Abstract
The case of a 4-month-old girl with familial hemophagocytic lymphohistiocytosis is described. The patient underwent stem cell transplantation from her haploidentical mother 2 months after receiving a living-related liver transplant from the same donor for acute hepatic failure. Conditioning regimen consisted of 16 mg/kg busulfan, 200 mg/kg cyclophosphamide, 10 mg/kg thiothepa, and antithymocyte globulin. Myeloid engraftment occurred on day +10, but CD3+ cells of recipient origin remained. To convert the T-cell chimerism, the patient received donor lymphocyte infusion on day +43, and subsequently the allelic pattern changed to complete donor genotype on day +57. Four months after stem cell transplantation the patient is disease free, with complete donor chimerism in bone marrow and stable hepatic graft function without any immunosuppressive therapy.
Introduction
Familial hemophagocytic lymphohistiocytosis (FHL) is a rare fatal genetic disorder in early infancy that is characterized by hypercytokinemia; activation of T cells and macrophages; and the presence of hemophagocytosis predominantly in bone marrow, liver, and spleen.1 Central nervous system (CNS) involvement can be observed in more than 70% of patients.2 Hepatic dysfunction is commonly observed, and fulminant hepatorenal failure may be a fatal complication of FHL.3 Diagnosis is mostly established late, often only at autopsy. Chemotherapy, including corticosteroids or cyclosporine A and etoposide, may lead to stabilization, but the overall 5-year survival is poor (10%). The only curative approach is allogeneic stem cell transplantation (SCT), in which there is an overall survival of 66%.1,4-6,7 Living-related liver transplantation has become a therapeutic option for young children with acute hepatic failure.8,9 Successful orthotopic cadaveric or living-related liver transplantation for veno-occlusive disease occurring as a complication of bone marrow transplantation has been reported during the last years.10,11 SCT following unrelated orthotopic liver transplantation has been reported in 3 cases. There has been one reported case of human leukocyte antigen (HLA)–identical sibling SCT unrelated cadaveric liver transplantation, and one case of matched unrelated bone marrow transplantation was performed shortly after cadaveric liver transplantation in a patient with hyperimmunoglobulin M (hyper-IgM) syndrome and hepatic failure.12,13 Recently Ringden et al14reported a combined transplantation of bone marrow and liver from a cadaveric donor.
Study design
A 4-month-old girl with the diagnosis of FHL who had received a living-related liver transplantation from her mother as an emergency procedure for acute hepatic failure at the University Hospital of Innsbruck, Innsbruck, Austria, was transferred to our center for SCT. The diagnosis of FHL had been made from the histology of the explanted liver and from bone marrow biopsies. Following the liver transplantation (LTX), immunosuppression comprised tacrolimus and methylprednisolone, and the patient received conventional treatment for FHL according to the HLH-94 protocol. On admission the girl presented with a markedly poor general condition with reduced muscular tone, no spontaneous motor activity, poor head control, and weak crying. Reactivation of the underlying disease was confirmed by hypertriglyceridemia of 411 mg/dL, serum ferritin level of 2226 μg/L, and active bone marrow disease with 13% histiomonocytic cells and phagocytosis. Before SCT, microchimerism analysis by allele-specific polymerase chain reaction (PCR) amplification of a donor-derived HLA-allele (DRB*107) revealed the presence of maternal cells in the peripheral blood.
Chemotherapy (150 mg/m2 etoposide once weekly and 0.6 mg/kg per day dexamethasone) for the underlying disease was continued until day −10 before SCT. The conditioning regimen consisted of 12 mg/kg busulfan, 10 mg/kg thiothepa, 200 mg/kg cyclophosphamide, and 30 mg/kg/d antithymocyte globulin (ATG) (rabbit). Immunosuppression with tacrolimus was continued, and an additional 30 mg/kg mycophenolate mofetil was administered starting from day −1. Except for mucositis and WHO grade 2I skin toxicity, the conditioning regimen was well tolerated, and liver function tests remained within the pretransplant range.
The patient's mother, who had an HLA-mismatch of 3 of 6 antigens, was stimulated with 10 μg/kg/d granulocyte colony-stimulating factor (G-CSF) subcutaneously for 4 days and underwent stem cell apheresis on day zero. CD34+ cells were prepared by CD34+ CD4/CD8/CD19− selection on an Isolex 300i (Nexell Therapeutics Inc, Irvine, CA) with a purity of 98.6%.15 On day +70 after LTX, the patient received 2.5 × 104 T cells and 25.6 × 106CD34+ cells per kg body weight.
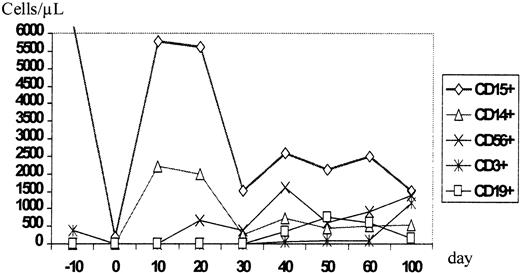
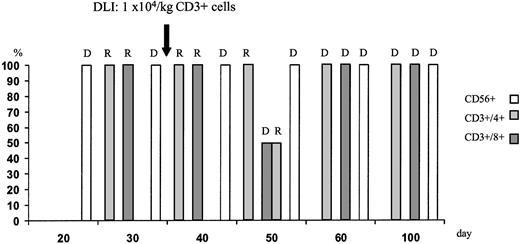
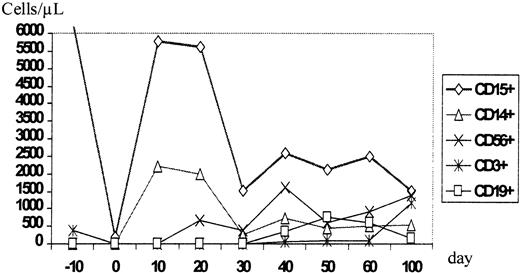
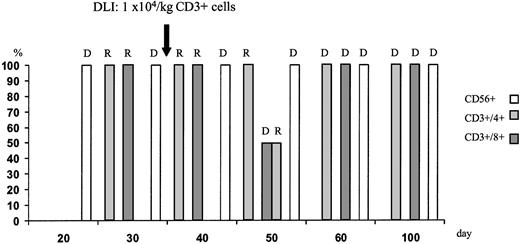
During aplasia, the girl experienced one septic episode, which was readily controlled by adequate antibiotic treatment. Engraftment was monitored by fluorescence-activated cell sorter (FACS) analysis and by short tandem repeats (STR)-PCR analysis for donor-recipient chimerism from FACS-sorted cell populations as described elsewhere.16 The patient showed prompt engraftment with a leukocyte count of 1.7 × 109/L on day +10. Engraftment data are shown in Figure 1, and chimerism kinetics are shown in Figure2. Natural killer (NK) cells, monocytes, and granulocytes were of pure donor origin, but CD3+/CD4+ and CD3+/CD8+cells in peripheral blood, detectable on day +29, were of recipient origin. To convert the T-cell chimerism, the patient received donor lymphocyte infusion (DLI) with 1 × 104 CD3+cells per kg on day +43. By day +52 the allelic pattern had changed to mixed chimerism, and subsequently the pattern changed to complete donor genotype on day +57.
Time course of the reconstitution of the leukocyte subsets monitored by flow cytometric phenotype analysis.
DLI was given on day +43. Chimerism data are shown in Figure2.
Time course of the reconstitution of the leukocyte subsets monitored by flow cytometric phenotype analysis.
DLI was given on day +43. Chimerism data are shown in Figure2.
T-cell chimerism following haploidentical STC and DLI on day +43 monitored by STR-PCR analysis of FACS-sorted cell populations.
Neutrophils and NK cells were of pure donor genotype. After DLI, the allelic pattern of CD3+ cells changed to mixed chimerism and subsequently to complete donor genotype. R indicates recipient; D, donor.
T-cell chimerism following haploidentical STC and DLI on day +43 monitored by STR-PCR analysis of FACS-sorted cell populations.
Neutrophils and NK cells were of pure donor genotype. After DLI, the allelic pattern of CD3+ cells changed to mixed chimerism and subsequently to complete donor genotype. R indicates recipient; D, donor.
Immunosuppression with tacrolimus and mycophenolate mofetil was maintained until stable, complete donor-recipient chimerism was documented. Three weeks after DLI, the girl developed grade I graft-versus-host disease (GVHD) of the skin, which resolved completely after 4 days of prednisolone treatment. Steroid therapy was discontinued 2 weeks later.
A liver biopsy was performed on day +44 after SCT (day +114 after LTX), and bone marrow biopsies were performed on days +28 and +100; both revealed no signs of FHL. During the first 2 months after SCT, the girl showed a surprisingly good improvement in her neuropsychological performance, with normalization of spontaneous motor activity and social development. She was discharged on day +100 in excellent clinical condition.
Results and discussion
Living-related liver transplantation has been established as a life-saving therapy, particularly for children younger than 1 year of age. However, Epstein-Barr virus (EBV)–associated lymphoproliferative disorders due to prolonged immunosuppression are a major posttransplantation complication, occurring in 19% of the patients.17,18 There is some evidence that organ transplantation with simultaneous infusion of donor bone marrow might be able to induce tolerance and thus reduce the incidence of rejection.14 19
In the case reported, diagnosis of FHL was established only after living-related liver transplantation, which had been performed for the treatment of acute liver failure. Haploidentical SCT has turned out to be a feasible alternative for children with FHL lacking an HLA-identical donor.5,6,20 In this particular patient, an unrelated donor search was not initiated, and haploidentical SCT from the organ donor was considered the first choice for 2 reasons: (1) Microchimerism after liver transplantation might facilitate donor stem cell engraftment. (2) Complete bone marrow donor chimerism in the organ recipient might not only cure the underlying disease, but the chimerism might also prevent organ rejection without the necessity of prolonged immunosuppression, thus reducing the risk of EBV-associated lymphoproliferative disease. In the case of persistent mixed chimerism after allogeneic SCT, DLI is able to displace residual host cells.14,21 22 Because the circulating CD8+and CD4+ T cells were of recipient origin on day +29, a DLI was given to promote donor T-cell engraftment. We have shown that SCT after living-related liver transplantation from the same haploidentical donor is feasible, and LTX might provide an attractive option for young children with acute or chronic liver failure due to diseases that can be cured by allogeneic SCT such as FHL, hyper-IgM syndrome, or generalized Langerhans cell histiocytosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Helmut Gadner, St Anna Kinderspital, Kinderspitalgasse 6, A-1090 Vienna, Austria; e-mail:gadner@ccri.univie.ac.at.