 1:20 or 1:40. IL-12 p40 and IL-12 p70 production was compared in culture supernatants at the end of DC maturation and after a further coculture with the TT-specific CD4+ T-cell line for 1, 2, or 3 additional days (Figure 7). We found that the IL-12 heterodimer p70 (biologically active IL-12) was selectively produced by TT-pulsed DCs in the presence of the TT-specific CD4+ T-cell line from day 1, and to a lesser extent on day 2 and 3, probably due to consumption of IL-12 p70 by T cells (Figure7B). IL-12 p70 was not detected in the supernatant of mature DCs in the absence of T cells (DC culture). IL-12 p70 production was Ag-specific (Figure 7B), because culture of T cells with unpulsed DCs produced only a low level of IL-12 p70. Also, a higher level of IL-12 p70 was produced if the ratio of DCs to T cells was increased (Figure 7B). In contrast, IL-12 p40 production was not dependent on the presence of T cells (Figure 7A). Production of IL-12 by the TT-specific CD4+ T-cell line was ruled out, as neither IL-12 p70 nor IL-12 p40 was detected after stimulation of the T cells with anti-CD3 and anti-CD28 mAbs or with PMA and ionomycin (data not shown).
Bioactive IL-12 p70 production by DCs, in contrast to IL-12 p40, is specifically induced by Ag-specific interactions with T cells.
DCs either unpulsed or pulsed with TT were used to activate an autologous TT-specific CD4+ T-cell line. Secretion of IL-12 p40 (top) and IL-12 p70 (bottom) was measured in supernatants of DC culture or supernatants of DC/T-cell cocultures at day 1, 2, and 3. For the coculture experiment, results for 2.5 × 104 and 5 × 104 DCs per well, at T/DC ratios of 40:1 or 20:1, respectively, are shown. The TT-specific CD4+ T-cell line did not produce IL-12 when stimulated by PMA + ionomycin or with anti-CD3/anti-CD28 coated beads (not shown). These results are representative of 2 different experiments.
Bioactive IL-12 p70 production by DCs, in contrast to IL-12 p40, is specifically induced by Ag-specific interactions with T cells.
DCs either unpulsed or pulsed with TT were used to activate an autologous TT-specific CD4+ T-cell line. Secretion of IL-12 p40 (top) and IL-12 p70 (bottom) was measured in supernatants of DC culture or supernatants of DC/T-cell cocultures at day 1, 2, and 3. For the coculture experiment, results for 2.5 × 104 and 5 × 104 DCs per well, at T/DC ratios of 40:1 or 20:1, respectively, are shown. The TT-specific CD4+ T-cell line did not produce IL-12 when stimulated by PMA + ionomycin or with anti-CD3/anti-CD28 coated beads (not shown). These results are representative of 2 different experiments.
Thus, in our system, mature DCs produce IL-12 p40 in the absence of T cells. In contrast, biologically active IL-12 is produced by mature DCs and only after activation by an Ag-dependent interaction between DCs and T cells. This underscores the importance of bidirectional interactions between DCs and T cells in determining the outcome of cellular immune responses.
Discussion
Several stimuli were tested in the present studies for their ability to induce functional and phenotypic maturation of peripheral blood monocyte-derived DCs, and TNF-α and CD40 ligand trimer were found to be similar in efficiency to prime CD4+ T cells. Most importantly, we have shown that these mature monocyte-derived DCs can sensitize naive CD4+ T cells to neoAg in a primary proliferation assay and that this ability is critically dependent on the timing and duration of the neoAg pulse. The magnitude of proliferation to neoAgs such as KLH and HIV p24 was moderate during the primary assay, as predicted from the low frequency of precursor T cells to neoAg expected in a diverse repertoire of naive CD4+CD45RA+ T-cell population. However, vigorous proliferation of the Ag-specific T-cell lines was observed following a second round of stimulation, consistent with continued efficient and specific priming of the naive CD4+ T cells.
Pulsing DCs with Ag prior to maturation strongly enhanced the neoAg priming process. In immature DCs, MHC class II molecules are mostly intracellular; they are only briefly expressed at the cell surface and are rapidly recycled.9,33 In contrast, maturation induced by inflammatory stimuli such as TNF-α results in a boost of MHC class II molecule synthesis and in the rapid association and accumulation of a large number of MHC class II–peptide complexes at the DC surface.9,33,34 The recycling phenomenon observed in immature DCs may contribute to their previously reported ability10 to capture and process Ag. The lower precursor frequency of naive precursor T cells to neoAg emphasizes the greater dependence on precise timing of the neoAg pulse if optimal T-cell proliferation is to be obtained. Thus, pulsing DCs with Ag prior to their maturation appears to be the optimal method of priming naive CD4+ T cells in vitro.
Previous studies have shown that a variety of APCs can activate memory T cells,2,10,35 and our studies suggest that full maturation of DCs may be critical for sensitizing naive CD4+ T cells to neoAgs. The MHC class II–peptide complexes expressed on mature DCs are sufficiently stable to stimulate T cells for more than 4 days9; as mentioned above, MHC class II–peptide complexes on immature DCs are highly unstable. Naive T cells stimulated with Ag become committed to proliferation only after 15 to 30 hours, depending on the amount of Ag, whereas effector T cells become committed within 1 hour of exposure, even with low doses of Ag.36 Thus, the stable long-term expression of MHC class II–peptide complexes found only on mature DCs may be required to induce proliferation of naive T cells. In contrast, a proliferative response to a recall Ag such as TT can be generated by pulsing mature DCs during the last day of their culture, even if Ag capture is markedly less efficient,25 because only low-level MHC/Ag expression is needed for activation.36 We found that immature DCs could be used as well to activate TT-specific memory CD4+ T cells, perhaps because only 1 hour of T-cell receptor ligation is required.36 The observation that memory and effector T cells can be activated by a wider variety of predominately immature DCs than would be found in peripheral tissues is consistent with the notion that these recall responses would be activated in the periphery.
Several stimuli have been used to induce monocyte-derived DC maturation in vitro. Maturation by monocyte-conditioned medium induces the irreversible acquisition of mature DC characteristics and the strong ability to stimulate CD8+ T cells.12-15TNF-α is a potent inducer of DC maturation, and our work confirms previous studies that indicate low amounts of IL-12 production by these dendritic cells.30 However, obtaining fully activated DCs before coculture with T cells might not be crucial. Indeed, a third step of DC differentiation has been described, in which dendritic cells “activated” by exposure to CD40L expressed by activated CD4+ T cells or transfected fibroblasts,30 or a soluble CD40L trimer,23 produce large amounts of IL-12. Furthermore, after contact with CD4+ T cells, DCs are “licensed” by CD40-CD40L interactions and acquire the ability to activate cytotoxic T cells.29,37-40 In our system, we demonstrated that monocyte-derived DCs acquired the ability to secrete IL-12 p70, the biologically active IL-12 heterodimer, only after contact with Ag-specific CD4+ T cells, confirming a previous study.30
Priming and expansion of Ag-specific CD4+ T cells to neoAg has numerous applications for cellular vaccination against microbial infections and tumors. DCs directly isolated from peripheral blood have been shown to elicit primary responses to KLH and HIV gp160 from the naive CD4+CD45RA+ T-cell subpopulation21 or from total CD4+ T cells.20,22 However, mature DCs are relatively scarce in human blood, composing less than 1% of the mononuclear cell population.11 In contrast, monocyte-derived DCs can be obtained in large quantities.12,13,15 On average, we found that the yield of viable, mature DCs generated from fresh monocytes after differentiation with TNF-α was 20% of the input number (data not shown). In previous studies, when monocyte-derived DCs have been used to sensitize total CD4+ T cells in vitro, proliferation to neoAg could be demonstrated only after 2 cycles of priming.41 Multiple previous studies indicate that CD4+ and CD8+ T-cell proliferation to recall Ag has been widely documented with monocyte-derived DCs.2,10,41 42 In the present study, we clearly demonstrated that a CD4+ T-cell response to neoAg can be obtained with TNF-α–treated monocyte-derived DCs, and this appears to be efficient because the response can be detected after a single round of stimulation.
Strategies using DCs to develop and expand ex vivo Ag-specific T cells have yet to be fully explored and defined. However, it is clear that DCs can elicit, in a few cycles of stimulation, large quantities of T cells specific to a neoAg. Indeed, the use of DCs may permit cell-based vaccination by reinfusion of autologous Ag-specific CD4+ T cells obtained from unprimed donors.
Acknowledgments
We thank Dr Thomas Davis for helpful discussions, Dr Richard Carroll for critical reading of the manuscript, Julio Cotte for assistance with the apheresis, Dr Elaine Thomas for CD40LT, and Doug Smoot for excellent technical assistance with FACS analysis.
Supported by Defense Advanced Research Projects Agency grant 97-023.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
C. H. June, University of Pennsylvania, Rm 554 Biomedical Research Bldg II/III, 421 Curie Blvd, Philadelphia, PA 19104-6160; e-mail: cjune@mail.med.upenn.edu.


![Fig. 4. Only mature monocyte-derived DCs can initiate a CD4+ T-cell response to a neoAg in a primary proliferation assay. / Immature DCs (top) or mature DCs (bottom) were pulsed on day 3 of culture with TT as a recall Ag or KLH as a neoAg, and were plated at day 8 with autologous CD4+ T cells at various T/APC ratios. T-cell Ag-specific proliferation was measured by [3H]-thymidine incorporation at day 6 after coculture initiation for TT, or at day 7 for KLH. Unpulsed DCs were used as negative controls for the 6- and 7-day assays, and these controls are shown separately. Each point was done in triplicate. These results are representative of 3 experiments with 3 different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3490/5/m_h82100326004.jpeg?Expires=1770377927&Signature=aIlmHEvyEW3hRVvi5O3Sg2a1OnCJITXAJPE6D~04TchK7G-0rS-F15rCZbBUTjfC3Q1Hkxoz5yz-NhTqBT~FFmEfX4cpRwzsFkuSuaF7xR5E5dBzqoNbfMTXc-4Y3RQgpwdLComlSftapl77KFhcDGmFiw8sSx6P-jDJzy2iRmHD8WvUQ1-jVVOm1kEr0AtzkO2hzh-MsjvEhJgyIESREqxbhtT-rLTjubxAju-6CrzQDzrlxFOhseN6LzPlH54~~1sYajjCJVJZxftGCT-kGwDoO6Fi6ftdX5spJ8lTCjO9DFHiSJZ5U3xZ48xzdoq5DZj5hB8W5Op8fNarCeB6Lg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Pulsing DCs with Ag prior to their maturation is required to prime CD4+ T cells but not to elicit a recall response. / (A) Experimental design. DCs were pulsed with Ag on day 3, 6, or 7 of their culture, either before or during TNF-α–induced maturation. DCs were then harvested on day 8, irradiated, and used as stimulator cells in a primary proliferation assay with autologous CD4+ T cells at a T/DC ratio of 1:10. (B) Proliferation to recall Ag. DCs loaded with TT at indicated times were used to stimulate autologous CD4+ T cells. When indicated, Ag was removed from the DC culture after 4 hours of uptake (rem. 4h). [3H]-thymidine incorporation was measured at day 6 of the assay. (C) Proliferation to neoAg. DCs were incubated with KLH at indicated times and used at day 8 to stimulate autologous CD4+ T cells in a primary assay. When indicated, Ag was removed from the DC culture after 4 hours, 1 day, or 2 days of Ag uptake (rem. 4h, rem. d4, rem. d5). [3H]-thymidine incorporation by CD4+ T cells was measured in triplicate at day 7 of the assay. These results are representative of 3 experiments with 3 different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3490/5/m_h82100326005.jpeg?Expires=1770377927&Signature=U23EX-DRP2ayJsVvLF9t1snDHmCCapeyCww~~3SvzzsoRkD1pgbn4Z2juaGeX72RXnK4XqakQrxOkBlKkKB-aR-O~cQ3iqk6tQjEHm3GrubsmrgzvEbG-uS9P03uoqioyDb-fIF0EQ4J9YpCy1PUSIGt~l38lt-jyg2eJKjIfCqNFa8GEOipKZEFxy~1XGfKNWDeWlzVzXyR7yMUrjErljMDrmYKulqkvTShwIhgwon75A-xYqZejWAlV1oYTx2NfR9DfNM-7u0j5exT911TOzHLGbQcc1XCQA9PrbN7HX-Z6lDtZjzecahrTFT9nBbDlWoJpMxehsBhlIHr8c3jdA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Peripheral blood monocyte-derived DCs can prime KLH-specific and HIV-1 p24–specific precursors from naive CD4+CD45RA+ T cells. / (A) Autologous naive CD4+CD45RA+ T cells (left) or memory CD4+CD45RO+ T cells (right) freshly isolated from peripheral blood were stimulated with unpulsed DCs (noAg d6 and noAg d7) or with DCs pulsed with KLH, p24, or TT, at various T/DC ratios. [3H]-thymidine incorporation was measured on day 6 for TT and on day 7 for KLH and p24. Each point was done in triplicate. A representative experiment out of 3 with 3 different donors is shown. (B) Secondary stimulation. After 9 days of culture for the first stimulation, T-cell lines RA/p24, RA/KLH, and RO/TT, derived from the same donor shown in panel A, were restimulated for 3 days with DCs pulsed with p24, KLH, or TT, respectively, or with DCs pulsed with an irrelevant Ag (control), ie, KLH for RA/p24, TT for RA/KLH, and KLH for RO/TT. [3H]-thymidine was added for the last 18 hours of the assay. Each point was done in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3490/5/m_h82100326006.jpeg?Expires=1770377927&Signature=mFSsJgqYNC3YI8ucqwQa2ZX6qrh-OmaOzsxVhdJbHu0sDESpG4~WB3NthE3KbjyGiaOJRXYadmVc4Gr51NTqQYvzNOc-tNOee32np5MXoMUY3ngwCnTPktzaWv6IdWPW8QqwGxqHq0hljKr-DsPMboFJ0QKNEkeVvhxTVdX9ET-aib8HwrDm-ma4jAdjU5kzlFuEdWiMJM3T2h151z2vuFBiQue0m8SUe045JJ100vcwj8r8K2LboA99LvOydKmN7f4H6DDnP7PV1LKj1ItziMsrDIKxDo7E23wmN7OlITxACEFHovZgPJbqR4HPEb7zSpvCSm7mUM27290Nx8AJVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. Only mature monocyte-derived DCs can initiate a CD4+ T-cell response to a neoAg in a primary proliferation assay. / Immature DCs (top) or mature DCs (bottom) were pulsed on day 3 of culture with TT as a recall Ag or KLH as a neoAg, and were plated at day 8 with autologous CD4+ T cells at various T/APC ratios. T-cell Ag-specific proliferation was measured by [3H]-thymidine incorporation at day 6 after coculture initiation for TT, or at day 7 for KLH. Unpulsed DCs were used as negative controls for the 6- and 7-day assays, and these controls are shown separately. Each point was done in triplicate. These results are representative of 3 experiments with 3 different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3490/5/m_h82100326004.jpeg?Expires=1770377928&Signature=0971CeNb2XjLCjJFjyy7X39OteYf~6Cab0byYVUoGG2r0ZKh4ldDmioJid4v3Bx7KOFrye3jpxOH85D8Y34ZzyopTJzEEaKQQUDYpcnoI5XSPc6TD70OoFl8Te0faQkTvwY8VKY3ZYoyhGC~dNXZH9NbqT10gVY26Od04dBrb40ou9Sz~QfQ4wsBpn0p-5qbND~Q0XAzeHlTle1AdA2BjBZfqBnldA73kWoJzw1W~uxAVwgtnWeLFbXljV4-qre-Lwp4l7FAbjbhMTpURI2Q1vxnThax6iSb3VhUB8NPcHJ7KsMKefpKTVGLHhQ126cmdehO~~AbitoHCsne6FlQFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Pulsing DCs with Ag prior to their maturation is required to prime CD4+ T cells but not to elicit a recall response. / (A) Experimental design. DCs were pulsed with Ag on day 3, 6, or 7 of their culture, either before or during TNF-α–induced maturation. DCs were then harvested on day 8, irradiated, and used as stimulator cells in a primary proliferation assay with autologous CD4+ T cells at a T/DC ratio of 1:10. (B) Proliferation to recall Ag. DCs loaded with TT at indicated times were used to stimulate autologous CD4+ T cells. When indicated, Ag was removed from the DC culture after 4 hours of uptake (rem. 4h). [3H]-thymidine incorporation was measured at day 6 of the assay. (C) Proliferation to neoAg. DCs were incubated with KLH at indicated times and used at day 8 to stimulate autologous CD4+ T cells in a primary assay. When indicated, Ag was removed from the DC culture after 4 hours, 1 day, or 2 days of Ag uptake (rem. 4h, rem. d4, rem. d5). [3H]-thymidine incorporation by CD4+ T cells was measured in triplicate at day 7 of the assay. These results are representative of 3 experiments with 3 different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3490/5/m_h82100326005.jpeg?Expires=1770377928&Signature=OWFcse78mq-k9pDNBcvjBZzZgHWLmof2CLzqdITa6DYAQNnGBNcQiG8ouinBYjFO3ONDuHHwI29FExl5tzpf9bBAYYsAPZI5f96Ss1bpoWhWL5WSu~fFtbn40DX0XisXx-sNVRQcIa8-PZ3B2~3nXG7GhUPSx6lF20zxKCxF8twMAg3hPdholvXtbeN-unqrSlzGxU5i9kmYeFfiYBFShiMvBPKsKEqISmQtnjfAEui4pG79U3EgnyKqiIToXtqXDCaYYOHmG0X4g4YhEFwP9mTyaOVSxe2sBbcnArrfZhE4bWG4c40Hl0PbYIn0ma6EaRZccMU-6rCrl~-xcjoOvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Peripheral blood monocyte-derived DCs can prime KLH-specific and HIV-1 p24–specific precursors from naive CD4+CD45RA+ T cells. / (A) Autologous naive CD4+CD45RA+ T cells (left) or memory CD4+CD45RO+ T cells (right) freshly isolated from peripheral blood were stimulated with unpulsed DCs (noAg d6 and noAg d7) or with DCs pulsed with KLH, p24, or TT, at various T/DC ratios. [3H]-thymidine incorporation was measured on day 6 for TT and on day 7 for KLH and p24. Each point was done in triplicate. A representative experiment out of 3 with 3 different donors is shown. (B) Secondary stimulation. After 9 days of culture for the first stimulation, T-cell lines RA/p24, RA/KLH, and RO/TT, derived from the same donor shown in panel A, were restimulated for 3 days with DCs pulsed with p24, KLH, or TT, respectively, or with DCs pulsed with an irrelevant Ag (control), ie, KLH for RA/p24, TT for RA/KLH, and KLH for RO/TT. [3H]-thymidine was added for the last 18 hours of the assay. Each point was done in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3490/5/m_h82100326006.jpeg?Expires=1770377928&Signature=n1o~YVwJpkPLwCCIGyOT8kIJLRUY71j27wAWSVU7RmNSANdL630p8n2~z1NJyly8aKV3I1Q7KTTrxVlnPC7stMHaeLNEb9Ao4LKzZL4NwlPX8KetLEH6SvY9mAf9ZbVYM~bOfNYYZgmkfLnNR0Rd357pLHXUJ98QbcfTCY1GKvEGPBq9pjtl4hFzZLZeaafnfoEPT3g4HFMjCONu-0sqci9aakhoLcQS1xtc3zRwNSIbMd185cz88f9yAseydFgkOeDp-NM2ac8xr~Zk-WaPRfrI4oGDRuZIXOhlzOBWpIfLsVtb5yVtxfuWE3m2PSWBjXndXXZcJHhNmBJ-zb4UKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
