Abstract
Bisphosphonates are well-known inhibitors of osteoclastic bone resorption, but recent clinical reports support the possibility of direct or indirect antitumor effects by these compounds. Because bisphosphonates share structural homologies with recently identified γδ T-cell ligands, we examined the stimulatory capacity of bisphosphonates to γδ T cells and determined whether γδ T-cell stimulation by bisphosphonates could be exploited to generate antiplasma cell activity in multiple myeloma (MM). All tested aminobisphosphonates (alendronate, ibandronate, and pamidronate) induced significant expansion of γδ T cells (Vγ9Vδ2 subset) in peripheral blood mononuclear cell cultures of healthy donors at clinically relevant concentrations (half-maximal activity, 0.9-4 μmol/L). The proliferative response of γδ T cells to aminobisphosphonates was IL-2 dependent, whereas activation of γδ T cells (up-regulation of CD25 and CD69) occurred in the absence of exogenous cytokines. Pamidronate-activated γδ T cells produced cytokines (ie, interferon [IFN]-γ) and exhibited specific cytotoxicity against lymphoma (Daudi) and myeloma cell lines (RPMI 8226, U266). Pamidronate-treated bone marrow (BM) cultures of 24 patients with MM showed significantly reduced plasma cell survival compared with untreated cultures, especially in cultures in which activation of BM-γδ T cells was evident (14 of 24 patients with MM). γδ T-cell depletion from BM cultures completely abrogated the cytoreductive effect on myeloma cells in 2 of 3 tested patients with MM. These results show that aminobisphosphonates stimulating γδ T cells have pronounced effects on the immune system, which might contribute to the antitumor effects of these drugs.
Bisphosphonates are the treatment of choice for diseases that involve excessive bone resorption and have been shown to be effective in preventing osteolytic bone disease in several different malignancies, including multiple myeloma (MM).1,2Chemically, bisphosphonates are synthetic analogues of endogenous pyrophosphate. Variation of their side chains contributes to the different relative potency of bisphosphonates. However, the precise mechanisms whereby bisphosphonates inhibit bone resorption are still not completely understood.3
Recent data raise the possibility that certain bisphosphonates also exert antitumor effects. In this context, results of a large, randomized, double-blind, placebo-controlled study4 showed significant improvement in the survival rates of a subgroup of patients with MM who entered the trial receiving intravenous pamidronate treatment in addition to salvage chemotherapy. In addition, objective remission or inhibition of disease progression has been reported in patients with MM who underwent pamidronate treatment alone.5 Furthermore, in experimental models of human breast cancer, bisphosphonates were found to reduce tumor burden in skeleton.6
Several studies demonstrate the presence of a T-cell–mediated immune response against MM.7,8 Most experiments have focused on αβ T cells and the idiotype of myeloma cells as a target for a specific immune response.9 The role of γδ T cells as possible antimyeloma effector cells has never been investigated. T cells bearing the T-cell receptor (TCR)-γδ represent a minor subset of human peripheral T cells (1%-10%), differing from αβ T cells in cell surface phenotype, in limited combinatorial diversity of TCR, and in a human leukocyte antigen-unrestricted antigen recognition. In adults, most of these γδ T cells display a disulfide-linked Vγ9/Vδ2 TCR.10 The physiologic function of γδ T cells remains elusive, though some evidence has been accumulated indicating that γδ T cells play a role in the “first line of defense” against a broad spectrum of invasive microorganisms such as mycobacteria. In addition, certain hematopoietic tumor cells (eg, Burkitt lymphoma cell line Daudi or myeloma cell line RPMI 8226) are specifically recognized and lysed by these T cells in vitro.11,12 In contrast to αβ T cells, γδ T cells (Vγ9Vδ2+ subset) recognize nonpeptide compounds of low molecular weight (100-600 d) with an essential phosphate residue. So far only 1 natural ligand has been isolated from mycobacteria and characterized as isopentenylpyrophosphate (IPP).13 However, γδ T cells exhibit a broad cross-reactivity with a variety of phosphorylated metabolites, such as nucleotidic phosphates,14 phosphorylated sugars,15 and synthetic pyrophosphates.16 The recognition of ubiquitous nonpeptide antigens by γδ T cells suggests a surveillance function of these T cells for infected or transformed cells.17
The structural relationship between bisphosphonates and defined γδ T-cell ligands prompted us to investigate whether certain bisphosphonates can stimulate γδ T cells and to determine the functional consequences of this stimulation. An increase of peripheral blood γδ T cells in patients with acute-phase reaction after their first pamidronate treatment has already been shown in vivo.18 The current study will record that only aminobisphosphonates (alendronate, ibandronate, and pamidronate) induce the activation and IL-2–dependent proliferation of Vγ9Vδ2 T cells. Stimulation of γδ T cells by aminobisphosphonates (ie, pamidronate) resulted in the induction of γδ T-cell–mediated antiplasma cell activity in vitro. Therefore, γδ T cells are new targets of aminobisphosphonate action, which might contribute to an immune response-mediated survival benefit for myeloma patients.
Patients, materials, and methods
Patients
We studied in vitro effects of bisphosphonates and IPP on peripheral blood mononuclear cells (PBMC) of 6 healthy donors and on bone marrow mononuclear cells (BMMC) of 24 patients with multiple myeloma (MM), after obtaining their informed consent. MM was classified according to the Durie and Salmon staging system. Six patients were in stage I, 7 were in stage II, 9 were in stage IIIA, and 2 were in stage IIIB. Sixteen patients were classified as IgG, 6 as IgA, and 2 as Bence–Jones MM subtype. Ten patients were evaluated at diagnosis, 5 patients were administered first-line chemotherapy with melphalan–prednisone, 8 patients were administered second-line chemotherapy, and 1 patient underwent a relapse after high-dose chemotherapy with autologous stem cell transplantation. All patients receiving treatment were studied at least 4 weeks after the last day of chemotherapy or corticosteroid treatment. Patients were not taking antibiotics, nor did they show any sign of infection. Eight patients had received bisphosphonates at least once before the in vitro studies: 6 were given pamidronate (Aredia; Novartis, Nuernberg, Germany) treatment (90 mg intravenously every 4 weeks), 1 patient was given clodronate (Bonefos; Astra, Wedel, Germany) treatment (900 mg intravenously every 4 weeks), and 1 patient was given ibandronate (Bondronat; Roche AG, Grenzach-Wyhlen, Germany) treatment (2 mg intravenously every 8 weeks).
Reagents
The following compounds were used for stimulation assays: isopentenylpyrophosphate (IPP; Sigma, Deisenhofen, Germany), 3-amino-1-hydroxypropylidene-1, 1-bisphosphonic acid (pamidronate; Novartis), 4-amino-1-hydroxybutylidene-1, 1-bisphosphonic acid (alendronate; Fosamax; MSD Sharp and Dohme, Haar, Germany), 1-hydroxy-3-(methylpentylamino) propylidenebisphosphonic acid (ibandronate; Bondronat; Roche AG), disodium dichloromethylidene-1, 1 bisphosphonic acid (clodronate; Astra GmbH), and 1-hydroxyethylidene-1, 1-bisphosphonic acid (etidronate; Gehe Medica, Stuttgart, Germany).
Cytofluorometric analysis
For evaluation of cell expansion, PBMC were harvested after a 7-day culture period and were analyzed using 2-color flow cytometry (FACScan; Becton Dickinson, Heidelberg, Germany). In some experiments, cell number per well was counted to calculate the expansion of absolute cell numbers. Monoclonal antibodies (mAb) used included fluorescein isothiocyanate (FITC)-conjugated antipan γδ TCR (TCRδ1), anti-Vγ9 variable light chain (TiγA), anti-Vδ2 variable light chain (BB3) (all from Coulter–Immunotech, Hamburg, Germany), and anti-αβ TCR (WT3; Becton Dickinson, Mountain View, CA) or phycoerythrin (PE)-conjugated anti-CD3, anti-CD19, anti-CD14, and anti-CD16/CD56 (all from Coulter–Immunotech). To identify activated peripheral blood (PB) or bone marrow (BM) γδ T cells, FITC-conjugated antipan γδ TCR (TCRδ1) and PE-conjugated anti-CD25 (α chain IL-2 receptor; Coulter–Immunotech) or PE-conjugated anti-CD69 (Coulter–Immunotech) mAb were used for 2-color flow cytometry analysis. For identification of malignant BM plasma cells, the following panel of mAbs was used: FITC-conjugated anti-CD45 (Coulter–Immunotech), PE-conjugated anti-CD38 (Becton Dickinson, Heidelberg, Germany), FITC-conjugated anti-CD19 (Coulter–Immunotech), and PE-conjugated anti-CD138 (B-B4/Syndecan-1; Biermann, Bad Nauheim, Germany). Myeloma plasma cells were defined as CD45low,(+)/CD38bright,++ and CD19−/ CD138++ cells. In all experiments, 10 000 viable cells were analyzed using forward/side-scatter gating. Isotype-matched mAbs were used as controls.
Cell preparations and culture
PBMC and BMMC were obtained by centrifugation of heparinized peripheral blood or bone marrow aspirates over Ficoll–Hypaque gradients (Pharmacia, Uppsala, Sweden); 1×105 cells were cultured in 96-well round-bottom microtiter wells (Nunc, Wiesbaden, Germany) for indicated time intervals (3 to 7 days) at 37°C in humidified atmosphere (5% CO2). Medium consisted of RPMI 1640 (Gibco, Life Technologies, Karsruhe, Germany) supplemented with 10% pooled human AB serum,L-glutamine (Gibco; 2 mmol/L), 1% penicillin–streptomycin (Seromed, Berlin, Germany), and, when indicated, 10 U/mL IL-2 (generously provided by W. Sebald; Theodor–Boveri Institute, University of Wuerzburg, Germany). For γδ T-cell depletion experiments, BMMC were depleted of γδ T cells by negative selection procedures using magnetic-activated cell sorting (MACS). In brief, BMMC were incubated with FITC-labeled anti-TCR γδ (TCRδ1) mAb or IgG-isotype control mAb followed by anti-FITC magnetic microparticles (MACS system; Miltenyi Biotec, Bergisch Gladbach, Germany). After 2 washing steps, the cells were passed through a strong magnetic field, and effluent cells were evaluated for residual γδ T cells by staining with PE-labeled anti-TCR γδ (TCRδ1). The negative selected BMMC consisted of less than 0.5% γδ TCR positive cells, whereas cell viability (by trypan blue exclusion test) and number of BM plasma cells (as identified by CD45low,(+)/CD38bright,++ and CD19−/CD138++ expression) remained unchanged after this procedure. Purification and enrichment of γδ T cells for proliferation assays was performed by similar negative-selection procedures. To deplete PBMC from αβ T cells and natural killer (NK) cells, PBMC were preincubated with FITC-labeled anti-αβ TCR (T Cell Diagnostics) and anti-CD 16 (Coulter–Immunotech) mAb before they were labeled with anti-FITC magnetic microparticles and MACS separation. Isolated cells consisted of 25% to 30% γδ T cells and less than 0.5% αβ T cells or NK cells, as determined by FACS analysis. Viability was confirmed by trypan blue exclusion test and forward/side-scatter gating.
Proliferation assay
In round-bottom microtiter wells, 4×104 purified (αβ T cell− and NK cell−) PBMC were cultured with pamidronate (4 μmol/L), IPP (4 μmol/L), phytohemagglutinin (PHA; 1 μg/mL), or medium alone for 96 hours in a 5% CO2 humidified atmosphere. Absolute cell number of γδ T cells in these PBMC cultures was 1.0 to 1.2 × 104 γδ T cells per well. Medium consisted of RPMI 1640 supplemented with 10% pooled human AB serum, L-glutamine (2 mmol/L), and 1% penicillin–streptomycin. After a culture period of 48 hours, IL-2 (10 U/mL) was added. During the last 12 hours of the 96-hour culture period, cells were pulsed with 1 μCi [3H] thymidine (Amersham, Braunschweig, Germany). Cells were harvested with a semiautomated sample harvester, and [3H] thymidine incorporation was measured in a liquid scintillation counter (Beckman, München, Germany). Results are shown as cpm (geometric mean + SD) of triplicate cultures.
Cytoine analysis
Quantification of cytokines (IFN-γ, granulocyte macrophage–colony-stimulating factor [GM-CSF], tumor necrosis factor [TNF]-α) in PBMC supernatants was performed by enzyme-linked immunosorbent assay (Endogen, Woburn, MA). Supernatants were collected after 4, 12, 24, 48, and 72 hours and stored at −80°C after centrifugation (5000g for 10 minutes) until analysis was performed according to the manufacturer's instructions. Samples were analyzed in triplicate. The sensitivity of the assays used was less than 2 pg/mL for IFN-γ, less than 2 pg/mL for GM-CSF, and less than 5 pg/mL for TNF-α, respectively. Intracellular cytokine staining was performed to determine IFN-γ production of γδ T cells at the single-cell level. Monensin (2 μmol/L; Sigma) was added for 2 hours to the cells in culture to cause intracellular accumulation of newly synthesized proteins. Cells were harvested and stained for surface expression of TCR-γδ by PE-conjugated anti-γδ TCR (TCRδ1). After they were washed with PBS/2% fetal calf serum (FCS), cells were fixed with 4% paraformaldehyde in PBS for 30 minutes at room temperature. Cells were washed with PBS/2% FCS and permeabilized with 0.5% saponin (Sigma) in PBS for 30 minutes at room temperature. FITC-conjugated anti–IFN-γ (Coulter–Immunotech) was added to permeabilized cells and incubated for 30 minutes. Afterward cells were washed with PBS/0.5% saponin and finally with PBS/2% FCS. Samples were analyzed on a FACScan flow cytometer. For control, samples were incubated with an irrelevant isoptype-matched mAb. Specificity of anti–IFN-γ mAb was demonstrated by preincubation of a 100- to 1000-fold molar excess of recombinant IFN-γ, together with the anti–IFN-γ mAb for 1 hour before it was added to the sample. This procedure resulted in greater than 90% inhibition of IFN-γ detection.
Cell lines
γδ T-cell lines were established by culturing 1 × 105 freshly isolated PBMC in standard medium (RPMI 1640 media supplemented with 10% pooled AB human serum, 2 mmol/LL-glutamine, and 1% antibiotics) with a single dose of aminobisphosphonate (40 μmol/L pamidronate). After 48 hours, IL-2 (50 U/mL) was added to the cultures, and cells were periodically restimulated with IL-2 (50 U/mL) every 4 to 6 days. After 2 to 3 weeks, more than 90% of the cells expressed the Vγ9Vδ2 TCR, as determined by flow cytometry. Daudi, U266, and RPMI 8226 cell lines were obtained from the ATCC (Rockville, MD). All cell lines were grown in RPMI 1640 media supplemented with 10% FCS and 2 mmol/LL-glutamine.
Quantification of plasma cell number
Number of plasma cells in BMMC of patients with MM was calculated by counting the number of viable cells per well and by cytofluorometric identification of plasma cells using FACS analysis (CD45low,(+)/CD38bright,++, CD19−/CD138++). On day 5 of culture, plasma cell numbers in treated and control (medium alone) cultures were counted, and results were shown as percentage of control culture according to the following calculation: [plasma cell number in treated (IPP or pamidronate) culture]/[plasma cell number in control culture (medium alone)] × 100.
Cytotoxicity assay
A standard 4-hour chromium Cr 51 release assay was performed. In brief, target cell lines (Daudi, RPMI 8226, U266, and allogeneic PHA blasts) were labeled with 100 μCi 51Cr, and 5000 cells/well were incubated in round-bottom triplicate wells with the pamidronate-reactive Vγ9Vδ2 T-cell line at the indicated effector/target (E/T) ratios. After 4 hours, the amount of51Cr released into supernatant was measured as cpm and expressed as specific lysis according to the following formula: % specific lysis = % specific 51Cr release = (effector induced cpm − spontaneous cpm/maximum cpm − spontaneous cpm) × 100. Spontaneous cpm represents the amount of51Cr released by target cells incubated without effector cells, and maximum cpm was obtained by lysis with 1 mol/L HCl.
Statistical analysis
Results are expressed as mean ± SD. The Student t test was used to determine statistical significance of detected differences.P < .05 was considered significant.
Results
Comparison of different bisphosphonates to stimulate γδ T cells
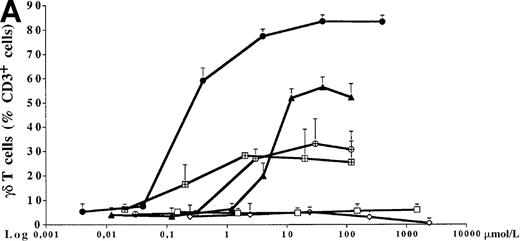
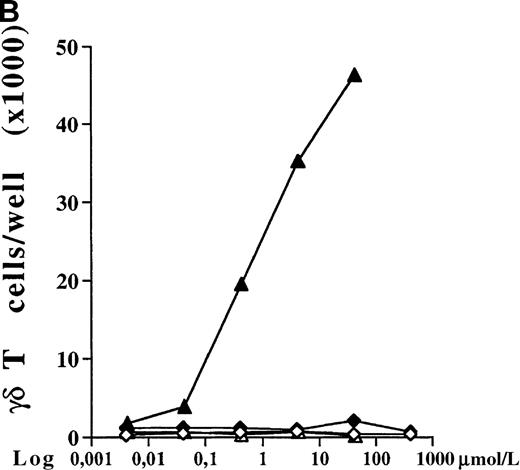
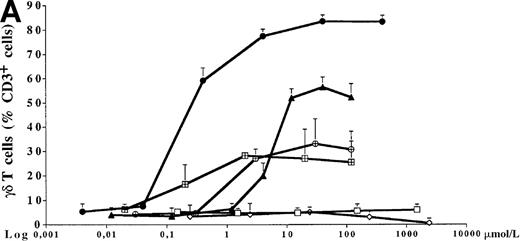
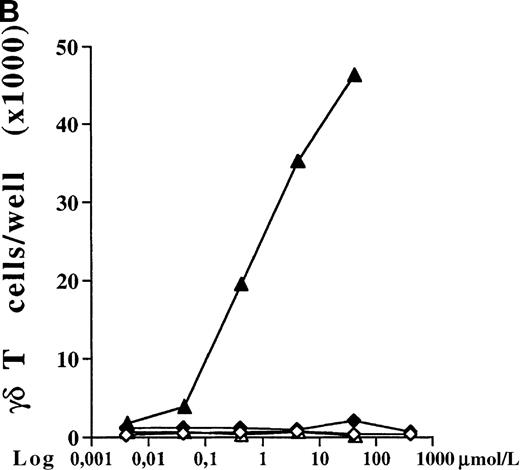
PBMC cultures of healthy donors were incubated for 7 days with increasing concentrations of 5 different bisphosphonates in the presence of low doses of exogenous IL-2 (10 U/mL). As shown for 1 representative donor in Figure 1A, all tested aminobisphosphonates (alendronate, ibandronate, and pamidronate) induced significant dose-dependent expansion of γδ T cells. In contrast, the non-aminobisphosphonates (clodronate and etidronate) were inactive even at very high concentrations (greater than 1000 μmol/L). All aminobisphosphonates exhibited a lower stimulating activity than IPP, which is known as a potent natural γδ T-cell ligand (half-maximal activities: IPP, 0.2 μmol/L; alendronate, 0.9 μmol/L; ibandronate, 1.0 μmol/L; pamidronate, 4 μmol/L). Flow cytometric analysis revealed no significant expansion of other PBMC subpopulations (monocytes, B cells, NK cells, and αβ T cells) after a 7-day culture in the presence of the bisphosphonates under study (data not shown). The relative increase of CD3+ γδ T lymphocytes in response to IPP or aminobisphosphonates (as shown in Figure 1A) also reflects an increase in absolute cell numbers, as determined by counting the number of γδ T cells per well on day 7. Figure 1B shows a dose-dependent absolute increase of γδ T cells in the presence of pamidronate in 1 representative experiment, whereas the nonaminobisphosphonate clodronate failed to expand γδ T cells. This selective γδ T-cell outgrowth required the presence of low doses of IL-2 (10 U/mL).
Comparison of different bisphosphonates to stimulate γδ T cells.
(A) Capacity of different bisphosphonates to stimulate γδ T cells. Primary PBMC of healthy donors were incubated with increasing concentrations of different bisphosphonates and IPP as a positive control and with low doses of IL-2 (10 U/mL). Percentage of γδ T cells was determined by FACS analysis using anti-CD3 and anti-γδ TCR mAb after 7 days of culture. Results are shown as mean ± SD of triplicate cultures in one representative donor. Similar dose–response curves were observed in 5 healthy donors. Maximum γδ T-cell increase in aminobisphosphonate-stimulated cultures of different donors ranged from 20% to 70%. PBMC cultures with bisphosphonates or IPP alone (without exogenous IL-2) or cultures with IL-2 alone exhibited no significant increase of the γδ T-cell proportion (always less than 10%). • = IPP; ▴ = pamidronate; □ = etidronate; ◊ = clodranate; ⊞ = alendronate; and ⊕ = ibandronate. (B) Proliferation of γδ T cells stimulated by aminobisphosphonates. Primary PBMC were incubated with increasing concentrations of the non-aminobisphosphonate clodronate or the aminobisphosphonate pamidronate in the presence or absence of IL-2 (10 U/mL). Absolute numbers of γδ T cells were calculated on day 7 by counting the absolute number of viable cells per well and measuring the percentage of γδ T cells by FACS analysis. Control cultures revealed the following absolute γδ T-cell numbers: medium alone = 0.44 × 103; medium + IL-2 = 0.90 × 103; IPP (4 μmol/L) alone = 0.33 × 103; IPP (4 μmol/L) + IL-2 = 45 × 103. Results are shown as mean values of triplicate cultures in 1 donor and are representative of similar experiments with 3 normal donors (range of γδ T-cell expansion in pamidronate/IL-2 cultures between 50- and 100-fold compared with medium or clodronate/IL-2 cultures). ◊ = clodranate; ⧫ = clodranate + IL-2; ▵ = pamidronate; and ▴ = pamidronate + IL-2.
Comparison of different bisphosphonates to stimulate γδ T cells.
(A) Capacity of different bisphosphonates to stimulate γδ T cells. Primary PBMC of healthy donors were incubated with increasing concentrations of different bisphosphonates and IPP as a positive control and with low doses of IL-2 (10 U/mL). Percentage of γδ T cells was determined by FACS analysis using anti-CD3 and anti-γδ TCR mAb after 7 days of culture. Results are shown as mean ± SD of triplicate cultures in one representative donor. Similar dose–response curves were observed in 5 healthy donors. Maximum γδ T-cell increase in aminobisphosphonate-stimulated cultures of different donors ranged from 20% to 70%. PBMC cultures with bisphosphonates or IPP alone (without exogenous IL-2) or cultures with IL-2 alone exhibited no significant increase of the γδ T-cell proportion (always less than 10%). • = IPP; ▴ = pamidronate; □ = etidronate; ◊ = clodranate; ⊞ = alendronate; and ⊕ = ibandronate. (B) Proliferation of γδ T cells stimulated by aminobisphosphonates. Primary PBMC were incubated with increasing concentrations of the non-aminobisphosphonate clodronate or the aminobisphosphonate pamidronate in the presence or absence of IL-2 (10 U/mL). Absolute numbers of γδ T cells were calculated on day 7 by counting the absolute number of viable cells per well and measuring the percentage of γδ T cells by FACS analysis. Control cultures revealed the following absolute γδ T-cell numbers: medium alone = 0.44 × 103; medium + IL-2 = 0.90 × 103; IPP (4 μmol/L) alone = 0.33 × 103; IPP (4 μmol/L) + IL-2 = 45 × 103. Results are shown as mean values of triplicate cultures in 1 donor and are representative of similar experiments with 3 normal donors (range of γδ T-cell expansion in pamidronate/IL-2 cultures between 50- and 100-fold compared with medium or clodronate/IL-2 cultures). ◊ = clodranate; ⧫ = clodranate + IL-2; ▵ = pamidronate; and ▴ = pamidronate + IL-2.
Expansion of Vγ9Vδ2 T cells by aminobisphosphonates in primary PBMC cultures
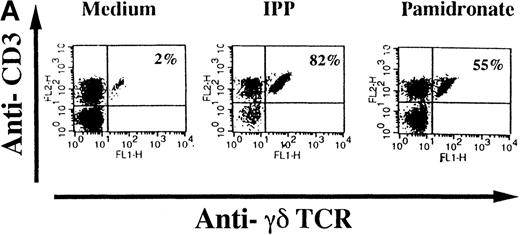
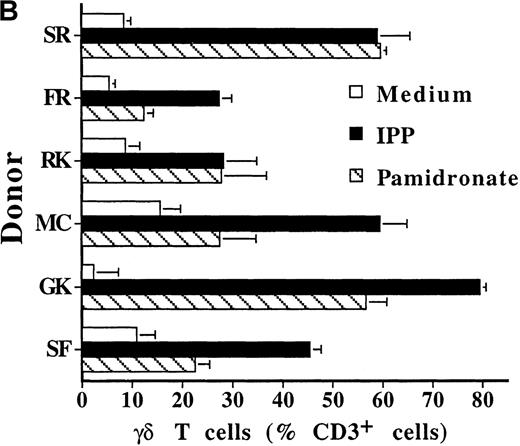
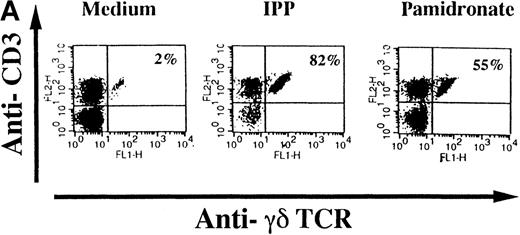
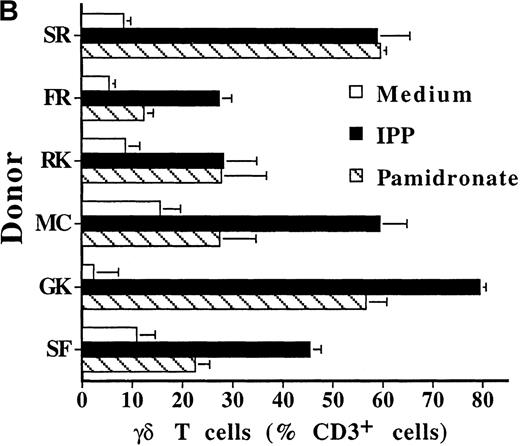
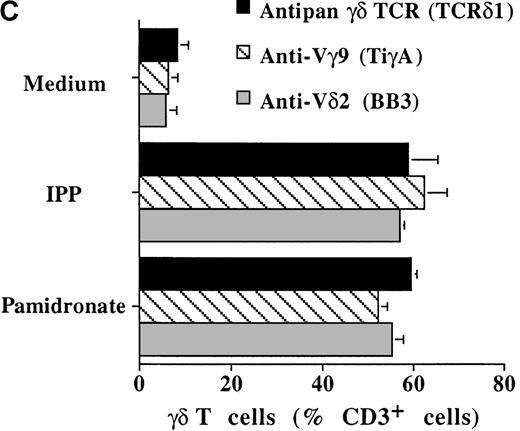
To investigate interindividual differences in the γδ T-cell–stimulating capacity of aminobisphosphonates, primary cultures of freshly isolated PBMC from 6 different healthy donors were analyzed for γδ T-cell stimulation by pamidronate. Determination of the percentage of γδ T cells after 7 days demonstrated that pamidronate induced a significant expansion of γδ T cells in all donors tested, though some interindividual differences could be observed (Figure2A,B). Previous studies have demonstrated that phosphorylated nonpeptidic ligands for γδ T cells (eg, IPP) preferentially induce an expansion of the Vγ9Vδ2 subpopulation.19 To determine γδ T-cell subsets that are stimulated by aminobisphosphonates, primary γδ T cells from healthy persons were incubated with pamidronate, and V gene expression was determined by 2-color FACS analysis after 7 days of culture. Results show that γδ T cells, which were expanded in the presence of pamidronate, exclusively expressed the Vγ9 and the Vδ2 genes (Figure 2B); no significant proliferation of T cells expressing other variable genes was detected.
Selective outgrowth of Vγ9Vδ2 T cells on stimulation with IPP or pamidronate.
Representative 2-color FACS analysis of PBMC after 7-day culture in the presence of medium, IPP (4 μmol/L), or pamidronate (4 μmol/L) using FITC anti-γδ TCR and PE anti-CD3 mAb (A). Expansion of γδ T cells in 7-day primary PBMC cultures of 6 different healthy donors by IPP (40 μmol/L) or pamidronate (40 μmol/L), as determined by 2-color FACS analysis. Results are expressed as mean ± SD of triplicates of 1 representative experiment (out of 3) for each donor (B). Analysis of TCR V gene expression of γδ T cells with specific mAb for Vγ9 and Vδ2 variable genes of the γδ TCR. Bars are mean values ± SD of triplicates of donor S.R. In the 5 other donors tested, the range of Vγ9Vδ2 TCR expression varied from 85% to 95% of all γδ T cells (C).
Selective outgrowth of Vγ9Vδ2 T cells on stimulation with IPP or pamidronate.
Representative 2-color FACS analysis of PBMC after 7-day culture in the presence of medium, IPP (4 μmol/L), or pamidronate (4 μmol/L) using FITC anti-γδ TCR and PE anti-CD3 mAb (A). Expansion of γδ T cells in 7-day primary PBMC cultures of 6 different healthy donors by IPP (40 μmol/L) or pamidronate (40 μmol/L), as determined by 2-color FACS analysis. Results are expressed as mean ± SD of triplicates of 1 representative experiment (out of 3) for each donor (B). Analysis of TCR V gene expression of γδ T cells with specific mAb for Vγ9 and Vδ2 variable genes of the γδ TCR. Bars are mean values ± SD of triplicates of donor S.R. In the 5 other donors tested, the range of Vγ9Vδ2 TCR expression varied from 85% to 95% of all γδ T cells (C).
Proliferative response of naive γδ T cells to pamidronate
In additional experiments, we assessed the proliferative response of purified γδ T cells to IPP and pamidronate by [3H] thymidine incorporation. For this purpose, αβ T-cell– and NK-cell–depleted PBMC of healthy donors were incubated with IPP or pamidronate for 96 hours. After 48 hours, IL-2 (10 U/mL) was added, and cells were exposed to [3H] thymidine during the last 12 hours of the culture period. In line with the results obtained with unpurified PBMC cultures, IPP and pamidronate induced a significant proliferation of purified γδ T cells in the presence of IL-2 (Figure 3). In contrast, purified γδ T cells did not proliferate in response to nonaminobisphosphonates or in the absence of exogenous IL-2 (data not shown).
Proliferative response of purified γδ T cells on stimulation with pamidronate.
Purified PBMC (4 × 104/well) were cultured in the presence of medium (IL-2 alone), IPP (4 μmol/L), pamidronate (4 μmol/L), or PHA (1 μg/mL). Low doses of IL-2 (10 U/mL) were added after 48 hours of culture. After 84 hours, cells were pulsed with [3H] thymidine (1 μCi) for 12 hours. Results are shown as cpm (mean ± SD of triplicate cultures).
Proliferative response of purified γδ T cells on stimulation with pamidronate.
Purified PBMC (4 × 104/well) were cultured in the presence of medium (IL-2 alone), IPP (4 μmol/L), pamidronate (4 μmol/L), or PHA (1 μg/mL). Low doses of IL-2 (10 U/mL) were added after 48 hours of culture. After 84 hours, cells were pulsed with [3H] thymidine (1 μCi) for 12 hours. Results are shown as cpm (mean ± SD of triplicate cultures).
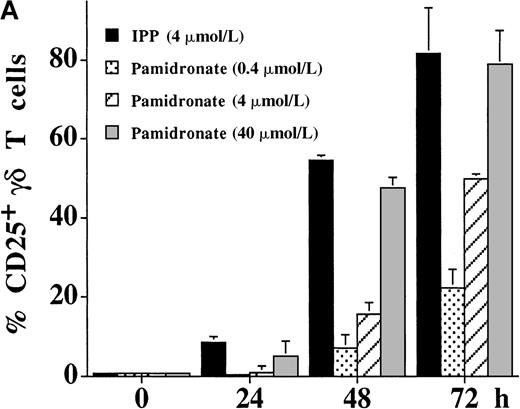
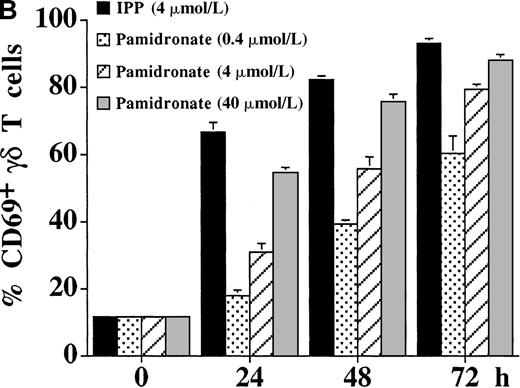
Kinetics of IL-2–independent γδ T-cell stimulation by aminobisphosphonates
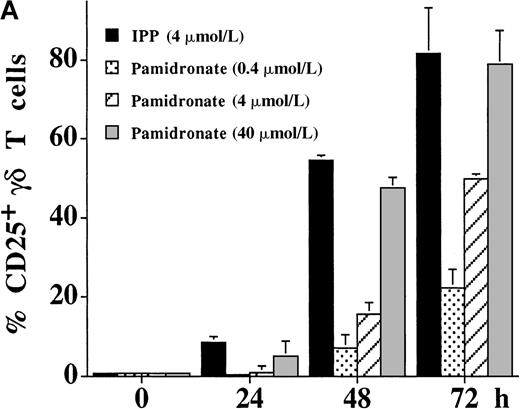
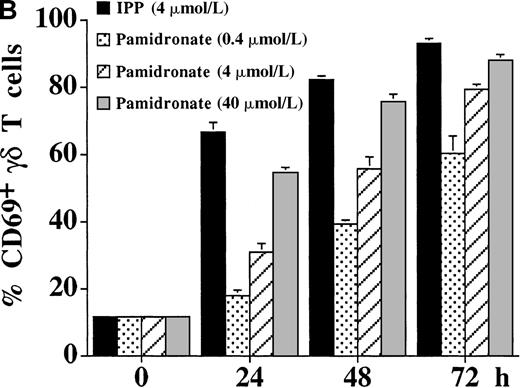
The activation of γδ T cells by aminobisphosphonates was followed by the determination of CD25 (α-chain of IL-2R) and CD69 expression on γδ T cells during a 72-hour culture period of PBMC cultures. As shown in Figure 4, both IPP and pamidronate induced CD25 (Figure 4A) and CD69 (Figure 4B) expression on a large fraction of γδ T cells in the absence of exogenous IL-2. Induction of CD25 and CD69 on γδ T cells by pamidronate was dose dependent, with significant up-regulation at concentrations as low as 0.4 μmol/L. Similar to αβ T cells, the induction of CD69 expression on γδ T cells was more rapid starting at 24 hours, whereas CD25 expression was first seen after 48 hours of the culture period. These results confirm that γδ T cells can recognize aminobisphosphonates such as pamidronate in the absence of exogenous cytokines such as IL-2. In contrast, proliferative responses of γδ T cells (as shown in Figure 1A,B and Figure 3) was dependent on exogenous IL-2, indicating that the proliferation of γδ T cells requires additional signals.
IL-2–independent activation of γδ T cells induced by IPP or pamidronate.
Expression of the activation markers CD25 (A) and CD69 (B) was measured on γδ T cells after stimulation of primary PBMC with IPP (4 μmol/L) or 3 different pamidronate concentrations (0.4 μmol/L, 4 μmol/L, 40 μmol/L) without exogenous IL-2. Percentage of CD25+ or CD69+ γδ T cells was determined by 2-color FACS analysis using anti-CD25 or anti-CD69 mAb and anti-γδ TCR mAb before (0 hour) and after (24, 48, and 72 hours) culture. In control cultures (medium alone) no significant up-regulation of CD 25 or CD69 was detected during the culture period (data not shown). Results represent mean values ± SD of triplicate cultures of 1 representative PBMC donor. Similar activation profiles were observed in 6 healthy donors.
IL-2–independent activation of γδ T cells induced by IPP or pamidronate.
Expression of the activation markers CD25 (A) and CD69 (B) was measured on γδ T cells after stimulation of primary PBMC with IPP (4 μmol/L) or 3 different pamidronate concentrations (0.4 μmol/L, 4 μmol/L, 40 μmol/L) without exogenous IL-2. Percentage of CD25+ or CD69+ γδ T cells was determined by 2-color FACS analysis using anti-CD25 or anti-CD69 mAb and anti-γδ TCR mAb before (0 hour) and after (24, 48, and 72 hours) culture. In control cultures (medium alone) no significant up-regulation of CD 25 or CD69 was detected during the culture period (data not shown). Results represent mean values ± SD of triplicate cultures of 1 representative PBMC donor. Similar activation profiles were observed in 6 healthy donors.
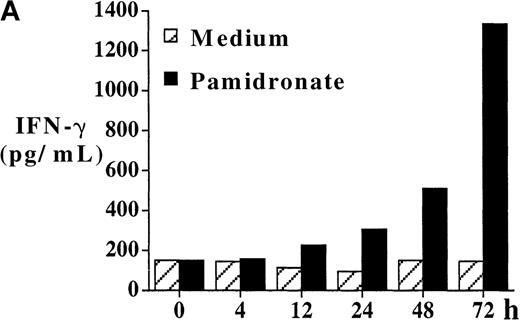
Cytokine production by aminobisphosphonate-activated γδ T cells
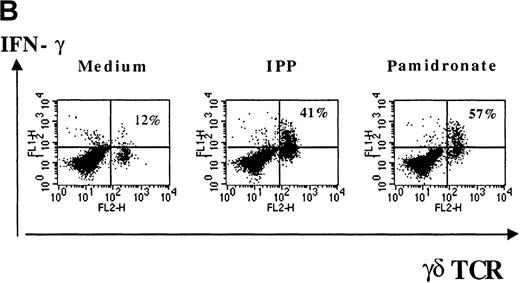
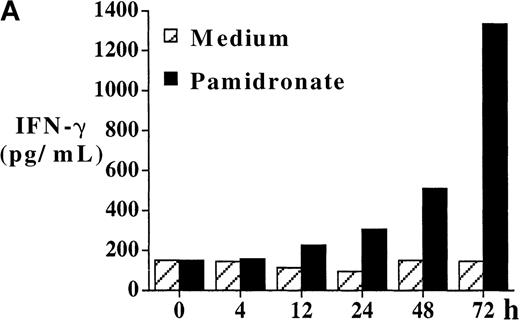
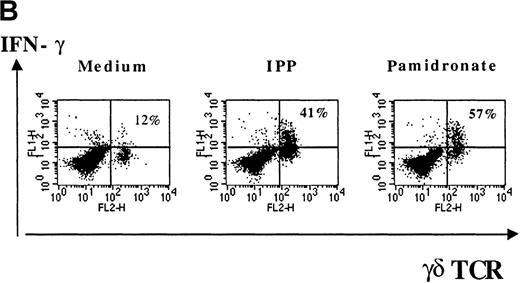
Several studies have shown that γδ T-cell stimulation induces the release of a variety of cytokines (IFN-γ, TNF-α, IL-2, and GM-CSF), particularly Th1-type cytokines.10 To investigate the functional consequences of γδ T-cell stimulation by aminobisphosphonates, cytokine concentrations in supernatants of pamidronate-treated PBMC were measured. Results show a significant increase of IFN-γ concentrations detectable after 24 to 48 hours of culture (Figure 5A). A similar secretion pattern for GM-CSF and a slight increase of TNF-α (not significant) concentrations was observed in pamidronate-treated culture supernatants, whereas cytokine concentrations in control cultures (medium alone) remained at a low level (data not shown). In addition, IL-4 concentrations did not change during the culture period (data not shown). Because cytokine production by other mononuclear cells in pamidronate-treated PBMC cultures could not be excluded, single-cell analysis of cytokine production was performed by intracellular staining of IFN-γ. As shown for a representative donor, few (12%) γδ T cells cultured with medium alone expressed significant intracellular levels of IFN-γ, whereas 41% and 57% of the γδ T cells, respectively, were positive for IFN-γ on stimulation with pamidronate or IPP (Figure 5B).
IFN-γ production of activated γδ T cells.
PBMC were incubated with pamidronate (40 μmol/L) or medium alone. For a determination of the kinetics of IFN-γ secretion, supernatants were collected at indicated time points, and cytokine concentration was measured by ELISA. Each bar represents the mean values in triplicate for 1 representative donor (A). Intracellular IFN-γ expression of γδ T cells in response to IPP or pamidronate was measured by single-cell analysis after PBMC culture in medium alone or in the presence of IPP (40 μmol/L) or pamidronate (40 μmol/L) for 72 hours. After surface staining with a γδ TCR mAb, cells were fixed, permeabilized, and intracellularly stained with mAb against IFN-γ. Percentages of IFN-γ+ γδ T cells are given in the upper right panels (B). Controls using an isotype-matched control mAb in the presence of medium alone, IPP, or pamidronate always revealed less than 10% positive γδ T cells (data not shown). Results shown are representative for 3 independent experiments with different healthy donors.
IFN-γ production of activated γδ T cells.
PBMC were incubated with pamidronate (40 μmol/L) or medium alone. For a determination of the kinetics of IFN-γ secretion, supernatants were collected at indicated time points, and cytokine concentration was measured by ELISA. Each bar represents the mean values in triplicate for 1 representative donor (A). Intracellular IFN-γ expression of γδ T cells in response to IPP or pamidronate was measured by single-cell analysis after PBMC culture in medium alone or in the presence of IPP (40 μmol/L) or pamidronate (40 μmol/L) for 72 hours. After surface staining with a γδ TCR mAb, cells were fixed, permeabilized, and intracellularly stained with mAb against IFN-γ. Percentages of IFN-γ+ γδ T cells are given in the upper right panels (B). Controls using an isotype-matched control mAb in the presence of medium alone, IPP, or pamidronate always revealed less than 10% positive γδ T cells (data not shown). Results shown are representative for 3 independent experiments with different healthy donors.
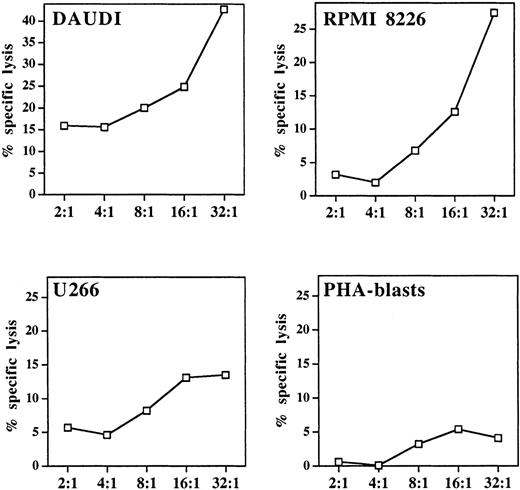
Cytotoxicity of pamidronate-activated γδ T cells
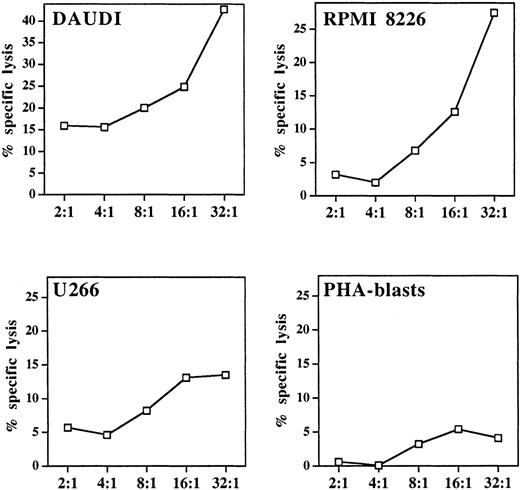
A well-defined functional characteristic of stimulated γδ T cells is their nonmajor histocompatibility complex (MHC)-restricted cytolytic activity against various tumor targets, particularly of hematopoietic origin.10 For determination of the lytic potential of aminobisphosphonate-activated γδ T cells, pamidronate-induced γδ T-cell lines were generated from PBMC (as described in “Materials and methods”). Cytotoxicity against 2 previously known γδ T-cell targets (Burkitt lymphoma cell line Daudi and myeloma cell line RPMI 8226) and another myeloma cell line (U266) was investigated in a 4-hour standard 51Cr release assay, in which allogeneic PHA-induced peripheral blood leucocyte blasts served as a control. Results showed that the pamidronate-stimulated γδ T-cell line exhibited strong lytic activity against Daudi and RPMI 8226 targets and intermediate cytotoxicity against U 266 targets. However, no significant killing of allogeneic PHA blasts was observed (Figure6).
Cytolytic response of pamidronate-activated γδ T cells against lymphoma or myeloma targets.
In a standard 4-hour chromium release assay, a pamidronate-induced γδ T-cell line was incubated with the Burkitt lymphoma cell line Daudi, 2 myeloma cell lines (RPMI 8226, U266), or allogeneic PHA-induced peripheral blood leukocyte blasts at indicated E:T ratios in the presence of low-dose IL-2 (10 U/mL). Cytotoxicity is expressed as percentage specific lysis of triplicate cultures.
Cytolytic response of pamidronate-activated γδ T cells against lymphoma or myeloma targets.
In a standard 4-hour chromium release assay, a pamidronate-induced γδ T-cell line was incubated with the Burkitt lymphoma cell line Daudi, 2 myeloma cell lines (RPMI 8226, U266), or allogeneic PHA-induced peripheral blood leukocyte blasts at indicated E:T ratios in the presence of low-dose IL-2 (10 U/mL). Cytotoxicity is expressed as percentage specific lysis of triplicate cultures.
Activation by aminobisphosphonates of bone marrow γδ T cells from patients with multiple myeloma
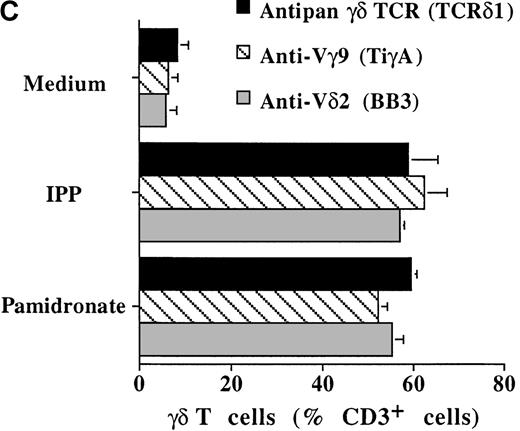
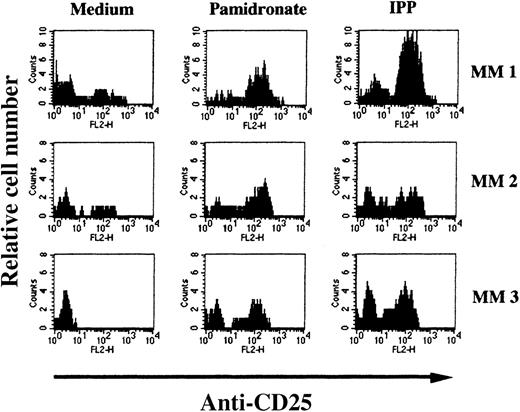
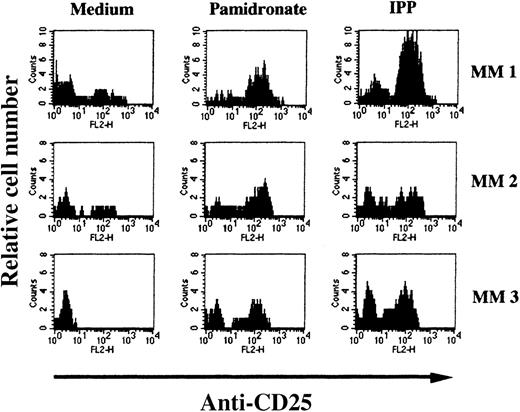
To determine the stimulating capacity of aminobisphosphonates on BM-γδ T cells from patients with MM, BMMC from 24 patients with MM were cultured with pamidronate, IPP, or medium alone. After 72 hours, the percentage of CD25 expressing γδ T cells was evaluated by FACS analysis. In 14 of 24 (58%) patients, a significant increase of CD25 expression on BM-γδ T cells was observed in both pamidronate- and IPP-treated BMMC cultures. Results of 3 representative patients are shown in Figure 7. Similar to the PBMC of healthy donors, CD25 expression on other mononuclear cell populations (eg, αβ T cells and NK cells) remained stable during the culture period. Therefore, BM-γδ T-cell stimulation could be induced by pamidronate in a significant proportion of patients with MM.
Induction of CD25 (IL-2 receptor chain) expression on BM γδ T cells of patients with MM.
BMMC of patients with MM were incubated with IPP (4 μmol/L) or pamidronate (4 μmol/L), and the relative cell number of CD25+ γδ T cells was determined by 2-color FACS analysis using anti-CD25 and anti-γδ TCR mAb after 72 hours of culture. Solid areas represent the fluorescence distribution of CD25 expression after gating on γδ TCR+ cells in 3 representative patients with MM.
Induction of CD25 (IL-2 receptor chain) expression on BM γδ T cells of patients with MM.
BMMC of patients with MM were incubated with IPP (4 μmol/L) or pamidronate (4 μmol/L), and the relative cell number of CD25+ γδ T cells was determined by 2-color FACS analysis using anti-CD25 and anti-γδ TCR mAb after 72 hours of culture. Solid areas represent the fluorescence distribution of CD25 expression after gating on γδ TCR+ cells in 3 representative patients with MM.
Cytoreductive effects of IPP and pamidronate in multiple myeloma
Our previous unpublished experiments have shown that in vitro culture of the bone marrow biopsy specimens from patients with MM, taken 24 hours after pamidronate infusion (90 mg intravenously) revealed a significant outgrowth of γδ T cells in the presence of low-dose IL-2 (10 U/mL). In addition, the quantification of viable plasma cells before and after 1 week of culture showed a significant decrease (30%-40%) of plasma cell number. This cytoreductive effect could not be observed in bone marrow biopsy specimens cultured without IL-2 or in specimens from patients with MM who have not received pamidronate before BM biopsy (data not shown). To confirm these preliminary observations, the effect of IPP and pamidronate on autologous plasma cells was determined by counting the total number of viable plasma cells on day 5 in BMMC cultures of 24 patients with MM. As illustrated in Table 1, IPP and pamidronate induced a significant reduction of plasma cells in BMMC cultures (IPP, P = .0345; pamidronate, P = .0002) compared with control cultures ( = medium with 10 U/mL IL-2). Although the range of plasma cell decrease was relatively wide, there seemed to be a correlation with γδ T-cell activation. Patients with MM who had significant up-regulation of CD25 expression on γδ T cells during BMMC culture had more prominent plasma cell decreases (% plasma cells after 5 days compared to control cultures: IPP, 87.0% ± 28.4%; pamidronate, 65.9% ± 38.4%; pamidronate in patients with CD25+ γδ T cells, 54.8% ± 28.8%). Plasma cell decrease was independent of the initial BM plasma cell number because the effect was observed in patients with high and low levels of BM plasma cell infiltration.
Antiplasma cell activity by pamidronate is mediated by γδ T-cell–dependent and γδ T-cell–independent mechanisms
To investigate the role of γδ T cells in pamidronate-mediated antiplasma cell activity, BMMC cultures from 3 patients with MM were performed under standard conditions and after depletion of γδ T cells. These BMMC and BMMC (γδ−) cultures were challenged with increasing concentrations of pamidronate, and the percentage of viable plasma cells was compared to that of control cultures (medium with 10 U/mL IL-2) after 5 days of exposure (Figure8). Pamidronate induced a dose-dependent reduction of plasma cells in all BMMC cultures without γδ T-cell depletion. In contrast, γδ T-cell depletion abrogated the antiplasma cell effect in 2 patients (patients 1 and 2) but had no effect on the BMMC cultures of the third patient (patient 3). Interestingly, the activation of BM-γδ T cells (increase of CD25 expression) was demonstrated only in patients 1 and 2, whereas no γδ T-cell activation was observed in patient 3 (data not shown). These data confirm the important role of γδ T cells in pamidronate-mediated plasma cell cytotoxicity, but they indicate that in certain patients additional mechanisms may contribute to this effect.
Effect of γδ T-cell depletion on pamidronate-induced autologous plasma cell decrease in BMMC cultures.
BMMC of 3 patients with MM (patients 1-3) were cultured under standard conditions (medium with 10 U/mL IL-2) or after depletion of γδ T cells by MACS (patients 1-3 γδ−) in the presence of different pamidronate concentrations (0.4 μmol/L, 4 μmol/L, 40 μmol/L). After 5 days, the number of viable plasma cells was determined as described in “Materials and methods.” Results are expressed as percentage of plasma cells according to the following calculation: [plasma cell number in pamidronate-treated cultures]/[plasma cell number in control cultures (medium alone)] × 100. Each bar represents the mean value ± SD of triplicate cultures.
Effect of γδ T-cell depletion on pamidronate-induced autologous plasma cell decrease in BMMC cultures.
BMMC of 3 patients with MM (patients 1-3) were cultured under standard conditions (medium with 10 U/mL IL-2) or after depletion of γδ T cells by MACS (patients 1-3 γδ−) in the presence of different pamidronate concentrations (0.4 μmol/L, 4 μmol/L, 40 μmol/L). After 5 days, the number of viable plasma cells was determined as described in “Materials and methods.” Results are expressed as percentage of plasma cells according to the following calculation: [plasma cell number in pamidronate-treated cultures]/[plasma cell number in control cultures (medium alone)] × 100. Each bar represents the mean value ± SD of triplicate cultures.
Discussion
Our results demonstrate that aminobisphosphonates (alendronate, ibandronate, and pamidronate) induce a dose-dependent activation (CD25 and CD69 expression) and expansion of γδ T cells in primary PBMC cultures of healthy donors at clinically relevant concentrations, whereas nonaminobisphosphonates (clodronate and etidronate) were inactive. Aminobisphosphonates revealed a lower γδ T-cell–stimulatory capacity than an already described potent natural antigen (IPP). However, the concentrations necessary for T-cell activation are relevant for patients treated with aminobisphosphonates because the range of half-maximal activity in vitro (0.9-4 μmol/L) reflects peak plasma concentrations in patients after aminobisphosphonate infusion.20 Given that these compounds are preferentially bound to skeletal sites of bone resorption, bisphosphonate concentrations in bone marrow have been shown to be much higher.21 Therefore, γδ T cells in the bone marrow compartment represent an interesting target of aminobisphosphonate action in vivo.
Induction of CD25 and CD69 expression on γδ T cells occurred in the absence of exogenous cytokines, whereas the proliferative response of γδ T cells was dependent on low doses of exogenous IL-2 (10 U/mL). Therefore, additional costimulatory signals such as IL-2 contribute to cellular expansion of aminobisphosphonate-reactive γδ T cells. In previous studies, an IL-2 requirement for the γδ T cell proliferative response to other phosphorylated ligands, such as IPP, has also been demonstrated.22
An important question remains whether phosphorylated γδ T-cell antigens bind directly to the γδ TCR or act by indirect mechanisms. Analysis of V gene expression by flow cytometry confirmed the preferential expansion of the Vγ9Vδ2 subset by aminobisphosphonates, which is also expanded by all other known phosphorylated γδ T-cell antigens.17 It should be noted that all known Vγ9Vδ2 T-cell ligands are very small (200-600 d), like haptens. Recent findings support direct γδ TCR participation, as shown by TCR gene transfer experiments,12 and a crucial role for the TCRγ chain junctional region in IPP recognition of Vγ9Vδ2 T cells.23 Interestingly, in our experiments the non–nitrogen-containing bisphosphonates (clodronate and etidronate) did not exhibit γδ T-cell–stimulating effects, indicating an essential role of the aminoalkane group for γδ T-cell stimulation by bisphosphonates. Earlier reports demonstrate that aminobisphosphonates appear to have different mechanisms of action in osteoclasts than non-aminobisphosphonates.3Nitrogen-containing bisphosphonates have been shown to inhibit bone resorption and to cause apoptosis in osteoclasts by inhibiting a rate-limiting step in the cholesterol biosynthesis pathway (mevalonate pathway),24,25 whereas clodronate acts by the accumulation of a nonhydrolyzable toxic analogue of adenosine triphosphate.26 The inhibition of enzymes in the mevalonate pathway might result in an accumulation of upstream prenyl pyrophosphate metabolites such as IPP or geranylpyrophosphat, which can both stimulate Vγ9Vδ2 T cells.16 Therefore, aminobisphosphonates might activate γδ T cells simply by an increase of stimulating prenyl pyrophosphate concentrations upstream of the inhibited target enzyme of the mevalonate pathway. However, further studies must characterize the exact mechanisms of γδ T-cell stimulation by aminobisphosphonates and the involvement of the γδ TCR in recognition of these and other phosphorylated antigens.
The functional significance of γδ T-cell stimulation by aminobisphosphonates was demonstrated by the increased secretion of cytokines (ie, IFN-γ) into supernatants of PBMC cultures. Furthermore, we provided evidence that IFN-γ is directly derived from activated γδ T cells. Increased cytokine plasma levels (TNF-α, IL-6) have been observed in patients after the first aminobisphosphonate treatment, and they have been associated with the clinically observed acute-phase reaction that occurs in 20% to 50% of patients almost exclusively after the first infusion.27 The cell population responsible for this cytokine production has not been defined thus far, but the lack of changes in IL-1 plasma levels in vivo argues against the monocyte/macrophage lineage as the cytokine source.28 We have previously shown an increase of peripheral blood γδ T cells in patients with acute-phase reactions after the first pamidronate treatment.18 Consistent with this observation, the activation of T lymphocytes (increase of CD69 expression) after pamidronate, but not clodronate, treatment in vivo was reported, though the TCR phenotype of these T lymphocytes was not further investigated in this study.29 Therefore, an acute-phase reaction as a side effect of the first aminobisphosphonate treatment might be mediated by the activation and cytokine production of γδ T cells. The failure of subsequent pamidronate administrations to induce either acute-phase reactions or increases of peripheral blood γδ T cells can be explained by a lack of sufficient costimulation (eg, IL-2). Alternatively, bisphosphonates such as γδ T-cell ligands might function as agonists during the first contact and act as antagonists after repetitive stimulation. This mechanism has recently been described for other γδ T-cell antigens.30
It has been thought that γδ T cells have a surveillance function against tumors. Although the molecular basis for the distinction between normal and malignant cells by γδ T lymphocytes is unknown, they can exhibit a human leukocyte antigen-unrestricted lytic activity against different tumor cells, especially of hematopoietic origin.10,11 Cytotoxic γδ T-cell clones with specific reactivity against autologous leukemic blasts have been isolated from patients with acute lymphoblastic leukemia,31-33 and γδ T cells have been found with increased frequency in disease-free survivors of acute leukemia after allogeneic bone marrow transplantation.34 Our data confirm the lytic potential of activated γδ T cells because pamidronate-induced γδ T-cell lines from healthy donors exhibited cytotoxic activity against 2 already characterized γδ T-cell targets, the lymphoma cell line Daudi and the myeloma-derived cell line RPMI 8226. In addition, pamidronate-activated γδ T cells killed the myeloma cell line U266, which has not yet been described as a target of cytotoxic γδ T cells.
Furthermore, this study showed that BM γδ T cells could be stimulated by pamidronate in 14 of 24 tested patients with MM, and their activation was associated with a significant decrease in the number of autologous BM plasma cells. The failure to stimulate BM γδ T cells in all patients with MM might reflect a defective T-cell immunity in some patients with MM, as occurs in other malignancies. Additionally, heterogeneity in disease stage and prior chemotherapy treatment might interfere with the γδ T-cell reactivity against pamidronate. However, previous aminobisphosphonate treatment was not correlated with the failure to stimulate BM γδ T cells because pamidronate-naive patients and patients who underwent repetitive pamidronate infusions were among those patients with MM who responded.
Several other groups tried to induce effective antiplasma cell activity by the stimulation of autologous T cells.7,8,35 They used broad and unspecific T-cell stimulation strategies such as anti-CD3 mAb, IL-2 or both. In contrast, our study indicated that the selective activation of a small T-cell subpopulation (Vγ9Vδ2 T cells) could be achieved by aminobisphosphonates, which could be further enhanced by costimulatory signaling by exogenous IL-2. The plasma cell decrease observed in our BM cultures treated with pamidronate is remarkable because BM γδ T cells as possible effector cells for pamidronate-induced cytoreductive effects contribute to only 0.1% to 5% of all bone marrow mononuclear cells. γδ T cells may exert their antiplasma cell activity through direct cell contact-dependent lysis or by secreting inhibitory cytokines such IFN-γ, which is produced in large amounts by activated γδ T cells.36,37IFN-γ was reported to inhibit IL-6–dependent proliferation38 and to activate Fas-mediated apoptosis of MM cells.39 γδ T-cell depletion experiments demonstrated that the cytoreductive effects of aminobisphosphonates seem to be mediated by γδ T-cell–dependent and –independent mechanisms. Possible additional mechanisms may involve the induction of apoptosis in myeloma cells by aminobisphosphonates, as shown in 2 recent studies.40,41 Similar to the molecular action of aminobisphosphonates in osteoclasts, the induction of apoptosis in myeloma cells might be mediated by the inhibition of enzymes of the mevalonate pathway.42 However, the described apoptotic effects were seen at aminobisphosphonate concentrations above peak plasma levels in patients (LD50 for pamidronate, 40-60 μmol/L),41 whereas γδ T-cell stimulation occurred at significantly lower concentrations (half-maximal activity for pamidronate, 4 μmol/L). Another mechanism could be the suppression of IL-6 release from osteoblast-like cells43 or BM stromal cells.44
In conclusion, our results demonstrate that aminobisphosphonates have the ability to stimulate human γδ T cells, which may induce antiplasma cell effects in patients with MM. Together with other potential cytoreductive effects of aminobisphosphonates, this T-cell activation may contribute to the recently described remissions or inhibition of disease progression by pamidronate infusion5in patients with MM and the survival advantage described in a subgroup of patients with MM.4 Our observation might be of importance for immune-based therapeutic strategies in MM and other malignancies. To improve the efficacy of γδ T-cell stimulation by aminobisphosphonates, a pilot phase I/II study has already been initiated to evaluate the concomitant administration of pamidronate and IL-2 in myeloma patients. Further in vitro investigations with more potent aminobisphosphonates and identification of high-affinity phosphorylated γδ T-cell ligands will help to refine this immunologic strategy and to develop compounds that combine maximal bone resorption inhibition with maximal γδ T-cell stimulation.
Acknowledgment
We would like to thank M. Knezevic for her expert technical assistance.
Supported by the Interdisziplinäres Zentrum für Klinische Forschung Wuerzburg (V.K., J.F., H.-P.T., M.W.) and by a fellowship from the Deutsche Forschungsgemeinschaft (E.B.).
Reprints:Martin Wilhelm, Medizinische Poliklinik Wuerzburg, Julius–Maximilians Universität Würzburg, Klinikstraße 6-8, 97070 Würzburg, Germany; e-mail:wilhelm.medpoli@mail.uni-wuerzburg.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 3. Proliferative response of purified γδ T cells on stimulation with pamidronate. / Purified PBMC (4 × 104/well) were cultured in the presence of medium (IL-2 alone), IPP (4 μmol/L), pamidronate (4 μmol/L), or PHA (1 μg/mL). Low doses of IL-2 (10 U/mL) were added after 48 hours of culture. After 84 hours, cells were pulsed with [3H] thymidine (1 μCi) for 12 hours. Results are shown as cpm (mean ± SD of triplicate cultures).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.384/5/m_bloo01307003x.jpeg?Expires=1768000654&Signature=T4uTGOnbX2xadC2xy4CrUtKoRdsOZkeHPoT8YtRSvz90oKV7u-S~d~LXSc40NtxJIFNP1SZ8AdmWe~TcifnT~AU3c9lEiEp8rloYkfch6C0t3Rtfv2~YdvyoX77gycRNSz1YO1W33OgaeWfr02LC-XxjwJ0KG7YM3HjnUdT~NdDl-04Ek-k2UiFRBFZIJ3uvkH00ZlcXFiSWTnXRqpekK917XrPgGte1ekt9JGg9RohK7f7gHzoacsVWtbBr5YVi~g9v2i94GcYB0Tofo~TnFS3PcYM6-2PsWa-G-LHQRFdnB9w6hCmOPgG43fQcDoxJr6LG0yaoO1XSGFo7Pwy5eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effect of γδ T-cell depletion on pamidronate-induced autologous plasma cell decrease in BMMC cultures. / BMMC of 3 patients with MM (patients 1-3) were cultured under standard conditions (medium with 10 U/mL IL-2) or after depletion of γδ T cells by MACS (patients 1-3 γδ−) in the presence of different pamidronate concentrations (0.4 μmol/L, 4 μmol/L, 40 μmol/L). After 5 days, the number of viable plasma cells was determined as described in “Materials and methods.” Results are expressed as percentage of plasma cells according to the following calculation: [plasma cell number in pamidronate-treated cultures]/[plasma cell number in control cultures (medium alone)] × 100. Each bar represents the mean value ± SD of triplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.384/5/m_bloo01307008x.jpeg?Expires=1768000654&Signature=YW92125zjrrlEBK1qrGL8HExWRj1QRdNxkL5OD6V0o29W3KU9ZTefuif8RMPtiZxwIg9Ma-VzdpcnjRHxYPHx4wsHll4r9JtYseXQWKteQLdokrYHSWyvBcdaF0oxKtFUX2mLG48UfP7R1uIfdcX75xHmQ7HMXXVodg9WqlQ3sdaJiDBPR40ltflNTvmaMCgtaSjXOHPW4IS8J-0RODgUTR851JiJY9XMZa365Y1YEggBIoUYLR3XF3LOsdzwp3D1FUPJWs7NZ0r-E6q35MWhFATqc-~P31iAMPystA6tEqpsid3o96o2qeyyQyWzrDE3O3-xn6vK5QBpw7IPk66HA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 3. Proliferative response of purified γδ T cells on stimulation with pamidronate. / Purified PBMC (4 × 104/well) were cultured in the presence of medium (IL-2 alone), IPP (4 μmol/L), pamidronate (4 μmol/L), or PHA (1 μg/mL). Low doses of IL-2 (10 U/mL) were added after 48 hours of culture. After 84 hours, cells were pulsed with [3H] thymidine (1 μCi) for 12 hours. Results are shown as cpm (mean ± SD of triplicate cultures).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.384/5/m_bloo01307003x.jpeg?Expires=1768325729&Signature=jb6fO9kJD0wxxdHmeQ0M~TNR4NNCDIYTLtjqs1ifp05LdSRPJLo6vpjTCWcTiPq-V8mSY0TidQt6hAh9Elh6SruPzhOjyXDs4MmSYH1qMJuEWzDReiZXVAndaKdQZyGhNEDDyQuPucTBhc6n17boq2rEtVOo8Z~YN93-qBB2r~9MfZnv4PN1A-iAt0eXF7NZrgCvQ9RpH2819hiXrsmYhLHroR9gfQEWCiXt8zVUpN2GhdGjtKGdplvYHIdcb0NKwTPwIBj8x3UeVLedMHGrO-qG4IKZvrd6wC5BWKxCRDvPDVeDrRpeCrN-esboBUpX8xnjNGJYV-9QHv2MYrMQdw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effect of γδ T-cell depletion on pamidronate-induced autologous plasma cell decrease in BMMC cultures. / BMMC of 3 patients with MM (patients 1-3) were cultured under standard conditions (medium with 10 U/mL IL-2) or after depletion of γδ T cells by MACS (patients 1-3 γδ−) in the presence of different pamidronate concentrations (0.4 μmol/L, 4 μmol/L, 40 μmol/L). After 5 days, the number of viable plasma cells was determined as described in “Materials and methods.” Results are expressed as percentage of plasma cells according to the following calculation: [plasma cell number in pamidronate-treated cultures]/[plasma cell number in control cultures (medium alone)] × 100. Each bar represents the mean value ± SD of triplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.384/5/m_bloo01307008x.jpeg?Expires=1768325729&Signature=n8xpYPuo-tZV-9XciuC6z1McRVwigTZRJ~4gwT9b76f2o6G4mkHqBvdeBcuv4f1wOeVhUf8QxgcOjWL6DzZI0c4tOMmw-e-S3ZVD8Atr32i027WFPr0G6XBIXmL-RqWhxa4XzcxWf3kz8OJKy4v75mqMXgxdXs-oxosUJU8~R~iSuAJ-BtoTOLjNuA5RrvgCdxRnFuAgRXTJ-atkrhQzaVaza9DfXjXmO8BlsiL4VVJZXSkG6RGrcwF9YsuCbhACYkuii6hO9W-MrE1wxLtwFri94gtpmTir4UY07s4tpu0OBqWAjeoaWBHWHjGRoJ9OgjrGSg2vaHLdfTLX778p8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)