Abstract
L-selectin is an adhesion molecule that plays an essential role in the early events of the inflammatory response. Our group has recently described that several nonsteroidal anti-inflammatory drugs (NSAIDs) are able to induce both in vivo and in vitro the shedding of L-selectin in neutrophils through an unknown mechanism. In this work, we have studied potential mechanisms involved in the shedding of L-selectin induced by NSAIDs. This effect of NSAIDs did not involve any detectable intracellular calcium flux. Pretreatment of neutrophils either with Ro 31-8220 and H7, 2 specific inhibitors of protein kinase C (PKC), or with inhibitors of protein tyrosine kinases such as tyrphostin A25 or herbimycin A did not prevent the NSAID-mediated L-selectin shedding. However, the KD-IX-73-4, an inhibitor of L-selectin proteolysis was able to block the effect of NSAIDs on L-selectin expression. Remarkably, NSAIDs caused a variable reduction in the neutrophil intracellular ATP concentration that highly correlated with the differential ability of NSAIDs to trigger L-selectin shedding (r = 0.8, P < .01). In agreement with this finding, azide plus 2-deoxy-D-glucose, 2 metabolic blockers, also induced a rapid L-selectin shedding (65% ± 8%) without affecting the neutrophil viability, activation, or expression level of other surface molecules with soluble isoforms such as CD16 and CD59. These data indicate that the maintenance of L-selectin on the neutrophil surface requires energy consumption, which suggests that L-selectin is shed in neutrophils by default. Interestingly, NSAIDs seem to cause the shedding of L-selectin, at least in part, through the reduction of the intracellular ATP concentration.
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a group of therapeutic agents widely used for symptomatic treatment of nonsevere human inflammatory disorders. Since the early 1970s, it is accepted that the mechanism of action of these agents is the inhibition of prostaglandin synthesis1 through the blockade of cyclooxygenase (COX). However, several clinical2-4 and experimental5,6 evidences suggest that COX inhibition might not adequately explain the fully anti-inflammatory action of this group of compounds. In the last decade, different studies have described several prostaglandin-independent actions of NSAIDs, such as the inhibition of oxidative phosphorylation in mitochondria7-9 or the blockade of transcription factor NF-kB,10 among many others.2,11-14 In this regard, our group has reported that leukocyte adhesion molecules essential for the inflammatory response may be potential targets for NSAIDs.15-18
The accumulation of leukocytes into tissues is a cardinal event in the inflammatory response that requires a complex coordination of adhesive interactions between flowing leukocytes and endothelial cells. The selectin family is a group of 3 adhesion receptors: L-, E-, and P-selectin. Selectins recognize carbohydrate moieties expressed by both leukocytes and activated endothelial cells, causing the slow down of flowing leukocytes.19 This selectin-mediated reduction in the leukocyte speed plays an important role in the inflammatory response because it facilitates the action of other important adhesion receptors such as integrins and members of the immunoglobulin superfamily.20 Unlike E- and P-selectins, the cellular mechanisms involved in the regulation of L-selectin (CD62L) expression are not fully defined. L-selectin is constitutively expressed on the cell surface of most leukocytes and is enzymatically cleaved and released after cell activation both in vitro21,22 and in vivo.23,24 Although the protease responsible for the cleavage of L-selectin has not been yet characterized, recent observations suggest that it may be a member of the ADAM (a desintegrin and metalloprotease) family of surface metalloproteases.25Several evidences support the prominent role of L-selectin in the inflammatory response. A human congenital disorder termedleukocyte adhesion deficiency (LAD) type II characterized by an aberrant inflammatory response is due to the lack of a class of cell-surface carbohydrates, which includes ligands of selectins.26 In addition, many groups have confirmed in gene-deficient animals the relevant role of L-selectin in the recruitment of leukocytes into inflamed tissues.27-29On this basis, many efforts are currently concentrated in the development of new anti-inflammatory agents that selectively promote the shedding of L-selectin.30 This antiselectin therapy has been already proved beneficial in several animal models of inflammation.31-33
Our group has recently described that commonly prescribed NSAIDs such as indomethacin, diclofenac, ketoprofen, sodium salicylate, aspirin, and aceclofenac, but not others such as piroxicam, are able to induce the shedding of L-selectin from the neutrophil surface.15,16 This effect of NSAIDs on L-selectin expression has been also confirmed in vivo; standard anti-inflammatory doses of indomethacin cause a significant reduction of L-selectin expression in flowing neutrophils of healthy volunteers.15However, the mechanism through which NSAIDs cause the L-selectin shedding in neutrophils is still unknown.
The aim of this article was to study the mechanism involved in the NSAID-mediated L-selectin shedding in neutrophils. We found that the intracellular ATP levels play an important role in the maintenance of L-selectin on the neutrophil surface. A group of NSAIDs seems to cause the shedding of L-selectin, at least in part, through its ability to reduce the intracellular ATP concentration.
Materials and methods
Antibodies and reagents
The following monoclonal antibodies (MAbs) were used: Bear 1 anti-CD11b, D3/9 anti-CD45,34 VJ1/12 anti-CD59, KD1 anti-CD1635, and P3X63 myeloma culture supernatant as a negative control. The Leu-8 anti-L-selectin MAb was purchased from Becton Dickinson Immunocytometry Systems (Mountain View, CA).
Aceclofenac was provided by Almirall-Prodesfarma (Barcelona, Spain) and meloxicam from Boëhringer Ingelheim GmbH (Ingelheim, Germany). Diclofenac, mefenamic acid, flufenamic acid, aspirin (acetylsalicylic acid), sodium salicylate, piroxicam, phenylbutazone, and indomethacin were purchased from Sigma Chemical Co (St Louis, MO). The 2-deoxy-D-glucose (2-DG), azide, ionophore A23187, N-formyl-met-leu-phe (f-MLP), phorbol myristate acetate (PMA), and prostaglandin E2 (PGE2) were purchased from Sigma Chemical Co.
The protein tyrosine kinase inhibitors tyrphostin A25 and herbimycin A and the protein kinase C (PKC) inhibitors H-7 and Ro 31-8220 were purchased from Calbiochem-Novabiochem (San Diego, CA). The protein kinase A inhibitor H-89 and the tyrosine phosphatase inhibitor sodium orthovanadate were from Calbiochem-Novabiochem. NS-398, a selective inhibitor of COX-2 was also obtained from Calbiochem-Novabiochem. Fluo-3 am was from Molecular Probes Inc (Eugene, OR).
The hydroxamic acid-based L-selectin shedding inhibitor, KD-IX-73-4,36 was a gift from Dr Takashi Kei Kishimoto (Boëhringer Ingelheim Pharmaceuticals, Inc, Ridgefield, CT). Hanks' balanced salt solution (HBSS) was obtained from BioWhittaker (Walkersville, MD).
Cell isolation and treatments
Human neutrophils were isolated from peripheral blood of healthy donors by Ficoll-Hypaque (Pharmacia Diagnostics AB, Uppsala, Sweden) density- gradient centrifugation for 30 minutes at 1800 rpm, followed by sedimentation at 1g in 1.3% (wt/vol) dextran (Sigma Chemical Co) at room temperature. The neutrophil enriched fraction was further purified by hypotonic lysis of erythrocytes, giving purity higher than 95%. In experiments with lymphocytes, after the Ficoll-Hypaque density centrifugation, the mononuclear band was washed once with phosphate buffer solution (PBS) and mononuclear cells were used without further purification.
Experiments on the effect of NSAIDs on the expression of adhesion molecules were carried out in 15 mL disposable polypropylene tubes (Falcon Labware, Oxnard, CA). Neutrophils were resuspended in PBS and incubated either alone or in the presence of 20 μg/mL of piroxicam, meloxicam, phenylbutazone, aceclofenac, diclofenac, indomethacin, mefenamic acid, and flufenamic acid for 30 minutes at 37°C. Aspirin and sodium salicylate were used at 200 μg/mL. Dose-response experiments were performed under the same experimental conditions. Flufenamic acid, diclofenac, aceclofenac, and indomethacin were used at doses ranging from 1 to 50 μg/mL, whereas aspirin and sodium salicylate from 100 to 500 μg/mL. Some experiments with NSAIDs were performed in the presence of 3 g/L of human albumin (Grifols, Barcelona, Spain). PMA was used at 20 ng/mL, azide at 10 nmol/L and 2-DG at 50 nmol/L. In experiments with inhibitors, neutrophils were pretreated with the specific inhibitor for 15 minutes at 37°C before adding the different NSAIDs. All NSAIDs and kinase/phosphatase inhibitors were solubilized in dimethyl sulfoxide (DMSO) (Panreac, Barcelona, Spain) and added to the cell solution in a final concentration below 0.2% DMSO. In experiments with prostaglandins, neutrophils were treated with PGE2 1 μmol/L and incubated for 30 minutes at 37°C. All control data were obtained in the presence of 0.2% DMSO.
Flow cytometry analysis
Cells were treated as described previously and then incubated with the different MAbs at 4°C for 30 minutes. After washing in PBS, the cells were labeled with fluorescein isothiocyanate (FITC)-labeled goat antimouse immunoglobulin (Ig) (Dako, Salstrup, Denmark). At least 5 × 103 cells of each sample were analyzed in a FACScan flow cytometer (Becton Dickinson) and the data were collected in both linear and logarithmic scales. In experiments with mononuclear cells, the L-selectin expression was analyzed in cells with side and forward scatter characteristics of lymphocytes. Mean fluorescence intensity (MFI) in linear scale was obtained by adjusting the fluorescence gain so that about 5% of the cells of the sample with the greatest fluorescence were positive in the highest fluorescence channel. The fluorescence produced by the myeloma P3X63 supernatant was considered background. Because the fluorescence conditions were different from experiment to experiment, data were normalized to express relative mean fluorescence intensity (rMFI), as follows: rMFI = (MFI NSAID − MFI P3X63) / (MFI medium − MFI P3X63) × 100.
In all experiments with metabolic inhibitors and in some ATP quantification assays, cell viability was determined in the FACScan through the spontaneous uptake of propidium iodide (10 μg/mL final concentration) (Sigma Chemical Co).
Soluble L-selectin enzyme-linked immunosorbent assay
Neutrophils (7 × 106 cells/mL) were incubated alone or with the different NSAIDs in PBS at doses and times indicated. After 30 minutes at 37°C, cells were centrifuged and the cell-free supernatants were tested for soluble L-selectin (sL-selectin) by an enzyme-linked immunosorbent assay (ELISA) kit (Bender Medsystem, Vienna, Austria) according to the manufacturer's instructions.
ATP quantification assays
Intracellular ATP concentration in neutrophils was measured with an ATP Bioluminiscence Assay Kit obtained from Boëhringer Mannheim (Mannheim, Germany). Neutrophils (7 × 106cells/mL) were incubated in PBS alone and in the presence of metabolic inhibitors, PGE2, or different NSAIDs at doses indicated. After times indicated at 37°C, cells were pelleted and resuspended in the lysis buffer provided with the kit. ATP was assayed following the manufacturer's instructions and measured in a luminophotometer Lumat LB9501 (Berthold, Wildbad, Germany).
Intracellular calcium assays
Neutrophils (3 × 106 cells/mL) were loaded with Fluo-3 am 3 μmol/L in PBS for 15 minutes at 37°C. After washing twice in PBS, cells were resuspended in HBSS at 1 × 106 cells/mL final concentration. Calcium influx was analyzed in a FACScan studying the variations in the basal fluorescence after the addition of the respective NSAID along 6 minutes. Ionophore (0.5 μmol/L) and f-MLP (0.1 μmol/L) were used as positive controls.
Statistical analysis
Results were expressed as arithmetic mean ± SD or standard error (SE) of the mean. Student t test for paired samples was used to determine significant differences between means.
Results
NSAIDs cause the shedding of L-selectin in neutrophils through a physiologic mechanism
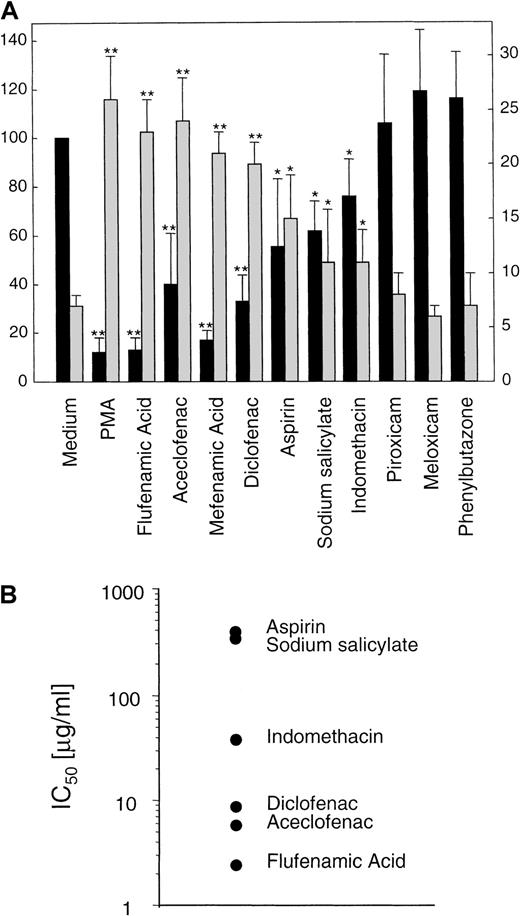
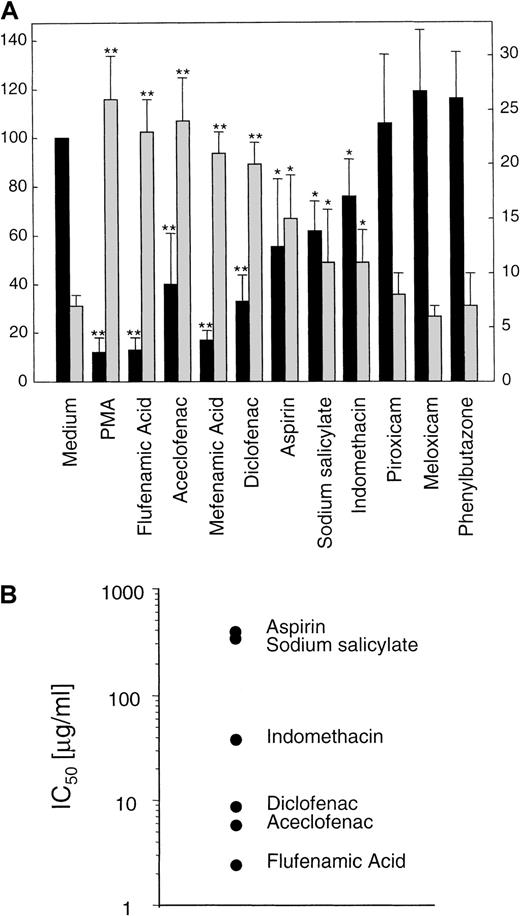
To complement previous data from our group,15 16 we decided to test the effect of an ample collection of NSAIDs on the expression of L-selectin. We examined by flow cytometry, the effect of sodium salicylate, aspirin, indomethacin, aceclofenac, diclofenac, piroxicam, meloxicam, flufenamic acid, mefenamic acid, and phenylbutazone on the basal expression of L-selectin in human neutrophils. As shown in Figure1, flufenamic acid, mefenamic acid, diclofenac, aceclofenac, aspirin, or indomethacin, caused, in this order of potency, a significant decrement in the basal surface expression of L-selectin. In contrast, neutrophils incubated with piroxicam, meloxicam, or phenylbutazone did not show any change in the basal expression of this adhesion molecule. In some experiments sodium salicylate, the circulating aspirin metabolite at 200 μg/mL was effective in triggering L-selectin shedding, even in the presence of human albumin (data not shown).
NSAIDs are able to induce differentially the shedding of L-selectin in neutrophils.
(A) Surface expression of L-selectin (▪) and supernatant concentration of its soluble isoform (sL-selectin, ░) in neutrophils treated with different NSAIDs. Neutrophils isolated from peripheral blood were incubated for 30 minutes at 37°C in medium alone, with 20 ng/mL of PMA, or with 20 μg/mL of the different NSAIDs, except aspirin and sodium salicylate that were used at 200 μg/mL. After incubation, cell solutions were centrifuged, and supernatant fluids were tested for sL-selectin by an ELISA. Values were obtained in duplicate determinations for each sample. Data represent the mean (ng/106 cells) ± SD (░) from 3 independent experiments (left y axis). Simultaneously, the surface expression level of L-selectin in neutrophils was assessed by flow cytometry and expressed as the relative mean fluorescence (rMFI) as described in “Materials and methods.” Data represent the mean ± SD of rMFI (▪) from 5 independent experiments (right yaxis). PMA was used as positive control of L-selectin shedding. * = P < .05 and ** = P < .01 versus medium, by Student paired t test. (B) Graphical display of IC50 values of several NSAIDs on basal expression of L-selectin in neutrophils. Data were obtained from a representative dose-response experiment of 3.
NSAIDs are able to induce differentially the shedding of L-selectin in neutrophils.
(A) Surface expression of L-selectin (▪) and supernatant concentration of its soluble isoform (sL-selectin, ░) in neutrophils treated with different NSAIDs. Neutrophils isolated from peripheral blood were incubated for 30 minutes at 37°C in medium alone, with 20 ng/mL of PMA, or with 20 μg/mL of the different NSAIDs, except aspirin and sodium salicylate that were used at 200 μg/mL. After incubation, cell solutions were centrifuged, and supernatant fluids were tested for sL-selectin by an ELISA. Values were obtained in duplicate determinations for each sample. Data represent the mean (ng/106 cells) ± SD (░) from 3 independent experiments (left y axis). Simultaneously, the surface expression level of L-selectin in neutrophils was assessed by flow cytometry and expressed as the relative mean fluorescence (rMFI) as described in “Materials and methods.” Data represent the mean ± SD of rMFI (▪) from 5 independent experiments (right yaxis). PMA was used as positive control of L-selectin shedding. * = P < .05 and ** = P < .01 versus medium, by Student paired t test. (B) Graphical display of IC50 values of several NSAIDs on basal expression of L-selectin in neutrophils. Data were obtained from a representative dose-response experiment of 3.
L-selectin is cleaved and released from the cell surface to plasma where it is detected as a soluble isoform (sL-selectin) at high concentrations.37 An ELISA detected a significant increment in the sL-selectin concentration ranging from 26 to 11 ng/106 cells in the cell-free supernatant of neutrophils incubated with flufenamic acid, aceclofenac, mefenamic acid, diclofenac, sodium salicylate, aspirin, and indomethacin. However, the supernatant of neutrophils treated with meloxicam, piroxicam, and phenylbutazone did not show any variation in the concentration of sL-selectin respect to cells incubated with the medium alone (Figure1A). The vehicle DMSO had no effect on L-selectin shedding at the concentration tested. Figure 1B shows the concentrations of different NSAIDs that caused the 50% of decrement in the basal expression of L-selectin in neutrophils (IC50).
Under physiologic stimuli, L-selectin is proteolytically cleaved by a still not fully characterized metalloprotease. Several reports have shown that such protease is a surface molecule that can be blocked by hydroxamic acid-based inhibitors.36,38 One of such compound is the KD-IX-73-4, recently described as a protease inhibitor that blocks L-selectin shedding from stimulated neutrophil surface without affecting CD11b mobilization or general neutrophil activation.36 39 To rule out the possibility of an inherent proteolytic activity of NSAIDs on L-selectin, we tested the effect of KD-IX-73-4 on NSAID-dependent L-selectin down-regulation in neutrophils. As shown in Table 1, pretreatment of neutrophils with 20 μmol/L of KD-IX-73-4 prevented the loss of L-selectin expression induced by flufenamic acid, aceclofenac, mefenamic acid, diclofenac, aspirin, and indomethacin.
These data indicate that a group of NSAIDs differentially induces the shedding of L-selectin through a mechanism that requires a functionally active KD-IX-73-4 sensitive-protease.
NSAIDs induce L-selectin shedding in neutrophils through a PKC and tyrosine kinase-independent mechanism
L-selectin is constitutively expressed on essentially all leukocytes and is rapidly released from the cell surface in response to a variety of stimuli.19 However, neither the physiologic significance of L-selectin shedding nor the signal transduction pathways involved in its regulation have been fully elucidated. Several recent reports suggest that, in a cell type-specific fashion, the PKC system and tyrosine phosphorylation and dephosphorylation cascades are involved in the regulation of L-selectin surface expression.40-43 Therefore, we decided to ascertain the role of both signaling pathways in the L-selectin down-regulation induced by NSAIDs in neutrophils.
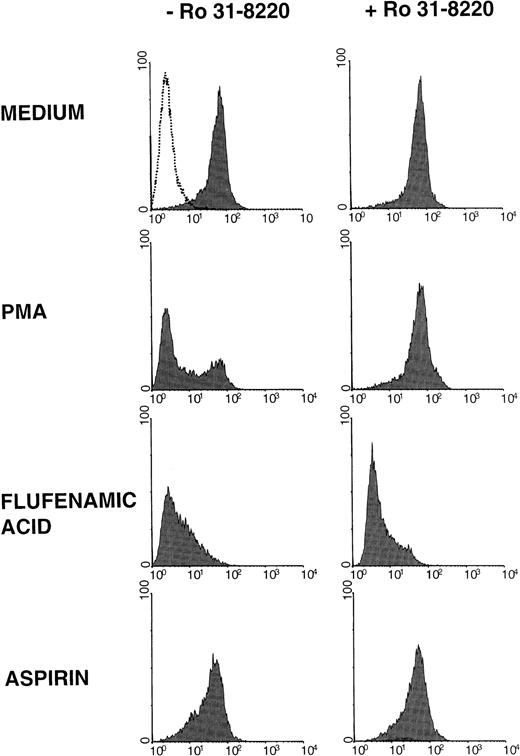
Ro 31-8220, a new and selective PKC inhibitor (Figure2), and H7, a broad-based serine-threonine kinase inhibitor, reverted the rapid L-selectin shedding induced by the activation of PKC by PMA in neutrophils (data not shown). However, none of these inhibitors was able to prevent the down-regulation of L-selectin in neutrophils induced by flufenamic acid and aspirin (Figure 2, and data not shown). In a similar manner to PKC inhibitors, the incubation of resting neutrophils with tyrphostin A25 and herbimycin A, 2 tyrosine kinase inhibitors, did not prevent the effect of NSAIDs on L-selectin expression (data not shown). It has been described that some MAbs against the tyrosine phosphatase CD45 elicit L-selectin down-regulation in lymphocytes,42 suggesting that the phosphorylation of tyrosine might play a role in the maintenance of L-selectin on the cell membrane. Thus, if NSAIDs induce the L-selectin shedding in neutrophils through a tyrosine dephosphorylation mechanism, an inhibitor of tyrosine phosphatase should rescue the down-regulation of L-selectin induced by NSAIDs. However, as shown in Table 2, pretreatment of neutrophils with sodium orthovanadate, an inhibitor of tyrosine phosphatases, did not prevent the loss of L-selectin expression induced by flufenamic acid and aspirin.
NSAIDs induce the shedding of L-selectin in neutrophils by a PKC-independent mechanism.
Effect of the PKC inhibitor Ro 31-8220 on the down-regulation of L-selectin induced by NSAIDs. Neutrophils were preincubated in medium alone (−Ro 31-8220) or in the presence of Ro 31-8220 2 μmol/L. After 15 minutes at room temperature, cells were treated with PMA, flufenamic acid, and aspirin at the concentrations and conditions indicated in Figure 1. The expression of L-selectin was estimated by flow cytometry as described in “Materials and methods.” Shaded histograms represent the L-selectin expression; the dotted line is the negative control of immunostaining (fluorescence produced by the supernatant of P3X63 myeloma). One representative experiment of 3 is shown.
NSAIDs induce the shedding of L-selectin in neutrophils by a PKC-independent mechanism.
Effect of the PKC inhibitor Ro 31-8220 on the down-regulation of L-selectin induced by NSAIDs. Neutrophils were preincubated in medium alone (−Ro 31-8220) or in the presence of Ro 31-8220 2 μmol/L. After 15 minutes at room temperature, cells were treated with PMA, flufenamic acid, and aspirin at the concentrations and conditions indicated in Figure 1. The expression of L-selectin was estimated by flow cytometry as described in “Materials and methods.” Shaded histograms represent the L-selectin expression; the dotted line is the negative control of immunostaining (fluorescence produced by the supernatant of P3X63 myeloma). One representative experiment of 3 is shown.
Recently, it has been suggested that calcium influx might be involved in the L-selectin shedding through the induction of conformational changes in the calmodulin.44 Consequently, we performed experiments to investigate whether NSAIDs were able to induce directly variations in the intracellular calcium concentration in resting neutrophils, as described in “Materials and methods.” Flufenamic acid, aceclofenac, diclofenac, and indomethacin at 20 μg/mL did not significantly modify the intracellular calcium levels in neutrophils preloaded with the calcium indicator fluo-3 (data not shown).
These results suggest that NSAIDs-induced L-selectin shedding occurs in neutrophils through a likely PKC and tyrosine kinase/phosphatase-independent pathway. In addition, the effect of NSAIDs on L-selectin expression is not associated with an increment in intracellular calcium concentration, an event that used to follow neutrophil activation.45 46
Correlation between L-selectin expression and intracellular ATP concentration in neutrophils treated with NSAIDs
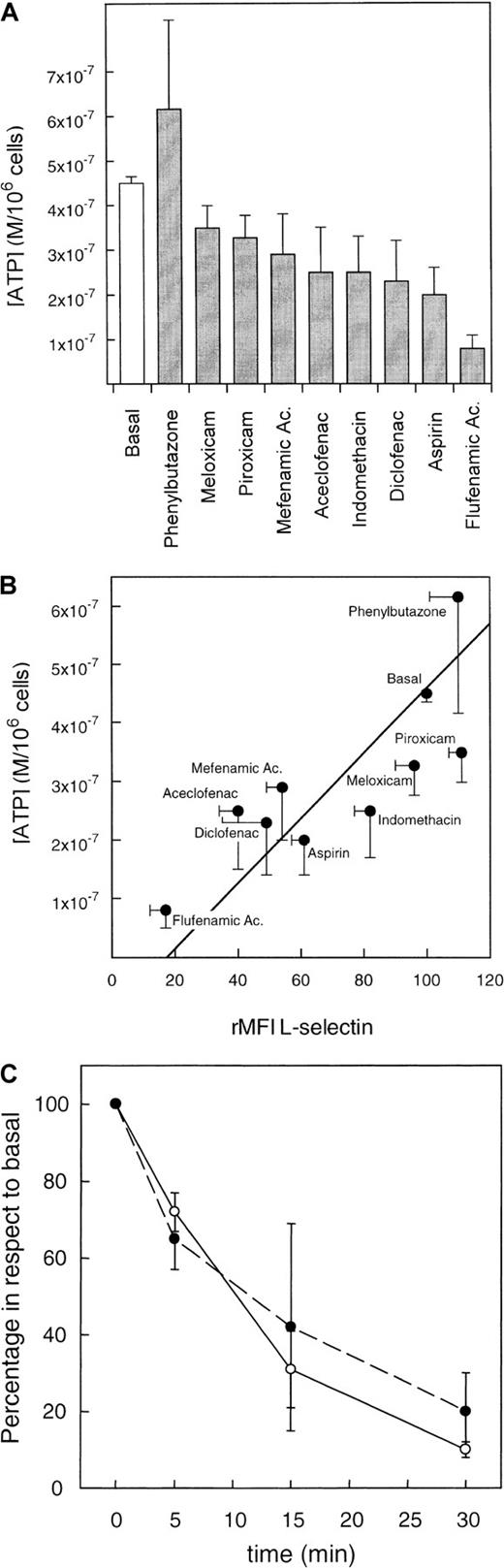
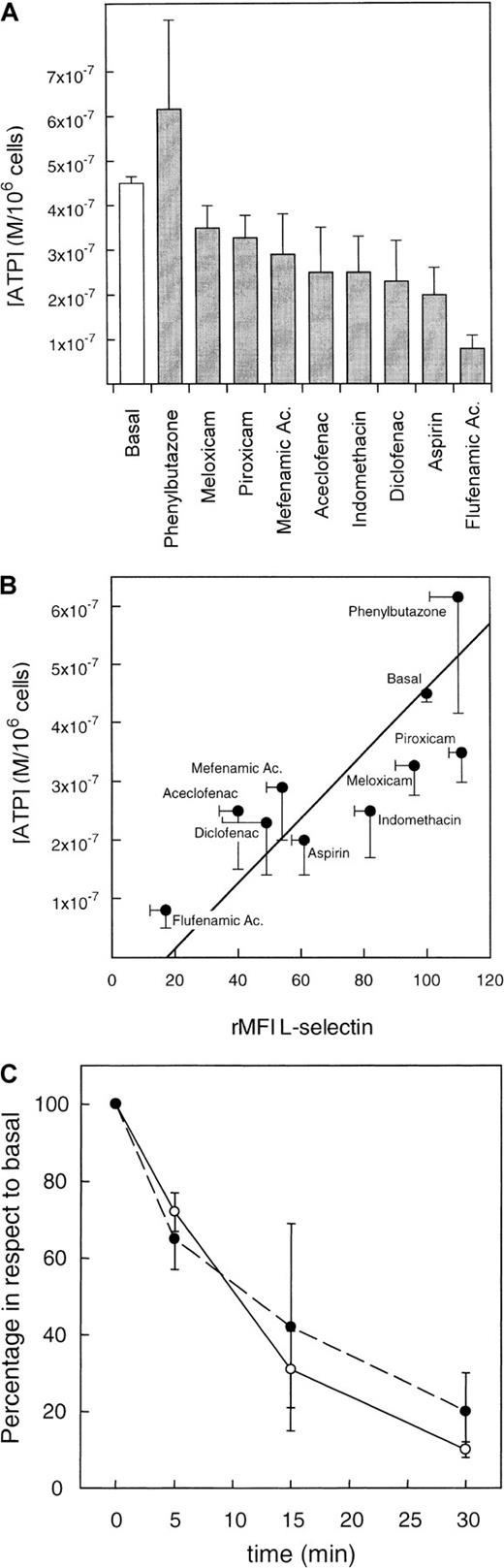
Studies with several NSAIDs indicate that these agents are able to reduce the intracellular ATP concentration through a mechanism that affects the mitochondrial respiration.7-9 We therefore decided to test the potential relationship between the NSAIDs-mediated reduction of intracellular ATP concentration and L-selectin shedding in neutrophils. Figure 3A shows the effects of different NSAIDs in the intracellular concentration of ATP in neutrophils. Although NSAIDs such as piroxicam, meloxicam, or phenylbutazone did not modify the concentration of ATP, the flufenamic acid was able to reduce 4 times the basal ATP concentration in neutrophils. When data of ATP concentration and L-selectin expression in NSAID-treated neutrophils were plotted together, a highly significant direct correlation between reduction of ATP intracellular and surface expression of L-selectin was observed (Figure 3B) (correlation coefficient r = 0. 8,P < .01). Moreover, kinetics studies showed that intracellular ATP concentration decreased in correlation with L-selectin expression in neutrophils incubated with flufenamic acid (Figure 3C). In some kinetics experiments, the neutrophil viability was assessed as described in “Materials and methods” without showing any significant changes (data not shown).
NSAIDs cause the shedding of L-selectin proportionally to the reduction of intracellular ATP concentration.
(A) Effect of NSAIDs on intracellular concentration of ATP. Neutrophils were incubated with the different NSAIDs under the same conditions described in Figure 1. After centrifugation, cell pellets were lysed and the ATP concentration was tested by a commercial kit as described in “Materials and methods.” Values were obtained in duplicate determinations for each sample. The results represent the mean (M/106 cells) ± SE from 6 independent experiments. (B) Correlation between the reduction of ATP concentration and surface expression of L-selectin induced by NSAIDs in neutrophils. Neutrophils were incubated with the different NSAIDs under the same conditions described in Figure 1. After centrifugation, cell pellets were divided in 2 parts; one was used for ATP determination, which results are depicted in A, and the other part was used for the quantification of the L-selectin surface expression by flow cytometry as described in “Materials and methods.” There was a highly significant direct correlation between reduction of ATP and surface expression of L-selectin (r = 0. 8, P < .01; n = 6). (C) Correlation between the time-dependent variation of surface L-selectin expression (○) and intracellular ATP concentration (●) in neutrophils incubated with 20 μg/mL of flufenamic acid. Values are percentage of expression and concentration of L-selectin and ATP in respect to the basal conditions (medium alone) in each time. Data represent the mean ± SD of 3 independent experiments.
NSAIDs cause the shedding of L-selectin proportionally to the reduction of intracellular ATP concentration.
(A) Effect of NSAIDs on intracellular concentration of ATP. Neutrophils were incubated with the different NSAIDs under the same conditions described in Figure 1. After centrifugation, cell pellets were lysed and the ATP concentration was tested by a commercial kit as described in “Materials and methods.” Values were obtained in duplicate determinations for each sample. The results represent the mean (M/106 cells) ± SE from 6 independent experiments. (B) Correlation between the reduction of ATP concentration and surface expression of L-selectin induced by NSAIDs in neutrophils. Neutrophils were incubated with the different NSAIDs under the same conditions described in Figure 1. After centrifugation, cell pellets were divided in 2 parts; one was used for ATP determination, which results are depicted in A, and the other part was used for the quantification of the L-selectin surface expression by flow cytometry as described in “Materials and methods.” There was a highly significant direct correlation between reduction of ATP and surface expression of L-selectin (r = 0. 8, P < .01; n = 6). (C) Correlation between the time-dependent variation of surface L-selectin expression (○) and intracellular ATP concentration (●) in neutrophils incubated with 20 μg/mL of flufenamic acid. Values are percentage of expression and concentration of L-selectin and ATP in respect to the basal conditions (medium alone) in each time. Data represent the mean ± SD of 3 independent experiments.
To rule out the potential role of cyclooxygenase-derived products in the regulation of ATP by NSAIDs, we tested the effect of PGE2 in the intracellular ATP levels in neutrophils. The incubation of neutrophils with PGE2 1 μmol/L neither modified the basal levels of ATP concentration nor the L-selectin surface expression. In addition, pretreatment with PGE2 showed no effect in the reduction of ATP and L-selectin induced by flufenamic acid in neutrophils. Moreover, the neutrophil incubation with NS-398, a selective COX-2 inhibitor, did not either significantly modify the basal expression of L-selectin or prevent the shedding of L-selectin induced by flufenamic acid (data not shown).
These data suggest that the ability of NSAIDs to induce both the shedding of L-selectin and the reduction of the intracellular ATP concentration may be linked effects.
Inhibition of cell metabolism causes the shedding of L-selectin in neutrophils
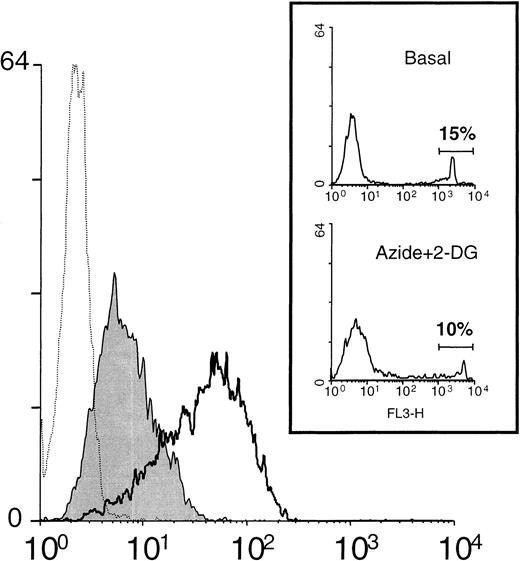
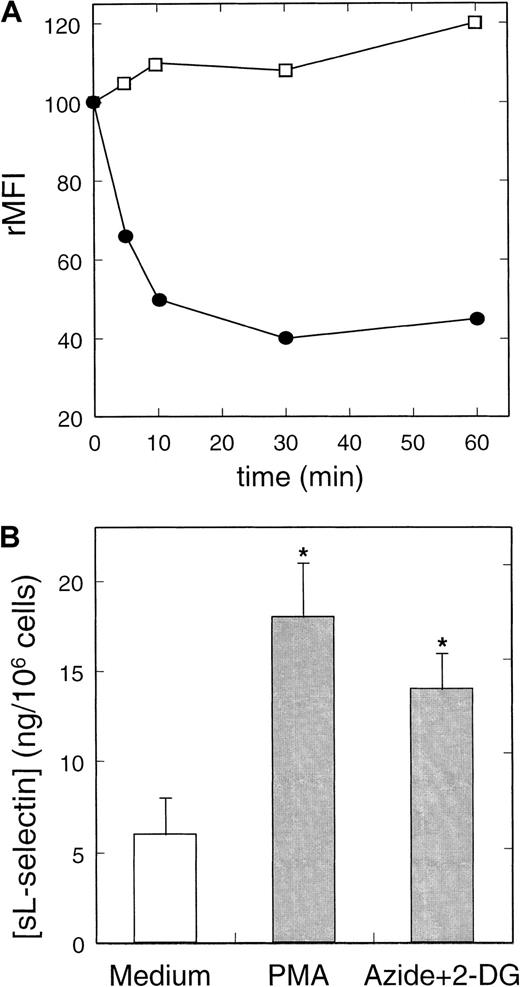
The foregoing experiments suggested a potential relationship between intracellular ATP reduction and L-selectin shedding in neutrophils. To further characterize this phenomenon, we tested the effect of standard metabolic inhibitors in the expression of L-selectin in neutrophils (Figure 4). The incubation of resting neutrophils with a combination of inhibitors of oxidative phosphorylation (azide) and anaerobic glycolysis (2-deoxy-D-glucose [2-DG]) induced a reduction in the basal intracellular ATP concentration of about 2 orders of magnitude (data not shown). Under those conditions, neutrophils displayed a reduction of 65% ± 8% (n = 8) in the basal surface expression of L-selectin (see Figure 5). Pretreatment of neutrophils with KD-IX-73-4 fully prevented the loss of L-selectin expression induced by the metabolic inhibitors (data not shown). To rule out the possibility that a decrement in cell viability might be responsible of such effects, the percentage of dead cells was assayed in all experiments in which metabolic inhibitors were used, as described in “Materials and methods.” The inset of Figure4 shows that under our experimental conditions, the neutrophil viability, assessed by the uptake of propidium iodide, was not modified by azide and 2-DG.
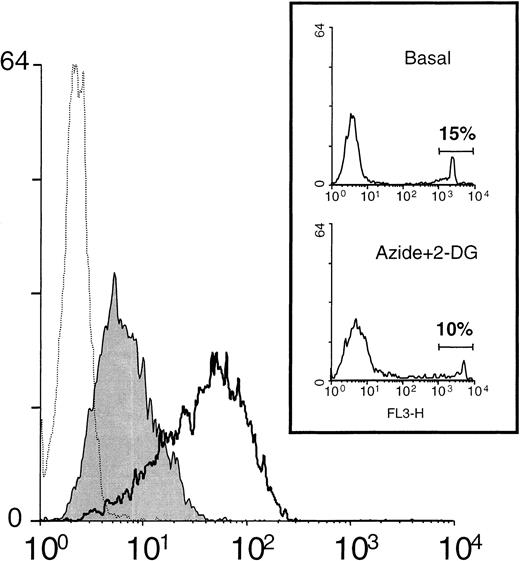
Metabolic inhibitors induce the down-regulation of L-selectin expression.
Neutrophils were incubated in medium alone or in the presence of the metabolic inhibitors: azide 10 nmol/L and 2-DG 50 nmol/L. After 30 minutes at 37°C, the surface expression of L-selectin was assessed by flow cytometry as described in “Materials and methods.” A representative histogram is shown. Unshaded histogram represents basal expression of L-selectin in neutrophils incubated in medium alone; shaded histogram represents the L-selectin expression in the presence of metabolic inhibitors; and dotted histogram represents the negative control (P3X63 myeloma). Histograms shown in the inset represent the spontaneous uptake of propidium iodide by neutrophils incubated in the absence (Basal) and presence of metabolic inhibitors (Azide+2-DG). Numbers correspond to the percentage of positive cells (dead cells).
Metabolic inhibitors induce the down-regulation of L-selectin expression.
Neutrophils were incubated in medium alone or in the presence of the metabolic inhibitors: azide 10 nmol/L and 2-DG 50 nmol/L. After 30 minutes at 37°C, the surface expression of L-selectin was assessed by flow cytometry as described in “Materials and methods.” A representative histogram is shown. Unshaded histogram represents basal expression of L-selectin in neutrophils incubated in medium alone; shaded histogram represents the L-selectin expression in the presence of metabolic inhibitors; and dotted histogram represents the negative control (P3X63 myeloma). Histograms shown in the inset represent the spontaneous uptake of propidium iodide by neutrophils incubated in the absence (Basal) and presence of metabolic inhibitors (Azide+2-DG). Numbers correspond to the percentage of positive cells (dead cells).
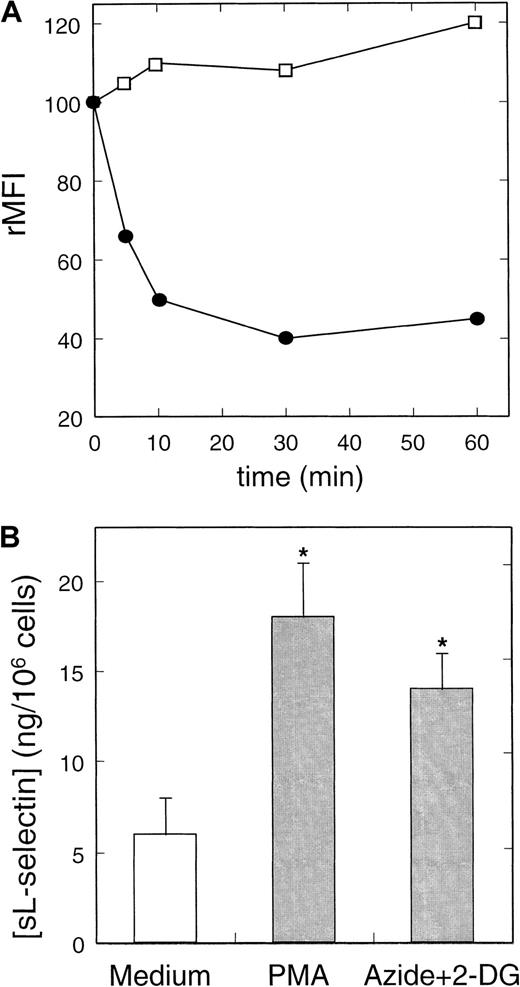
Metabolic inhibitors induce in a time-dependent manner the shedding of L-selectin.
(A) Kinetics of the effect of metabolic inhibitors on the expression of L-selectin (●) and CD11b (■) in neutrophils. Cells were cultured in the presence or absence of metabolic inhibitors at concentrations described in Figure 4 for different periods. The rMFI of L-selectin and CD11b was related to the expression by cultured cells in medium in each time. A representative experiment of 3 is shown. (B) Quantification of neutrophil-shed L-selectin induced by NSAIDs. Neutrophils were incubated with azide+2-DG, PMA, and medium alone for 30 minutes at 37°C. After centrifugation, neutrophil-free supernatants were tested for sL-selectin by ELISA as described in “Materials and methods.” Values represent the mean (ng/106 cells) ± SD from 3 independent experiments. * = P < .01 versus medium, by Student pairedt test.
Metabolic inhibitors induce in a time-dependent manner the shedding of L-selectin.
(A) Kinetics of the effect of metabolic inhibitors on the expression of L-selectin (●) and CD11b (■) in neutrophils. Cells were cultured in the presence or absence of metabolic inhibitors at concentrations described in Figure 4 for different periods. The rMFI of L-selectin and CD11b was related to the expression by cultured cells in medium in each time. A representative experiment of 3 is shown. (B) Quantification of neutrophil-shed L-selectin induced by NSAIDs. Neutrophils were incubated with azide+2-DG, PMA, and medium alone for 30 minutes at 37°C. After centrifugation, neutrophil-free supernatants were tested for sL-selectin by ELISA as described in “Materials and methods.” Values represent the mean (ng/106 cells) ± SD from 3 independent experiments. * = P < .01 versus medium, by Student pairedt test.
The effect of azide and 2-DG on L-selectin expression on neutrophils was time-dependent. Figure 5A shows that metabolic blockers induced a maximum down-regulation of L-selectin expression in neutrophils after 10 to 30 minutes of treatment. On activation, L-selectin is shed from neutrophils both in vivo23,24 and in vitro.22,47 Variations in the expression of CD11b and CD45, 2 glycoproteins contained in the membrane of secretory granules of neutrophils, can be considered as an indirect measurement of neutrophils degranulation (activation).34 48 As shown in Figure 5A, the inhibition of cell metabolism did not cause any significant effect on CD11b basal expression on neutrophils. Similar results were obtained when the CD45 expression was studied (data not shown) that indicate the L-selectin shedding induced by metabolic inhibitors is not due to nonspecific cell activation. Azide and 2-DG caused the loss of L-selectin expression in neutrophils by inducing the shedding of the molecule from the cell surface. An ELISA detected a significant increment in the concentration of sL-selectin in the cell-free supernatant of neutrophils treated with azide and 2-DG (14 ± 2 ng/mL) in respect to the medium (6 ± 2 ng/mL) (Figure 5B). On peripheral blood lymphocytes, these metabolic inhibitors did not cause any effect on the basal expression of L-selectin, even as long as 90 minutes after treatment (data not shown).
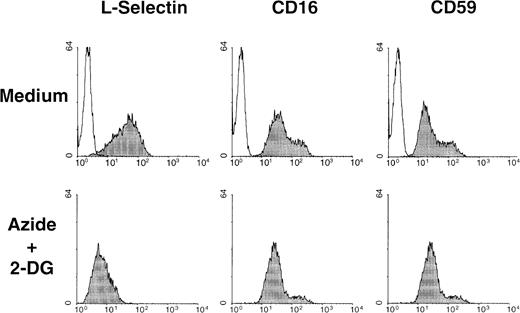
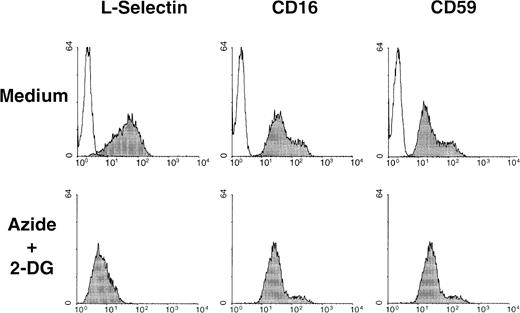
In addition to L-selectin, numerous other integral plasma membrane proteins are released from the cell membrane by proteolysis.49 CD16 and CD59 are 2 glycophosphoinositol lipid-anchored surface molecules that can be shed from cell surface during cell activation.50 51 The basal expression of these 2 surface molecules in neutrophils was not altered on exposure to the metabolic inhibitors. A representative experiment of the effect of azide plus 2-DG on the basal expression of L-selectin, CD16, and CD59 on neutrophils is shown in Figure 6.
Metabolic inhibitors do not induce down-regulation of other surface molecules with soluble isoforms expressed in neutrophils.
Surface expression of CD16 and CD59, 2 glycophosphoinositol lipid-anchored surface molecules, and L-selectin were determined by flow cytometry in neutrophils incubated in the absence (medium) of presence of metabolic inhibitors (azide+2-DG) for 30 minutes at 37°C. Shaded histograms represent the expression of each surface molecule; unshaded histograms represent the negative control (P3X63 myeloma). A representative experiment of 3 is shown.
Metabolic inhibitors do not induce down-regulation of other surface molecules with soluble isoforms expressed in neutrophils.
Surface expression of CD16 and CD59, 2 glycophosphoinositol lipid-anchored surface molecules, and L-selectin were determined by flow cytometry in neutrophils incubated in the absence (medium) of presence of metabolic inhibitors (azide+2-DG) for 30 minutes at 37°C. Shaded histograms represent the expression of each surface molecule; unshaded histograms represent the negative control (P3X63 myeloma). A representative experiment of 3 is shown.
These data indicate that the maintenance of L-selectin in the surface of neutrophils is an energy-dependent process.
Discussion
The major findings of this study are as follows: (1) Some NSAIDs cause the down-regulation of L-selectin expression through the action of the physiologic membrane metalloprotease responsible of the L-selectin cleavage in neutrophils; (2) this effect seems to be intracellular calcium concentration and protein kinase and phosphatase-independent; (3) our data also indicate that the maintenance of L-selectin on the surface of neutrophils is an energy-dependent process, suggesting that L-selectin is shed in neutrophils by default; and (4) interestingly, that the capability of NSAIDs to reduce the intracellular ATP concentration could account for their ability to induce the shedding of L-selectin in neutrophils.
It has been widely accepted that the mechanism of action of NSAIDs is the inhibition of prostaglandin synthesis. However, a significant body of evidence suggests that prostaglandins, as the sole target of anti-inflammatory action of NSAIDs is no longer tenable. The fact that certain prostaglandins have anti-inflammatory activity in animal models of arthritis52 supports this contention, as does the failure of some NSAIDs to act as COX inhibitors, despite their anti-inflammatory activity.4 Furthermore, there is no correlation between the small doses of aspirin that inhibit prostaglandin synthesis and the higher doses required to exert an anti-inflammatory effect in vivo.2 All these data, in addition to the high variations in clinical response of patients to NSAIDs,3 support the idea of many groups, including ours that these agents must have more than just one anti-inflammatory mechanism of action. In this regard, it has been reported that some NSAIDs are able to inhibit membrane-associated processes, to promote synthesis of anti-inflammatory eicosanoids as well as to block a number of intracellular events, including transmembrane anion transport, oxidative phosphorylation in mitochondria, and activation of transcription factor NF-kB.2,9,11,13 53 However, none of these prostaglandin-independent effects fully explains the in vivo anti-inflammatory action of NSAIDs.
Our group has recently described that leukocyte adhesion molecules may be potential targets for the anti-inflammatory action of NSAIDs.54 Several, but not all of these therapeutic agents induce a rapid loss of L-selectin expression15,16 through an unknown prostaglandin-independent mechanism.54 Other compounds with anti-inflammatory properties such as colchicine and leumedins have also shown able to reduce the expression of L-selectin in neutrophils.55,56 A group of NSAIDs composed by flufenamic acid, mefenamic acid, aceclofenac, diclofenac, sodium salicylate, aspirin, and indomethacin induced the shedding of L-selectin in neutrophils. This effect was detected at reported therapeutic concentrations.57 The NSAIDs that display a high L-selectin shedding activity seem to share the chemical structure diphenylamine. Indeed, the diphenylamine is able to induce the shedding of L-selectin in neutrophils (González-Alvaro et al, unpublished observations). However, other NSAIDs such as piroxicam, meloxicam and phenylbutazone, clinically as effective as members from the first group, did not show any effect on the expression level of this adhesion molecule; instead, this group of compounds affects the activation-dependent integrin-mediated adhesion of leukocytes to endothelial cell.17 Preliminary data from our group suggest that only those NSAIDs based in the diphenylamine display a high L-selectin shedding activity in neutrophils. The importance of L-selectin shedding as a mechanism of action of NSAIDs in vivo has not been established. However, the relevant role that L-selectin plays in inflammation,27-29 in addition to the fact that indomethacin decreases in vivo the L-selectin expression in human neutrophils,15 make this effect of NSAIDs, at least in potency, biologically relevant.
L-selectin is a member of the selectin family of surface adhesion receptors constitutively expressed by most leukocytes. The interaction between L-selectin and its ligands, carbohydrates expressed by activated endothelial cells, plays an important role in the inflammatory response mediating the initial rolling and arrest of flowing leukocytes to the endothelium surface.20,58Membrane-anchored adhesion molecules can modify its activity through several mechanisms.59 One of those mechanisms is the proteolytic processing of the extracellular domain of the adhesion molecules. L-selectin is proteolytically cleaved from the cell surface by the action of a not-yet-well-characterized cell membrane metalloprotease that can be blocked by hydroxamic acid-based protease inhibitors.38 39 The incubation of neutrophils with the L-selectin shedding inhibitor KD-IX-73-4 prevented the down-regulation of L-selectin expression induced by all NSAIDs tested. These data rule out the possibility of an intrinsic proteolytic activity of NSAIDs on L-selectin and demonstrate that the shedding of L-selectin induced by these agents is dependent on the activity of the physiologic metalloprotease responsible of the L-selectin cleavage in neutrophils.
Recently, it has been proposed that calcium-induced conformational changes in the calmodulin, an intracellular calcium regulatory protein, might be involved in the control of L-selectin shedding.44 Therefore, we analyzed the possibility that NSAIDs were able to trigger cytosolic calcium oscillations in resting neutrophils as a potential explanation for the L-selectin down-regulation induced by these agents. However, neither the flufenamic acid,60 the most powerful inductor of L-selectin shedding, nor any of the other NSAIDs tested were able to cause a detectable increment in the intracellular calcium concentration in neutrophils. These data suggest that the NSAID-mediated down-regulation of L-selectin cannot be consistently explained by calcium signals in the neutrophils.
The signaling mechanisms involved in the regulation of L-selectin expression in leukocytes are largely unknown. Several studies using protein kinases and phosphatases inhibitors suggest that, in a cell type-specific fashion, the PKC and tyrosine phosphorylation and dephosphorylation cascade may be involved in the regulation of L-selectin surface expression.40-43 Interestingly, it has been reported that some NSAIDs activate the PKC signaling pathway in leukocytes.61,62 However, neither Ro 31-8220, a member of a highly specific new family of PKC inhibitors, nor H7, a broad-based serine-threonine kinase inhibitor, was able to prevent the NSAID-induced down-regulation of L-selectin in neutrophils. Likewise, protein tyrosine kinase inhibitors, herbimycin A and tyrphostin A25, did not reverse the down-regulation of L-selectin induced by NSAIDs. Alternatively, some MAbs against the tyrosine phosphatase CD45 are able to promote L-selectin down-regulation in both lymphocytes42 and neutrophils (Gómez-Gaviro et al, unpublished observation), suggesting that NSAIDs may induce the L-selectin shedding through a tyrosine phosphatase-dependent system. However, the sodium orthovanadate, a tyrosine phosphatase inhibitor, did not modify the activity of NSAIDs on L-selectin expression in neutrophils. Although results obtained in living cells with inhibitors neither support nor preclude definitive conclusions, these data indicate that the NSAIDs-mediated L-selectin shedding involves a protein phosphorylation/dephosphorylation-independent mechanism.
NSAIDs are able to interfere with the mitochondrial oxidative phosphorylation reducing the intracellular ATP synthesis in vivo.8 This effect has been proposed as an early pathogenic event in the NSAID-mediated enteropathy.9,63The incubation of neutrophils with the different NSAIDs showed a differential effect on the intracellular ATP concentration regardless of the presence of cyclooxygenase products (PGE2) in the cell suspension. The effect of NSAIDs on the level of intracellular ATP strongly correlated with the ability of these agents to reduce the basal expression of L-selectin in neutrophils, which suggests that both events may be functionally related. Remarkably, the metabolic inhibitors, azide and 2-DG, also caused the shedding of L-selectin without an evident variation in the neutrophil activation state, as assessed by the CD11b and CD45 expression. These observations suggest that the maintenance of L-selectin in the membrane of neutrophils is an energy-dependent process, raising the idea that, in resting neutrophils but not in lymphocytes, the L-selectin tends to be shed by default. The fact that serum from healthy humans presents high levels of sL-selectin supports this latter contention.37 Other molecules expressed by neutrophils that can be shed from cell surface such as CD16 and CD59 did not modify their basal expression in the presence of azide plus 2-DG. This fact excludes that the shedding of L-selectin is an unspecific effect of metabolic inhibitors on neutrophils. All these data strongly suggest that NSAIDs are able to induce, at least in part, the shedding of L-selectin in neutrophils through the reduction of the energy capacity of the cell.
Recently, a cell-surface metalloprotease responsible of the cleavage of the surface form of TNF-α, the TACE, has shown a mild proteolytic activity on L-selectin.25 This metalloprotease is a member of a protein family characterized by a disintegrin and a metalloprotease domain (ADAM)64 also known as ADAM 17. Although the low L-selectin cleavage activity described by Peschon et al25 almost excludes ADAM 17 as the physiologic regulator of L-selectin in neutrophils, general characteristics of this family of proteases make their members very good candidates for this role. Although the mechanism of L-selectin recognition by the surface metalloprotease is undefined, it seems that intracellular signals either activate the metalloprotease or induce a conformational change in the extracellular domain of L-selectin that exposes the cleavage site.65 Our data suggest that probably the metalloprotease is active in neutrophils by default but, in resting cells, its ability to release L-selectin is highly attenuated through an inhibitory mechanism that requires ATP consumption. These results may help to a better comprehension of physiologic mechanisms involved in the regulation of L-selectin expression in neutrophils. In addition, all these data might be useful for the development of new nonprostaglandin-dependent anti-inflammatory agents that specifically interfere with L-selectin–related functions and, consequently, with a more favorable safety profile.
Supported in part by Boëhringer Ingelheim, by grant SAF 96/0332 from Ministerio de Educación y Cultura and by FIS 99/0222 from Fondo de Investigaciones Sanitarias de la Seguridad Social (F.D-G) and by BEFI 99/9012 from Fondo de Investigaciones Sanitarias de la Seguridad Social (M.V.G-G).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Federico Dı́az-González, Servicio de Reumatologı́a, Hospital Universitario de Canarias, Universidad de La Laguna, Ofra s/n, La Cuesta, 38320 La Laguna, Santa Cruz de Tenerife, Spain; e-mail: fdiaz@cnb.uam.es.