Abstract
The phosphatidylinositol 3-kinase (PI3K) signaling pathway is important for the regulation of a number of cellular responses. Serine/threonine kinase Akt (protein kinase B; PKB) is downstream of PI3K and activated by growth factors. This study found that erythropoietin (EPO) induced tyrosine phosphorylation of Akt in a time- and dose-dependent manner in EPO-dependent human leukemia cell line UT-7/EPO. In vitro kinase assay using histone H2B and glucose synthase kinase as substrates demonstrated that Akt was actually activated by EPO. EPO-induced phosphorylation of Akt was completely blocked by a PI3K-specific inhibitor, LY294002, at 10 μmol/L, indicating that activation of Akt by EPO is dependent on PI3K activity. In addition, overexpression of the constitutively active form of Akt on UT-7/EPO cells partially blocked apoptosis induced by withdrawal of EPO from the culture medium. This finding suggested that the PI3K-Akt activation pathway plays some role in the antiapoptotic effect of EPO. EPO induced phosphorylation of a member of the trancription factor Forkhead family, FKHRL1, at threonine 32 and serine 253 in a dose- and time-dependent manner in UT-7/EPO cells. Moreover, results showed that Akt kinase activated by EPO directly phosphorylated FKHRL1 protein and that FKHRL1 phosphorylation was completely dependent on PI3K activity as is the case for Akt. In conjunction with the evidence that FKHRL1 is expressed in normal human erythroid progenitor cells and erythroblasts, the results suggest that FKHRL1 plays an important role in erythropoiesis as one of the downstream target molecules of PI3K-Akt.
Erythropoietin (EPO) is the major regulator of the proliferation and differentiation of erythroid progenitors.1 EPO exerts its action through interaction with its receptor (EPOR). Recent studies have revealed that several transducing molecules including Jak2 tyrosine kinase, signal transducers and activators of transcription (Stat) proteins, p85 subunit of phosphatidylinisitol 3-kinase (PI3K), mitogen-activated protein kinases (MAPKs), and phospholipase C-γ1 are activated by EPO-EPOR interaction.2-5 However, the biologic significance of the postreceptor signaling pathways is still not fully understood. Especially, the mechanism by which EPO inhibits DNA breakdown and prevents apoptosis in erythroid progenitor cells remains unknown.6
Akt, the serine/threonine kinase protein kinase B (PKB), was identified as a downstream component in survival signaling through PI3K.7 It was shown that Akt phosphorylates the proapoptotic factor, Bad, on a serine residue and the phosphorylated Bad dissociates from a cell survival factor, Bcl-XL, resulting in protection from apoptosis.8-10 Subsequently, it was reported that Akt-induced phosphorylation of a death protease called caspase-9 prevents apoptosis by inhibiting the protease activity directly.11 Moreover, Akt induces the degradation of IκB by promoting IKKα activity and subsequently increases the activity of nuclear factor-κB (NF-κB), leading to increased synthesis of antiapoptotic proteins.12,13 Thus, these observations indicate that Akt is a key factor of cell survival. Very recently, Bao and coworkers reported that EPO induced activation of Akt in the murine EPO-responsive cell line HCD57.14 In addition, they also found that constitutive activation of Akt occurred in an apoptosis-resistant HCD57 subclone.14 Moreover, Haseyama and associates reported that the PI3 kinase inhibitor LY294002 induced apoptosis of human erythroid progenitor cells, accompanied by suppression of Akt kinase activity.15 Collectively, these observations suggested that Akt and its downstream molecules play some role in the EPO-induced antiapoptotic effect. Therefore, it would be important to identify downstream target molecule(s) of Akt kinase in the EPO signaling pathway.
Recently it was shown that members of the transcription factor Forkhead family (FH), AFX, FKHR, and FKHRL1, are substrates of Akt kinase in vitro.16-20 These members are mammalian counterparts of DAF-16, which plays an important role in regulating the life span ofCaenorhabditis elegans.21 When AFX, FKHR, and FKHRL1 are phosphorylated by Akt in the presence of survival factors, they are retained in the cytoplasm and interact with 14-3-3 proteins. On the other hand, when these molecules become nonphosphorylated in the absence of survival factors, they translocate into nucleus and activate the transcription of target genes.16-20 Thus, Akt negatively regulates the transcription activity of AFX, FKHR, and FKHRL1 by phosphorylation. Because the Fas ligand (FasL) promoter contains 3 FH-responsive elements that bind FKHRL1, the nonphosphorylated form of FKHRL1 can activate the FasL promoter in vitro and indeed induce apoptosis in cerebellar neurons, fibroblasts, and Jurkat T lymphoma.16 Thus, the nonphosphorylated form of FKHRL1 triggers apoptosis at least in part by a FasL-dependent mechanism. Considering that FasL messenger RNA (mRNA) is induced to express at the stage of erythroid maturation,22 23 FKHRL1 may play a role in the antiapoptotic effect of EPO on erythroid cells, although it has never been reported that FKHRL1 is expressed in erythroid cells.
The purpose of the study was to elucidate the involvement of PI3K-Akt-FKHRL1 activation pathway in the EPO signal transduction. We show here that EPO activates Akt kinase in a human erythroleukemia cell line, UT-7/EPO, which is absolutely dependent on EPO for growth and survival.24 In addition, we identify FKHRL1 as one of the downstream target molecules of Akt kinase in the EPO signaling pathway. Finally, we demonstrate that Akt and FKHRL1 are expressed and actually phosphorylated by EPO in primary erythroid cells. Collectively, these results suggest a potential role for FKHRL1 in hematopoiesis, at least in erythropoiesis.
Materials and methods
Hematopoietic growth factors and reagents
Recombinant human EPO was a gift from the Life Science Research Institute of Snow Brand Milk Company (Tochigi, Japan). A polyclonal antibody against Akt (C-20) was purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Antibodies against phosphothreonine 32 (T32) FKHRL1, phosphoserine 253 (S253) FKHRL1, and native FKHRL1, and GST-FKHRL1 fusion protein were kindly provided by Dr Anne Brunet (Children's Hospital, Harvard Medical School, Boston, MA).16 A monoclonal antibody (MoAb) raised against human FasL was purchased from Pharmingen/Transduction Laboratories (San Diego, CA). Phosphoplus AKT (T308 and Ser473) Antibody Kits and AKT Kinase Assay Kit were purchased from New England BioLabs Inc (Beverly, MA). MEBCYTO-Apoptosis Kit was purchased from MBL (Nagoya, Japan). Neomycin (G418) was purchased from Gibco BRL Life Technologies (Gaithersburg, MD). Wild-type (WT) Akt and pleckstrin homology (PH) domain Akt mutant (E40K) complimentary DNAs (cDNAs) were kindly provided by Dr Alfonso Bellacosa (Fox Chase Cancer Research Center, Philadelphia, PA).25
Cell culture of UT-7/EPO cell line and generation of transfectants
UT-7/EPO was maintained in liquid culture with Iscove's modified Dulbecco's medium (IMDM; Gibco Laboratories, Grand Island, NY) containing 10% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT) and 1 U EPO/mL.24 UT-7/EPO cells were transfected with mammalian expression vector (pcDNA3.1; Invitrogen, Carlsbad, CA) alone or pcDNA3.1 containing human WT Akt or E40K by the lipofectin method according to the manufacturer's instructions (Promega, Madison, WI). We selected 3 independent clones resistant to neomycin (1.0 mg/mL).
Colorimetric MTT assay for cell proliferation
Cell growth was examined by a colorimetric assay according to Mosmann with some modifications.26 Briefly, cells were incubated at a density of 1 × 104/100 μL in 96-well plates in IMDM containing 10% FCS in the presence of EPO (10 U/mL). After 72 hours of culture at 37°C, 20 μL of sterilized 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, St Louis, MO) was added to each well. Following 2-hour incubation at 37°C, 100 μL of 10% sodium dodecyl sulfate (SDS) was added to each well to dissolve the dark-blue crystal product. The optical density was measured at a wavelength of 595 nm using a microplate reader (model 3550; Bio Rad, Richmond, CA).
Preparation of cell lysates, immunoprecipitation, and Western blotting
The UT-7/EPO cells were deprived of growth factor for 24 hours. After stimulation with EPO at 37°C for a given period, cells were washed and suspended in lysis buffer composed of 20 mmol/L Tris (pH 7.4), 137 mmol/L NaCl, 10% glycerol, 1% NP-40, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 15 μg/mL aprotinin, and 2 mmol/L sodium orthovanadate. After 20 minutes of incubation on ice, insoluble materials were removed by centrifugation at 15 000gfor 10 minutes. The supernatants were immunoprecipitated with anti-Akt (C-20) attached to protein G sepharose for 4 hours at 4°C in an Eppendorf shaker. Immunoprecipitates were collected by a brief centrifugation and washed 4 times with 1 mL of lysis buffer. The immunoprecipitated proteins were boiled for 5 minutes in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer. After a brief centrifugation, the supernatants were resolved by SDS-PAGE and electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Bio Rad). The blots were blocked with 5% skim milk in Tris-buffered saline (TBS) for 1 hour, then incubated with the appropriate concentration of primary antibodies including antiphospho Akt (S473) polyclonal antibody overnight at 4°C. After a wash with TBS containing Tween 20, 1:1000, the blots were probed with a 1:2000 dilution of antirabbit horseradish peroxidase-conjugated secondary antibodies for 20 minutes at room temperature (RT). After a second wash, the blots were incubated with an enhanced chemiluminescence substrate according to the instruction manual (New England BioLabs). In some experiments, the supernatants were boiled for 5 minutes in SDS-PAGE sample buffer containing 20 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1% NP-40, 5 mmol/L EDTA, 1 mmol/L PMSF, 15 μg/mL aprotinin, 20 μg/mL leupeptin, 2 mmol/L sodium orthovanadate, and 20 mmol/L sodium fluoride. After a brief centrifugation, the supernatants were resolved by SDS-PAGE, then electroblotted onto a PVDF membrane. The blots were blocked with 5% skim milk in TBS for 1 hour at RT, then incubated with the appropriate concentration of primary antibody against phospho-T32 FKHRL1, phospho-S253 FKHRL1, native FKHRL1, or FasL overnight at 4°C. After washing with TBS containing Tween 20, 1:2000, the blots were probed with a 1:5000 dilution of antirabbit or antimouse horseradish peroxidase-conjugated second antibodies for 90 minutes at RT. After a second wash, the blots were incubated with an enhanced chemiluminescence substrate (Amersham, Buckinghamshire, England) and exposed to Hyperfilm ECL to visualize immunoreactive bands. The blots were stripped with 62.5 mmol/L Tris-HCI, pH 6.8, 2% SDS, and 100 mmol/L β-mercaptoethanol (ME) at 50°C for 30 minutes, washed, blocked, and reprobed.
In vitro kinase assay
Immunoprecipitates were washed 3 times with lysis buffer, and once with the Akt kinase buffer: 20 mmol/L HEPES-NaOH (pH7.4), 10 mmol/L MgCl2, and 10 mmol/L MnCl2. The kinase assays were carried out in the presence of 10 μCi [γ-32P] adenosine triphosphate (ATP) (3000 Ci/mmol, NEN Research Products, Boston, MA) and 30 μL of the Akt kinase buffer. The exogenous substrate histone H2B was resuspended in water at a concentration of 5 mg/mL, then used at a concentration of 0.1 mg/mL. The reaction mixtures were incubated at RT for 30 minutes and stopped by the addition of 30 μL of Laemmli sample buffer. Reaction products were resolved by SDS-PAGE and visualized by autoradiography. In vitro kinase experiments were also performed by a commercial kit (AKT Kinase Assay Kit) using glycogen synthase kinase-3 (GSK-3) or GST-FKHRL1 fusion protein16 as another substrate of Akt.
Detection of apoptotic cells
Apoptotic cells were detected according to the manufacturer's instructions. In brief, the cells were washed with phosphate-buffered saline (PBS) and resuspended in binding buffer. After 15 minutes of incubation with Annexin V-FITC and propidium iodide, the cell samples were measured by flow cytometry (FACScan, Becton Dickinson, Franklin Lakes, NJ) using a single laser emitting excitation light at 488 nm.
Purification of erythroid progenitor cells and erythroblasts
Erythroid progenitor cells (colony-forming unit erythroid; CFU-E) and erythroblasts were purified as described previously.27In brief, recombinant human granulocyte colony-stimulating factor (G-CSF; Chugai Pharmaceutical Co and Kyowa Hakko Pharmaceutical Co, Tokyo, Japan) was administered to healthy subjects who previously signed consent forms approved by the Hokkaido University School of Medicine and the Hokkaido Red Cross Blood Center Committee for the Protection of Human Subjects. The mobilized peripheral blood (PB) CD34+ cells were isolated using immunomagnetic beads. The cells were then cryopreserved and stored until use in liquid nitrogen. The frozen PB CD34+ cells were thawed, suspended in IMDM containing 30% FCS and 100 U/mL DNase, and then centrifuged at 400g for 5 minutes at 4°C. The cells were washed twice with IMDM containing 20% FCS and then resuspended in IMDM containing 0.3% deionized bovine serum albumin (BSA). The cells were next cultured in liquid phase as described elsewhere. In brief, cells at 0.5 × 104 to 2.0 × 104 cells/mL were suspended in a mixture containing 20% FCS, 10% heat-inactivated pooled human AB serum, 1% BSA, 10 μg/mL insulin, 10 μg/mL vitamin B12, 15 μg/mL folic acid, 100 U/mL interleukin (IL)-3, 100 ng/mL stem cell factor (SCF), and 4 U/mL EPO in the presence of 5 × 10−5 mol/mL β-ME, 50 U/mL penicillin, 50-U/mL streptomycin, and IMDM in a 50-mL polystyrene flask (Corning Coster Corp, Cambridge, MA). After incubation for the periods indicated at 37°C in a 5% CO2/95% O2atmosphere, the cells were collected and washed twice with IMDM containing 0.3% BSA.
Reverse transcription-polymerase chain reactions (RT-PCRs)
The RT-PCRs were performed using oligonucleotide primers as follows. The cDNA was synthesized by reverse transcription using a commercial kit (Roche Molecular Biochemicals, Mannheim, Germany). Total cellular RNA (1 μg) isolated from cells according to the method of Chomczynski and Sacchi28 was reverse transcribed using oligo-dT primers followed by 35 PCR amplification cycles (94°C for 20 seconds, primer annealing at 56°C for 30 seconds, extension at 72°C for 40 seconds) in a Perkin-Elmer Cetus Thermal Cycler (GeneAmp PCR System 9600), and a final incubation at 60°C for 7 minutes. Amplification products were separated on 2% agarose TAE gels stained with ethidium bromide and photographed. The FKHRL1 forward 5′-ATG AGG GAA CTG GCA AGA G-3′ (nucleotides 1598-1616) and reverse 5′-GAG AGC TGG GAG GGA CTG T-3′ (nucleotides 1790-1772) primers amplified a 193-bp fragment of the FKHRL1cDNA.29 FKHRL1 is 99% identical to pseudogene FKHRL1P1 over about 2.4 kb of related sequence.29 To distinguish between FKHRL1 and FKHRL1P1, PCR products (193 bp) were purified with a QIAquick PCR purification kit (Qiagen Inc, Tokyo, Japan) and then digested with HhaI. The HhaI restriction site is present only in FKHRL1 and the digestion results in 2 bands (68 and 125 bp in size).29
Results
EPO-induced phosphorylation of Akt in a dose- and time-dependent manner
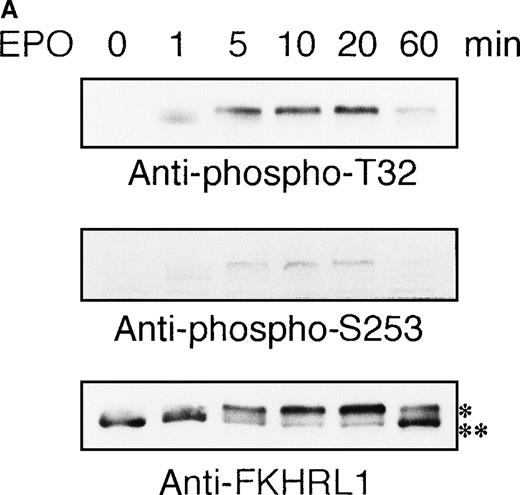
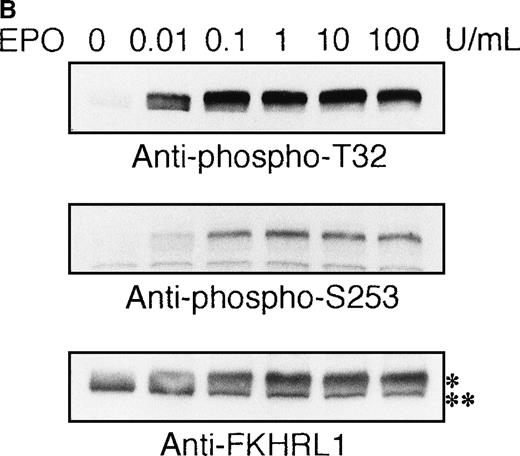
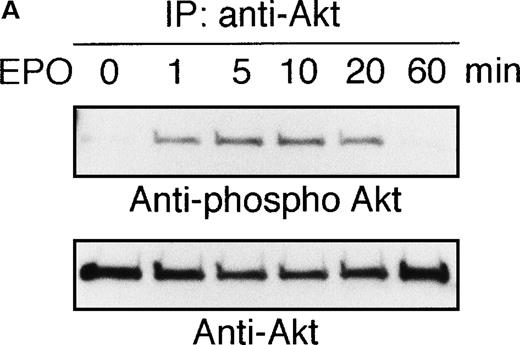
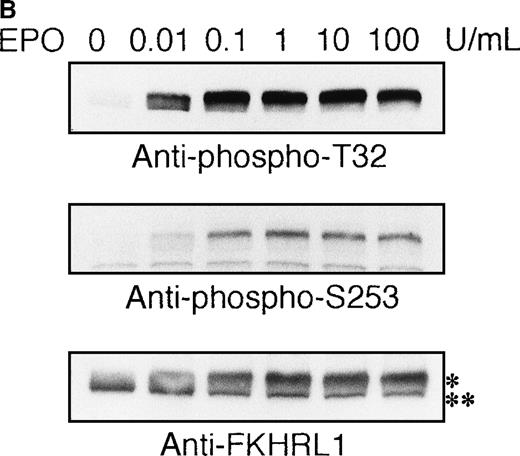
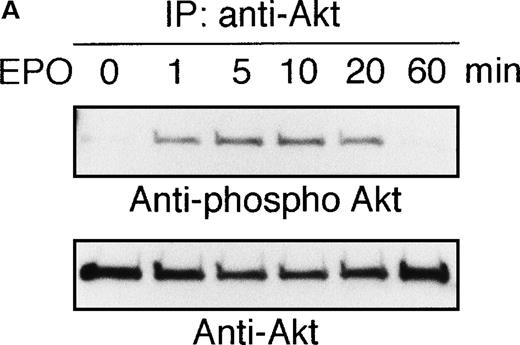
To elucidate the functional role of Akt in EPO-induced prevention of apoptosis, we initially examined whether or not EPO activates Akt kinase using a human leukemia cell line, UT-7/EPO. Growth factor-deprived UT-7/EPO cells were exposed to EPO (10 U/mL) for given periods of up to 60 minutes and then harvested for immunoprecipitation with anti-Akt antibody. Western blotting analysis was performed using anti-phosphoAkt (Ser473) antibody that recognizes a phosphorylated serine 473, 1 of 2 sites on Akt phosphorylated in its active form. As shown in Figure 1A, phosphorylated Akt appeared within 1 minute, and its level reached a maximum at 5 to 10 minutes and had diminished by 60 minutes. Moreover, growth factor-deprived UT-7/EPO cells were exposed to increasing concentrations of EPO (0.01-100 U/mL). As shown in Figure 1B, phosphorylated Akt was detected at 0.1 U/mL of EPO, and the level reached a plateau at 1 U/mL of EPO. These findings indicate that EPO induced phosphorylation of Akt in a dose- and time-dependent manner.
EPO induces serine phosphorylation of Akt protein in time- and dose-dependent fashions in UT-7/EPO cells.
EPO was removed from UT-7/EPO cells for 24 hours. The cells were then stimulated with EPO (10 U/mL) for the periods indicated (A), or with increasing concentrations of EPO (0.01-100 U/mL) for 10 minutes (B). After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Immunoprecipitates were eluted with buffer containing SDS and resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane. Upper panel: immunoblotting with antiphospho Akt antibody. Lower panel: the blot was reprobed with anti-Akt serum to confirm equal loading of protein.
EPO induces serine phosphorylation of Akt protein in time- and dose-dependent fashions in UT-7/EPO cells.
EPO was removed from UT-7/EPO cells for 24 hours. The cells were then stimulated with EPO (10 U/mL) for the periods indicated (A), or with increasing concentrations of EPO (0.01-100 U/mL) for 10 minutes (B). After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Immunoprecipitates were eluted with buffer containing SDS and resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane. Upper panel: immunoblotting with antiphospho Akt antibody. Lower panel: the blot was reprobed with anti-Akt serum to confirm equal loading of protein.
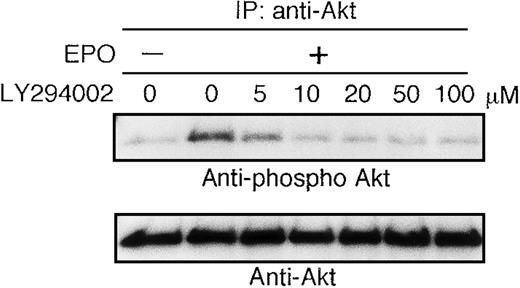
EPO-induced phosphorylation of Akt is absolutely dependent on PI3K activity
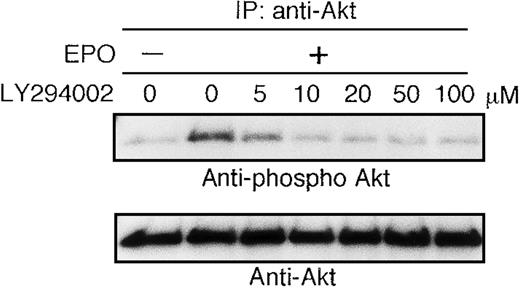
The growth factor-deprived UT-7/EPO cells were pretreated with increasing concentrations of the PI3K-specific inhibitor LY294002 (5-100 μmol/L) for 45 minutes and then stimulated with EPO (10 U/mL). Ten minutes later, the cells were harvested for immunoprecipitation with anti-Akt antibody. Western blotting analysis was performed using antiphospho Akt (S473) antibody. As shown in Figure2, the phosphorylation density of Akt was slightly diminished at 5 μmol/L of LY294002 and equal to the basal level at 10 μmol/L of LY294002. This result suggested that EPO-induced phosphorylation of Akt is perfectly mediated via PI3K activity.
Activation of AKT by EPO is absolutely dependent on PI3K activity.
EPO was removed from UT-7/EPO cells for 24 hours. The cells were pretreated with increasing concentrations of LY294002 (10-100 μmol/L) and then stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Immunoprecipitates were eluted with buffer containing SDS and resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane. Upper panel: immunoblotting with antiphospho Akt antibody. Lower panel: the blot was reprobed with anti-Akt serum to confirm equal loading of protein.
Activation of AKT by EPO is absolutely dependent on PI3K activity.
EPO was removed from UT-7/EPO cells for 24 hours. The cells were pretreated with increasing concentrations of LY294002 (10-100 μmol/L) and then stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Immunoprecipitates were eluted with buffer containing SDS and resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane. Upper panel: immunoblotting with antiphospho Akt antibody. Lower panel: the blot was reprobed with anti-Akt serum to confirm equal loading of protein.
In vitro kinase assay revealed that EPO induces activation of Akt
To confirm the activation of Akt by EPO, we performed in vitro kinase assay using histone H2B and GSK-3 as substrates (Figure3). After 10 minutes of exposure to EPO (10 U/mL), the cells were immunoprecipitated with anti-Akt antibody for in vitro kinase assay. As shown in Figure 3A, a radiolabeled band of histone H2B was clearly enhanced by EPO treatment. In addition, when we used GSK-3 as a substrate of Akt, phosphorylated GSK-3 was detected by EPO treatment and its density was returned to the basal level by treatment with LY294002 (50 μmol/L). These observations indicate that EPO indeed induced Akt kinase activation via PI3 kinase activity.
In vitro kinase assay revealed that AKT kinase is activated by EPO stimulation.
(A) EPO was removed from UT-7/EPO cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (B) EPO was removed from UT-7/EPO cells for 24 hours, then the cells were incubated with 50 μmol/L LY294002 for 45 minutes and stimulated with EPO (10 U/mL) for 10 minutes. Cells were lysed and immunoprecipitated with anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 200 μmol/L ATP using GSK-3 as a substrate (0.025 mg/mL) . The reactions were incubated at 30°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and immunoblotted with antiphospho GSK-3 antibody.
In vitro kinase assay revealed that AKT kinase is activated by EPO stimulation.
(A) EPO was removed from UT-7/EPO cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (B) EPO was removed from UT-7/EPO cells for 24 hours, then the cells were incubated with 50 μmol/L LY294002 for 45 minutes and stimulated with EPO (10 U/mL) for 10 minutes. Cells were lysed and immunoprecipitated with anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 200 μmol/L ATP using GSK-3 as a substrate (0.025 mg/mL) . The reactions were incubated at 30°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and immunoblotted with antiphospho GSK-3 antibody.
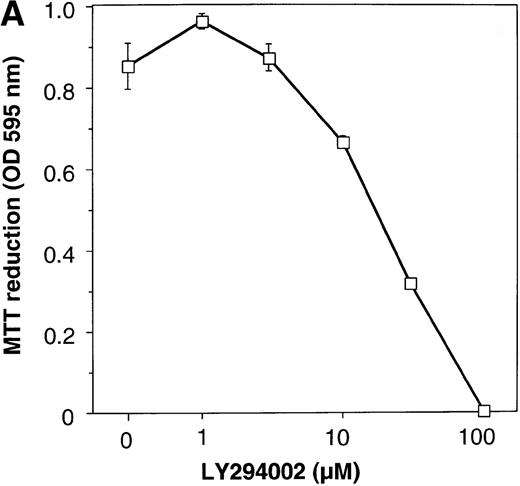
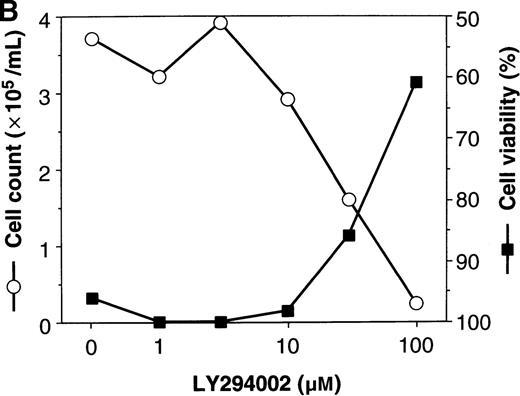
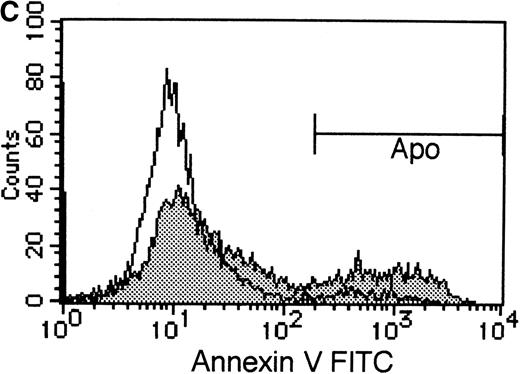
PIK3-Akt pathway is in part involved in the survival effect of EPO on erythroid cells
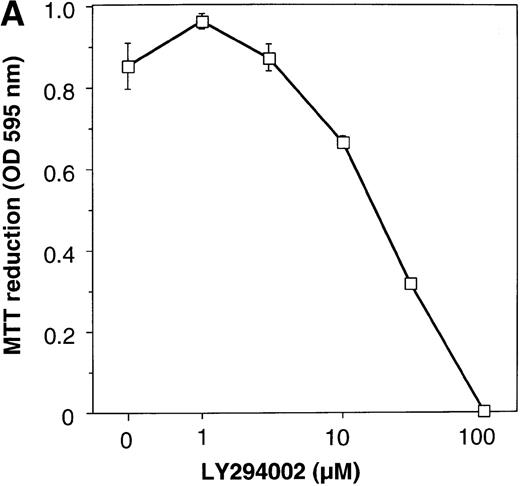
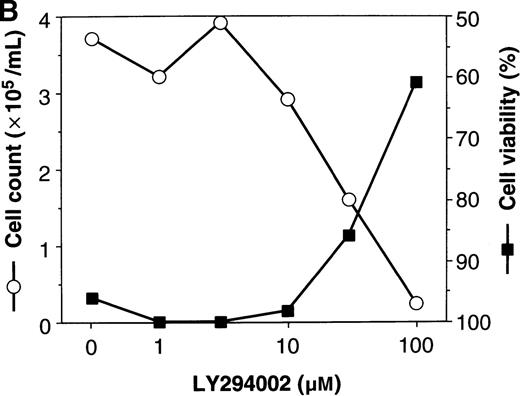
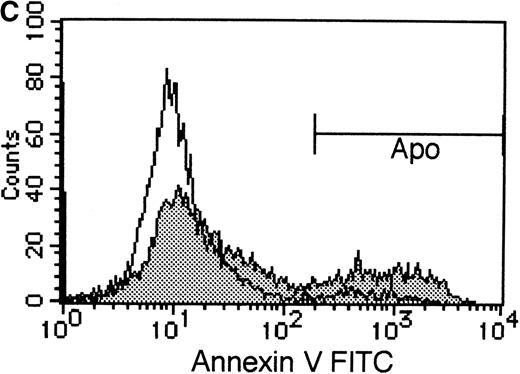
To elucidate the biologic role of PI3K-Akt activation in the EPO signaling pathway, we examined the effect of LY294002 on the proliferation and survival of UT-7/EPO cells. As shown in Figure4A, LY294002 suppressed the MTT incorporation into UT-7/EPO cells in a dose-dependent manner after 3 days of culture. Consistent with this result, the number of viable cells significantly decreased on treatment with increasing concentrations of LY294002 (Figure 4B). At 100 μmol/L of LY294002, cell viability was about 60%. To examine whether or not the decrease in cell viability is due to apoptosis, we detected apoptotic cells by FACS analysis with annexin-V-FITC. As shown in Figure 4C, more than 20% of cells were positive for annexin-V, suggesting that a high dose of LY294002 (100 μmol/L) induced apoptosis in UT-7/EPO cells. These observations suggested that EPO exerts its effect on cell survival at least in part via the PI3K-Akt activation pathway.
Inhibitory effect of increasing concentrations of LY294002 on EPO-induced proliferation and anti-apoptosis in UT-7/EPO cells.
UT-7/EPO cells were plated at a density of 10 000 cells/well in IMDM supplemented with 5% FCS and cultured with increasing concentrations of LY294002 in the presence of 1 U/mL of EPO. MTT reduction assay (A) and cell counting (B) were performed after 3 days of culture. Cell viability was assessed by trypan blue dye exclusion (B). The values represent the mean ± SD from triplicate cultures. (C) Induction of apoptosis by LY294002. UT-7/EPO cells were cultured with 100 μmol/L of LY294002 in the presence of EPO (1 U/mL). Three days later, the cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan.
Inhibitory effect of increasing concentrations of LY294002 on EPO-induced proliferation and anti-apoptosis in UT-7/EPO cells.
UT-7/EPO cells were plated at a density of 10 000 cells/well in IMDM supplemented with 5% FCS and cultured with increasing concentrations of LY294002 in the presence of 1 U/mL of EPO. MTT reduction assay (A) and cell counting (B) were performed after 3 days of culture. Cell viability was assessed by trypan blue dye exclusion (B). The values represent the mean ± SD from triplicate cultures. (C) Induction of apoptosis by LY294002. UT-7/EPO cells were cultured with 100 μmol/L of LY294002 in the presence of EPO (1 U/mL). Three days later, the cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan.
To further elucidate the role of Akt in EPO signaling, we generated transfectant cells expressing constitutively active Akt (E40K) or WT Akt. E40K is a PH domain Akt mutant that exhibits enhanced basal kinase activity, but also responds to physiologic stimuli.25 We selected at least 3 independent clones by expression of tag protein HA (Figure 5A and data not shown). In these transfectant cells, we performed in vitro kinase assay using histone H2B as a substrate of Akt kinase. As shown in Figure 5B, even in the absence of EPO, a strongly phosphorylated band was detected in UT-7/EPO-E40K but not in UT-7/EPO-WT cells, indicating that E40K Akt mutant exhibited enhanced basal activity in UT-7/EPO-E40K cells. In addition, this mutant responded to physiologic stimuli (in this study EPO) as reported previously.25 Using these transfectant cells, we evaluated the ratio of viable cells after deprivation of EPO for 5 days. As shown in Figure 5C, although the majority of the cells expressing WT or vector alone died on the fifth day of culture, about 40% of the UT-7/EPO-E40K cells survived. This observation suggested that constitutively active Akt could in part prevent the cell death induced by withdrawal of EPO from UT-7/EPO cells. In addition, FACS analysis with annexin-V-FITC revealed that the ratio of apoptotic cells significantly decreased in UT-7/EPO-E40K cells, compared with parent UT-7/EPO cells and UT-7/EPO-WT cells (Figure 5D). Thus, our results indicate that Akt kinase plays some role in EPO-dependent cell survival of UT-7/EPO cells.
Activated form of Akt prevents cell death induced by withdrawal of EPO.
(A) Generation of transfectant cells expressing HA-tagged Akt. The transfectant cells and the parent cells were harvested for detection of HA-tagged Akt proteins. Cell lysates were resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane and immunoblotted with anti HA-antibody. (B) EPO was removed from the transfectant cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (C) EPO was removed from the culture medium and the cell viability was assessed by trypan dye exclusion during the observation periods. (D) EPO was removed from the culture medium and the transfectant cells were cultured without any growth factors (shade). Three days later, cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan. The cells cultured with EPO (1 U/mL) were used as a negative controls (light).
Activated form of Akt prevents cell death induced by withdrawal of EPO.
(A) Generation of transfectant cells expressing HA-tagged Akt. The transfectant cells and the parent cells were harvested for detection of HA-tagged Akt proteins. Cell lysates were resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane and immunoblotted with anti HA-antibody. (B) EPO was removed from the transfectant cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (C) EPO was removed from the culture medium and the cell viability was assessed by trypan dye exclusion during the observation periods. (D) EPO was removed from the culture medium and the transfectant cells were cultured without any growth factors (shade). Three days later, cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan. The cells cultured with EPO (1 U/mL) were used as a negative controls (light).
FKHRL1 is one of the target molecules of AKT kinase activated by EPO
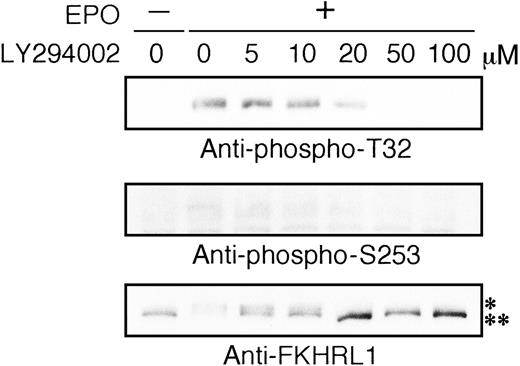
Most recently, it was found that human FKHRL1 is directly phosphorylated by Akt kinase in vitro and in vivo.16 Based on this observation, we examined whether or not EPO induced phosphorylation of FKHRL1 protein using antiphospho T32 and antiphospho S253 antibodies. As shown in Figure 6A, FKHRL1 was phosphorylated at T32 and S253 at 1 minute, the level reaching a plateau at 5 to 20 minutes and declining thereafter. Phosphorylation of FKHRL1 was observed at 0.01 U/mL of EPO and the level reached a plateau at 0.1 U/mL of EPO (Figure 6B). LY294002 inhibited phosphorylation of FKHRL1 in a dose-dependent manner. Phosphorylation of FKHRL1 was slightly suppressed at 20 μmol/L and completely suppressed at 50 μmol/L of LY294002 (Figure7), suggesting that phosphorylation of FKHRL1 is absolutely dependent on PI3K activity. This was supported by the evidence that MEK1 inhibitor PD98059 did not inhibit the phosphorylation of FKHRL1 by EPO (data not shown). In addition, even in the absence of EPO, FKHRL1 phosphorylated at T32 and S253 was detected in UT-7/EPO-E40K cells but not in parent UT-7/EPO and UT-7/EPO-WT cells (data not shown). Taken together, these findings strongly suggested that FKHRL1 is one of the downstream target molecules of Akt kinase in the EPO signaling pathway.
EPO induces phosphorylation of FKHRL1 protein in time- and dose-dependent fashions in UT-7/EPO cells.
EPO was removed from UT-7/EPO cells for 24 hours. The cells were then stimulated with EPO (10 U/mL) for the periods indicated (A), or with increasing concentrations of EPO (0.01-100 U/mL) for 10 minutes (B). After solubilization, cell extracts were resolved by 7.5% SDS-PAGE and immunoblotted with the antibodies directed against phospho-T32 (top panel) or phospho-S253 (middle panel). The blot was reprobed with anti-FKHRL1 antibody to confirm equal loading of protein (bottom panel). Anti-FKHRL1 antibody recognizes 2 bands; upper band (*) is the phosphorylated form, and the lower band (**), the unphosphorylated form.
EPO induces phosphorylation of FKHRL1 protein in time- and dose-dependent fashions in UT-7/EPO cells.
EPO was removed from UT-7/EPO cells for 24 hours. The cells were then stimulated with EPO (10 U/mL) for the periods indicated (A), or with increasing concentrations of EPO (0.01-100 U/mL) for 10 minutes (B). After solubilization, cell extracts were resolved by 7.5% SDS-PAGE and immunoblotted with the antibodies directed against phospho-T32 (top panel) or phospho-S253 (middle panel). The blot was reprobed with anti-FKHRL1 antibody to confirm equal loading of protein (bottom panel). Anti-FKHRL1 antibody recognizes 2 bands; upper band (*) is the phosphorylated form, and the lower band (**), the unphosphorylated form.
Phosphorylation of FKHRL1 by EPO is absolutely dependent on PI3K activity.
EPO was removed from UT-7/EPO cells for 24 hours. The cells were pretreated with increasing concentrations of LY294002 (1-100 μmol/L) and then stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell extracts were resolved by 7.5% SDS-PAGE and immunoblotted with the antibodies directed against phospho-T32 (top panel) or phospho-S253 (middle panel). The blot was reprobed with anti-FKHRL1 antibody to confirm equal loading of protein (bottom panel). Anti-FKHRL1 antibody recognizes 2 bands; upper band (*) is the phosphorylated form, and the lower band (**), the unphosphorylated form.
Phosphorylation of FKHRL1 by EPO is absolutely dependent on PI3K activity.
EPO was removed from UT-7/EPO cells for 24 hours. The cells were pretreated with increasing concentrations of LY294002 (1-100 μmol/L) and then stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell extracts were resolved by 7.5% SDS-PAGE and immunoblotted with the antibodies directed against phospho-T32 (top panel) or phospho-S253 (middle panel). The blot was reprobed with anti-FKHRL1 antibody to confirm equal loading of protein (bottom panel). Anti-FKHRL1 antibody recognizes 2 bands; upper band (*) is the phosphorylated form, and the lower band (**), the unphosphorylated form.
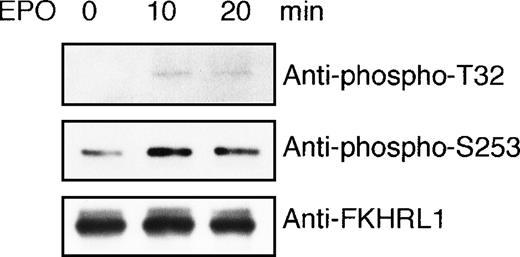
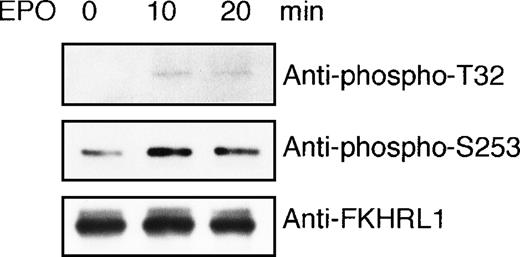
To demonstrate that FKHRL1 is directly targeted by AKT kinase activated by EPO in vivo, we performed an in vitro kinase assay using GST-FKHRL1 fusion protein as a substrate. EPO-deprived UT-7/EPO cells were exposed to EPO for 10 minutes and then harvested for immunoprecipitation with anti-Akt antibody. Immunoprecipitates were incubated with GST-FKHRL1 fusion protein according to the instructions of the AKT Kinase Assay Kit. As shown in Figure 8, a phosphorylated FKHRL1 band was obtained with antibody that recognizes phosphorylated T32 and phosphorylated S253, respectively, indicating that Akt activated by EPO directly phosphorylated FKHRL1 at T32 and S253.
FKHRL1 is directly phosphorylated by EPO-activated Akt.
(A) EPO was removed from UT-7/EPO cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 and 20 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 200 μmol/L ATP using GST-FKHRL1 fusion protein as a substrate (25 μg/mL). The reactions were incubated at 30°C for 30 minutes. Reaction products were resolved by 7.5% SDS-PAGE and immunoblotted with the antibodies directed against phospho-T32 (top panel) or phospho-S253 (middle panel). The blot was reprobed with anti-FKHRL1 antibody to confirm equal loading of protein (bottom panel).
FKHRL1 is directly phosphorylated by EPO-activated Akt.
(A) EPO was removed from UT-7/EPO cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 and 20 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 200 μmol/L ATP using GST-FKHRL1 fusion protein as a substrate (25 μg/mL). The reactions were incubated at 30°C for 30 minutes. Reaction products were resolved by 7.5% SDS-PAGE and immunoblotted with the antibodies directed against phospho-T32 (top panel) or phospho-S253 (middle panel). The blot was reprobed with anti-FKHRL1 antibody to confirm equal loading of protein (bottom panel).
FKHRL1 is present in normal erythroid progenitor cells and erythroblasts
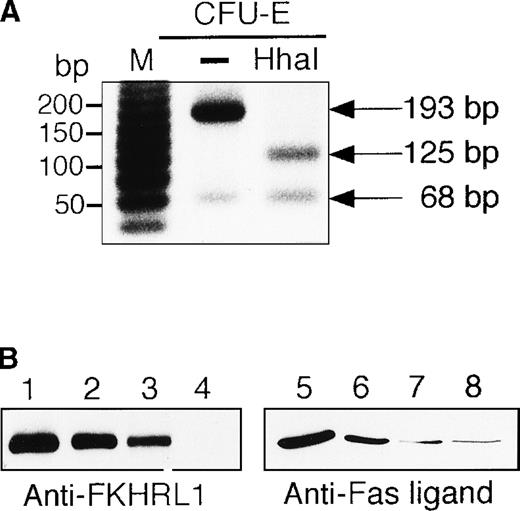
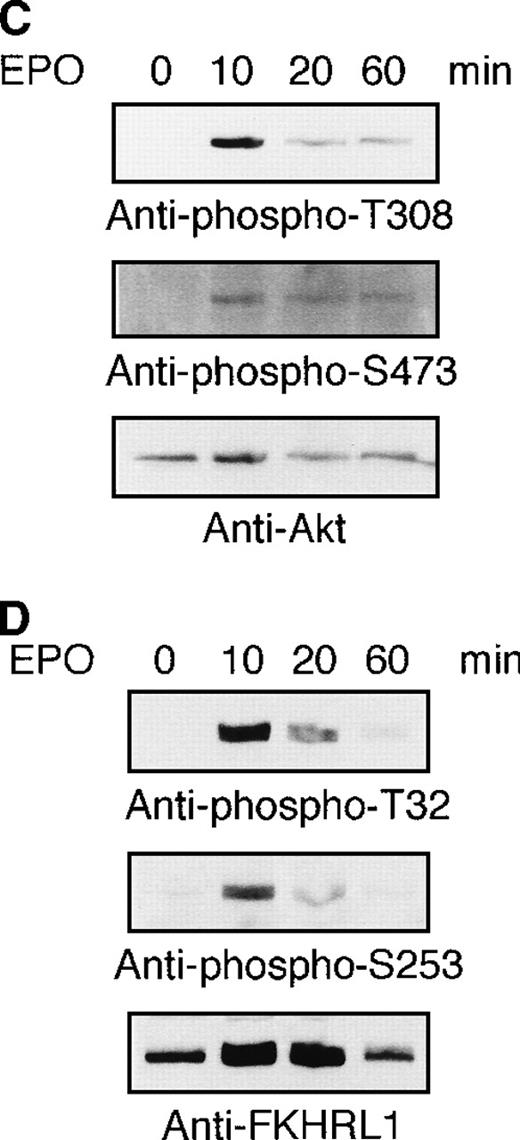
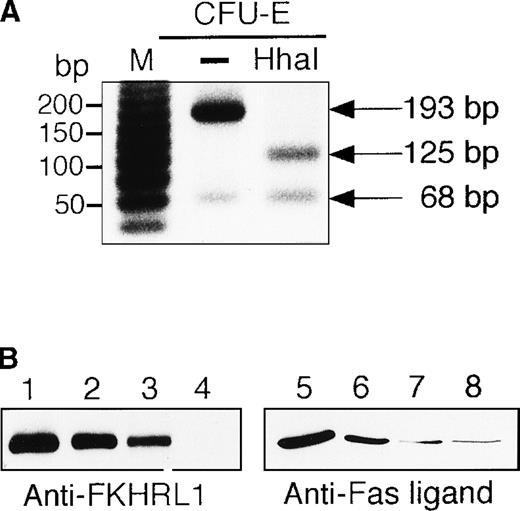
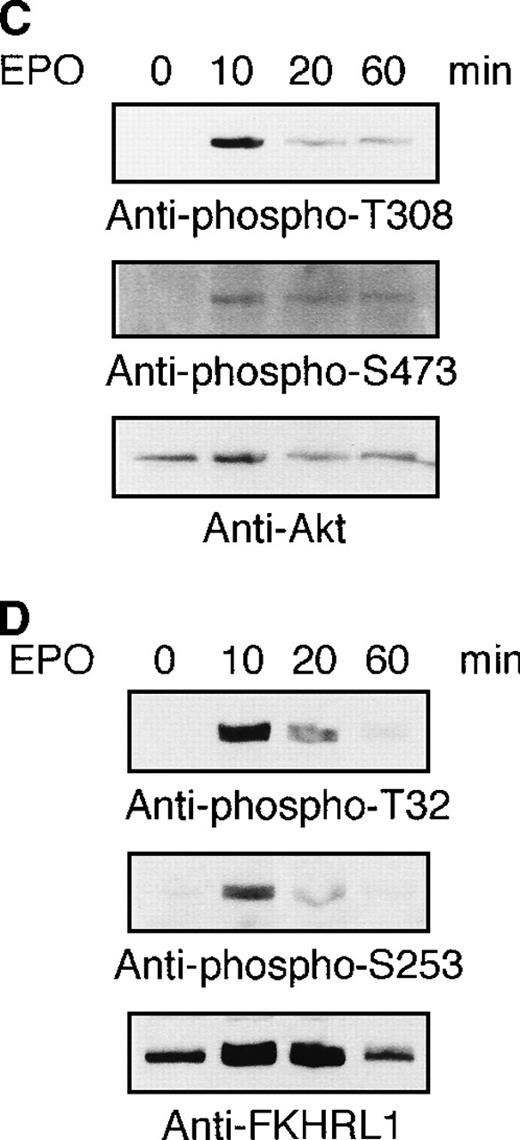
To examine whether or not the FKHRL1 is present in normal erythroid cells, human CD34+ cells were cultured in the presence of IL-3, SCF, and EPO. Erythroid progenitor cells and erythroblasts were isolated from the cultures at the periods indicated (Table1). To detect FHKRL1 mRNA from a small amount of the cells, we used RT-PCR. However, because there is extremely high DNA conservation between FKHRL1 and FKHRL1P1, we chose to amplify a region where FKHRL1 has a single base difference and gives rise to a HhaI restriction site.29 As shown in Figure9A, RT-PCR products (193 bp) were detected in normal CFU-E progenitor cells (day 7), and the products were divided into 2 bands (68 and 125 bp in size) after HhaI digestion, indicating that FKHRL1 mRNA was actually expressed in erythroid progenitor cells. In addition, Western blot analysis with anti-FKHRL1 antibody revealed that FKHRL1 protein is indeed expressed in normal erythroid progenitor cells and immature erythroblasts but not in orthochromatic erythroblasts (Figure 9B and Table 1). Concomitantly, FasL protein was highly expressed in normal erythroid progenitor cells and faintly expressed in relatively mature erythroblasts including orthochromatic erythroblasts (Figure 9B; lanes 7 and 8). To confirm that Akt and its downstream molecule FKHRL1 are indeed phosphorylated in primary erythroid cells, we prepared CFU-E cells from normal volunteers as described in “Materials and methods”. After 2 hours of deprivation of growth factors, the cells were stimulated with EPO (10 U/mL) for the periods indicated and then harvested for Western blotting analysis. As shown in Figure9C and D, Akt and FKHRL1 were phosphorylated in a time-dependent manner, as in the case for UT-7/EPO cells.
Detection of FKHRL1 in primary erythroid progenitor cells and erythroblasts.
(A) CFU-E cells were obtained as described in “Materials and methods.” Total cellular RNA extracted was reverse transcribed and amplified by RT-PCR (see “Materials and methods”). The PCR products were digested with HhaI and resolved by agarose/formaldehyde gel electrophoresis and the gel was stained with ethidium bromide. (B) Fraction 1 (lanes 1 and 5), fraction 2 (lanes 2 and 6), fraction 3 (lanes 3 and 7), and fraction 4 (lanes 4 and 8) were obtained as described in “Materials and methods” and Table 1. After solubilization, cell extracts (2 × 106 cells/1 lane) were resolved by 7.5% or 15% SDS-PAGE and immunoblotted with anti-FKHRL1 antibody (left panel) or anti-FasL antibody (right panel). (C) Phosphorylation of Akt by EPO stimulation in CFU-E cells. CFU-E cells were obtained as described in “Materials and methods” and subsequently cultured without EPO for 2 hours. The cells were then stimulated with EPO (10 U/mL) for the periods indicated. After solubilization, cell extracts (2 × 106 cells/1 lane) were resolved by 7.5% SDS-PAGE and immunoblotted with Akt antibodies directed against phospho-T308 (top panel) or phospho-S473 (middle panel). The blot was reprobed with anti-Akt antibody to confirm equal loading of protein (bottom panel). (D) Phosphorylation of FKHRL1 by EPO stimulation in CFU-E cells. CFU-E cells were prepared as described above. Cell extracts (2 × 106 cells/1 lane) were resolved by 7.5% SDS-PAGE and immunoblotted with FKHRL1 antibodies directed against phospho-T32 (top panel) or phospho-S253 (middle panel). The blot was reprobed with anti-FKHRL1 antibody to confirm equal loading of protein (bottom panel).
Detection of FKHRL1 in primary erythroid progenitor cells and erythroblasts.
(A) CFU-E cells were obtained as described in “Materials and methods.” Total cellular RNA extracted was reverse transcribed and amplified by RT-PCR (see “Materials and methods”). The PCR products were digested with HhaI and resolved by agarose/formaldehyde gel electrophoresis and the gel was stained with ethidium bromide. (B) Fraction 1 (lanes 1 and 5), fraction 2 (lanes 2 and 6), fraction 3 (lanes 3 and 7), and fraction 4 (lanes 4 and 8) were obtained as described in “Materials and methods” and Table 1. After solubilization, cell extracts (2 × 106 cells/1 lane) were resolved by 7.5% or 15% SDS-PAGE and immunoblotted with anti-FKHRL1 antibody (left panel) or anti-FasL antibody (right panel). (C) Phosphorylation of Akt by EPO stimulation in CFU-E cells. CFU-E cells were obtained as described in “Materials and methods” and subsequently cultured without EPO for 2 hours. The cells were then stimulated with EPO (10 U/mL) for the periods indicated. After solubilization, cell extracts (2 × 106 cells/1 lane) were resolved by 7.5% SDS-PAGE and immunoblotted with Akt antibodies directed against phospho-T308 (top panel) or phospho-S473 (middle panel). The blot was reprobed with anti-Akt antibody to confirm equal loading of protein (bottom panel). (D) Phosphorylation of FKHRL1 by EPO stimulation in CFU-E cells. CFU-E cells were prepared as described above. Cell extracts (2 × 106 cells/1 lane) were resolved by 7.5% SDS-PAGE and immunoblotted with FKHRL1 antibodies directed against phospho-T32 (top panel) or phospho-S253 (middle panel). The blot was reprobed with anti-FKHRL1 antibody to confirm equal loading of protein (bottom panel).
Discussion
In this study we have demonstrated that Akt kinase is activated by EPO in a dose- and time-dependent manner in EPO-dependent human erythroleukemia cell line UT-7/EPO, and that the activation was absolutely dependent on functional PI3K activity. In addition, we found that FKHRL1 is rapidly and transiently phosphorylated by EPO in a PI3K-dependent fashion. In vitro kinase assay revealed that Akt kinase activated by EPO directly phosphorylated FKHRL1 protein. Finally, we have demonstrated that Akt and FKHRL1 are expressed and actually phosphorylated by EPO in primary erythroid cells.
FKHRL1 is a member of the Forkhead transcription factor family, which is characterized by the presence of a highly conserved forkhead domain having a winged-helix motif and DNA binding activity. It was originally reported that the homeotic gene forkhead controls morphogenesis in Drosophila.30 To date, members of the Forkhead family have been described in a wide range of organisms from yeast to humans. Among them, AFX, FKHR, and FKHRL1 are known to be mammalian counterparts of DAF-16. DAF-16 is a molecule downstream of DAF-2, a homologue of the insulin and insulin-like growth factor receptors. Because the life-span extension caused by DAF-2 mutations requires the gene DAF-16,21 DAF-16 plays an important role in the regulation of the life span of C. elegans. Therefore, human homologues of DAF-16, AFX, FKHR, and FKHRL1, may function as regulators of cell survival. If so, because EPO has an antiapoptotic effect on erythroid cells, it would not be surprising if FKHRL1 is one of the downstream molecules in the EPO signaling pathway.
FKHRL1 has 3 putative Akt consensus phosphorylation sites (RXRXXS/T); T32 (RPRSCT32), S253 (RRRAVS253), and S315 (RSRTNS315). Among them, T32 and S253 sites were phosphorylated by EPO stimulation in a dose- and time-dependent manner in UT-7/EPO cells. Moreover, an in vitro kinase assay revealed that both sites were directly phosphorylated by Akt activated by EPO. However, we could not demonstrate the phosphorylation of S315 induced by EPO because inadequate antiphospho S315 antibody was available. To overcome this obstacle, we took advantage of the finding that phosphorylation of FKHRL1 at S315 but not T32 or S253 had a significant effect on the mobility of FKHRL1 on an SDS gel.16 As shown in Figures 6 through 9, EPO induced a shift up in the mobility of FKHRL1. This result strongly suggested that FKHRL1 at S315 was phosphorylated by EPO stimulation, although it is still unknown whether or not Akt directly phosphorylated S315.
Because it has not been determined whether or not FKHRL1 is expressed in hematopoietic cells, it is noteworthy that this molecule is expressed in UT-7/EPO cells and normal erythroid cells. This strongly suggested that FKHRL1 plays an important role in erythropoiesis, although the exact function of this molecule is still unknown. The finding that the nonphosphorylated form of FKHRL1 can activate the FasL promoter16 and that FasL mRNA is expressed in erythroid cells22,23 suggests that FKHRL1 is involved in regulating the expression of the FasL gene in erythroid cells. This notion is also supported by our finding that FKHRL1 and FasL proteins are concomitantly detectable in CFU-E cells and immature erythroblasts (Figure 9B). Mature erythroid progenitor cells are highly sensitive to EPO and the cells gradually become insensitive to EPO during erythroid maturation,31 presumably resulting in down-regulation of functional PI3K-Akt activity in mature erythroid cells. If so, it is possible that FKHRL1 is dephosphorylated, translocates into the nucleus, and finally activates FasL promoter during erythroid maturation.
Although a high dose of LY294002 (100 μmol/L) completely abrogated EPO-induced activation of Akt and phosphorylation of FKHRL1, it induced apoptosis in only 25% of the UT-7/EPO cells. In addition, the constitutively active form of Akt E40K delayed the time to apoptosis but did not completely protect the cells from apoptosis induced by EPO deprivation. Therefore, the PI3K-Akt-FKHRL1-independent pathway may also have a significant effect on cell survival.32 In our preliminary experiments, PD98059, a specific inhibitor of the MAPK/extracellular signal-regulated kinase kinase 1 (MEK1), induced apoptosis in about 15% of UT-7/EPO cells. Thus, the antiapoptotic effect of EPO should be controlled via complicated networks including Jak2-Stat, Ras-MAPK, and PI3K-Akt activation pathways.12,13,33 34
Because Akt is reportedly activated by phosphorylation of 2 residues, T308 and S473,35 we also performed Western blotting analysis using a specific antibody that recognizes Akt only when phosphorylated at T308. Akt was phosphorylated by EPO at not only S473 but also T308, and LY294002 completely inhibited the phosphorylation of T308 like S473 at 10 μmol/L at which dosage Akt activity was suppressed in an in vitro kinase assay (Figure 3B and data not shown). Therefore, these results suggested that phosphorylation of both T308 and S473 induced by EPO is dependent on functional PI3K activity and accurately reflects the activation of Akt.35 However, the constitutively phosphorylated Akt was detected in the absence of EPO and the level was unchanged even after treatment with a high dose of LY294002 (Figure 2). These observations suggest that Akt phosphorylation is in part independent of PI3K activity.
There was a discrepancy between Akt and FKHRL1 in the dosage of LY294002 needed for the inhibition of their phosphorylation. Phosphorylation of Akt by EPO was completely blocked at 10 μmol/L LY294002, whereas even in the presence of 20 μmol/L LY294002, FKHRL1 was phosphorylated by EPO. In addition, the in vitro kinase assay revealed that the phosphorylation level of FKHRL1 was much weaker than that in vivo. Although we cannot completely exclude the possibility that the discrepancy in phosphorylation level between in vitro and in vivo is due to technical problems, the results suggest that not only Akt but also other protein kinase(s) are involved in the phosphorylation of FKHRL1 protein.
It is important to identify the RT-PCR product as that ofFKHRL1 because this gene has ultimately high homology toFKHRL1P1 over about 2.4 kb of related sequence.29Because FKHRL1P1 lacks the 2 introns found inFKHR and FKHRL1 and contains a single nucleotide mutation (G1541A) in the middle of the Forkhead domain that leads to a stop codon and lacks about a half of the Forkhead domain, this gene can be considered a pseudogene.29 To demonstrate that the RT-PCR product is identical to FKHRL1 but not FKHRL1P1, we digested amplified RT-PCR products with HhaI because the HhaI restriction site is present only in the RT-PCR product fromFKHRL1.29 As predicted, the products (193 bp) were divided into 2 bands 68bp and 123bp (Figure 9A), indicating that normal erythroid progenitor cells actually express the FKHRL1 gene but not the pseudogene FKHRL1P1. This was confirmed by Western blotting analysis with anti-FKHRL1 antibody that specifically recognizes FKHRL1 protein (Figure 9B).
In summary, we identified FKHRL1 as one of the downstream target molecules of Akt kinase in the EPO signaling pathway. Although we did not demonstrate the direct involvement of FKHRL1 in FasL gene expression, our finding that FKHRL1 and FasL proteins are concomitantly expressed in normal erythroid progenitors and erythroblasts may suggest that EPO exerts its antiapoptotic effect on erythroid cells, at least in part via regulation of the FasL gene by FKHRL1. In addition, the evidence that there was a discrepancy in the respective dosage of LY294002 needed for inducible apoptosis and the inhibition of FKHRL1 phosphorylation might suggest that FKHRL1 has also distinct functions from a mediator of cell survival.
Acknowledgments
We thank A. Brunet and M. E. Greenberg, Division of Neuroscience, Children's Hospital, Harvard Medical School, for antibodies against phospho-T32 FKHRL1, phospho-S253 FKHRL1 and native FKHRL1, and GST-FKHRL1 fusion protein; A. Bellacosa, Fox Chase Cancer Research Center, for wild-type Akt and E40K cDNAs; and T. Nagai and Y. Gunji for critical reading of the manuscript.
Supported by Grants-in-Aid for Cancer Research and Scientific Research from the Ministry of Education, Science and Culture of Japan.
Reprints:Norio Komatsu, Department of Hematology, Jichi Medical School, Minamikawachi-machi, Kawachi-gun, Tochigi-ken 329-0498, Japan; e-mail: nkomatsu@ms.jichi.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 3. In vitro kinase assay revealed that AKT kinase is activated by EPO stimulation. / (A) EPO was removed from UT-7/EPO cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (B) EPO was removed from UT-7/EPO cells for 24 hours, then the cells were incubated with 50 μmol/L LY294002 for 45 minutes and stimulated with EPO (10 U/mL) for 10 minutes. Cells were lysed and immunoprecipitated with anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 200 μmol/L ATP using GSK-3 as a substrate (0.025 mg/mL) . The reactions were incubated at 30°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and immunoblotted with antiphospho GSK-3 antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.941/5/m_bloo01514003w.jpeg?Expires=1763463535&Signature=kgz~y4F69CDFZGUBXkvOzO3xbBKYksPE1tO-rUNQRdTwET89WHhz1CWuNJfBtO6Qh72zwT2cfDF5NRHYb9JenrRRBK4g1NggJ2wYrtKEkB219TEegLbECjwl2rBN4X5IRDL3m18uv8hDwgvDR0nFL5yuYWHBoeOVqh-g4c6c9ByErJY6U2q3HZTLNH-r5kGGFrNGCTEAkLPZCYJaRVlZJ9mXyrFerc~kBL41vUZ-ohYSNsyv~IeJFXheBkir2uGSP~fs0uZli6M~WVmO2lDQCfLvlYu8TR1mumNcq--8Mb38fgKG8Q3NsqDOSPboBcG4zP4sEDlZVXfrUG~4eoqEqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activated form of Akt prevents cell death induced by withdrawal of EPO. / (A) Generation of transfectant cells expressing HA-tagged Akt. The transfectant cells and the parent cells were harvested for detection of HA-tagged Akt proteins. Cell lysates were resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane and immunoblotted with anti HA-antibody. (B) EPO was removed from the transfectant cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (C) EPO was removed from the culture medium and the cell viability was assessed by trypan dye exclusion during the observation periods. (D) EPO was removed from the culture medium and the transfectant cells were cultured without any growth factors (shade). Three days later, cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan. The cells cultured with EPO (1 U/mL) were used as a negative controls (light).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.941/5/m_bloo01514005aw.jpeg?Expires=1763463535&Signature=kOT0chQCYXlVaIdM2kJaPKI~gQjydrp0KwV5dPsE1mGS9Nw60AdPfLv6hsH3zVjmToByuJvm0WeC25jMSZgO7gY7WT2WpUsFkM1LXRdLeQP3XPBNqjSBupD1hC~6dBuZCYM4Lbu6RoI2vr~nFroSauowsdgjb~ESltJdZ~CQWo00A00A2800v85JkcDFrQ2rja0NGBf61BoRf4nEGvDKPSU9PjmsJDteDjl59m97XgAESmCKwpI~PSDXXq3O-9ppiLDp82vC3i3Eqh70XsJKCzgsd2QhdXKW63324zcaD~4c9XUK9PBNhJCu3iiIpMmK9RHfdleLlQGhpPnF3LUeiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activated form of Akt prevents cell death induced by withdrawal of EPO. / (A) Generation of transfectant cells expressing HA-tagged Akt. The transfectant cells and the parent cells were harvested for detection of HA-tagged Akt proteins. Cell lysates were resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane and immunoblotted with anti HA-antibody. (B) EPO was removed from the transfectant cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (C) EPO was removed from the culture medium and the cell viability was assessed by trypan dye exclusion during the observation periods. (D) EPO was removed from the culture medium and the transfectant cells were cultured without any growth factors (shade). Three days later, cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan. The cells cultured with EPO (1 U/mL) were used as a negative controls (light).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.941/5/m_bloo01514005bw.jpeg?Expires=1763463535&Signature=QJKKorCCYsFuR4F9LlbvjBNzC-Aae6dnOU6tOt~4Jywh3KuVH3yKEmA9bZ24MKibfI7ba4K~A1rTvQB4JwaL7KfAEup6XWNeA4HNfaNofcuartISe6uA3iZjAmrqHdUJ2EHjJ~m6NGfF8XpnuvVp~6L1rBkR0EcJGtxD2Q~FZlgX-aZo~oicmCO8d6x0aRMnYLSDD~mmyt6jB0VJ9YCS3Fawh8cUBQ45m-7LQtCISMsiVRsLo-eyrYHAsHuS9o9Htj272N33d-cpIPGHLeFahUpSqCv~1eRdXRyUXjkaHwS-aV-mr7y6jh6T4fQmsjDull6DBLKo6Pk~fnSGRwc-yA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activated form of Akt prevents cell death induced by withdrawal of EPO. / (A) Generation of transfectant cells expressing HA-tagged Akt. The transfectant cells and the parent cells were harvested for detection of HA-tagged Akt proteins. Cell lysates were resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane and immunoblotted with anti HA-antibody. (B) EPO was removed from the transfectant cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (C) EPO was removed from the culture medium and the cell viability was assessed by trypan dye exclusion during the observation periods. (D) EPO was removed from the culture medium and the transfectant cells were cultured without any growth factors (shade). Three days later, cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan. The cells cultured with EPO (1 U/mL) were used as a negative controls (light).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.941/5/m_bloo01514005cx.jpeg?Expires=1763463535&Signature=rYmchz-n01MzbslnYiog9zsdY6QXXG7mTVw7sOixoC1XuvYf-X8qnOYyucEyMwypwXDf2c5lAUAIstSXM-GbRdddgYlyW8~SqmQhcZ-jIknvXxVS7l4jqeNAXnV0RjA-A2-yL-SGOOWa9gmtu8U~BoxizhlYponVUZH1blcHM1xVXBdJihqQQd-DVFyIwsN7uhqHd-IxhpeoUhMh5Yby4IrqUr75Sumz8cp4bbr87iOLeZFmY4ub72Kz7V6OCAP3CUm6GS9iz7Nk0zSciIjyS5Wx~vQ6BFqycMq6vKrZZT6OuNXwIEYPJvoMS92NUaRRQRZoaR3ePzS-aTExayNdPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activated form of Akt prevents cell death induced by withdrawal of EPO. / (A) Generation of transfectant cells expressing HA-tagged Akt. The transfectant cells and the parent cells were harvested for detection of HA-tagged Akt proteins. Cell lysates were resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane and immunoblotted with anti HA-antibody. (B) EPO was removed from the transfectant cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (C) EPO was removed from the culture medium and the cell viability was assessed by trypan dye exclusion during the observation periods. (D) EPO was removed from the culture medium and the transfectant cells were cultured without any growth factors (shade). Three days later, cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan. The cells cultured with EPO (1 U/mL) were used as a negative controls (light).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.941/5/m_bloo01514005dx.jpeg?Expires=1763463535&Signature=EVT63soQ04a3PiAo-zpUCRd4p7DgjhsVXlSkNY5Fc-TnSz6I8JdVqdcjtG-CYMjugeeXVgnNhZGNWLtX6dOTFYSDdDzlTqRH0BmGnJoAkdVyvCh8xJIlvwBMptzOS273tIULNUUQ1vd1k89Y4tcPUpzdQXmgB9FVCzATgvti8tWE1vmQsM~8tvGd78dlaWBChYxLDkqjszoaPDaiuj4TCUGabVvxaVQnJORM01VsNooseUrU26kjhMscDRk1czeM2QpWsWLZaoilx9jnQWjMhGFwORX7UewVO6GM4A0EKWrESR2P5A-84tiQtpF8D-OSnY6J~CCqWHWFExUxzMvspA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 3. In vitro kinase assay revealed that AKT kinase is activated by EPO stimulation. / (A) EPO was removed from UT-7/EPO cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (B) EPO was removed from UT-7/EPO cells for 24 hours, then the cells were incubated with 50 μmol/L LY294002 for 45 minutes and stimulated with EPO (10 U/mL) for 10 minutes. Cells were lysed and immunoprecipitated with anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 200 μmol/L ATP using GSK-3 as a substrate (0.025 mg/mL) . The reactions were incubated at 30°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and immunoblotted with antiphospho GSK-3 antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.941/5/m_bloo01514003w.jpeg?Expires=1763463536&Signature=XcEJX2E-oKZElJ3meRn4nssLLW1FCmpf5FoGczhqsQ4rSnuGtPe~gD28gjFymmvtsouTgeupfBQd3q~F4VGOniQgttWnv2y06NGPHuKbxfteBbxHkEXs1dNuosrtNJ3VTt9Ov-heMMYJQ4i8gKDfcYFfGsTJsJk~VBzDKfh91NZdlGJwQ9kEbEEueFIsqZzK1rWF~GzJsWe6XDyia8~r93bQRbdWLkwr-Y8hAKmN2byjLMIq6T1987GDDhqMhuw4idYESoAgJhCW0rIHaVgdJSv1ACwqd6~SkmGgL7-jmXxxr1ljvaBQc8I0-BlnYVLg3Vg5Me8sNAYWU7MZcvEJng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activated form of Akt prevents cell death induced by withdrawal of EPO. / (A) Generation of transfectant cells expressing HA-tagged Akt. The transfectant cells and the parent cells were harvested for detection of HA-tagged Akt proteins. Cell lysates were resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane and immunoblotted with anti HA-antibody. (B) EPO was removed from the transfectant cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (C) EPO was removed from the culture medium and the cell viability was assessed by trypan dye exclusion during the observation periods. (D) EPO was removed from the culture medium and the transfectant cells were cultured without any growth factors (shade). Three days later, cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan. The cells cultured with EPO (1 U/mL) were used as a negative controls (light).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.941/5/m_bloo01514005aw.jpeg?Expires=1763463536&Signature=IkNnsLYpMhZkD9x08fzPmIJghci~8MAB9xGGRqvIouOjuZeDYPQ3RquEDVUykcwcrvywhOn3WJDL2ifWO~aYucbl6uhJUgNQYBRY6SiAPzjIS060bjTOkTurntFpiWQoOznur6JLTf6r8zgrTGZpxvQgEOqB7Z3vwrh6BHqT9SinXrEvqS0P~i510AtFme1tmnCjNqwSOVl8Pjwh077I0yQjtW~kcSEWMgPliaYlVuAiI7FJBD-NzZiVhp7hSWYbCwFV10FhIOeHnOEOFoG2O-I9FfdQ3gVRcg4rkE814TuT1Bqsxc22qC4MEX3T5GqcQty0k69cUIrI-GbvHHwyLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activated form of Akt prevents cell death induced by withdrawal of EPO. / (A) Generation of transfectant cells expressing HA-tagged Akt. The transfectant cells and the parent cells were harvested for detection of HA-tagged Akt proteins. Cell lysates were resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane and immunoblotted with anti HA-antibody. (B) EPO was removed from the transfectant cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (C) EPO was removed from the culture medium and the cell viability was assessed by trypan dye exclusion during the observation periods. (D) EPO was removed from the culture medium and the transfectant cells were cultured without any growth factors (shade). Three days later, cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan. The cells cultured with EPO (1 U/mL) were used as a negative controls (light).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.941/5/m_bloo01514005bw.jpeg?Expires=1763463536&Signature=C7UZoMZ3BYvXkr4u~-cfLTQaYI-~FSE38soPVYBRqJjnrL0STMiirvH1nhyTBC5IIg98nJWEd-Nlc7PaLkxT-AItYiwz4G-pidLNqxgV8N2VVNRacU7na2RbzifVZapr7HjLJG~tY2ddx~FYkSncEjiJCj41X9RSINZkGKwDj7eIVapTOcFryHofUUthPye6O~lcr6e2EQP9~4bt-6ANJ7PB5x4VEeN8s7lV~1mf8AdPBRFTxovHrHA6M98laeNZUY23hqbCHcpzDnijUhdkOE412mjNvYqcNTZwQhBeds3LEOFF6H7h-zW0vGYQk~KIbZ2QdWG1Pow3yhr25HPoOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activated form of Akt prevents cell death induced by withdrawal of EPO. / (A) Generation of transfectant cells expressing HA-tagged Akt. The transfectant cells and the parent cells were harvested for detection of HA-tagged Akt proteins. Cell lysates were resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane and immunoblotted with anti HA-antibody. (B) EPO was removed from the transfectant cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (C) EPO was removed from the culture medium and the cell viability was assessed by trypan dye exclusion during the observation periods. (D) EPO was removed from the culture medium and the transfectant cells were cultured without any growth factors (shade). Three days later, cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan. The cells cultured with EPO (1 U/mL) were used as a negative controls (light).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.941/5/m_bloo01514005cx.jpeg?Expires=1763463536&Signature=pp73YWO~9P~BDVZkfOTk42qLudNqL8zJySFnN7IJl2qOuB1R9RTS7tQdpt3N9nl7pDlwqnIHU~vWfBBnq2mVBiXfTY2XEsPVL8KTv5clw72OVaZ0RXlCJAmreEEwIdZQDM-ebMO6Km1Rwciv1v7kp-vyav0-qJYxO~07AJCjXoCj-UvIwyM6J-Eq1~T7~tmimlfhli2-LVRAMVYo750wHEj2f33JA-TKuX7Q5jxRLl5w9Fjc1y5wnrDDEpvUyrWDnRW0Chwrsb8YDHCCoBwEPVUU40WI2uOXpL5MrNmXZ7eyjA4HBAUtBYi4DkmKg02dtC1862C0wKVGRz4hhlyI-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activated form of Akt prevents cell death induced by withdrawal of EPO. / (A) Generation of transfectant cells expressing HA-tagged Akt. The transfectant cells and the parent cells were harvested for detection of HA-tagged Akt proteins. Cell lysates were resolved by 10% SDS-PAGE. Proteins were transferred onto a PVDF membrane and immunoblotted with anti HA-antibody. (B) EPO was removed from the transfectant cells for 24 hours. Then the cells were stimulated with EPO (10 U/mL) for 10 minutes. After solubilization, cell lysates were immunoprecipitated with protein G-conjugated anti-Akt antibody. Then immunoprecipitates were subjected to an in vitro kinase assay. The kinase assays were carried out in the presence of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) using histone H2B as a substrate (0.1 mg/mL). The reactions were incubated at 25°C for 30 minutes. Reaction products were resolved by 15% SDS-PAGE and visualized by autoradiography. (C) EPO was removed from the culture medium and the cell viability was assessed by trypan dye exclusion during the observation periods. (D) EPO was removed from the culture medium and the transfectant cells were cultured without any growth factors (shade). Three days later, cells were harvested and stained with annexin-V-FITC for detection of apoptotic cells (Apo) using FACScan. The cells cultured with EPO (1 U/mL) were used as a negative controls (light).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.941/5/m_bloo01514005dx.jpeg?Expires=1763463536&Signature=5EXVuezKSDKmw1hxsjhNvqsKbsAlBvIl3kk5Vz2vEK4utpWiQqT24DcwZK4VezM07RPZCMyEOQfvOFpVwHZAOiiHpwlmJkYyLrUa0wCSxsLAkusoOZ8PVYLxzHc6zT4IGHQ3VBFJOC3EPNZ62CwI1EtaWlpRlEdPJxIxSwLEh10hMdKFs-eyRiC~xSGIy7J5hs2paz9~Sf6CNO0R-00WjcIpymSkHdV2LGWmVHUjjxkyBydOBnzNItH5Rdd9NGg0fzDhjdCF~Tm8U041JASUBi8bcERbczsUWwyFxCwawgyxqi0ocSCwnzTTpeP9Qevud8zkerlrLidJ-M7ygn~ytg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)