Abstract
Herein is described the case of a young woman presenting with iron overload and macrocytosis. The initial diagnosis was hereditary hemochromatosis. Severe anemia developed after a few phlebotomies, and she was also found to have congenital dyserythropoietic anemia that, though not completely typical, resembled type II. Only genetic testing allowed the definition of the coexistence of the 2 diseases, both responsible for the iron overload. This report points out the need to consider congenital dyserythropoietic anemia in patients with hemochromatosis and unexplained macrocytosis and, conversely, to check for the presence of hereditary hemochromatosis in patients with congenital dyserythropoietic anemia and severe iron overload. To the authors' knowledge, this is the first report of homozygosity for the C282Y mutation of the HFE gene in a patient affected by congenital dyserythropoietic anemia.
Introduction
Congenital dyserythropoietic anemias (CDAs) are a rare and heterogeneous group of chronic anemias characterized by ineffective erythropoiesis, dyserythropoiesis, mild hemolysis, and severe iron overload related to repeated transfusions or to ineffective erythropoiesis.1 However, because clinical and laboratory findings may be subtle, patients with CDAs are easily unrecognized or misdiagnosed.
CDA type II, an autosomal recessive disease, is the most common form; the responsible gene, CDAN2, was mapped to 20q11.2.2 It may be asymptomatic or may present in childhood with a clinical picture of macrocytic or normocytic anemia, splenomegaly, and intermittent jaundice.3 Patients are at high risk of hemochromatosis developing in middle age,4-6and iron chelation therapy is usually necessary. Despite severe anemia, a few patients have been successfully treated with phlebotomy.7
Hereditary hemochromatosis (HHC), an autosomal recessive disease with a prevalence of 1 of 220, is the most frequent cause of iron overload in white persons. It is characterized by progressive iron accumulation in the parenchymal tissues because of a defect in the regulation of duodenal iron absorption. In 1996 the gene responsible for HHC (HFE) was identified and 2 point mutations (C282Y, H63D) were associated with the disease.8
Several cases of chronic anemias with severe iron overload due to the association of hemolytic anemias and HHC have been described, including hereditary spherocytosis,9 acanthocytosis,10sickle cell disease,11 and thalassemia.12 13In contrast, the severe iron overload that often accompanies CDAs has been ascribed to the erythropoietic defect.
In this paper we present evidence of an association between CDA and homozygous HHC, suggest that HHC may aggravate iron overload in a number of patients with CDAs, and point out the potential benefit of genetic counseling of other family members for both CDA and HHC.
Study design
In 1986 a 26-year-old woman underwent routine biochemical analysis. She was found to have increased transferrin saturation, hyperferritinemia, and mild anemia. Since 1993 she reported oligomenorrhea. In 1994 her blood tests revealed a transferrin saturation of 98%, ferritin 1080 ng/mL, and Hb 11.8 g/dL. Liver histology showed a normal structure and no inflammatory cells; Perls staining demonstrated diffuse iron deposition in periportal hepatocytes and Kupffer cells, and a diagnosis of HHC was made. The patient was referred to our hemochromatosis outpatient service.
A complete reevaluation confirmed the presence of macrocytosis (MCV 104), mild anemia (Hb 11.6), and iron overload; white cell and platelet counts, bilirubin, LDH, and liver biochemistry were in the normal range. She reported a diet poor in fresh vegetables and mild alcohol intake (less than 30 g/d) and denied previous therapy with iron supplements. Vitamin B12 and folic acid serum levels were measured; folic acid concentration was decreased, whereas B12 was normal. Despite the parenteral administration of folic acid with normalization of serum folate, no decrease in macrocytosis was observed.
The patient was put on weekly phlebotomy (350 mL/wk), but after 9 phlebotomies, Hb concentration fell to 9.7 g/dL in the presence of transferrin saturation 68% and serum ferritin 1000 ng/mL. Phlebotomies were stopped; Hb returned to pretreatment levels within 8 weeks, but a second and a third phlebotomy cycle also had to be interrupted because of symptomatic anemia.
Ham and sucrose hemolysis tests were performed, erythropoietin serum levels dosed, and autoantibodies and alloantibodies searched for. All tests resulted normal. The young age of the patient and the absence of a worsening of the anemia made myelodysplastic syndrome unlikely.
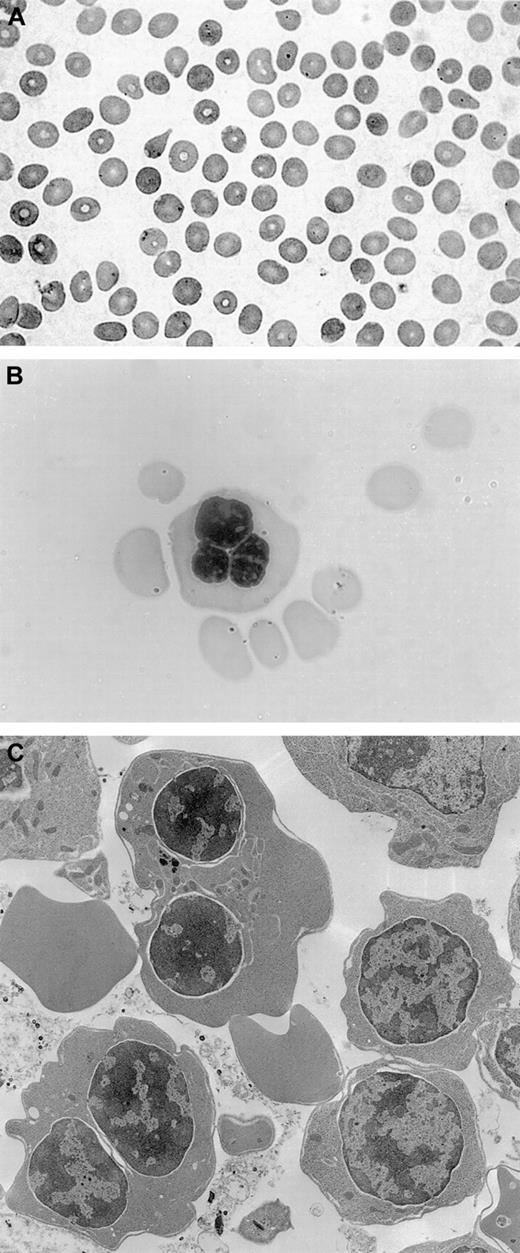
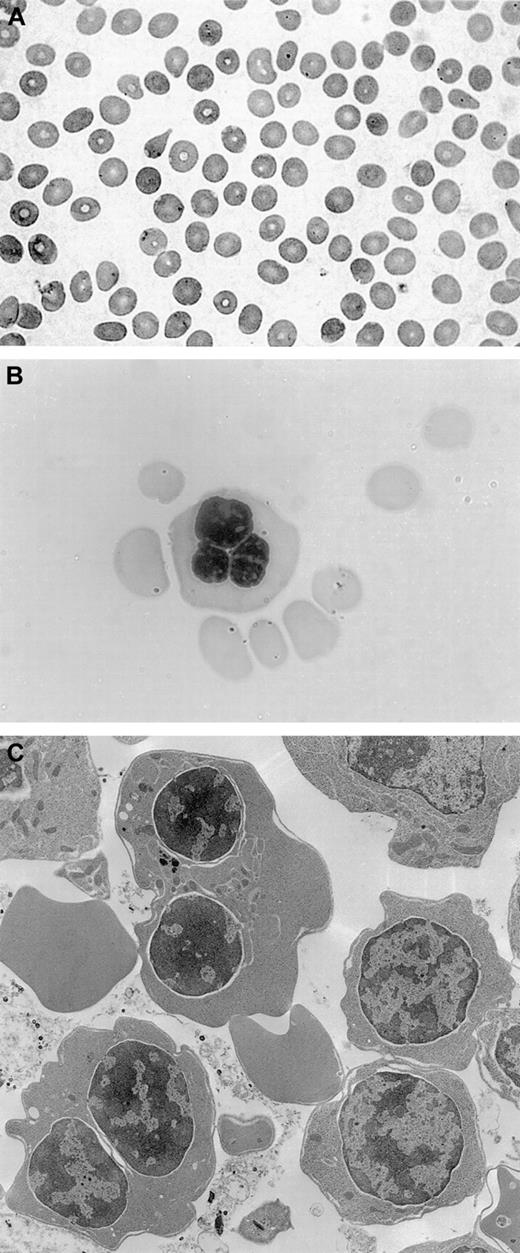
The patient underwent a bone marrow aspirate and biopsy, which showed normal myelopoiesis, dyserythropoiesis, and more than 20% binucleated erythroblasts. No karyotypic alterations were detected. Electron microscopy analysis revealed double membranes on the majority of erythroid precursors; the negativity of the Ham test oriented to a diagnosis of CDA type II with variant features.14Peripheral blood smear, bone marrow aspirate, and electron microscopy findings are shown in Figure 1.
Peripheral blood smear, bone marrow aspirate, and electron microscopy findings.
(A) Peripheral blood smear, showing macrocytosis. (B) Bone marrow aspirate with a multinucleated erythroblast. (C) Electron microscopy findings with evidence of double membranes on erythroid precursors.
Peripheral blood smear, bone marrow aspirate, and electron microscopy findings.
(A) Peripheral blood smear, showing macrocytosis. (B) Bone marrow aspirate with a multinucleated erythroblast. (C) Electron microscopy findings with evidence of double membranes on erythroid precursors.
Since 1996, the patient has been treated with desferrioxamine 1 g twice daily intramuscularly and a diet poor in iron was advised. After15 months of regular treatment, ferritin concentration decreased from 1248 to 20 and transferrin saturation from 100% to 22%. Interestingly, her 75-year-old mother also had evidence of iron overload (transferrin saturation 98%, ferritin 719 ng/mL), with normal Hb (14.5) and MCV (93.5) values. Liver biochemistry and ultrasonography were normal. She suffered from non-insulin–dependent diabetes mellitus, hypertension, and ischemic heart disease.
In the hypothesis of the coexistence of CDA and HHC, HFEgene mutations were searched for in the patient and her mother.
The C282Y HFE mutation was searched for in genomic DNA extracted from peripheral leukocytes as previously described8 by using the following primers: forward HLAHF282 (GTT CGA ACC TAA AGA CGT ATT GCC) and reverse R282NEW (CCT AAC AAA GAG CAG ATC CTC).
Results and discussion
Analysis of HFE mutations revealed that the patient and her mother were homozygous for the C282Y mutation.
Characteristics of the patient and her mother, at presentation, are shown in Table 1.
Severe hemochromatosis is the predominant clinical manifestation of CDA type II,15 and in some never-transfused patients, clinical manifestations of iron overload precede the diagnosis of dyserythropoietic anemia.
A diagnosis of CDA, by itself, could potentially explain all the pathologic findings of our patient. In fact, liver siderosis was found in both Kupffer cells and hepatocytes, the former location reflecting erythrophagocytosis and the latter the increased iron absorption due to ineffective erythropoiesis. In contrast, the diagnosis of HHC, given the early stage of the disease, could not explain siderosis in Kupffer cells.
Genetic testing was required to make the diagnosis of HHC and to clarify that iron overload was the result of a synergistic effect of HHC and CDA on iron absorption. Low penetrance of HHC was present in the proband and her mother; the latter had clinical manifestations of angina and non-insulin–dependent diabetes mellitus, which were not necessarily related to iron overload, as she was 75 years old.
In conclusion, this case describes an unusual association between dyserythropoietic anemia and HHC. It points out the need to test for CDA in patients with hemochromatosis and unexplained macrocytosis, mainly when iron is present in Kupffer cells and vice versa, to check for the presence of HHC in patients with CDA and severe iron overload. This will provide, in addition to a better diagnostic definition and therapeutical decision for iron removal, a potential benefit in genetic counseling for other family members, both for CDA and HHC.
Supported in part by MURST (ex 40%) 1998; Ricerca finalizzata IRCCS 1998; MURST 60% 1999.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Silvia Fargion, Dipartimento di Medicina Interna, Ospedale Maggiore IRCCS, Pad Granelli, Via F Sforza 35, 20122 Milano, Italy; e-mail: silvia.fargion@unimi.it.