Abstract
The 5-lipoxygenase (5-LO) pathway in human CD34+ hematopoietic progenitor cells, which were induced to differentiate into dendritic cells (DCs) by cytokines in vitro and in DCs of lymphoid tissues in situ, was examined. Extracts prepared from HPCs contained low levels of 5-LO or 5-LO–activating protein. Granulocyte-macrophage colony-stimulating factor (GM-CSF) plus tumor necrosis factor–α (TNF-α) promoted DC differentiation and induced a strong rise in 5-LO and FLAP expression. Fluorescence-activated cell sorter (FACS) analyses identified a major DC population coexpressing human leukocyte antigen (HLA)-DR/CD80 and monocytic or Langerhans cell markers. Transforming growth factor–β1 (TGF-β–1), added to support DC maturation, strongly promoted the appearance of CD1a+/Lag+ Langerhans-type cells as well as mature CD83+ DCs. TGF-β–1 further increased 5-LO and FLAP expression, recruited additional cells into the 5-LO+DC population, and promoted production of 5-hydroxyeicosatetraenoic acid and leukotriene B4 in response to calcium (Ca++) ionophore A23187. These in vitro findings were corroborated by 5-LO expression in distinct DC phenotypes in vivo. Scattered 5-LO and FLAP in situ hybridization signals were recorded in cells of paracortical T-lymphocyte–rich areas and germinal centers (GCs) of lymph nodes (LNs) and tonsil and in cells of mucosae overlying the Waldeyer tonsillar ring. 5-LO protein localized to both CD1a+ immature DCs and to CD83+ mature interdigitating DCs of T-lymphocyte–rich areas of LNs and tonsil. As DCs have the unique ability to initiate naive lymphocyte activation, our data support the hypothesis that leukotrienes act at proximal steps of adaptive immune responses.
Introduction
Leukocyte lineages of the innate immune system, including neutrophils, monocyte-macrophages, mast cells, and eosinophils, express the 5-lipoxygenase (5-LO; arachidonate:oxygen 5-oxidoreductase, EC 1.13.11.34) pathway and produce leukotrienes (LTs) in response to inflammatory agonists in vitro.1 Investigators have therefore considered LTs as inflammation- and/or allergy-associated molecules.2,3Several lines of evidence, however, support an expanded role of the 5-LO pathway in the regulation of antigen-specific adaptive immunity. Epidermal Langerhans cells (LCs), ie, immature members of the dendritic cell (DC) family capable of initiating antigen-specific immune responses in naive lymphocytes, markedly express the 5-LO pathway.4 Among human tissues studied by Northern blot analysis, LTB4 receptor messenger RNA (mRNA) is strongly expressed in both thymus5 and lymph nodes (LNs) (R.S., unpublished data, October 1998), while cysteinyl LT1 receptor mRNA is expressed in spleen.6Probably most significantly, 5-LO–deficient mice show altered ovalbumin-dependent cellular and humoral immune responses.2,3,7 8
Although these studies have provided substantial support for an indispensable role of the 5-LO pathway in the regulation of adaptive immunity in general, important questions remain. The identities of the 5-LO pathway– and LT receptor–expressing cells in lymphoid tissues are not known. Moreover, the significance of 5-LO expression by the 2 other professional antigen-presenting cells (APCs), B lymphocytes9,10 and monocyte-macrophages,11for the regulation of immune responses is far from clear. Accordingly, the impact of LTs within the adaptive immune system is presently difficult to conceive at cellular and molecular levels.
Antigens invade the host through epithelial surfaces, yet primary immune reactions require prolonged interaction of DCs with naive T lymphocytes in the extrafollicular (paracortical) areas of lymphoid organs.11 Hence, antigens must be translocated to these areas to trigger immunity. Moreover, T-cell activation by DCs requires the antigen to be taken up, processed, and presented at the DC surface in an immunogenic form on major histocompatibility complex (MHC) molecules. It has been established that these tasks are carried out by LCs. LCs form a network of initially quiescent sentinel DCs that are located throughout epithelial surfaces. They are endowed with high antigen-binding capacities and thus can effectively monitor their environment for antigen. Upon antigen capture, they become activated, migrate via afferent lymph vessels as veiled cells, home in paracortical areas of draining LNs, and eventually mature into interdigitating DCs (IDCs). IDCs have largely lost antigen-binding activity, but they have acquired the ability to attract, cluster, and activate naive T lymphocytes. In addition to DCs and T cells, lymphoid organs harbor other cells that participate in the regulation of immune reactions within anatomically and functionally highly organized compartments including B lymphocytes at various stages of differentiation, tingible body macrophages (TBMs), germinal center (GC) DCs, and follicular DCs.11 Nothing is known about 5-LO expression in these cells, the anatomy of the 5-LO pathway, or 5-LO pathway target cells in any lymphoid organ.4 We reasoned that analyses of in vitro DC systems, together with anatomical studies, would conceivably enhance understanding of the potential role of 5-LO in immunity.
To establish in vitro models of 5-LO in DCs, we studied DC differentiation systems from human CD34+ hematopoietic progenitor cells (HPCs) isolated from cord blood (CB). These systems have permitted us to generate DCs at different stages of differentiation and at sufficient quantities to explore LT formation in intact DCs. In parallel studies, we have begun to outline the anatomy of the 5-LO pathway in human LNs, tonsil, oral mucosa, and the Waldeyer tonsillar ring by 5-LO and 5-LO–activating protein (FLAP) in situ hybridization (ISH) and fluorescence immunohistochemistry. We show that (1) cytokines known to affect the immune response in vivo up-regulate the 5-LO pathway in DCs in vitro, (2) these DCs are capable of producing large amounts of 5-hydroxyeicosatetraenoic acid (5-HETE) and LTB4, and (3) distinct DC phenotypes in vivo, as well as GC TBMs, express the 5-LO gene at marked levels.
Materials and methods
Materials
The following reagents (Sigma Chemical, St Louis, MO, unless otherwise noted) were used in this study: 37 × 1012 Bq/mmol (1000 Ci/mmol) sulfur 35 cytidine 5′-triphosphate (35S-CTP) (Amersham Life Sciences, Uppsala, Sweden); avian myeloblastosis virus reverse transcriptase (RT) and Thermus aquaticus DNA polymerase (Roche Diagnostic Systems, Basel, Switzerland); antirabbit immunoglobulin-G (IgG)-Cy3 (red), IgG phycoerythrin (PE) (Dianova, Hamburg, Germany), and IgG fluorescein isothiocyanate (FITC) (Coulter Immunotech, Miami, FL); antimouse IgG-Cy2 (green) and IgG-PE (Dianova, Hamburg, Germany); mouse monoclonal antisera against antigens CD1a, CD14, HLA-DR, CD83, CD34 (Coulter Immunotech, Miami, FL), CD80, CD86, mannose receptor, CD40, eosinophil peroxidase (PharMingen, San Diego, CA), CD68 (Dako, Glostrup, Denmark), and Ki67 (Dianova, Hamburg, Germany); rabbit anticollagen IV antiserum (gift from J.M. Foidart, Liege, France); and tumor necrosis factor-α (TNF-α), stem cell factor (SCF) (Bachem, Heidelberg, Germany), transforming growth factor-β-1 (TGF-β-1), and granulocyte-macrophage colony stimulating factor (GM-CSF) (R&D Systems, Heidelberg, Germany).
Cell culture
CD34+ HPCs were prepared and cultured as described12-14 from human CB, except that there were 2 rounds of CD34 bead absorption (Miltenyi Biotech, Bergisch Gladbach, Germany) which yielded a purity of more than 98%.
RT–polymerase chain reaction and ISH
5-LO and FLAP RT–polymerase chain reaction (RT-PCR) analyses were performed as described.4 For ISH, human 5-LO complementary DNA (cDNA) EcoRI fragment (nucleotides, 1-2496) and human FLAP ApaI-NotI fragment (nucleotides, 13-504) were subcloned into pBluescript II KS vectors (Stratagene, La Jolla, CA). Frozen sections of normal human LNs were obtained during routine surgery from axillae of patients undergoing neck dissection and were free of metastases. Mucosal tissue and tonsil were obtained during routine surgery from patients undergoing tonsillectomy. Details of tissue preparation, hybridization conditions, probe specificity, and PCR parameters are available upon request.
Fluorescence immunohistochemistry
Frozen tissue sections or cytocentrifuged cells were fixed in 80% ice-cold methanol followed by 1 minute in 100% acetone at −20°C. Before use, anti–5-LO antiserum 1550 was affinity-purified on a 5-LO Sepharose column. For 2-color immunofluorescence, slides were incubated with donkey antirabbit IgG F(ab′)2 conjugated with Cy3 and goat antimouse IgG F(ab′)2 conjugated with Cy2 for one hour. Additional details are available upon request.
Fluorescence-activated cell sorter analyses
Fluorescence-activated cell sorter (FACS) analyses were performed using anti–5-LO antiserum 1550 as described.4Unconjugated antibodies (5-LO, Lag, mannose receptor, and eosinophil peroxidase) were added for 2 hours, then washed twice in saponin buffer. Fluorochrome-conjugated monoclonal antibodies (mAbs) (CD1a-PE, CD80-PE, CD83-PE, CD86-PE, CD40-PE, CD14-FITC, and HLA-DR–FITC) and fluorochrome-conjugated secondary antibodies (goat antirabbit IgG-FITC, donkey antirabbit IgG-PE, and donkey antimouse IgG-PE) were added in saponin buffer for one hour. Negative controls were performed with unrelated mouse mAbs and rabbit anticollagen IV antiserum. Fluorescence analyses were performed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Data were analyzed with the CellQuest program (Becton Dickinson). 5-LO mean fluorescence intensity (MFI) was calculated by 5-LO MFI minus collagen MFI.
Assays
Immunoblots were performed on 10 μg total cell protein as described using anti–5-LO antiserum or a polyclonal anti-FLAP antiserum (gift from J. Evans, Merck Frosst, Canada).45-HETE and LTB4 formation in intact cells were determined upon stimulation with 10 μmol/L Ca++ ionophore A23187 and 40 μmol/L arachidonic acid or in cell-free systems in the presence of 40 μmol/L arachidonic acid as described.15
Results
Freshly isolated highly purified CD34+ HPCs contain a small number of immature 5-LO+/HLA-DR+cells
Transcript and protein levels of 5-LO pathway constituents in CD34+ HPCs were determined by RT-PCR, immunoblot, and immunohistochemical analyses. When compared with buffy-coat cells or CD14+ blood monocytes, HPCs showed low but detectable levels of 5-LO and FLAP transcripts (Figure1; additional data not shown). Consistent with low transcript levels, 5-LO and FLAP proteins were below the detection limit in immunoblots of 10 μg protein (Figure2). These data indicated the presence of small numbers of cells with significant expression of 5-LO mRNA and protein or low-level expression in larger numbers of cells. To detect single rare 5-LO protein-expressing cells, we applied double-fluorescence immunohistochemical analyses. CD14+monocytes and CD66b+ myeloid precursor/granulocytes, ie, leukocytes that could possibly have contaminated the HPC preparations, were used to evaluate the assay. We found that the great majority of cells expressed CD34 and HLA-DR but were 5-LO− (Figure3; see below). However, rare cells (less than 2%) did show significant nuclear 5-LO fluorescence (Figure 3C, arrow). These 5-LO+ cells resembled HPCs by several criteria including CD14 and CD66b negativity, small size, scarcity of cytoplasm, and round or moderately lobulated nuclei (Figure 3D).
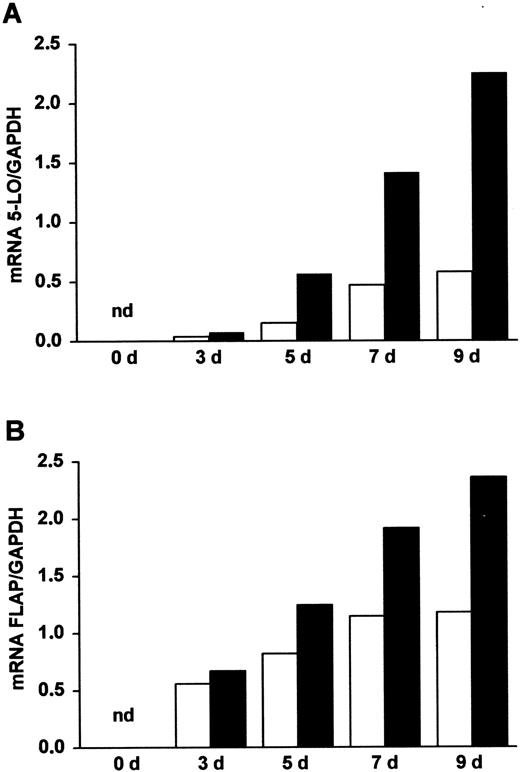
Appearance of 5-LO and FLAP transcripts during DC differentiation of HPCs.
Highly purified HPCs (more than 98%) were prepared from CB as described in “Materials and methods.” The cells were maintained in culture in the presence of SCF/GM-CSF/TNF-α (open columns), referred to as standard medium, or in the presence of SCF/GM-CSF/TNF-α/TGF-β–1 (closed columns), referred to as standard medium plus TGF-β–1, for increasing time periods (days, d). Transcript levels of (A) 5-LO and (B) FLAP were determined by RT-PCR analyses as described.4 Data are expressed as ratios of densitometry units of 5-LO (27 cycles) or FLAP (25 cycles) transcripts relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts (22 cycles). nd indicates not detectable.
Appearance of 5-LO and FLAP transcripts during DC differentiation of HPCs.
Highly purified HPCs (more than 98%) were prepared from CB as described in “Materials and methods.” The cells were maintained in culture in the presence of SCF/GM-CSF/TNF-α (open columns), referred to as standard medium, or in the presence of SCF/GM-CSF/TNF-α/TGF-β–1 (closed columns), referred to as standard medium plus TGF-β–1, for increasing time periods (days, d). Transcript levels of (A) 5-LO and (B) FLAP were determined by RT-PCR analyses as described.4 Data are expressed as ratios of densitometry units of 5-LO (27 cycles) or FLAP (25 cycles) transcripts relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts (22 cycles). nd indicates not detectable.
Appearance of 5-LO and FLAP proteins during DC differentiation of HPCs.
HPCs were maintained in culture as described in Figure 1 in medium supplemented with standard medium (S) or in the presence of standard medium plus TGF-β–1 (S + T) for 11 days. Immunoblots were performed on 10 μg total cell protein using anti–5-LO antiserum, LO-32, or a polyclonal anti-FLAP antiserum. To obtain sufficient protein from freshly isolated HPCs at day (d) 0, pools of 3 separate CB preparations were used. Arrows at right represent the positions of molecular weight standards; asterisks indicate the position of (A) recombinant 5-LO standard or (B) leukocyte membrane protein for FLAP.
Appearance of 5-LO and FLAP proteins during DC differentiation of HPCs.
HPCs were maintained in culture as described in Figure 1 in medium supplemented with standard medium (S) or in the presence of standard medium plus TGF-β–1 (S + T) for 11 days. Immunoblots were performed on 10 μg total cell protein using anti–5-LO antiserum, LO-32, or a polyclonal anti-FLAP antiserum. To obtain sufficient protein from freshly isolated HPCs at day (d) 0, pools of 3 separate CB preparations were used. Arrows at right represent the positions of molecular weight standards; asterisks indicate the position of (A) recombinant 5-LO standard or (B) leukocyte membrane protein for FLAP.
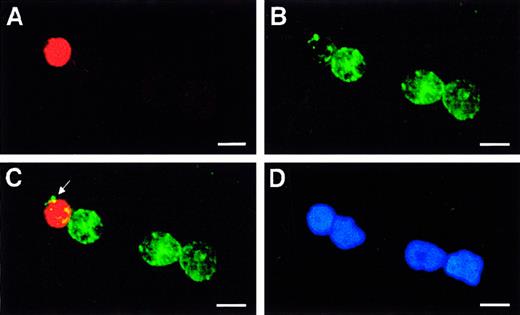
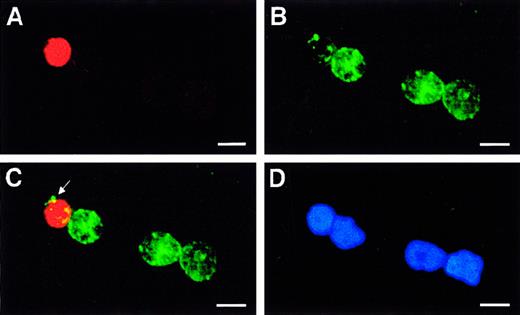
Identification of immature 5-LO+/CD34dim cells in highly purified freshly prepared HPCs.
Fresh HPCs were isolated to high purity, and fluorescence immunohistochemistry was performed as described in “Materials and methods” using (A) anti–5-LO antiserum, 1550 (red, Cy3), (B) CD34 (green, Cy2), (C) merge of A and B, and (D) DNA stain Hoechst 33258 on an identical field. The arrow in panel C indicates patches of CD34 antigen in a single 5-LO+/CD34dim immature leukocyte close to 3 other 5-LO−/CD34+ HPCs. Bars indicate 10 μm.
Identification of immature 5-LO+/CD34dim cells in highly purified freshly prepared HPCs.
Fresh HPCs were isolated to high purity, and fluorescence immunohistochemistry was performed as described in “Materials and methods” using (A) anti–5-LO antiserum, 1550 (red, Cy3), (B) CD34 (green, Cy2), (C) merge of A and B, and (D) DNA stain Hoechst 33258 on an identical field. The arrow in panel C indicates patches of CD34 antigen in a single 5-LO+/CD34dim immature leukocyte close to 3 other 5-LO−/CD34+ HPCs. Bars indicate 10 μm.
However, while CD34 antigen was detectable, the immature 5-LObright cells displayed a CD34dim phenotype (Figure 3C, arrow at patches of CD34 signals). It is noteworthy that all 5-LObright HPCs were also HLA-DR+, whereas we failed to detect 5-LObright cells that were HLA-DR−. In addition to this 5-LObrightpopulation, however, we observed 5-LOdim/HLA-DR+/CD34+ cells. Thus, we identified a spectrum of cells ranging from 5-LO−/HLA-DR−/CD34+ to 5-LOdim/HLA-DR+/CD34+ to 5-LObright/HLA-DR+/CD34dim cells. By contrast, we never observed 5-LObright/CD34bright cells, indicating that 5-LO is not expressed in hematopoietic stem cells (HSCs). Bone marrow–derived as well as CB-derived CD34+ HPCs have previously been shown to represent a heterogenous yet immature cell population consisting of lineage marker–negative uncommitted hematopoietic stem cells and other immature cells expressing HLA-DR, myeloperoxidase, or markers of lymphoid precursors.16 17These data indicate that the small 5-LObright cells (Figure3) are immature leukocyte precursors and that up-regulation of the5-LO gene is an early event during embryonic leukopoiesis.
Induction of the 5-LO pathway during generation of DCs from HPCs in response to SCF, GM-CSF, and TNF-α
We next studied 5-LO pathway expression during cytokine-dependent DC differentiation. For this purpose, a combination of cytokines consisting of SCF/GM-CSF/TNF-α, known to promote DC differentiation from CB-derived CD34+HPCs,12-14 was chosen.18 Upon addition of cytokines, 5-LO and FLAP transcripts rose within 3 days (Figure 1). The increase of 5-LO and FLAP transcripts was largely caused by the combination of GM-CSF and TNF-α because either cytokine alone was much less effective, and SCF alone had only small effects. The other major human hematopoietic SCG factor, the fms-like tyrosine protein kinase ligand 3,19 yielded similar results. This indicates that SCF and fms-like tyrosine protein kinase ligand 3 act primarily to promote proliferation of HPCs rather than to influence the expression of the 5-LO pathway. The rise in 5-LO and FLAP transcripts continued for up to 22 days and was accompanied by a large increase in 5-LO and FLAP proteins (Figure 2; additional data not shown).
TGF-β–1 added to SCF/GM-CSF/TNF-α to support DC maturation enhances 5-LO and FLAP gene expression and alters expression of leukocyte lineage markers
Among cytokines affecting DC differentiation from CD34+ HPCs in vitro, only TGF-β–1 has been demonstrated to promote LC maturation in vivo, as shown by the absence of epidermal LCs in TGF-β–1 knockout mice.20-22 When we added TGF-β–1 to SCF/GM-CSF/TNF-α, we observed a further strong increase in 5-LO and FLAP transcript and protein levels in the total cell population (Figures 1 and 2). The effect on transcripts became significant within 3 days and continued for up to 22 days. Using RT-PCR analysis, we determined whether TGF-β–1 alters transcript levels of leukocyte and DC markers and those of other constituents of the arachidonic acid cascade. TGF-β–1 decreased transcript levels of LTA4 hydrolase and CD14 while it increased transcript levels of LTC4 synthase, 15-LO, cyclo-oxygenase 2, CD1a, and CD83. No change was observed in transcript levels of cyclo-oxygenase 1. Transcript levels of 12-LO were low at 35 cycles at all time points, but we cannot rule out 12-LO regulation in subpopulations of cells. Again, in view of the heterogeneity of leukocyte differentiation in SCF/GM-CSF/TNF-α–containing medium (see below), further work is needed to determine the leukocyte lineage in which each arachidonic acid cascade constituent is expressed and/or regulated.
Double-fluorescence immunohistochemistry of SCF/GM-CSF/TNF-α–treated HPCs reveals generation of distinct 5-LO+ leukocyte lineages and 5-LO+ DC phenotypes
Although it is well established that the cytokine cocktail SCF/GM-CSF/TNF-α specifically promotes the DC differentiation pathway,12 other leukocyte lineage differentiation programs may not be entirely suppressed. To identify 5-LO+cell lineages and DC phenotypes, we applied double-fluorescence immunohistochemistry using a combination of 5-LO antisera, the nuclear proliferation–associated antigen Ki67, antisera of leukocyte lineages, and DC maturation markers. In contrast to freshly isolated HPCs, the majority of their cytokine-derived progeny was strongly nuclear 5-LO+ (compare Figure4A-B). The majority of Ki67+cells were 5-LO−, indicating that 5-LO up-regulation during leukopoiesis occurs in cells that have left the cell cycle (data not shown). Of 5-LObright cells, many (but not all) were HLA-DR+, indicating that they are capable of antigen presentation; other cells were 5-LO−/HLA-DR−(Figure 4A-B, arrows at lower left) or 5-LO−/HLA-DR+ (Figure 4A-B, arrows in upper center). A small but significant number of 5-LO+ cells were HLA-DR−/CD66b+, revealing 5-LO expression in myeloid precursor/granulocytes (data not shown). In addition, a minor subpopulation of cells (less than 3%) was identified as eosinophil peroxidase–positive/5-LO+ eosinophil precursors (Figure4H). 5-LO+ eosinophils also showed strong cytoplasmic 15-LO positivity, revealing 5-LO/15-LO double-positive cells, yet no other cell subpopulation, including all DC phenotypes, expressed 15-LO protein (data not shown).
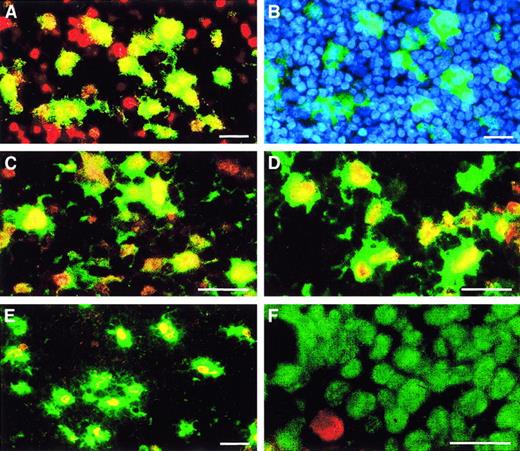
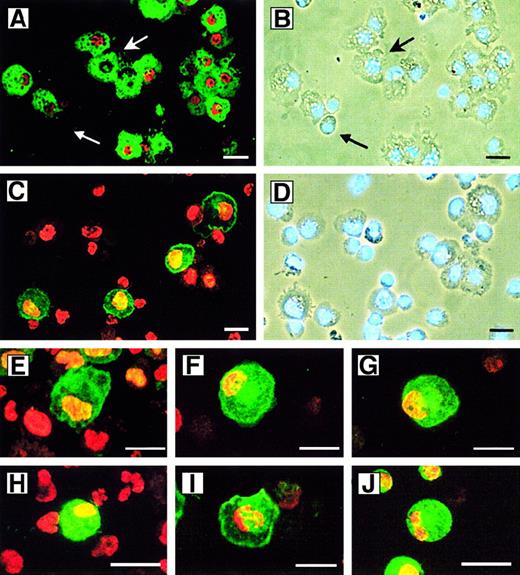
Heterogeneity of 5-LO+ leukocytes derived from CD34+ HPCs.
HPCs were maintained in culture for 15 days as described in Figure 2 in standard medium (panels A-D, F, I, and J) or standard medium plus TGF-β–1 (panels E, G, and H). Cytospins were stained with antisera as described in “Materials and methods”; 5-LO throughout Cy3 red, marker antisera throughout Cy2 green. (A) HLA-DR/5-LO, identical phase contrast field but stained with (B) DNA stain Hoechst 33258. Upper arrow indicates an HLA-DR+/5-LO− cell; lower arrow indicates an immature HLA-DR−/5-LO−cell. (C) CD1a/5-LO and identical phase contrast field but stained with (D) DNA stain Hoechst 33258, (E) CD40/5-LO, (F) CD83/5-LO, (G) Lag antigen/5-LO, (H) eosinophil peroxidase/5-LO, (I) CD14/5-LO, and (J) mannose receptor/5-LO. Bars indicate 20 μm.
Heterogeneity of 5-LO+ leukocytes derived from CD34+ HPCs.
HPCs were maintained in culture for 15 days as described in Figure 2 in standard medium (panels A-D, F, I, and J) or standard medium plus TGF-β–1 (panels E, G, and H). Cytospins were stained with antisera as described in “Materials and methods”; 5-LO throughout Cy3 red, marker antisera throughout Cy2 green. (A) HLA-DR/5-LO, identical phase contrast field but stained with (B) DNA stain Hoechst 33258. Upper arrow indicates an HLA-DR+/5-LO− cell; lower arrow indicates an immature HLA-DR−/5-LO−cell. (C) CD1a/5-LO and identical phase contrast field but stained with (D) DNA stain Hoechst 33258, (E) CD40/5-LO, (F) CD83/5-LO, (G) Lag antigen/5-LO, (H) eosinophil peroxidase/5-LO, (I) CD14/5-LO, and (J) mannose receptor/5-LO. Bars indicate 20 μm.
We next focused on the 5-LO+/HLA-DR+ DC population after extended periods of cytokine exposure. A large number of the HLA-DR+ progeny expressed the monocytic markers, CD14 (Figure 4I) and mannose receptor (Figure 4J), and the monocytic lysosome marker, CD68 (data not shown). A lesser number expressed the LC markers CD1a (compare Figure 4C-D) and Lag (Figure4G); the costimulatory molecule, CD40 (Figure 4E); and the marker for mature DCs, CD83 (Figure 4F). Caux et al13 14 described several DC populations in SCF/GM-CSF/TNF-α–treated CD34+HPCs. One was derived from a monocytic phenotype (CD14+/CD68+/mannose receptor–positive), and another displayed an HLA-DR+/CD1a+ LC phenotype. We found that 5-LO signals colocalized to all markers of monocytic DCs and to all markers of LC DCs (Figure 4; see below). These data indicate that the 5-LO+ cells in the HPC progeny have left the cell cycle, the cells are heterogenous regarding lineage specificity, a minor eosinophil peroxidase–expressing population is 5-LO/15-LO double-positive, and the majority of all cells represent HLA-DR+ DCs.
5-LO FACS analyses reveal profound TGF-β–1–dependent changes of 5-LO expression in leukocyte lineages and in DCs of different phenotypes and maturation stages
The immunohistochemical analyses provided evidence for considerable heterogeneity in the 5-LO+SCF/GM-CSF/TNF-α–derived HPC progeny (Figure 4). In addition, TGF-β–1 was found to strongly affect leukocyte lineage and DC maturation markers as well as 5-LO transcript and protein levels (Figures 1 and 2; additional data not shown). To characterize and quantitate 5-LO protein expression in the entire cell population at the single cell level, in each leukocyte lineage, and at distinct DC differentiation stages, we employed FACS analyses. A sensitive 5-LO FACS assay was established using the affinity-purified anti–5-LO antiserum 1550.4 In mixing experiments (confirmed by immunohistochemical analyses), we used CHO cells as negative controls and added RBL1 cells as positive controls. We noted that this antiserum detected less than 2% RBL1 cells in a suspension of greater than 98% CHO cells. When we applied the assay to the cytokine-derived HPC progeny, we observed that approximately 50% of all cells were 5-LO+ after 15 days of exposure to SCF/GM-CSF/TNF-α (Figure 5). At this time a significant number of cells were still proliferating due to the action of SCF. These cells were Ki67+ and largely lacked 5-LO, as shown by immunohistochemical analyses (data not shown). At 15 days of exposure to SCF/GM-CSF/TNF-α in the presence of TGF-β–1, 85% of all cells had been recruited into the 5-LO+ cell population (Figure 5).
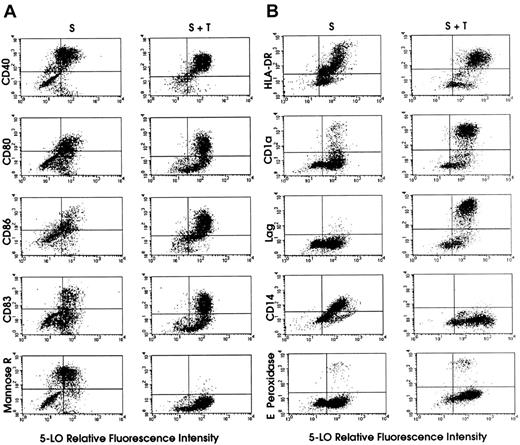
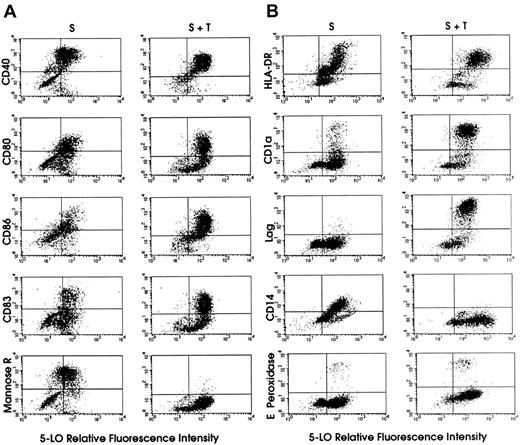
FACS dot-blot analyses of 5-LO protein-expressing cell populations derived from HPCs.
The y axes of the dot-blots show that TGF-β–1 increases the frequency of cells with high levels of CD80, CD86, CD83, CD1a, and Lag, and strongly reduces the frequency of cells expressing mannose receptor and CD14. HPCs were maintained in standard medium (S, left columns) or standard medium plus TGF-β–1 (S + T, right columns) for 15 days (Figure 1). 5-LO staining was performed as described in “Materials and methods.” Panels A and B represent 2 independent experiments. Secondary antibody for 5-LO staining was goat antirabbit IgG-FITC, except for HLA-DR–expressing and CD14-expressing cells in panel B, in which the secondary antibody was donkey antirabbit IgG-PE.
FACS dot-blot analyses of 5-LO protein-expressing cell populations derived from HPCs.
The y axes of the dot-blots show that TGF-β–1 increases the frequency of cells with high levels of CD80, CD86, CD83, CD1a, and Lag, and strongly reduces the frequency of cells expressing mannose receptor and CD14. HPCs were maintained in standard medium (S, left columns) or standard medium plus TGF-β–1 (S + T, right columns) for 15 days (Figure 1). 5-LO staining was performed as described in “Materials and methods.” Panels A and B represent 2 independent experiments. Secondary antibody for 5-LO staining was goat antirabbit IgG-FITC, except for HLA-DR–expressing and CD14-expressing cells in panel B, in which the secondary antibody was donkey antirabbit IgG-PE.
This effect of TGF-β–1 was largely due to recruitment from proliferating 5-LO− leukocyte progenitors (some of which may express CD40, CD80, and CD86; Figure 5A) or HSCs and, to a lesser extent, from 5-LO−/CD1a+ and 5-LO−/CD83+ cells (Figure 5). This interpretation was supported by TGF-β–1–dependent inhibition of the renewal rate of the entire cell population and by suppressive effects of TGF-β–1 on DNA synthesis in CD34+ HPCs. Interestingly, approximately 80% to 90% of all CD1a+ and CD83+ cells expressed 5-LO, and TGF-β–1 recruited an additional 10% into this population. However, although almost 100% of all Lag+ cells also expressed 5-LO in both cytokine cocktails, the total number of CD1a+ and Lag+cells was dramatically increased by the cytokine (Figure 5B). These results demonstrate that the TGF-β–1–dependent differentiation of immature DCs and their further maturation into mature DCs is closely associated with up-regulation of the 5-LO andFLAP genes. In studies performed in standard medium (Figure1) in which 5-LO MFI was determined at different time periods after the addition of cytokines, 5-LO MFI increased up to 5-fold between days 7 and 15 (data not shown), in part reflecting the increase in 5-LO protein and/or cells (Figure 2).
The effects of TGF-β–1 on leukocyte markers in relation to 5-LO expression are also noteworthy. Thus, TGF-β–1 obliterated the early myeloid/granulocyte marker, CD66b (data not shown), and the monocytic DC markers, mannose receptor (Figure 5A) and CD14 (Figure5B). Concomitantly, TGF-β–1 strongly promoted the generation of a 5-LO+/CD1a+/Lag+ (Figure 5B) and 5-LO+/CD83+ mature DCs (Figure 5A) and, to a lesser extent, CD86 (Figure 5A). However, TGF-β–1 did not suppress the expression of eosinophil peroxidase–positive cells (Figure 5B). TGF-β–1 has been shown previously to support DC maturation in vitro and in vivo and to be required for localization of LCs in the epidermis.20 We observed that TGF-β–1 significantly increased 5-LO MFI in the entire HLA-DR+ DC population and in CD40+, CD80+, CD86+, CD1a+, and CD83+ DCs (data not shown). It is known that a significant yet variable percentage of proliferating CD34+ HPCs already express HLA-DR.16 17 Thus, TGF-β–1 increases the frequency of cells with high CD80, CD86, CD83, CD1a, and Lag expression, and strongly reduces the frequency of cells expressing mannose receptor, CD68, and CD14. When taken together, TGF-β–1 recruits cells from proliferating (Ki67+) and other cell populations into the 5-LO+ cell population, suppresses leukocyte lineages other than DCs, supports the generation of a highly 5-LO+–mature DC phenotype at the expense of immature DCs (Figure 5), and increases the overall expression of5-LO and FLAP genes (Figures 1 and 2).
HPC progeny produce significant amounts of 5-HETE and LTB4 in response to Ca++ ionophore A23187 and arachidonic acid
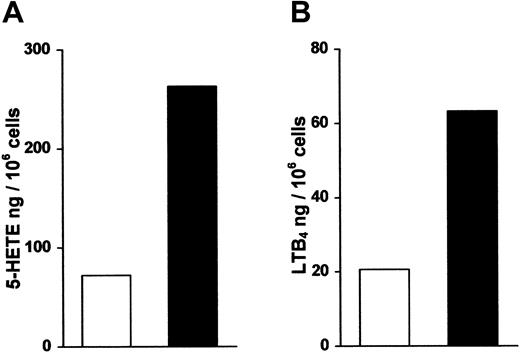
To assay the ability of HPC-derived progeny to produce LTs, we added Ca++ ionophore A23187 in the presence of arachidonic acid to intact cells that had differentiated in the presence of SCF/GM-CSF/TNF-α or SCF/GM-CSF/TNF-α/TGF-β–1. We observed that both populations of cells produced significant amounts of 5-HETE and LTB4 and that TGF-β–1–derived cells produced amounts of 5-LO pathway products that exceeded those of control cells by a factor of 3-4 (Figure 6). Similar amounts of 5-HETE and LTB4 were produced by cell-free systems (data not shown). In a single preliminary experiment we found that several immune agonists, CD40 ligand trimer (Immunex, Seattle, WA), 2 members of the chemokine family,23-25 macrophage inflammatory protein (MIP)-3–α and MIP-3–β, and prostaglandin E2 stimulated LTB4 formation in intact cells (data not shown). These data reveal that intact DCs can produce significant amounts of 5-HETE and LTB4.
Intact DCs form significant amounts of 5-HETE and LTB4.
HPCs were maintained as described in Figure 1 in medium supplemented in standard medium (open column) or standard medium plus TGF-β–1 (closed column). The cell populations were assayed for production of (A) 5-HETE and (B) LTB4 after the addition of 10 μmol/L Ca++ ionophore A 23187 and 40 μmol/L arachidonic acid as described.15 Data represent the mean of triplicate dishes prepared in parallel with a maximum SD of 4.7% for 5-HETE and 20% for LTB4. Similar data were obtained in a second experiment.
Intact DCs form significant amounts of 5-HETE and LTB4.
HPCs were maintained as described in Figure 1 in medium supplemented in standard medium (open column) or standard medium plus TGF-β–1 (closed column). The cell populations were assayed for production of (A) 5-HETE and (B) LTB4 after the addition of 10 μmol/L Ca++ ionophore A 23187 and 40 μmol/L arachidonic acid as described.15 Data represent the mean of triplicate dishes prepared in parallel with a maximum SD of 4.7% for 5-HETE and 20% for LTB4. Similar data were obtained in a second experiment.
In situ hybridization and double-fluorescence immunohistochemistry identify scattered 5-LO+ cells in the paracortex of LNs or tonsil, in DCs of the Waldeyer tonsillar ring, and in GC TBMs
We studied 5-LO and FLAP mRNA expression by in situ hybridization (ISH) in LNs, tonsil, oral mucosa, and mucosa overlying the Waldeyer tonsillar ring. Specific scattered 5-LO and FLAP ISH signals were recorded in paracortical areas (Figure7A,C-D) and tonsil (data not shown). Signals were not found when the sense RNAs were used as probes (Figure7B; data not shown). The distribution patterns of 5-LO and FLAP signals in these lymphoid organs were similar and consistent with the localization of LCs (peripheral paracortex) and IDCs (peripheral and central paracortex).11 There was no apparent difference in either the signal density/surface area or signal intensity/cell when peripheral and central paracortical areas were compared, which indicates that there was no major regulation of 5-LO during differentiation of immature (CD1a+) to mature DCs (CD83+). Scattered 5-LO and FLAP ISH signals were also found in cells of the mucosa of the Waldeyer tonsillar ring in proximity to the tonsil (Figure 7G-H). It is noteworthy that we had failed earlier to identify FLAP ISH signals in the normal human epidermis.4
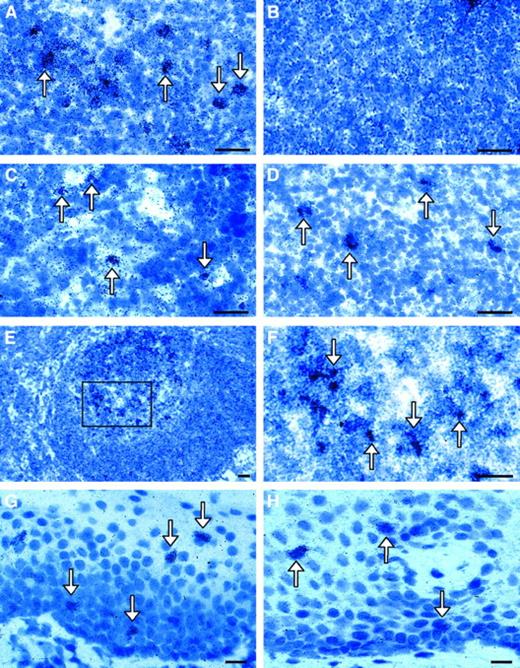
ISH of 5-LO and FLAP mRNA analyses in human LNs and mucosa of the Waldeyer tonsillar ring.
Bright fields of the (A) paracortical area of LN 5-LO antisense or (B) paracortical area of LN sense; (C) central paracortical area 5-LO antisense; (D) paracortical area FLAP antisense; (E) LN GC 5-LO antisense; (F) same as panel E, but with a higher magnification of inset; (G) mucosa overlying the Waldeyer tonsillar ring 5-LO antisense; or (H) mucosa overlying the Waldeyer tonsillar ring FLAP antisense. There were no specific ISH signals observed for 5-LO sense (panel B) or FLAP sense probes (data not shown). The arrows indicate 5-LO or FLAP mRNA+ cells. Bars indicate 20 μm.
ISH of 5-LO and FLAP mRNA analyses in human LNs and mucosa of the Waldeyer tonsillar ring.
Bright fields of the (A) paracortical area of LN 5-LO antisense or (B) paracortical area of LN sense; (C) central paracortical area 5-LO antisense; (D) paracortical area FLAP antisense; (E) LN GC 5-LO antisense; (F) same as panel E, but with a higher magnification of inset; (G) mucosa overlying the Waldeyer tonsillar ring 5-LO antisense; or (H) mucosa overlying the Waldeyer tonsillar ring FLAP antisense. There were no specific ISH signals observed for 5-LO sense (panel B) or FLAP sense probes (data not shown). The arrows indicate 5-LO or FLAP mRNA+ cells. Bars indicate 20 μm.
Immunohistochemical analyses of T-cell–rich paracortical areas of LNs and tonsil localized nuclear 5-LO to cells that were dendritic in morphology and expressed CD1a (Figure8A-B) and/or CD40 (data not shown) or CD83 (Figure 8C) and HLA-DR (Figure 8D). To show the T-lymphocyte cellularity of this area and the scattered location of the 5-LO+ DCs, we stained identical specimens with the DNA-binding dye, Hoechst 33258 (Figure 8B). Similar findings were obtained in human tonsil and the lymphoid tissue of the Waldeyer tonsillar ring. These data reveal that LCs of the oral mucosa and 2 phenotypes of DCs in T areas in normal human LNs, tonsil, and Waldeyer tonsillar ring express the 5-LO gene at significant levels. Most of the 5-LO protein-expressing cells in the oral mucosa were CD1a+, which indicates that the majority of ISH signals did not stem from cells of innate immunity such as mast cells, neutrophils, or macrophages (data not shown). These data extend the fact that 5-LO is expressed in epidermal LCs of epithelia other than the epidermis.4 Although we were unable to identify the FLAP-expressing cell lineage by immunohistochemistry, the distribution of FLAP and 5-LO ISH signals were similar, indicating that the majority of 5-LO–expressing cells in the normal oral mucosa were LCs. It is noteworthy in this connection that the LTB4receptor5 and LTC4receptor6 RT-PCR analyses revealed significant mRNA levels of both receptors in LNs and tonsil, thereby indicating the presence of LTB4 and LTC4receptor–expressing cells in secondary lymphoid organs. Further work is required to determine the identity of these cells.
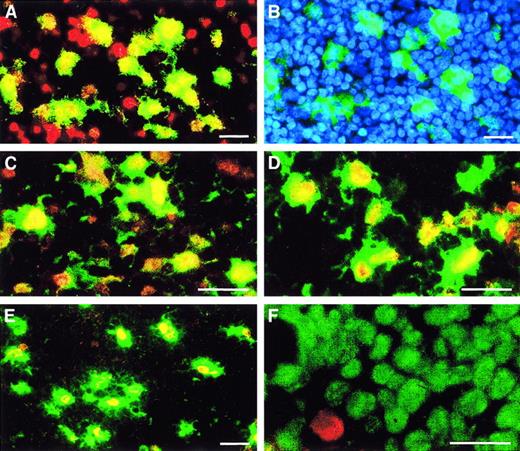
Immunohistochemistry of LNs localizes 5-LO protein to nuclei of CD1a+- and CD83+-expressing DCs in the paracortex and to CD68+ cells in GCs.
LNs were prepared as described in “Materials and methods,” then double-fluorescence immunohistochemistry was performed as described in Figure 4 (ie, 5-LO throughout Cy3 red, marker antisera throughout Cy2 green) and (A) stained for CD1a/5-LO in the peripheral paracortex, (B) CD1a and DNA stain Hoechst 33258 of the identical field as in panel A, (C) CD83/5-LO in the peripheral paracortex, (D) HLA-DR/5-LO in the peripheral paracortex, (E) CD68/5-LO in LN GC, and (F) Ki67/5-LO in LN GC. Bars indicate 25 μm.
Immunohistochemistry of LNs localizes 5-LO protein to nuclei of CD1a+- and CD83+-expressing DCs in the paracortex and to CD68+ cells in GCs.
LNs were prepared as described in “Materials and methods,” then double-fluorescence immunohistochemistry was performed as described in Figure 4 (ie, 5-LO throughout Cy3 red, marker antisera throughout Cy2 green) and (A) stained for CD1a/5-LO in the peripheral paracortex, (B) CD1a and DNA stain Hoechst 33258 of the identical field as in panel A, (C) CD83/5-LO in the peripheral paracortex, (D) HLA-DR/5-LO in the peripheral paracortex, (E) CD68/5-LO in LN GC, and (F) Ki67/5-LO in LN GC. Bars indicate 25 μm.
In addition to T-lymphocyte–rich areas, we observed scattered 5-LO and FLAP ISH signals in GCs of both tonsil and LNs (Figure 7E-F). However, while these 5-LO– expressing cells also displayed a dendritic morphology, they stained for CD68 (lysosomal marker of monocytes) and CD71 (transferrin receptor), ie, markers previously associated with follicular TBMs (Figure 8E). In some GC TBMs we found 5-LO protein fluorescence in patches within the cytoplasm (Figure 8E). In view of the known ability of TBMs to phagocytose apoptotic B cells, the origin of this 5-LO remains to be demonstrated. Because GCs largely consist of rapidly proliferating centroblasts and because B lymphocytes have been reported to express 5-LO,9,10 we double-stained GCs with Ki67 and 5-LO antisera. It became evident, however, that centroblasts did not express significant 5-LO protein, as Ki67 signals of centroblasts were clearly dissociated from 5-LO signals (Figure 8F). Moreover, using the affinity-purified antiserum 1550, we did observe cytoplasmic fluorescence in significant numbers of cells in the B-lymphocyte–rich GC mantle zones. These presumptive 5-LO+cells were small when compared to DCs and TBMs, did not have a dendritic morphology, and most likely represented differentiated B lymphocytes26 27 (data not shown).
These results raise the interesting possibility that the5-LO gene is up-regulated during differentiation of GC centroblasts into B lymphocytes. However, more direct evidence is needed to demonstrate that this assumption is legitimate. We are presently attempting to clarify these issues by using confocal laser scan microscopy and isolation of B lymphocytes from human lymph nodes at different stages of differentiation. Of note also, as single 5-LO+ cells in GCs expressed CD11c+, a marker previously found in GCDCs,14 it is possible that GCDCs express 5-LO. However, because fluorescence intensity of CD11c signals was found to be relatively weak in our preliminary studies, GCDC purification is required to substantiate this notion.
Discussion
Most investigators have favored the view that 5-LO pathway products act distally of a T-lymphocyte helper 2 type-dependent immune response that is governed by interleukin-4 (IL-4), IL-5, IL-6, and IL-13 involving eosinophils, mast cells, and macrophages, as typified by extrinsic asthma.3,28 This notion implies that the major function of LTs in diseased states is to contribute to the organization of inflammatory infiltrates or to trigger other inflammatory tissue reactions, such as contraction of bronchial smooth muscle, and to enhance mucus production by epithelial cells. Studies by other investigators29 have provided evidence that the 5-LO pathway plays a role in both cellular and humoral immunity, thereby implying its action at one or more proximal steps within the immune response cascade.2,7 8 However, the identity of immune cells that produce LTs and their target cells, as well as their functional impact, all remain to be defined.
Primary immune reactions are initiated by LC-type sentinel DCs within epithelial surfaces.11 These DCs first monitor and sample antigen, then are triggered to emigrate from the sites of antigen capture, pass through lymph vessels as “veiled” cells, home in T-lymphocyte–rich areas of draining lymphoid tissues, and finally present antigen to instruct naive lymphocytes during prolonged multiple-cell interactions. The complexity of these events and the lack of information regarding the anatomy of the 5-LO pathway in lymphoid tissues have precluded predictions of specific roles of LTs in the immune response.
Two lines of evidence detailed above are consistent with a proximal action of the 5-LO pathway within the immune-response cascade. DC differentiation and maturation from CD34+ HPCs is associated with strong up-regulation of the 5-LO pathway in vitro, and CD1a+ DCs of epithelia and the peripheral paracortex (immature) and central paracortical CD83+ IDCs of T-lymphocyte–rich areas of LNs and tonsil (mature) express both5-LO and FLAP genes in vivo.
While 5-LO expression in freshly prepared embryonic CB-derived CD34+ HPCs is restricted to a scarce immature leukocyte HLA-DR+ (Figure 3) subpopulation (indicating early up-regulation of 5-LO in APCs during hematopoiesis), SCF/GM-CSF/TNF-α–induced DC differentiation in vitro is associated with 5-LO and FLAP up-regulation in a major population of immature DCs (Figures 1 and 2). These 5-LO+ DCs express HLA-DR and the costimulatory molecules CD40, CD80, and CD86, and thus they are capable of naive T-lymphocyte activation (Figures 4 and 5; R.S., unpublished observations, December 1998). TGF-β–1, known to be required for DC maturation in vitro and in vivo,20 recruits further DCs into the 5-LO+progeny (Figure 5), strongly promotes generation of CD1a+/Lag+ LC-type DCs (Figure 5B) and mature CD83+ DCs (Figure 5A), enhances 5-LO MFI in all DC phenotypes, and increases the expression of FLAP mRNA and protein (Figures 1 and 2). Concomitantly, TGF-β–1 abolishes immature monocytic DCs by down-regulating CD14, the mannose receptor, CD68; and CD66b, an early marker of myeloid precursors/granulocytes (Figure 5B; additional data not shown).
The HPC progeny produce significant amounts of 5-HETE and LTB4 in response to arachidonic acid and Ca++ionophore A23187, although mature DCs generated in the presence of TGF-β–1 produce even higher amounts (Figure 6). As lengthy isolation procedures of primary DCs from tissues affect their properties, thereby hampering biochemical studies, our data have established in vitro DC models to identify immune agonists capable of triggering LT formation. Indeed, in preliminary studies we have observed that the CD40 ligand, 2 chemokines, MIP-3–α, MIP-3–β,23-25 and prostaglandin E2 trigger formation of LTB4 in TGF-β–1 pretreated DCs (data not shown). However, we failed in 2 experiments to observe an inhibition of T-lymphocyte DNA synthesis initiated by these DCs in the presence of the FLAP inhibitor, MK-886, in an allogeneic mixed lymphocyte reaction assay. To identify potential functional alterations in target lymphocytes induced by DC-derived LTs, the cytokine pattern of DC-activated T lymphocytes and activation parameters of B lymphocytes should now be defined in the presence of 5-LO inhibitors in similar assays.27 Moreover, the identification of LT receptor target cells will be important to identify potential sites of LT action in this in vitro–reconstituted immune reaction. These data reveal a close association between the 5-LO pathway and the DC differentiation and maturation pathways in vitro and are likely to mirror expression of 5-LO in both immature and mature DCs in vivo.
Our ISH and immunohistochemical data provide new information on the organization of the 5-LO pathway in epithelia and secondary lymphoid organs. Both immature CD1a+ DCs and mature CD83+ IDCs in paracortexes of normal LNs express the5-LO and FLAP genes (Figures 7 and 8). Similar results have been obtained in inflamed tonsil (data not shown) and the epithelium of the Waldeyer tonsillar ring (Figure 7). Other DCs, including those located in buccal mucosa, CD1a+ LCs in the dermis, and those in nonasthmatic bronchial epithelium of the lung (obtained from lung cancer patients), express 5-LO (data not shown). Moreover, scattered cells in LN GCs that are strongly 5-LO+and FLAP+, display a dendritic morphology, and stain for CD68 (Figure 8) and CD71 (the transferrin receptor; data not shown), probably represent GC TBMs. Together with previous studies of naive epidermal LCs,4 these data reveal that the5-LO gene is expressed in both immature and mature DCs and possibly in GC TBMs in vivo. They raise the possibility that the entire myeloid-derived human DC system11 expresses the 5-LO pathway. There is no information presently available on 5-LO expression in lymphoid-derived DCs.30 Paracortical CD1a+cells of LNs are believed to represent recently arrived LCs that are in the process of down-regulating CD1a and maturing into CD83+IDCs.11 18
What is the function of this prominent 5-LO pathway expression in several DC phenotypes located in the epidermis, epithelia, and lymphoid organs? Major roles of immature DCs are antigen sampling, processing, and antigen transport to sites of lymphocyte activation in LNs, whereas major functions of mature DCs are antigen presentation and initiation of primary immune reactions. Thus, the immune response involves multiple cell–cell interactions that are separated both in time and space and range from LC/keratinocyte interaction in the epidermis, DC/endothelial cell interactions during retroendothelial migration of DCs into dermal lymph vessels, homing events at specific sites within lymphoid organs, and DC/lymphocyte interactions in the paracortex and GCs. We hypothesize that LTs play a role in one or more of these events. Moreover, as the paracortex of LNs does not contain inflammatory cells of the innate immune system, such as neutrophils, mast cells, or eosinophils, it is unlikely that 5-LO products produced by LCs,4 IDCs, GC TBMs, or GCDCs31participate in any bona fide inflammatory/allergic tissue reaction previously associated with the action of 5-LO pathway products.1-3,7,8,28,29 Instead, it is more likely that these products affect one or more proximal steps of the immune response cascade either by acting on the DCs in an autocrine way or by acting on neighboring cells, possibly lymphocytes. The fact that LTB4can affect B-lymphocyte functions has already been shown in vitro.32 To delineate molecular mechanisms within immune responses that are affected by 5-LO pathway products, future studies should be directed toward DC/lymphocyte cocultures, the identification of LT receptor-expressing cells in lymphoid organs, and immune-response studies of 5-LO knockout mice.
Acknowledgments
We are grateful to Dr F.-X. Bosch, Ear Nose and Neck Clinic, University of Heidelberg, Heidelberg, Germany, for gifts of normal human LNs and tonsil, and to the nurses of the Department of Gynecology of the University of Heidelberg for providing CB. Antiserum 1550 was prepared by Drs Y.-Y. Zhang, T. Hammarberg, and H. Okamoto.
Supported by grant Ha 1083/12-1 from the German Research Council, Germany; grant 01GB 9401/6 from the German Bundesministerium für Forschung und Technologie, Germany; grants BMH4-CT96-0229 and BMH4-CT98-3191 from the EU concerted actions; grant TP5.11 from the Verbund für Klinische Forschung of the University of Jena, Erfurt, Germany; grant 03X-217 from the Swedish Medical Research Council, Stockholm, Sweden; grant A95067 from the Vårdal Foundation, Stockholm, Sweden; and the Stiftung VERUM, Munich, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rainer Spanbroek, Center for Vascular Medicine, Friedrich Schiller University of Jena, Nordhäuserstr. 78, 99089 Erfurt, Germany; e-mail: spanbroek@zmkh.ef.uni-jena.de.