Abstract
Liver-expressed chemokine (LEC) is an unusually large CC chemokine, which is also known as LMC, HCC-4, NCC-4, and CCL16. Previously, LEC was shown to induce leukocyte migration but the responsible signaling receptors were not characterized. We report chemotaxis and competitive binding studies that show LEC binds to and activates CCR1 and CCR8 transfected HEK-293 cells. LEC induced maximal migration of CCR1 and CCR8 transfected cells at 89.3 nmol/L and cell adhesion at 5.6 nmol/L. The molar concentration of LEC required to induce maximum cell migration is 20- to 200-fold greater than that required for RANTES or I309, respectively. All 3 chemokines induced maximal static adhesion at 5 to 7 nmol/L. A neutralizing polyclonal antibody to LEC was developed to demonstrate that the unusually high concentration of LEC required to induce chemotaxis was a property of LEC and not as a result of an irrelevant protein contamination. This study suggests that LEC may be a more effective inducer of cell adhesion than cell migration.
Chemokines are small, usually less than 10 kd, secreted chemoattractant cytokines.1,2 Chemokines can be divided into 4 subfamilies, based on a cysteine motif located close to the first 10 to 12 amino acids of the mature protein. The 4 subfamilies are C, CC, CXC, and CXXXC. The human chemokine LEC (liver-expressed chemokine), a CC chemokine, has a number of different names, including NCC-4, LMC, and HCC-4.3-5 LEC was originally found in an expressed sequence tag library and later the gene location was determined to be on human chromosome 17q in the CC chemokine cluster.3,6 Shoudai et al3 reported 2 sizes of LEC messenger RNA (mRNA), 1.8 and 0.8 kilobase (kb), both expressed by unstimulated liver. Hedrick et al5 detected a 0.5-kb message in most unstimulated lymphoid tissue, but did not detect the larger message until cells were stimulated with IL-10. These mRNA species encode a 120 amino acid protein corresponding to a 20 to 24 amino acid propeptide and a 100 amino acid mature protein. Thus, LEC is an unusually, large CC chemokine with a relatively restricted expression pattern that makes it difficult to predict its functional role.
Chemokines modulate cell function by interacting with 7 transmembrane G-protein coupled receptors (GPCRs). Currently, there are at least 9 characterized CC chemokine receptors, CCR1-9.1,7LEC has been shown to chemoattract both monocytes and lymphocytes but not neutrophils, which does not indicate what receptor is likely to be used by LEC.4,5 The maximal chemotactic response to LEC by either monocytes or lymphocytes was obtained at 89 nmol/L, a concentration that is 6 to 12 times greater than required for a typical CC chemokine.1 There is some controversy concerning the ability of LEC to induce calcium flux in leukocytes or myeloid cell lines. Youn et al4 did not observe LEC-induced calcium flux, whereas Hedrick et al5 did observe calcium flux in THP-1 cells. Although both groups used recombinant chemokines, the proteins differed at their amino termini. To address these concerns, we tested the ability of recombinant LEC to induce calcium flux in human monocytes. To further characterize LEC and identify a receptor (s), we performed chemotaxis, adhesion and competitive binding assays with individual CC receptor transfected HEK-293 cells and found that LEC uses 2 of the characterized CC receptors.
Materials and methods
Reagents and cells
Recombinant human LEC and anti-LEC were a kind gift from Pepro Tech Inc (Rocky Hill, NJ). All other chemokines and cytokines were obtained from the NIH cytokine repository. All reagents were purchased from Sigma (St Louis, MO), unless otherwise noted. Human peripheral blood monocytes were separated from normal blood donor samples obtained at the NIH Clinical Center, Transfusion Medicine Department, Bethesda, MD, by enrichment for mononuclear cells by using an iso-osmotic Percoll gradient as previously described.8 HEK-293 cells were cultured in DMEM (Biowhittaker, Walkersville, MD) containing 10% fetal bovine serum (Hyclone, Logan, UT), 2 mmol/L glutamine, and 100 U/mL penicillin and streptomycin (Quality Biologicals, Gaithersburg, MD). HEK-293 transfectants were maintained in tissue culture media containing 400 μg/mL Geneticin (Life Technologies, Gaithersburg, MD).
Calcium mobilization
Monocytes were loaded with Fura-2 in the following manner; 2 × 107 monocytes per milliliter in loading medium (DMEM, 10% fetal calf serum [FCS]) were incubated with 5 μmol/L Fura-2 AM (Molecular Probes, Eugene, OR) for 30 minutes at room temperature in the dark. The dye-loaded cells were washed 3 times and resuspended in saline buffer (138 mmol/L NaCl, 6 mmol/L KCl, 1 mmol/L CaCl2, 10 mmol/L HEPES pH 7.4, 5 mmol/L Glucose, 0.1% bovine serum albumin [BSA]). The cells were then transferred into quartz curettes (2 × 106 cells in 2 mL), which were placed in a luminescence spectrometer (LS-50B, Perkin-Elmer, Beaconsfield, England). Stimulants at different concentrations were added in a 20-μL volume to each curette at the indicated time points. The ratio of fluorescence at 340 and 380 nm wavelengths was calculated using FL WinLab program (Perkin Elmer).
Binding studies
Binding assays were performed in triplicate by adding increasing amounts of unlabeled competitor and constant125I-radiolabeled chemokine, 0.2 ng/assay (RANTES-NEX 292, MIP1α NEX-298, or MIP1β-NEX 299, New England Nuclear, Boston, MA; I309-IM313, Amersham Pharmacia Biotech, Piscataway, NJ) to individual 1.5-mL microfuge tubes. Two hundred milliliter samples of cells (5 × 106 cells/mL of monocytes or 2 × 106 cells/mL HEK-transfectants) suspended in binding media were added to the tubes and mixed by continuous rotation at room temperature for 45 minutes. After incubation, the cells were centrifuged through a 10% sucrose/phosphate-buffered saline (PBS) cushion and the cell-associated radioactivity was measured using a 1272 Wallac gamma counter. A minimum of 2 independent binding assays were performed in triplicate for each cell type and radiolabeled chemokine. Scatchard Analysis was performed using LIGAND (Peter Munson, Analytical Biostatistics section, NIH). The percentage maximal specific binding was determined in the following manner 1-[(maximum specific cpm)−(competitor cpm)/(maximum specific cpm)] × 100. Heterologous displacement was performed to provide apparent affinities for LEC.
Chemotaxis assay
Cell migration was assessed using the 48-well microchemotaxis chamber technique. Various chemoattractants, diluted in binding medium (BM) (RPMI 1640 with 1% BSA and 25 mmol/L HEPES), were placed in the lower wells of a chemotaxis chamber. A polycarbonate filter was placed over the chemoattractants; with 5-μm pore size for monocytes and lymphocytes, and 10-μm pore size for HEK-transfectants (Neuroprobe, Cabin John, MD). Membranes were precoated with 10 μg/mL fibronectin or 50 μg/mL rat tail collagen type I (Collaborative Biomedical Products, Bedford, MA) for lymphocytes, THP-1 or transfectants, respectively. A 50-μL cell suspension, 1 × 106cell/mL for monocytes and transfectants, 5 × 106cell/mL for lymphocytes, were placed in the upper compartment of the chamber. The chamber was incubated at 37°C (1.5 hours for monocytes, 3 hours for lymphocytes, and 5 hours for transfectants) in a humidified 5% CO2 incubator. At the end of the incubation, the filter was removed, fixed, and stained with Diff-Quik kit (Trends Scientific, Kalamazoo, MI). The migrated cells were counted by computer using the BIOQUANT 95 program (R & M Biometrics, Nashville, TN). The results are expressed as the chemotactic index (average number of cells exposed to chemokine divided by the average number of cells exposed to binding media alone ± SE) migration in triplicate samples and are representatives of at least 3 experiments performed.
Adhesion
Adhesion assays were adapted from a neutrophil adhesion assay.9 Established human embryonic kidney (HEK)-293 CCR1 or CCR8 transfectants, were suspended at 2 × 106cells/mL in RPMI-1640 and were labeled with 5 μL of 1 mmol/L calceinam (Molecular Probes, Eugene, OR) at 37°C for 30 minutes. The 96-well plates were coated with rat tail collagen 0.47 μg/mL, at 37°C for 2 hours, followed by adding adhesion buffer (0.05 mol/L HEPES, 0.15 mol/L NaCl, 1 mmol/L MgCl2, pH 7.4) containing 2% BSA and incubated at room temperature for 1 hour. THP-1 cells were suspended in RMPI-1640 at 5 × 106cells/mL and loaded with 5 μL of 1 mmol/L calcein am for 30 minutes at 37°C. The 96-well plates were coated with fibronectin 0.2 μg/mL 100 μL for 2 hours, followed by adding adhesion buffer containing 2% BSA for an additional 1 hour at room temperature incubation. After washing with RPMI-1640, the cells were resuspended in warm RPMI-1640 at the concentration of 1 × 106/mL. Calcein-loaded cell suspension was mixed with 100 μL 2X concentration of LEC, RANTES or chemokine, and anti-LEC and dispensed onto individual wells, followed by 30 minutes incubation at 37°C. Each condition was repeated in triplicate. After careful washing with a multichannel pipettor, the fluorescence of the adherent cells was determined by Cytofluor (PerSeptives Biosytems, Farmingham, MA) using emission wavelength/excitation wavelength 530/485.
Results
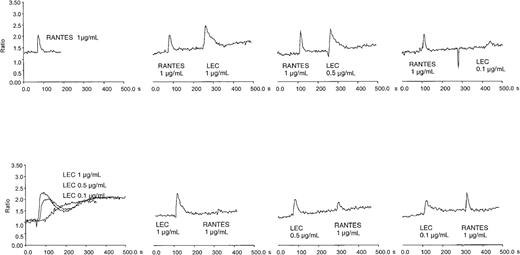
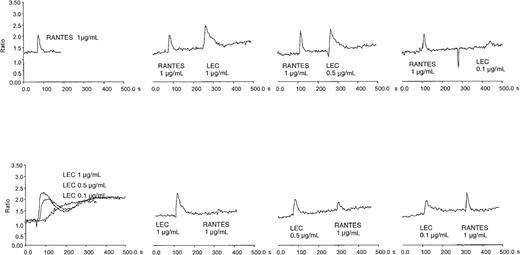
We confirmed that LEC is chemotactic and not chemokinetic for human monocytes, lymphocytes, and THP-1 cells (data not shown). Earlier studies indicated that LEC could induce calcium flux in a myeloid cell line, THP-1, and was desensitized by equivalent concentrations of RANTES.5 To confirm this observation and extend it to a relevant primary cell system, we determined the ability of LEC to induce a calcium flux in primary human monocytes. Human monocytes express high levels of a variety of 7 transmembrane receptors, including fMLP receptor, CCR1, CCR2 (MCP-1 receptor) CCR5, and CCR8; therefore, we examined whether the response of monocytes to fMLP, RANTES, MCP-1, I309 was desensitized by LEC or the reverse. As can be seen in Figure 1, LEC induced calcium flux over a broad concentration range. LEC did not reduce MCP-1 or fMLP-induced calcium flux and interestingly fMLP did not reduce LEC induced calcium flux (data not shown). I309 did not stimulate calcium flux in human monocytes. Pretreatment with a 10-fold higher concentration RANTES did reduce LEC-induced calcium flux, similar results were not observed at equivalent concentrations. However, pretreatment of monocytes with LEC from 1 μg/mL to 0.1 μg/mL did reduce RANTES-induced calcium flux. These data suggest that LEC shares at least one receptor with RANTES and uses another independent of RANTES.
Intracellular calcium flux in monocytes and cross-desensitization of LEC and RANTES.
Human monocytes were loaded with Fura-2 and subjected to treatment with varying amounts of recombinant chemokine. The chemokine concentrations are shown in each panel. This is a representative experiment of 3 performed.
Intracellular calcium flux in monocytes and cross-desensitization of LEC and RANTES.
Human monocytes were loaded with Fura-2 and subjected to treatment with varying amounts of recombinant chemokine. The chemokine concentrations are shown in each panel. This is a representative experiment of 3 performed.
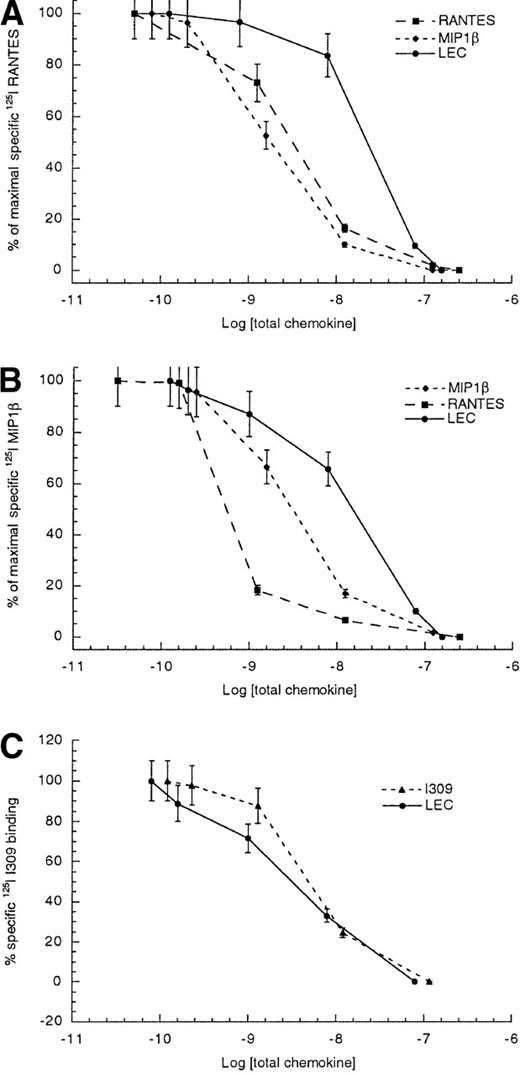
To further delineate which of the 3 RANTES receptors, CCR1, CCR3, and CCR5, found on monocytes might be shared with LEC, we performed competitive binding assays using primary monocytes. As can be seen in Figure 2, competition between radiolabeled RANTES and unlabeled RANTES or MIP1β yielded 50% inhibitory concentrations (IC50 ± STD) values of 1.8 ± 0.4 and 3.4 ± 0.7 nmol/L, respectively. The competition between radiolabeled RANTES and unlabeled LEC yielded an IC50 of 23.6 ± 3.8 nmol/L. The LEC data suggested that LEC is a low-affinity ligand for at least one of the RANTES receptors. In an effort to establish which of these 3 receptors might be used by LEC, we also determine the IC50 values for radiolabeled MIP1β binding to human monocytes. The IC50 for the competition between radiolabeled MIP1β and unlabeled MIP1β was 3.4 ± 0.8 nmol/L, whereas the competition between radiolabeled MIP1β and unlabeled LEC was 15.8 ± 3.7 nmol/L. The data suggested that LEC was a better competitor for MIP1β binding than for RANTES binding to monocytes. Earlier studies had shown at least 3 receptors for MIP1β are expressed on human monocytes; CCR1, CCR5, and CCR8.10-12 Thus, CCR3 appears to be ruled out but CCR1, CCR5, and CCR8 continued to be candidate receptors for LEC. Because the best characterized CCR8 ligand was I309, we also performed competition binding studies using unlabeled LEC and radiolabeled I309 binding to human monocytes. These studies yielded IC50values of 5.2 ± 1.1 nmol/L for unlabeled I309 and 3.1 ± 0.8 nmol/L for unlabeled LEC competed with radiolabeled I309, indicating that LEC is a strong competitor for a shared receptor with I309.
Competitive binding to human monocytes.
(A) Radiolabeled RANTES, (B) radiolabeled MIP1β, and (C) radiolabeled I309. The log of total chemokine concentration is shown on the x-axis, while percentage of inhibition of specific radiolabeled chemokine is shown on the y-axis. The competitive effect of unlabeled LEC is shown with closed circles, unlabeled MIP1β is shown with closed diamonds, unlabeled RANTES is shown with closed boxes and unlabeled I309 is shown with closed triangles. The error bars mark the standard deviation for each point. This is representative of 3 experiments.
Competitive binding to human monocytes.
(A) Radiolabeled RANTES, (B) radiolabeled MIP1β, and (C) radiolabeled I309. The log of total chemokine concentration is shown on the x-axis, while percentage of inhibition of specific radiolabeled chemokine is shown on the y-axis. The competitive effect of unlabeled LEC is shown with closed circles, unlabeled MIP1β is shown with closed diamonds, unlabeled RANTES is shown with closed boxes and unlabeled I309 is shown with closed triangles. The error bars mark the standard deviation for each point. This is representative of 3 experiments.
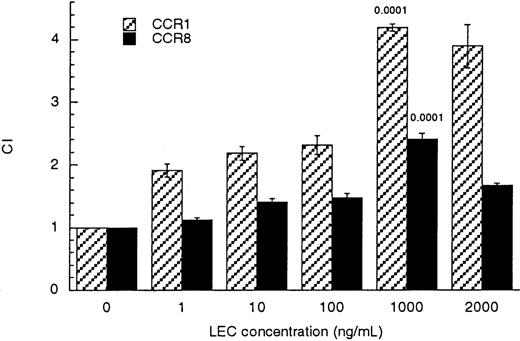
To establish which of the possible RANTES/MIP1β receptors was used by LEC, we tested the ability of individual receptors to transduce a chemotactic signal in response to LEC. Human embryonic kidney cells stably transfected to express either CCR1, CCR3, CCR5, or CCR8 were tested for their ability to respond chemotactically to LEC. HEK-CCR3 and HEK-CCR5 cells did not respond to LEC (data not shown). HEK-CCR1 and HEK-CCR8 cells were chemoattracted to LEC with a maximal response at 1000 ng/mL (83 nmol/L) similar to that observed for monocytes and lymphocytes (Figure 3). These values were statistically significant when compared with binding media controlP ≤ .001.
LEC induced migration of CCR1/HEK-293 and CCR8/HEK-293 transfectants.
Receptor transfected cells (CCR1 hatched bars) or (CCR8 solid bars) were subjected to increasing concentrations of LEC, in the lower wells (shown on the x-axis). The chemotactic index was determined by comparison with the binding media control, see equation in “Materials and methods.” The unpaired Student P values comparing the binding media (BM) to treated cells are indicated above each bar. This is a representative experiment of 3 performed.
LEC induced migration of CCR1/HEK-293 and CCR8/HEK-293 transfectants.
Receptor transfected cells (CCR1 hatched bars) or (CCR8 solid bars) were subjected to increasing concentrations of LEC, in the lower wells (shown on the x-axis). The chemotactic index was determined by comparison with the binding media control, see equation in “Materials and methods.” The unpaired Student P values comparing the binding media (BM) to treated cells are indicated above each bar. This is a representative experiment of 3 performed.
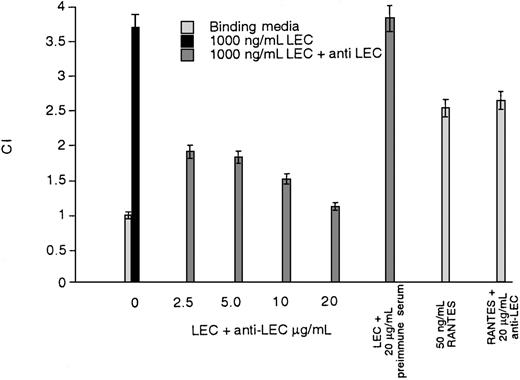
To demonstrate that the LEC-induced response was specific and inhibitable, we tested a polyclonal antibody against LEC for neutralizing activity in an HEK-CCR1 chemotaxis assay. As can be seen in Figure 4, increasing concentrations of anti-LEC progressively reduced the LEC-induced response. The maximum concentration of anti-LEC achievable in this assay was 20 μg/mL. The paired Student t test between 1000 ng/mL LEC and 1000 ng/mL LEC + 20 μg/mL anti-LEC was P < .001. Because the anti-LEC inhibited LEC activity, but had no effect on RANTES-induced chemotaxis and preimmune serum did not inhibit LEC activity, polyclonal anti-LEC has specific neutralizing activity. Similar results were obtained when CCR8-mediated chemotaxis was tested (data not shown). These data show that the shared LEC/RANTES receptor is CCR1 and the receptor shared between I309 and I309 is CCR8. Further, LEC-induced chemotaxis can be inhibited by polyclonal anti-LEC.
Inhibition of LEC-induced chemotaxis by anti-LEC.
Increasing concentrations of polyclonal LEC were added together with 1000 ng/mL of LEC or 50 ng/mL of RANTES to the lower wells of a chemotaxis chamber. The anti-LEC concentration in μg/mL can be seen on the x-axis. The average number of cells per high-powered field are shown on the y-axis. This is a representative experiment of 2 performed.
Inhibition of LEC-induced chemotaxis by anti-LEC.
Increasing concentrations of polyclonal LEC were added together with 1000 ng/mL of LEC or 50 ng/mL of RANTES to the lower wells of a chemotaxis chamber. The anti-LEC concentration in μg/mL can be seen on the x-axis. The average number of cells per high-powered field are shown on the y-axis. This is a representative experiment of 2 performed.
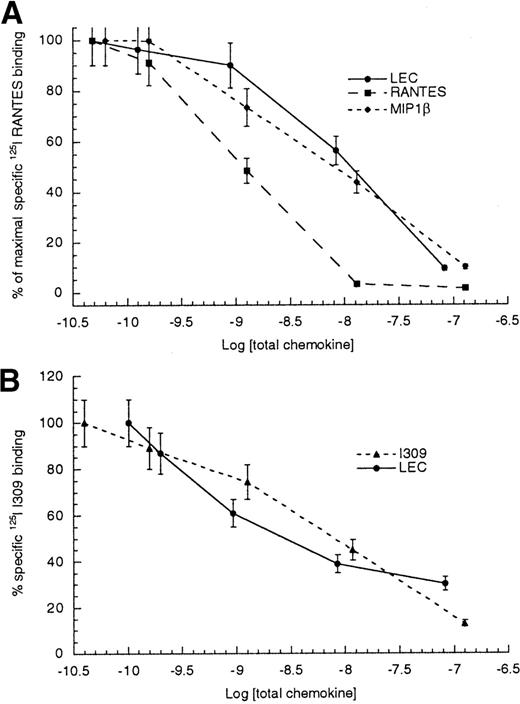
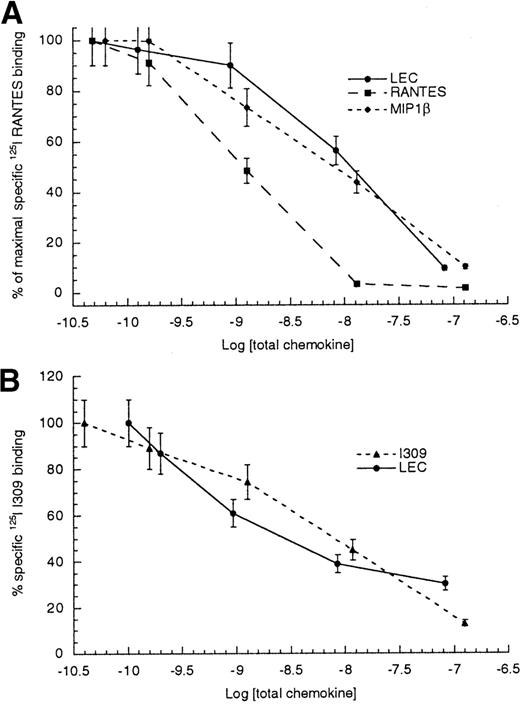
To determine a rank order for CCR1 ligands, we performed competitive binding assays using several CCR1 ligands. The results are shown in Table 1 and Figure5A. These data indicate that the order of affinity for CCR1 would be RANTES > MIP1α ≫ MIP1β ≫ LEC. The ability of LEC to compete with radiolabeled I309 binding to CCR8 transfected HEK-293 cells was performed. The resulting IC50values for competition with radiolabeled I309 were 8.5 ± 3.5 nmol/L for unlabeled I309 and 7.12 ± 4.7 nmol/L for unlabeled LEC (Table 2 and Figure 5B). TARC, another high- affinity CCR8 ligand, displayed comparable IC50values when competing for I309 binding to CCR8 transfected HEK-293 cells. The IC50 values determined for I309 binding to monocytes were very similar to those determined for CCR8 transfectants.
Competitive binding to HEK tranfectants.
(A) Radiolabeled RANTES binding to CCR1/HEK cells, (B) radiolabeled I309 binding to CCR8/HEK cells. The log of total chemokine concentration is shown on the x-axis, while percentage of inhibition of specific radiolabeled chemokine is shown on the y-axis. The competitive effect of unlabeled LEC is shown with closed circles, unlabeled MIP1β is shown with closed diamonds, unlabeled RANTES is shown with closed boxes and unlabeled I309 is shown with closed triangles. The error bars mark the standard deviation for each point. This is representative of 3 experiments.
Competitive binding to HEK tranfectants.
(A) Radiolabeled RANTES binding to CCR1/HEK cells, (B) radiolabeled I309 binding to CCR8/HEK cells. The log of total chemokine concentration is shown on the x-axis, while percentage of inhibition of specific radiolabeled chemokine is shown on the y-axis. The competitive effect of unlabeled LEC is shown with closed circles, unlabeled MIP1β is shown with closed diamonds, unlabeled RANTES is shown with closed boxes and unlabeled I309 is shown with closed triangles. The error bars mark the standard deviation for each point. This is representative of 3 experiments.
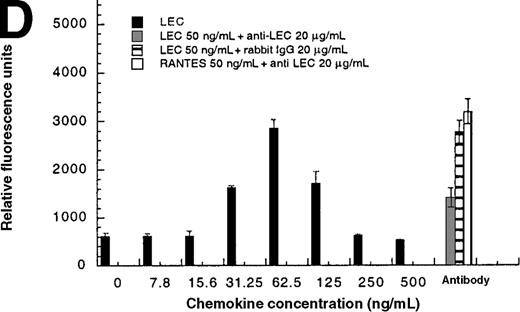
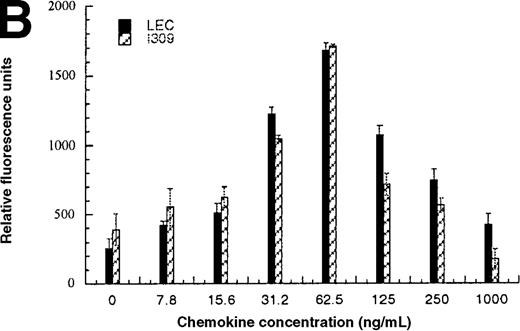
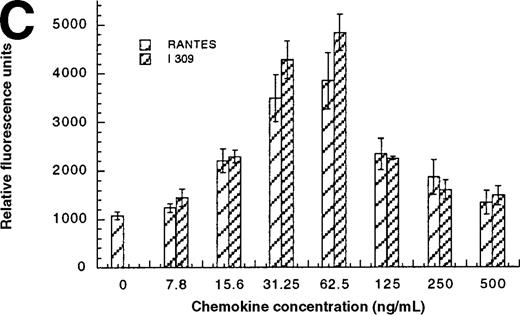
In addition to inducing cell migration, chemokines also induce cell adhesion. To further examine the consequences of the CCR1-LEC–induced signal, we developed a static adhesion assay using CCR1-transfected HEK-293 cells, the results are shown in Figure6. Initially, we determined which concentration of rat tail collagen, fibronectin, or BSA yielded the greatest increase in adherent cells, followed by determining the optimum incubation time. The optimum conditions are reported in the “Materials and methods” section. Our data show that 0.1 nmol/L PMA (data not shown) and RANTES (31.2-62.5 ng/mL) induced maximal HEK-CCR1 cell adhesion as measured by increased relative fluorescence units. The maximal adherence induced by LEC was observed at 62.5 ng/mL (5.6 nmol/L). The LEC-, but not RANTES-, induced CCR1 adhesion was inhibited by anti-LEC when they were preincubated together (Figure 6A). When CCR8 transfectants were tested for chemokine-induced adhesion, the maximum response was observed at 62.5 ng/mL for both chemokines (Figure6B). Thus, RANTES, I309, and LEC all induce transfected cell adhesion in the 5 to 8 nmol/L range. Next, we determined that these chemokines induced adhesion in a relevant cell model, THP-1 cells. As shown in Figure 6C and 6D, I309, RANTES and LEC induced THP-1 cell adhesion at 62.5 ng/mL. The LEC-induced adhesion of THP-1 cells was reduced by addition of anti-LEC.
Comparison of LEC and RANTES.
(A) Comparison of LEC and RANTES-induced CCR1/HEK-293 adhesion. (B) Comparison of LEC and I309-induced CCR8/HEK-293 adhesion. (C) Comparison of I309 and RANTES-induced THP-1 adhesion. (D) Demonstration of LEC-induced THP-1 adhesion and inhibition by anti-LEC. Receptor transfected cells were incubated for 30 minutes with varying concentrations of chemokines and antibodies. LEC-induced response is indicated with solid black bars, RANTES or I309-induced response is indicated with right-hand hatched bars. Treatment with LEC (1000 ng/mL) and 20 μg/mL anti-LEC is indicated by solid gray bars, treatment with RANTES (100 ng/mL) and 20 μg/mL anti-LEC is indicated by solid white bars. Preimmune serum treatment is indicated by a horizontal striped bar. Representative of 3 experiments.
Comparison of LEC and RANTES.
(A) Comparison of LEC and RANTES-induced CCR1/HEK-293 adhesion. (B) Comparison of LEC and I309-induced CCR8/HEK-293 adhesion. (C) Comparison of I309 and RANTES-induced THP-1 adhesion. (D) Demonstration of LEC-induced THP-1 adhesion and inhibition by anti-LEC. Receptor transfected cells were incubated for 30 minutes with varying concentrations of chemokines and antibodies. LEC-induced response is indicated with solid black bars, RANTES or I309-induced response is indicated with right-hand hatched bars. Treatment with LEC (1000 ng/mL) and 20 μg/mL anti-LEC is indicated by solid gray bars, treatment with RANTES (100 ng/mL) and 20 μg/mL anti-LEC is indicated by solid white bars. Preimmune serum treatment is indicated by a horizontal striped bar. Representative of 3 experiments.
Discussion
The chemokines and their receptors are promiscuous and CCR1 seems to interact with the largest number of ligands. Currently, CCR1 is documented to be a receptor for RANTES, MIP1α, MIP1β, HCC-1, HCC-2, MPIF-1, MCP-2, MCP-3, MCP-4, and, as reported here, LEC.1,13-19 A CCR1 ligand binding order has not been determined; however, our data indicate that LEC is a lower affinitiy ligand for CCR1. HCC-1 is another low-affinity ligand for CCR1 and it has the greatest amino acid identity (38%) to LEC. Both chemokines induce cell migration by CCR1 expressing cells with maximal response concentrations at approximately 100 nmol/L.14 Tsou et al14 performed competitive binding studies between radiolabeled MIP1α and unlabeled MIP1α or HCC-1. They observed a comparable IC50 value for MIP1α (1.3 nmol/L) and an IC50 3.8-fold greater for HCC-1 (93 nmol/L) than we observed for LEC (24.3 nmol/L). These earlier results in combination with our study suggest that LEC has a higher affinity for CCR1 than HCC-1 and both are low-affinity CCR1 ligands.
Our competitive binding studies of MIP1β and LEC to human monocytes suggested that MIP1β and LEC might share a monocyte receptor other than CCR1, CCR3, or CCR5, the most likely candidate being CCR8. When we tested single receptor HEK-293 transfectants for chemotactic response to LEC, CCR1 and CCR8 were shown to receptors but not CCR3 or CCR5. Like CCR1, CCR8 is promiscuous. Recent studies showed that the virally encoded chemokines, vMCC1, vMIPI, vMIPII, are high-affinity ligands for CCR8.20,21 There is some controversy concerning what other chemokines are ligands for CCR8. Bernardini et al12demonstrated that TARC and MIP1β induce CCR8HA/Jurkat transfectants to migrate. Dairaghi et al20 did not observe TARC or MIP1β binding to CCR8/NOS cells; however, they did observe eotaxin binding. We observed a high-affinity binding of TARC to CCR8/HEK-293 transfectants (J Leuk Biol, submitted). LEC has an apparent lower binding affinity for CCR1 than for CCR8 and induces chemotaxis by interacting with either receptor.
Chemokine interactions with their receptors and subsequent transmission of a chemotactic signal has been modeled on the basis of data derived from receptor chimera and point mutation of individual chemokines and chemokine receptors. One of the earliest models was of RANTES interaction with CCR1.22 This model clearly showed that the ligand binding site was distinct from a second, signaling site. Later studies of CCR1 and CCR3 chimera supported this model23 and suggested that the signaling sites might be unique for each ligand. Recently, Lu et al24 showed that the ligands for CXCR3 induced chemotaxis with a hierarchy of ITAC > IP10 = MIG, however the hierarchy of binding affinities, IP10 = ITAC > MIG, was different, further supporting the theory that each ligand interacts with its receptor in a unique manner. Thus, it is not unreasonable for LEC to interact with different receptors with apparently different affinities while inducing similar chemotactic signals.
Chemokines have been demonstrated to induce adhesion, which is an integral step in leukocyte migration.25-29 RANTES has been shown to increase T-cell adhesion to endothelial cells, rhICAM-1 or rhVCAM-1 in the 1 to 100 ng/mL range.30 This is very similar to our observations with CCR1/HEK-293 transfectants. The murine homolog of I309, TCA-3, has been shown to induce adhesion by monocytes and neutrophils.31 32 TCA-3 induced phagocytic cell adhesion to fibronectin beginning at 0.1 nmol/L. This is similar to our results with I309 and LEC in which we observed maximal adhesion at 5 to 7 nmol/L. The concentrations of RANTES and I309 needed to induce maximal adhesion were very similar to the concentrations needed to induce maximal chemotaxis. LEC is unique because it induced adhesion at molar concentrations that were 20-fold less than the concentrations needed to induce chemotaxis. Therefore, LEC appears to strongly induce adhesion and weakly induce migration.
To demonstrate that the recombinant protein used in these studies was responsible for the observed increased chemotaxis or adhesion, we tested a rabbit polyclonal anti-LEC for neutralizing activity. Both LEC-induced adhesion and chemotaxis were inhibited. Earlier studies suggested that the amino terminus of LEC could play an important role in the type of signal induced.4 5 The recombinant form of LEC used in these studies had the shorter QPKVPEW amino terminus and induced calcium flux, chemotaxis, and adhesion. Therefore, the shorter form of the protein is completely functional.
The LEC gene (SCYA16) is located in a gene minicluster with the genes for MPIF-1 (SCYA23), HCC-2 (SCYA15), HCC-1 (SCYA14) on human chromosome 17q11.2.33 The genes for HCC1 and LEC are found next to each other and these 2 chemokines share the greatest sequence homology. However, there is a very striking difference in the range of HCC-1 and LEC expression that suggests a difference in function. HCC-1 is constituently expressed by many tissues, although not leukocytes, and plasma levels of protein between 1 to 80 nmol/L have been detected.34 These data suggest that HCC-1 participates in maintenance of normal systemic homeostasis. Unlike HCC-1, LEC does not appear to be widely expressed and may require immune stimulation before being expressed.3-5Inducible LEC expression in the liver, which expresses increased IL-10 levels during acute toxic injury, would suggest that LEC could participate in the maintenance of a normal hepatic integrity perhaps by recruiting or increasing the adhesion of immature Kupffer cells and natural killer (NK) cells.35 Immature Kupffer cells express similar surface markers to immature dendritic cells,36suggesting that they might express similar CC chemokine receptors.37 38 LEC induces both NK and immature dendritic cells to migrate in vitro (data not shown). These data suggest that LEC has a very specialized role in the immune system. Proof of function awaits targeted disruption of the genes for HCC-1 or LEC.
In conclusion, LEC has an apparent low affinity for CCR1 and a higher apparent affinity for CCR8. By interacting with CCR1 or CCR8, LEC induces cell migration at 83 nmol/L and increased cell adhesion at 5.6 nmol/L. LEC induces cell adhesion in the same molar range that RANTES and I309 induce chemotaxis and adhesion, suggesting LEC maybe a more effective promotor of cellular adhesion than migration.
Acknowledgments
We would like to thank Dr Bob Goldman and his associates at Peprotech for making LEC and anti-LEC available for study.
Supported in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health under Contract No NO1-CO-56000.
The content does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Reprints:O. M. Zack Howard, PO Box B, Frederick, MD 21702; e-mail: howardz@mail.ncifcrf.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.