Abstract
Idiopathic pneumonia syndrome (IPS) is a significant complication following bone marrow transplantation (BMT). We have developed a murine model in which severe IPS is induced by pre-BMT conditioning and allogeneic T cells and is characterized by the recruitment of host monocytes and donor T cells into the lung by day 7 post-BMT. Chemokines regulate cellular recruitment and the migration of cells into inflammatory lesions. In this study, we examined the profiles of chemokines produced locally in the lung (parenchyma and bronchoalveolar lavage fluid) and systemically (serum) during the generation of IPS in the peri-BMT period. Protein and messenger RNA (mRNA) levels of CC chemokines (monocyte/lymphocyte attractants), especially monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1, RANTES (regulated upon activation normal T-cell expressed and secreted), and C10, were preferentially induced in the lung by day 7 postallogeneic BMT. In addition, there was an increase in mRNA for IP-10 (a monocyte and Th1-cell chemoattractant). The CXC chemokines MIP-2 and KC, known neutrophil attractants, were moderately elevated. For the most part, these increases in chemokines were dependent on the coinfusion of allogeneic T cells with the BM inoculum. Ribonuclease protection assay and in situ hybridization analyses post-BMT showed that the lung was a major producer of MCP-1, a potent inducer of monocyte chemotaxis. Increases in MCP-1 levels in the lung preceded host APC influx whereas MIP-1 levels accompanied donor T-cell infiltration. In summary, we have shown that monocyte- and T-cell–attracting chemokines are associated with monocyte and T-cell recruitment during IPS.
Our laboratory has focused upon the early peri–bone marrow transplant (BMT) inflammatory events that lead to the generation of idiopathic pneumonia syndrome (IPS), a significant cause of mortality following BMT.1 The risk of developing IPS is associated with the intensity of the conditioning regimens and the severity of graft-versus-host disease (GVHD).2-5 We reported, in our murine IPS model, that the severity of IPS and exaccerbation of host macrophage activation in the lung in the early post-BMT period were dependent on allogeneic T cells and potentiated by preconditioning with cyclophosphamide (Cy).6 The manifestations of lung injury included epithelial cell injury, increased wet and dry weights, decreased specific lung compliance and lung capacity, and an influx of host monocytes and allogeneic T cells.6 The frequency of cells expressing T-cell costimulatory B7 molecules increased in the lung, as did the frequency of cells expressing messenger RNA (mRNA) for transforming growth factor-β (a monocyte chemoattractant) and the cytolysin granzyme B.6,7 In addition, we reported that lung dysfunction post-BMT in mice was associated with increased bronchoalveolar lavage (BAL) levels of nitrite, lactate dehydrogenase, and protein, all indices of lung injury.8 Allogeneic T cells stimulate nitric oxide production whereas Cy stimulates the production of superoxide, the combination of which forms a tissue-damaging oxidant, peroxynitrite.8 Because all the above manifestations were seen during the first week post-BMT, the need for early intervention to circumvent the lung damage critical to the generation of IPS was implicated.
Chemokines are small (8 to 14 kd) proteins that can regulate leukocyte functioning and migration during inflammation. Chemokines are categorized based on the presence and position of the conserved cysteine residues.9,10 Most chemokines belong either to the CXC (α), CC (β), or C (γ) family. Although there are redundancies, as with cytokines, members of the α family (CXC) generally are chemotactic for polymorphonuclear cells (neutrophils, eosinophils, and basophils); members of the β family (CC) are chemotactic for mononuclear cells such as monocytes and lymphocytes; and the γ family (C) are chemotactic for lymphocytes.9Following tissue injury, the up-regulation of adhesion molecules on endothelial surfaces causes tethering and rolling of circulating leukocytes.11 These cells are then activated by chemokines immobilized on the luminal surface of endothelium, resulting in cytoskeletal remodeling, flattening of the cell with firmer adhesion via cell-surface integrins, and subsequent diapedesis and extravasation into the tissue.12,13 Chemokines then continue to act as migratory signals, guiding inflammatory cells to the site of injury. The cellular specificity depends on the presence of chemokine receptors on the cell surface (termed CXCRs, CCRs).9
It is not known whether chemokines produced locally in the lung, as opposed to systemic circulating levels, are increased in conditions that generate IPS and whether these chemokines increase in concordance with the influx of inflammatory cells. This would help to further define the mechanism(s) leading to the inflammatory cell influx and activation leading to lung injury and the generation of IPS following allogeneic BMT. Ultimately, treatments to interfere with chemokine signaling and thus hinder the infiltration process in the early post-BMT period may prove to be beneficial. The purpose of this study was to define the potential chemokines responsible for leukocyte recruitment into the lung leading to IPS injury. The chemokine production profile was examined in distinct lung compartments, ie, alveolar space, lung parenchyma, and systemic blood circulation, in relation to pre-BMT conditioning and allo-BMT infusion on these IPS-inducing inflammatory events in the lung in the early post-BMT period.
Materials and methods
Mice
B10.BR (H2k) and C57BL/6 (H2b) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in microisolator cages in the specific pathogen-free facility of the University of Minnesota and cared for according to the Research Animal Resources guidelines of our institution. For BMT, donors were 8 to 12 weeks of age, and recipients were used at 8 to 10 weeks of age.
Pre-BMT treatment and conditioning
B10.BR mice received phosphate-buffered saline (PBS) or Cy (Cytoxan, Bristol Myers Squibb, Seattle, WA), 120 mg/kg per day intraperitoneally, as a conditioning regimen pre-BMT on days −3 and −2. All mice were lethally irradiated on the day before BMT (7.5 Gy total body irradiation [TBI]) by x-ray at a dose rate of 0.41 Gy/min as described.14
Bone marrow transplantation
Our BMT protocol has been described previously.15Briefly, donor C57BL/6 BM was T-cell depleted with anti-Thy 1.2 monoclonal antibody (clone 30-H-12, rat IgG2b, kindly provided by Dr David Sachs, Cambridge, MA) plus complement (Nieffenegger, Woodland, CA). Recipient mice were transplanted via caudal vein with 20 × 106 T-cell–depleted C57BL/6 (H-2b) marrow with or without 15 × 106natural killer (NK) cell–depleted (PK136, anti-NK1.1 plus complement) spleen cells (BMS) as a source of IPS-causing T cells.
Bronchoalveolar lavage
Mice were euthanized with sodium pentobarbitol, and the thoracic cavity was partially dissected. The trachea was cannulated with a 19-gauge needle and infused with 0.5 mL of PBS and withdrawn. This was repeated 2 more times, and a total of 1.5 mL BAL fluid was collected per mouse. BAL fluid was centrifuged at 4°C for 10 minutes at 1000g to pellet the cells and stored at −80°C. The BAL fluid was analyzed for chemokine levels in the same manner used for serum (see “Chemokine level determination”).
Frozen tissue preparation
Following euthanasia on day 3 post-BMT, the thoracic cavity was partially dissected, and a mixture of 0.5 mL Optimal Cutting Temperature compound (OCT, Miles, Elkhart, IN) with PBS (3:1) was infused via the trachea into lungs. Lung tissue was snap-frozen in liquid nitrogen and stored at −80°C.
In situ hybridization
Cryosections (4 μm) were hybridized with digoxigenin-labeled antisense RNA probes. The corresponding ribonucleotide sequences used were 237 to 749 for monocyte chemoattractant protein (MCP)-1, 229 to 667 for macrophage inflammatory protein (MIP)-1α, 234 to 550 for MIP-1β, 761 to 987 for MIP-2, 179 to 467 for TCA-3, and 83 to 421 for RANTES (regulated upon activation normal T-cell expressed and secreted). Immunologic detection of digoxigenin-labeled RNA duplexes was accomplished with antidigoxigenin antibody (alkaline-phosphatase conjugated; Boehringer Mannheim, Indianapolis, IN) and fluorescent substrate using the ELF-97 system (excites at 370 nm and emits at 515 nm; Molecular Probes, Eugene, OR). Stained tissue was analyzed on an Olympus BX50 WI microscope with multiphoton confocal MRC-1024 imaging (Bio-Rad, Hercules, CA) using LaserSharp v3.1 software (Bio-Rad).
Serum collection
At the time of sacrifice (days 0, 3, and 7 post-BMT), blood was collected by cardiac puncture, placed immediately at 4°C, the serum separated at 4°C, and stored at −80°C.
Lung protein extracts
At the time of sacrifice (days 0, 3, and 7 post-BMT), after exsanguination and BAL, the left lung was homogenized in 1 mL PBS containing protease inhibitor cocktail (Boehringer Mannheim) and centrifuged at 3000 rpm for 10 minutes. The supernatant was filtered through a 1.2-μm syringe filter and stored at −80°C until assayed.
Chemokine level determination
Serum, BAL, and lung protein extract levels of MIP-1α, MIP-1β, MCP-1, MIP-2, eotaxin, KC, RANTES, and C10 were determined by sandwich enzyme-linked immunosorbent assay (ELISA) as previously described16 17 (sensitivity, 25 pg/mL) or using commercial kits (R & D Systems, Minneapolis, MN; sensitivity, 1.5 to 3 pg/mL).
Ribonuclease protection assay
Total lung RNA was isolated18 and hybridized with antisense RNA probes labeled with 32P-UTP using the mCK-5 chemokine template set from Pharmingen (San Diego, CA). The RNA duplexes were run on premade polyacrylamide gels (Novex, San Diego, CA) and autoradiographs scanned on a Bio-Rad GS700 with densitometry using Molecular Analyst. In some experiments, independent ribonuclease protection assay (RPA) analyses on the same samples were done for chemokines not represented in the mCK-5 template. The corresponding ribonucleotide sequences used were 112 to 241 for KC and 960 to 1143 for C10. Cyclophilin was used as a control to normalize mRNA levels. Following ribonuclease treatment (RPA II kit, Ambion, Austin, TX), RNA duplexes were run on 6% polyacrylamide gels. Following overnight exposure, autoradiographs were scanned on a PhosphorImager and band densities determined using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Statistical analysis
Data were analyzed by ANOVA or the Student t test. Probability values less than or equal to .05 were considered statistically significant. P values more than .05 and less than .1 were considered a statistical trend.
Results
Preferential elevation of monocyte– and T-cell–attracting chemokines in different lung compartments following allogeneic BMT is predominantly T-cell dependent
One of our central hypotheses is that the chemokine production that occurs during the early phase of chemoradiotherapy-induced injury is essential for the recruitment of leukocytes into the lung. Because the chemokine-receptor interactions necessary for IPS injury are unknown, a kinetic analysis of chemokine production was performed. The 3 time points selected corresponded to the 3 distinct phases of early tissue injury and cellular recruitment in this model: day 0 (post-chemoradiotherapy, predonor cell infusion), day 3 (end of first wave of host monocyte recruitment), and day 7 (donor CD4+and CD8+ T-cell and host monocyte recruitment, severe inflammation and destruction). Particular attention was devoted to the analysis of chemokines known to be important to the recruitment of monocytes (eg, MCP-1) and T cells (MIP-1α, MIP-1β, RANTES). Due to the potential importance of such data and the virtual absence of data regarding the role of chemokines in either IPS or GVH responses, we measured the levels of members of both the CC (MCP-1, MIP-1α, MIP-1β, RANTES, eotaxin, C10) and CXC (MIP-2, KC) chemokine families in 3 physically defined compartments of the lung, namely parenchyma (lung protein extracts), alveolar space (BAL fluid), and circulating serum in an allogeneic BMT setting.
To induce IPS, B10.BR recipients were conditioned with TBI with or without Cy and infused with C57BL/6 BM with or without spleen cells as described in “Materials and methods.” On day 0 of BMT (1 day post-TBI and prior to BM/BMS infusion), we did not find elevations in any of the CC chemokines we examined in the lung compartments (parenchyma and BAL fluid, data not shown) as assessed by ELISA, consistent with the lack of inflammatory cell infiltrates in the lung at this time point. The only CC chemokine elevated in the serum of TBI-conditioned mice was MCP-1 (6.5-fold increase vs normals,P = .001, data not shown). Cy treatment potentiated the increase in serum MCP-1 levels (9.1-fold vs normals,P = .000 05; and 1.6-fold vs TBI alone, P = .03; data not shown).
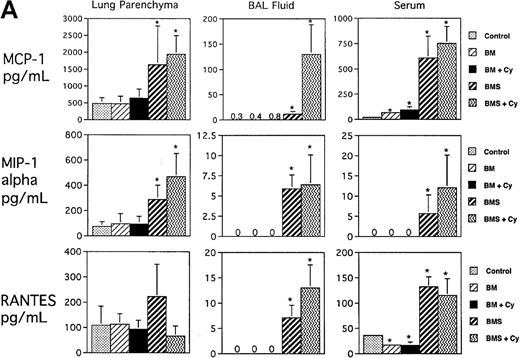
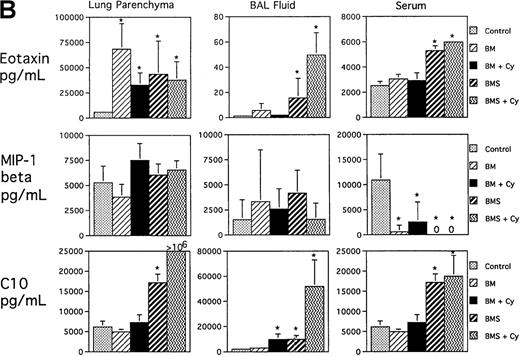
Regarding the day 3 and day 7 post-BMT time points examined, the more significant findings were seen for day 7 post-BMT consistent with the striking cellular recruitment seen at this time point. Figure1A shows that significant increases in 3 CC chemokines known to increase monocyte and T-cell migration were seen in all 3 compartments as measured by ELISA: MCP-1, MIP-1α, and RANTES (except RANTES in parenchyma). In general, the highest levels of these 3 chemokines were seen in the Cy/TBI/BMS groups that had the most severe degree of cellular infiltration and tissue injury in the lung.6 Potentiation by Cy was seen for MCP-1 in BAL fluid (P < .0007, BMS/Cy vs BMS). Figure 1B illustrates that T-cell–dependent increases for the CC chemokine eotaxin (major chemoattractant for eosinophils) were observed only in the BAL and serum. Cy potentiated the level of eotaxin to some degree in the BAL (P = .01, BMS/Cy vs BMS). T-cell–dependent increases for MIP-1β (a monocyte chemoattractant) were not observed. In fact, MIP-1β levels were unchanged in the lung (parenchyma and BAL) and were actually decreased in the serum compared with control mice regardless of treatment group. Figure 1B also shows that there was a T-cell–dependent increase in the level of C10 (monocyte and lymphocyte attractant) in lung parenchyma, BAL fluid, and serum. Cy and BMS, interestingly, induced a synergistic increase in C10, and this synergism was only seen in the 2 lung compartments. Overall, the T-cell–dependent increase of CC chemokines is consistent with our previous observations of the donor T-cell infiltration and the allogeneic T-cell–dependent second wave of host monocytic influx in the lung at this day 7 post-BMT time point.6
Expression of CC chemokines in lung parenchyma, BAL fluid, and serum on day 7 postallogeneic BMT as assessed by ELISA.
B10.BR recipient mice were preconditioned with TBI (7.5 Gy, day −1) with or without Cy (120 mg/kg per day, days −3, −2) as indicated and given C57BL/6 BM without (BM) or with 15 × 106 spleen cells (BMS) on the day of BMT (day 0). Lung protein extracts were obtained after exsanguination and BAL. (A ) Expression of MCP-1, MIP-1α, and RANTES. (B) Expression of eotaxin, MIP-1β, and C10. Mean values ± SD are indicated for 12 mice per group pooled from 2 experiments (except for RANTES and MIP-1β, n = 6 per group). *P < .05 vs control unmanipulated B10.BR mice; > 106 means all samples measured off-scale in the assay.
Expression of CC chemokines in lung parenchyma, BAL fluid, and serum on day 7 postallogeneic BMT as assessed by ELISA.
B10.BR recipient mice were preconditioned with TBI (7.5 Gy, day −1) with or without Cy (120 mg/kg per day, days −3, −2) as indicated and given C57BL/6 BM without (BM) or with 15 × 106 spleen cells (BMS) on the day of BMT (day 0). Lung protein extracts were obtained after exsanguination and BAL. (A ) Expression of MCP-1, MIP-1α, and RANTES. (B) Expression of eotaxin, MIP-1β, and C10. Mean values ± SD are indicated for 12 mice per group pooled from 2 experiments (except for RANTES and MIP-1β, n = 6 per group). *P < .05 vs control unmanipulated B10.BR mice; > 106 means all samples measured off-scale in the assay.
Because neutrophils make up approximately 20% of the pulmonary infiltrate by day 7 post-BMT in our model, we examined the profiles of CXC chemokines that are known to increase neutrophil migration. On day 0, the only CXC chemokine elevated in TBI-conditioned mice was KC in the BAL fluid (4.6-fold vs normals, P = .002, data not shown). Cy treatment caused an increase in serum KC levels (1.8-fold vs normals, P = .03; and 1.8-fold vs TBI alone,P = .01; data not shown).
Day 7 post-BMT levels were elevated in a T-cell–dependent manner for MIP-2 and, to a lesser extent, KC (data not shown), indicating that MIP-2 and KC may be involved in the migration of neutrophils into the lung during IPS generation post-BMT.
Lung tissue is a major source of T-cell– and monocyte-attracting chemokines post-BMT
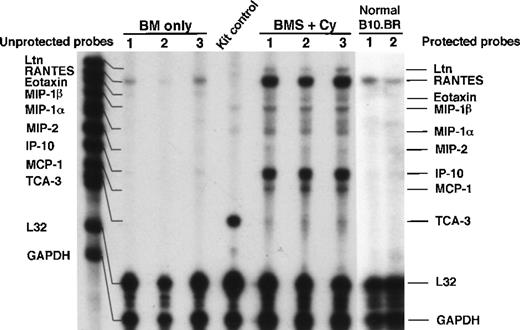
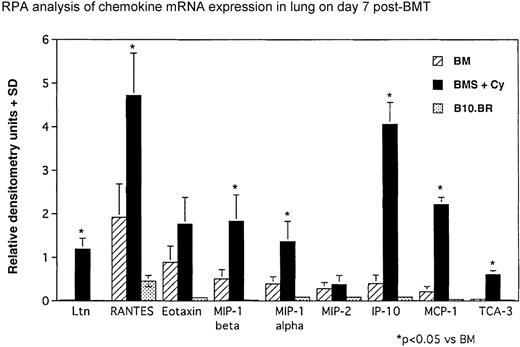
To better understand the relationship(s) between chemokine production and IPS generation post-BMT, we sought to determine whether the lung itself was a source of chemokines that may be responsible for the infiltration of inflammatory cells. As illustrated by RPA analysis of lung tissue homogenates from day 7 post-BMT (Figure2), messages for CC chemokines lymphotactin (Ltn), RANTES, eotaxin, MIP-1β, MIP-1α, MCP-1, and TCA-3 were significantly elevated in mice with severe IPS (ie, BMS plus Cy). In addition, the CXC chemokines MIP-2 and IP-10 were elevated in these same mice. To determine the relative abundance of these chemokines and their possible relevance to the T-cell–dependent lung injury seen in our model, densitometry was performed. Figure3 shows that the CCchemokines Ltn, MIP-1α, MCP-1, and TCA-3 and the CXC chemokine IP-10 were significantly elevated above control in mice receiving allogeneic T cells. The CC chemokines RANTES, eotaxin, and MIP-1β and the CXC MIP-2 also were significantly elevated in these mice, but these chemokines were also significantly elevated, albeit to a lesser degree, in mice not given allogeneic T cells. In separate RPA analyses, we also saw a significant increase in mRNA for C10 in BMS-plus-Cy mice (4.6-fold increase vs control, P < .05, data not shown). KC was transcribed at very low levels (not shown), but because different housekeeping genes were used to control RNA input, we are unable to make a direct comparison to the other chemokines. In summary, a predominant T-cell– and monocyte-attracting chemokine mRNA profile was induced in the lungs of mice exhibiting IPS injury on day 7 post-BMT consistent with our protein data and with the predominant monocyte and lymphocyte infiltrate we initally described for our IPS model.
Expression of chemokines in lung parenchyma on day 7 post-allogeneic BMT.
B10.BR recipient mice were preconditioned with TBI (7.5 Gy, day −1) with or without Cy (120 mg/kg per day, days −3, −2) as indicated and given C57BL/6 BM without (BM) or with 15 × 106 spleen cells (BMS) on the day of BMT (day 0). Lung tissue total RNA (obtained after exsanguination and BAL of lungs) on day 7 post-BMT was analyzed by RPA as described in “Materials and methods” using the CK5 template from Pharmingen. One of 3 representative experiments is shown. Densitometry data are shown in Figure 3.
Expression of chemokines in lung parenchyma on day 7 post-allogeneic BMT.
B10.BR recipient mice were preconditioned with TBI (7.5 Gy, day −1) with or without Cy (120 mg/kg per day, days −3, −2) as indicated and given C57BL/6 BM without (BM) or with 15 × 106 spleen cells (BMS) on the day of BMT (day 0). Lung tissue total RNA (obtained after exsanguination and BAL of lungs) on day 7 post-BMT was analyzed by RPA as described in “Materials and methods” using the CK5 template from Pharmingen. One of 3 representative experiments is shown. Densitometry data are shown in Figure 3.
IPS is associated with elevations in chemokines known to chemoattract monocytes and T cells.
Densitometry readings of RPA blots are shown. T-cell–dependent increases for Ltn, MIP-1α, MIP-1β, RANTES, IP-10, MCP-1, and TCA-3 were significantly elevated in mice receiving allogeneic splenocytes. Data (densitometry readings) are normalized to L32 mRNA levels and expressed as mean chemokine/L32 ± SD (n = 3 per group). *P < .05 vs control unmanipulated B10.BR control mice.
IPS is associated with elevations in chemokines known to chemoattract monocytes and T cells.
Densitometry readings of RPA blots are shown. T-cell–dependent increases for Ltn, MIP-1α, MIP-1β, RANTES, IP-10, MCP-1, and TCA-3 were significantly elevated in mice receiving allogeneic splenocytes. Data (densitometry readings) are normalized to L32 mRNA levels and expressed as mean chemokine/L32 ± SD (n = 3 per group). *P < .05 vs control unmanipulated B10.BR control mice.
The production of MCP-1 in the lung in response to injury is associated with the initial recruitment of monocytes post-BMT
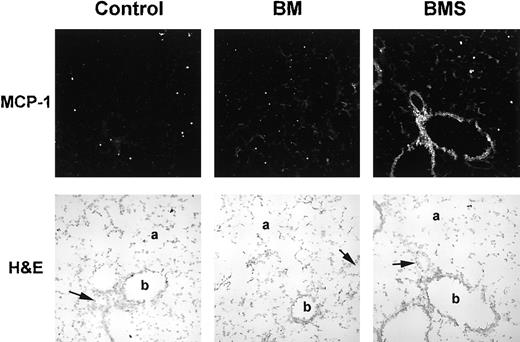
Because the first wave of monocyte recruitment into the lung is evident by day 3 post-BMT, studies were performed to confirm the expression of MCP-1 mRNA in the lung during this initial monocyte recruitment phase, prior to substantial donor T-cell migration. In situ hybridization analysis of cryosections of lungs taken on day 3 post-BMT confirmed the presence of MCP-1 mRNA-producing cells in the lung (Figure 4). Increased staining for MCP-1 mRNA in the BMS groups was seen for endothelial, bronchial epithelium, and alveolar cells, consistent with known sources of MCP-1.9 Therefore, it appears that the lung itself is responsible for the production of the MCP-1 chemotactic gradient. At this day 3 post-BMT time point, staining for MIP-1α, MIP-2, RANTES, and TCA-3 was not different from control consistent with our protein data for this time point (not shown). Therefore, of the chemokines we studied by in situ hybridization, we found only MCP-1 transcription to be induced in the lung at least by day 3 post-BMT, and this closely parallels the monocyte influx we previously demonstrated consistent with the target cell specificity of MCP-1.
MCP-1 mRNA transcription in the lung increases postirradiation and is potentiated by the presence of allogeneic T cells as assessed by in situ hybridization.
B10.BR recipient mice were preconditioned with TBI (day −1) and given C57BL/6 BM with 15 × 106 spleen cells (BMS) on the day of BMT (day 0). Lung tissues were harvested on day 3 post-BMT and cryosections hybridized with antisense digoxigenin-labeled riboprobe for MCP-1. Stained tissue (using the ELF-97 alkaline phosphatase substrate) was analyzed on an Olympus BX50 WI microscope under a 20 × objective lens with multiphoton confocal MRC-1024 imaging (tissue excited at 370 nm and emission collected at 515 nm). Light field images (stained by hematoxylin and eosin) were also photographed under a 20 × objective lens. Solid arrow indicates pulmonary arteriole; a, alveolar duct; b, bronchiole. Note the increased staining of vessel endothelium, bronchiolar epithelium, and alveolar cells in the BMS group.
MCP-1 mRNA transcription in the lung increases postirradiation and is potentiated by the presence of allogeneic T cells as assessed by in situ hybridization.
B10.BR recipient mice were preconditioned with TBI (day −1) and given C57BL/6 BM with 15 × 106 spleen cells (BMS) on the day of BMT (day 0). Lung tissues were harvested on day 3 post-BMT and cryosections hybridized with antisense digoxigenin-labeled riboprobe for MCP-1. Stained tissue (using the ELF-97 alkaline phosphatase substrate) was analyzed on an Olympus BX50 WI microscope under a 20 × objective lens with multiphoton confocal MRC-1024 imaging (tissue excited at 370 nm and emission collected at 515 nm). Light field images (stained by hematoxylin and eosin) were also photographed under a 20 × objective lens. Solid arrow indicates pulmonary arteriole; a, alveolar duct; b, bronchiole. Note the increased staining of vessel endothelium, bronchiolar epithelium, and alveolar cells in the BMS group.
Increases in MCP-1 levels in the lung precede host antigen-presenting cell (APC) influx whereas MIP-1 levels accompany donor T-cell infiltration
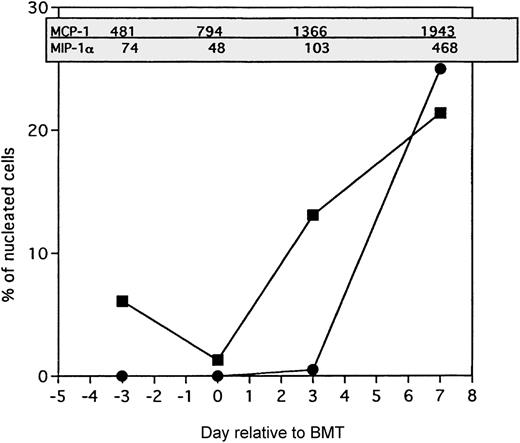
To better understand the association between the induction of the CC chemokines MCP-1 and MIP-1α in the lung and the presence of inflammatory infiltrate, the levels of these chemokines in lung protein extracts were compared with the frequencies of the predominant classes of infiltrating cells, namely the host monocytes and donor T cells, on a kinetic basis. Figure 5 shows that, in BMS mice conditioned with Cy/TBI that exhibit the most severe IPS injury, the increase of MCP-1 (as measured by ELISA) in the lung precedes the increase in host monocytes (APCs) as assessed by host major histocompatibility complex class II expression.6 The increase in MCP-1 did not correlate with the influx of other cell types such as neutrophils or T cells. In contrast, the levels of MIP-1α appeared to accompany the presence of infiltrating donor T cells, suggesting either that there is a short time lag between MIP-1α increase (from resident cells) and the recruitment of T cells or that the infiltrating T cells themselves are producing MIP-1α.
Comparison of MCP-1 and MIP-1 levels in the lung with frequencies of host monocytes and donor T cells.
▪ indicates host monocytes; •, donor T cells. B10.BR recipient mice were preconditioned with TBI (7.5 Gy, day −1) and Cy (120 mg/kg per day, days −3, −2) and given C57BL/6 BM with 15 × 106 spleen cells (BMS) on the day of BMT (day 0). Chemokine levels in picograms per milliliter of BAL fluid (in shaded box) were determined by ELISA of lung protein extracts as described in “Materials and methods” from mice killed on day −3 (prior to Cy administration), day 0 (prior to BMT), day 3, and day 7 after BMT. Frequencies of host monocytes and donor T cells (as a percentage of total nucleated cells) were determined by immunohistochemical staining of lung cryosections using biotinylated monoclonal antibodies as described previously.6
Comparison of MCP-1 and MIP-1 levels in the lung with frequencies of host monocytes and donor T cells.
▪ indicates host monocytes; •, donor T cells. B10.BR recipient mice were preconditioned with TBI (7.5 Gy, day −1) and Cy (120 mg/kg per day, days −3, −2) and given C57BL/6 BM with 15 × 106 spleen cells (BMS) on the day of BMT (day 0). Chemokine levels in picograms per milliliter of BAL fluid (in shaded box) were determined by ELISA of lung protein extracts as described in “Materials and methods” from mice killed on day −3 (prior to Cy administration), day 0 (prior to BMT), day 3, and day 7 after BMT. Frequencies of host monocytes and donor T cells (as a percentage of total nucleated cells) were determined by immunohistochemical staining of lung cryosections using biotinylated monoclonal antibodies as described previously.6
Discussion
This is the first comprehensive analysis of chemokines produced locally, in the lung, and systemically, during the generation of IPS in the peri-BMT period in our established murine model. Collectively, the data indicate that severe IPS injury is associated with increases of chemokines in the lung compartments. We found an induction of CC chemokines (especially MCP-1, MIP-1α, RANTES, and C10) and a CXC chemokine (IP-10) that attract T cells and monocytes in the lung by day 7 post-BMT. These increases, for the most part, were dependent on the coinfusion of allogeneic T cells with the BM inoculum. In contrast, CXC chemokines that attract neutrophils were only moderately elevated compared with normal. The inflammatory pattern observed correlates with the chemokine data and is consistent with our previous findings on the composition of the cellular infiltrate of the lung at this time point, ie, a predominance of monocytes and T cells, the target cells of CC chemokines.
On Day 0 at the time of BMT, we found an approximate 6.5-fold increased level of MCP-1 in the serum that is probably relevant in mobilizing monocytes into the circulation. MCP-1 is produced by many cell types, including endothelial cells, epithelial cells, smooth muscle cells, fibroblasts, lymphocytes, eosinophils, monocytes, and macrophages (peritoneal and alveolar)19,20 and is considered to be essential for monocyte recruitment21 probably by up-regulation of adhesion molecules on monocytes.22,23Damage to cells by irradiation causes the release of intracellular contents and acute-phase reactants. The increase in serum MCP-1 was potentiated by Cy increasing values by about 4-fold. This is probably due to the greater damage caused by the Cy/TBI combination and may be the cause of the Cy-mediated acceleration of the monocyte infiltration post-BMT.6 Monocytes preferentially use the chemokine receptor CCR2 for MCP-1–mediated migration,24 and we did find an increase in CCR2 mRNA in the lungs of IPS mice (data not shown). On day 7 post-BMT, there was a T-cell–dependent increase in the MCP-1 levels in the serum. This increase coincides with increases in the serum levels of the cytokines interleukin-1β, tumor necrosis factor-α, and interferon-γ,6 combinations of which can stimulate production of MCP-1 in endothelial cells.25 MCP-1 was highest in the mice with the most significant host monocyte influx. We determined, by both RPA and in situ hybridization analyses of mRNA, that the lung parenchyma and epithelium is a major source of MCP-1 as least as early as day 3 post-BMT. RPA analysis showed that MCP-1 mRNA transcription in the lung is significantly induced by irradiation and is further potentiated by allogeneic T cells. We found that MCP-1 protein levels in lung parenchyma and BAL continued to increase only in mice receiving allogeneic T cells (with potentiation of this effect by Cy only in BAL fluid). These data imply that there is a T-cell–dependent posttranscriptional regulation of MCP-1 in the lung. The increase of MCP-1 (as measured by ELISA) in the lung preceded the increase in host monocytes (APCs). These data support the hypothesis that MCP-1 produced in the lung by resident cells is responsible for generating the MCP-1 chemotactic gradient for the recruitment of host monocytes to the lung post-BMT.
MIP-1α, a CD8+ T-cell chemoattractant in vivo, was elevated in a T-dependent manner and to the greatest extent in the lung parencyma and BAL fluid under conditions that resulted in the highest influx of CD8+ T cells into the lung. The importance of donor T-cell–derived MIP-1α for the generation of GVHD has been recently reported for the lungs, liver, and intestine but only across the class I major histocompatibility complex barrier.26This is consistent with the preferential migration of CD8+T cells in response to MIP-1α.27,28 It is likely, in our IPS model, that MIP-1α is being produced by the donor T cells because the lung and BAL levels of MIP-1α did not rise significantly until day 7 post-BMT, concomitant with the influx of donor CD8+ T cells; however, we cannot exclude the posssibility that host alveolar macrophages may be producing MIP-1α, as has been described for bleomycin-induced lung injury in rodents.29 Also, other cells can make MIP-1α, including monocytes, neutrophils, fibroblasts, and bronchiolar epithelium. It has been shown that MIP-1α can decrease interleukin-4 production,30 and that may be a contributing factor skewing the CD4 response to a Th1-mediated one, because we have seen significant levels of interferon-γ in the BAL fluid of IPS mice. Th1 cells express CCR531,32 and therefore can respond to MIP-1α and RANTES,33 both of which were elevated in BAL fluid post-BMT. The increase in mRNA for IP-10 (a monocyte and Th1-cell chemoattractant) in the lungs of mice with IPS may be relevant, because it is induced by interferon-γ,34 which we also find elevated in IPS.6,7 35 In addition, we found an increase in the expression of CCR5 mRNA in the lungs of mice with IPS consistent with the influx of inflammatory Th1 cells (data not shown).
The CC chemokine C10 was also increased in a T-dependent manner in the lungs, BAL fluid, and sera post-BMT. The function of C10 is unknown, but it is presumed to play a role in hematopoiesis, because it is produced by cells newly derived from bone marrow36 and it is chemotactic for CD4+ T cells, B cells, monocytes, and NK cells. It is unique not only because of its genetic structure37 but also in that it is not induced by lipopolysaccharide stimulation and is distinctly regulated, requiring de novo protein synthesis for its induction.38 Recent evidence indicates that C10 is a chemoattractant for eosinophils,39 but eosinophil recruitment is not a characterisitc of our IPS model and, in addition, we could not demonstrate the presence in the lung of mRNA for CCR3 found on eosinophils (data not shown). The role of C10 in our IPS model remains unknown.
The demonstration of increases in chemokines that attract monocytes and T cells in the lungs and airway space post-BMT raises the question of whether these chemokines regulate GVHD responses in other GVHD target organs. This comprehensive analysis of the chemokines induced in the peri-BMT period will be beneficial in the development of strategies to circumvent the inflammatory events leading to GVHD and IPS.
Acknowledgments
The expert technical assistance of Naomi Fujioka, Marie Burdick, Sheila Scully, Alana Eli, Stacey Hermanson, Chris Lees, Erika Estevez, Nhien Bui, and Kelly Coffey is greatly appreciated. We thank Jerry Sedgewick and John Oja from the BioImaging Processing Lab at the University of Minnesota for help with microscopy imaging. We also thank Dr Patricia A. Taylor for helpful discussions.
Supported by National Institutes of Health (grant HL 55209), the Minnesota Medical Foundation, and the Viking Children's Fund.
Reprints:Angela Panoskaltsis-Mortari, University of Minnesota, Dept of Pediatrics, Division of Heme/Onc/Blood and Marrow Transplant Program, Box 366 Mayo, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: panos001@tc.umn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal