Abstract
A major limitation to the widespread use of hematopoietic stem cells (HSC) is the relatively crude level of our knowledge of how to maintain these cells in vitro without loss of the long-term multilineage growth and differentiation properties required for their clinical utility. An experimental and theoretical framework for predicting and controlling the outcome of HSC stimulation by exogenous cytokines would thus be useful. An emerging theme from recent HSC expansion studies is that a net gain in HSC numbers requires the maintenance of critical signaling ligand(s) above a threshold level. These ligand-receptor complex thresholds can be maintained, for example, by high concentrations of soluble cytokines or by extracellular matrix- or cell-bound cytokine presentation. According to such a model, when the relevant ligand-receptor interaction falls below a critical level, the probability of a differentiation response is increased; otherwise, self-renewal is favored. Thus, in addition to the identity of a particular receptor-ligand interaction being important to the regulation of stem cell responses, the quantitative nature of this interaction, as well as the dynamics of receptor expression, internalization, and signaling, may have a significant influence on stem cell fate decisions. This review uses examples from hematopoiesis and other tissue systems to examine existing evidence for a role of receptor activation thresholds in regulating hematopoietic stem cell self-renewal versus differentiation events.
Introduction
Transplantation of hematopoietic stem cells (HSC) has an established and unique position in the treatment of human disease. This promising approach is limited, however, by a lack of knowledge about how to maintain these cells in vitro without loss of the very long-term multilineage growth and differentiation properties required for their clinical utility. A theoretical framework for predicting and controlling the outcome of HSC stimulation by exogenous cytokines would be highly useful. Although the extent to which individual HSC are biologically amenable to cytokine-determined alteration of their fates remains to be clarified,1,2 data from some in vivo studies has been interpreted as indicating that sustained HSC self-renewal, resulting in a significant net expansion of HSC numbers, may be constrained by the tissue environment.3-6 Moreover, the observation that cytokines such as interleukin (IL)-3 and tumor necrosis factor (TNF) have both been shown to exert HSC differentiation-inducing activities under certain conditions in vitro7,8 or that (murine) HSC self-renewal probabilities may be increased by infection with Friend murine leukemia virus9 or by overexpression of HOXB4,10 have renewed interest in the possibility of defining in vitro conditions that might allow the controlled manipulation of HSC self-renewal. However, neither the conditions under which these manipulations may be exploited nor their mechanisms of action at the cellular level have been defined. Even modest advances in this area could have an important medical impact, for example, in cord blood transplantation and gene therapy.
The introduction of quantitative and specific assays for hematopoietic cells capable of long-term multilineage repopulation in vivo11-16 has been key to assessing the magnitude of changes in HSC populations in vivo and in vitro. The use of these assays has established that a significant net expansion of HSC occurs during ontogeny17 and that this process can be reactivated in the adult during marrow regeneration.3,4 18
Identification of in vitro conditions that will support a similar degree of HSC self-renewal activity has proven to be a major challenge, although recently, some success toward achieving this goal has occurred.6,12,15,19-24 Clues to why these groups have begun to succeed may lie in certain common features of their methodologies. These include the initiation of cultures with relatively low concentrations of cells that are enriched in their HSC content, frequent replacement of the medium, interactions of the HSC with particular stromal cell types, or the use of very high concentrations of selected cytokines. There are obviously certain intrinsic properties that can influence the magnitude of HSC amplification detected, including CXCR425 and VLA-426 expression and other parameters that may fluctuate during the cell cycle to specifically affect HSC engraftment in vivo,27,28 as well as factors that may limit the ultimate proliferative potential displayed by a given HSC without affecting its undifferentiated state (eg, telomere shortening29). However, an emerging theme from HSC expansion studies is that a net gain in HSC numbers requires the maintenance of some critical signaling ligand(s) above a threshold level. According to such a signaling-threshold model of stem cell differentiation control, when a relevant ligand-receptor interaction is kept above this threshold level, differentiation continues to be suppressed. When this threshold level is not maintained, the probability of a differentiation response being activated is increased. Thus, not only may the identity of a particular receptor-ligand interaction be important to the regulation of stem cell responses, the quantitative nature of this interaction may also have a significant influence on stem cell fate. In this review, we will examine some of the evidence that receptor activation thresholds, achieved through interaction with either soluble or surface-immobilized ligands, can regulate stem cell self-renewal versus differentiation responses.
Signaling complex threshold control of stem cell fate
Evidence from hematopoiesis
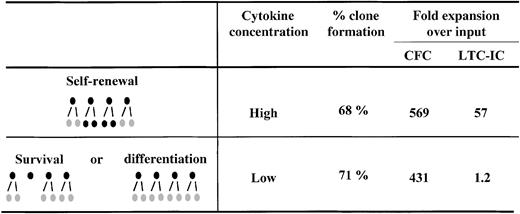
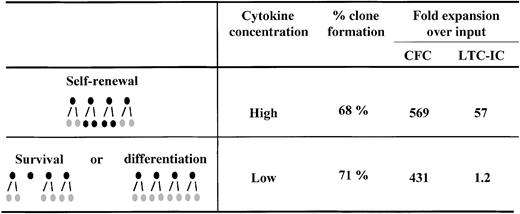
Several lines of evidence support the idea that quantitative changes in receptor/ligand signaling complex numbers may modulate HSC fate. Our recent comparison of the cytokine dose-response relationship for the amplification of hematopoietic progenitors (detected as colony-forming cells [CFC]) and HSC (detected as long-term culture-initiating cells [LTC-IC]) provided an important clue that these responses might involve pathways distinct from (or additional to) those responsible for blocking apoptosis and stimulating S-phase entry. The relevant key finding in these studies was the observation that to maximize LTC-IC expansion, it is necessary not only to stimulate cells with particular cytokines,30 but also to present these cytokines at very high concentrations—in fact more than 10-fold higher than the concentrations of the same cytokines that are sufficient to maximize the expansion of CFC numbers in the same cultures.8 Experiments were then performed with single CD34+CD38− cells to determine whether the reduced LTC-IC production at low cytokine concentrations was simply due to a failure of these conditions to stimulate a subset of CD34+CD38− cells with high LTC-IC generating potential. The results did not support such a model. We found no difference in the number of cells initially stimulated to divide (clone frequency) nor in the total number of progeny they generated (clone size distribution) under the 2 cytokine conditions, despite the expected difference in the number of progeny with LTC-IC activity (Figure 1). Similarly Bennaceur-Griscelli et al31 observed an enhanced preservation of LTC-IC in the presence of a stromal cell line, which was also independent of cellular survival and proliferation.
Clone formation and progenitor expansion from single cell cultures of CD34+CD38− adult human bone marrow cells.
CD34+CD38− cells were isolated and cultured as single cells in serum-free medium in the presence of 300 ng/mL stem cell factor, Flt-3 ligand, and 60 ng/mL IL-3 (High) or 30 ng/mL stem cell factor, Flt-3 ligand, and 6 ng/mL IL-3 (Low) for 10 days before analysis. Analysis consisted of determining the number of clones produced under each condition and then performing LTC-IC and CFC assays on a pool of each set of clones generated under the same condition. These studies showed that although the same number of clones and CFC were generated in the High versus the Low cytokine concentrations, the net generation of LTC-IC was dramatically affected by changes in cytokine concentration. Taken together these results suggested that self-renewal versus differentiation, not self-renewal versus survival, was being modulated. Results are from reference 8.
Clone formation and progenitor expansion from single cell cultures of CD34+CD38− adult human bone marrow cells.
CD34+CD38− cells were isolated and cultured as single cells in serum-free medium in the presence of 300 ng/mL stem cell factor, Flt-3 ligand, and 60 ng/mL IL-3 (High) or 30 ng/mL stem cell factor, Flt-3 ligand, and 6 ng/mL IL-3 (Low) for 10 days before analysis. Analysis consisted of determining the number of clones produced under each condition and then performing LTC-IC and CFC assays on a pool of each set of clones generated under the same condition. These studies showed that although the same number of clones and CFC were generated in the High versus the Low cytokine concentrations, the net generation of LTC-IC was dramatically affected by changes in cytokine concentration. Taken together these results suggested that self-renewal versus differentiation, not self-renewal versus survival, was being modulated. Results are from reference 8.
More recently, Ramsfjell et al32 have confirmed and extended our studies by showing that the cytokine concentrations required to amplify hematopoietic cells with extended LTC-IC (ELTC-IC) are significantly higher than the cytokine concentrations required to amplify LTC-IC that produce CFC after shorter periods of time. Using cell division tracking studies, they further showed that the observed cytokine concentration effect was independent of the rate or extent of cell proliferation.
These results are, of course, not the only ones to show that the differentiation of primitive hematopoietic cells can be affected by the types or levels of cytokines used to stimulate them. Metcalf et al first showed almost 20 years ago that the proliferative activity and lineage commitment of bipotent granulocyte-macrophage progenitors could be shifted according to the concentration and order of granulocyte-macrophage colony-stimulating factor (GM-CSF) or macrophage colony-stimulating factor (M-CSF) to which the cells were initially exposed.33,34 More recently, evidence of a differentiation-inducing effect of IL-5 or thrombopoietin, in combination with steel factor, on murine progenitor cells was presented.35 Extended “self-renewal” of erythroid progenitors stimulated by insulin-like growth factor rather than insulin36 and their accelerated differentiation by exposure to transforming growth factor (TGF)-β37 have also been reported. Finally, IL-3 concentration-dependent control of proliferation and differentiation of murine FDCP-mix cells38,39 and a concentration-dependent ability of IL-3 and IL-1 to suppress the self-renewal of murine stem cells40 have been observed.
A further message from a large number of studies is the ability of particular feeder cell types to improve stem cell maintenance in vitro.23,31,41,42 Although these observations may indicate the production by these feeders of novel cytokines or nontoxic inhibitors of differentiation, a capacity to stimulate more prolonged signaling by sustained receptor activation may also be important.43 In studies with (predominantly) membrane-bound steel factor (SF), prevention of internalization prolonged tyrosine kinase activation and the half-life of the SF receptor on the surface of the responsive cells.43,44Transmembrane SF is a more potent stimulant of primordial germ cell survival in vitro than soluble SF and, in vivo, the SLdmutation (which causes only a soluble form of SF to be produced45) results in significant hematopoietic and developmental abnormalities including severe anemia, sterility, and progenitor cell defects.46 In fact, the positive effects of cell surface–bound ligand presentation may derive from both the high effective local concentration achieved47 and the inhibition of receptor internalization that occurs after soluble ligand-receptor interactions.48
It is interesting to note that thresholds in receptor expression/activation have also been shown to be important in T- and B-cell development/lineage commitment.49-51 In fact, similar to our studies with HSC, it is not the presence or absence of a particular receptor that acts as a developmental switch, but the relative levels of surface expression that appear to govern developmental potential.51 Particularly interesting in this regard are data that show a clear difference in the sensitivity of responses of mature and immature B-cells.50 In these studies, low activation signals to immature B cells (induced by low antigen concentrations) resulted in clonal elimination (differentiation), whereas higher signal thresholds were required for clonal expansion. In the T-cell system, Smith52 has shown that the cell cycle progression of T cells can be predicted based on changes in IL-2 concentration, IL-2 receptor density, and the duration of receptor activation. Particularly noteworthy is the finding that gaussian distributions in cycle progression times closely correlate with parallel differences in IL-2 receptor expression, even within otherwise identical clonal T-cell populations. This suggests that the rate-limiting step in the IL-2–stimulated expansion of T-cell populations is the interaction of IL-2 with its receptor. From studies of the responses of separated T-cell subpopulations isolated on the basis of their individual IL-2 receptor densities, evidence has been obtained to indicate that some finite number of ligand/receptor interactions must occur before the cell replicates its DNA.53 Under the same conditions, this threshold may be reached earlier in cells expressing a high number of receptors than in cells expressing a low number of receptors. Moreover, a recent study has shown that the potency of IL-2 for stimulation of T-cell proliferation is enhanced by a ligand mutation that reduces its endocytic degradation54 (thereby resulting in prolonged receptor stimulation).
Evidence from nonhematopoietic systems
The role of inductive membrane-associated or soluble concentration gradients in activating distinct genetic programs during embryonic development and tissue specification is also well documented (for recent reviews see references 55 and 56). Cell secreted factors—morphogens—form concentration gradients over distances of more than 300 μm and thereby elicit positional information that dictates tissue patterning. Current evidence indicates that these gradients are relatively stable and may involve the diffusion of soluble factors across many cells, as well as juxtacrine57and transcytotic58 cell relay. Examples of molecules that form gradients resulting in spatially distinct tissue specification during vertebrate development include members of the TGF-β family (ie, activin, TGF-β, bone morphogenic proteins [BMPs]). Mesoderm development in Xenopus is induced by treating presumptive ectoderm with activin. Low concentrations result in the induction of hematopoietic tissue, whereas very high concentrations induce the development of notochord.59 The ability of cells to respond to many factors, or factor complexes with overlapping activities, as well as the existence of complex intersecting concentration gradients of these factors suggests that relative concentrations of factors, not just their absolute magnitudes, are important. The observation that dorsal-ventral patterning inDrosophila is positionally mediated by a morphogen called short gastrulation (Sog) and its interaction with decapentaplegic (Dpp), a secreted protein that belongs to the family of BMPs, exemplifies the types of elaborate mechanisms for the control of signaling thresholds that exist.60 Similarly, lineage determination during neural crest stem cell differentiation is instructively influenced by the timing and relative dosage of growth factor encountered (reviewed in Morrison et al61).
Recently, much attention has been directed toward the possible roles of fibroblast growth factor (FGF) signaling in early mammalian development. For example, signaling by the FGF receptor is required for the normal development of multiple organs during embryogenesis62 and may be spatially and directionally modulated by secretion and presentation of FGF by the extraembryonic trophectoderm.63 Further complexity is generated by the presence of membrane-bound and secreted receptor isoforms and by the interaction of FGF ligands with heparin sulfate proteoglycans on the cell surface and extracellular matrix.64 The availability of multiple, highly developed ways of controlling the local concentration of FGF in fetal tissues points to the importance of a threshold-based mechanism of FGF control of developmental processes.
Perhaps the most experimentally accessible model for the threshold-dependent regulation of stem cell self-renewal and differentiation is provided from in vitro studies of embryonic stem (ES) cell responses. A well-established feature of ES cells is their ability to be maintained in culture in an undifferentiated state in the presence of high concentrations of cytokines from the IL-6 family,65 the biologic action of which is mediated by multiple subunit cell surface receptors that share the gp130 protein. In fact, the concentration of the IL-6 type of cytokines required to prevent ES cell differentiation is dependent on the identity of the ligand.66 Like the HSC regulation by different concentrations of soluble cytokines mentioned above, analysis of ES cell responses to leukemia inhibitory factor (LIF) indicates that changes in extracellular ligand concentration directly influence the probability of differentiation independent of effects on the rate of ES cell proliferation.67 Our more recent studies using the J1 and D3 ES cell lines have extended these results by showing that the potency of the mitogenic stimulus required to maintain ES cell pluripotency may be related to the numbers of LIF receptors expressed by each ES cell line.81 In fact, in ES cells, as in PC12 neuronal cells, the level of receptor occupancy appears to determine the self-renewal versus differentiation decision.67
A ligand-receptor signaling threshold (LIST) model of stem cell regulation
What might be the mechanisms by which high levels of cytokines selectively promote HSC self-renewal? We know that the continual stimulation of responsive cells by cytokines is a dynamic process with significant changes occurring over many different time scales. Formation of ligand-receptor complexes results in the recruitment and activation of specific intracellular molecules that then initiate different signaling pathways. At any specific point in time and spatial position, the number of ligand-receptor complexes per cell depends on 2 variables—the number of unoccupied receptors available and the ligand concentration—and one parameter—the ligand-receptor binding affinity. However, the 2 variables can change as a function of time or spatial position (or both), so that the number of ligand-receptor complexes can consequently change. It is also conceivable that accessory molecular factors, either inside (eg, signaling intermediates) or outside (eg, receptor agonists/antagonists) the cell can influence the parametric value of the ligand/receptor binding affinity. Thus, mechanisms that govern cell receptor number and ligand concentration can be predicted to correspondingly govern whether or not the resulting complex-activated signal(s) remain(s) above a threshold level.
If binding affinity is sufficiently great that the ligand/receptor complex remains stable during the usually relatively brief time required to internalize the receptor (about 10 minutes),68the ligand may be rapidly depleted from the extracellular milieu by cellular endocytic degradation of receptor-ligand complexes.48,54,69 This internalization and degradation of receptors can also result in their down-regulation.48,70Simultaneously, on the time scale of a cell division cycle, newly synthesized or recycled receptors (or both) can typically be re-expressed on the cell surface.71,72 Sustained signal propagation, itself, can result in protein-mediated desensitization of the complex to further signaling73 or perhaps the depletion of intracellular signaling intermediates. Thus, some metric characterizing the number of ligand-receptor complexes per cell is likely to determine the types and magnitude of the signals activated. (Indeed, it might be important to further characterize the homogeneity of ligand-receptor complexes on the cell surface and between the cell surface and intracellular compartments, because signaling pathway fluxes may also be altered by differences in these distributions.72) Hence, by careful quantitation of changes in receptor levels to improve understanding of the parameters that influence these changes, it may be possible to develop rational strategies to enhance HSC self-renewal divisions.
It is important to note that the kinetics of ligand-stimulated receptor-mediated action depend exquisitely on the molecular properties of the receptor-ligand pair. Our investigations into the kinetics of epidermal growth factor (EGF) binding to its receptor are consistent with the concept that information essential for regulating cellular changes can be found in both the magnitude and the persistence of the cytokine signal (not simply the presence of the signal per se).74 When the response of wild-type cells was compared with cells expressing a carboxy-terminal truncated EGF receptor mutant that is deficient in ligand-induced internalization,75proliferation rather than death of the 2 cell types was similar only when EGF depletion was minimized by medium replenishment,76 or when a mutant EGF with a significantly reduced binding affinity was used.71,77 These results suggest that a dynamic integration of the kinetics of receptor binding, internalization, and degradation may be important to relate cellular proliferation/death decisions to the number of ligand-receptor complexes activated. Similar findings have emerged more recently for control of T-lymphocyte proliferation by IL-2.54,69Moreover, results showing than a similar model is applicable to the regulation of hepatocyte differentiation by IL-6 and soluble IL-6 receptor (sIL-6R) (and “hyper-IL-6”—a fusion protein of Il-6 and sIL-6R78) have been reported.70 Taken together, these findings support the application of these concepts to other systems where proliferation may be associated with a change in cellular phenotype.
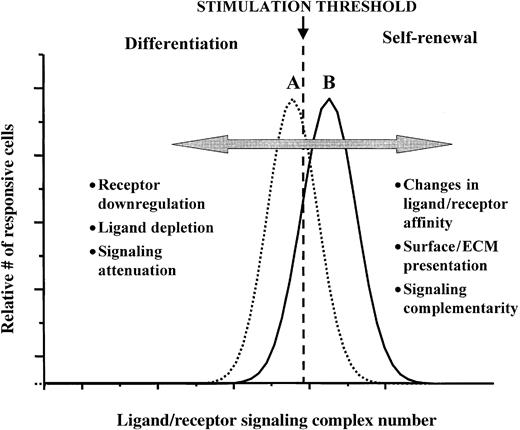
The model we are proposing (summarized diagrammatically in Figure2) envisages that the fraction of cells undergoing a self-renewal division depends on the numbers of signaling ligand-receptor complexes as compared to a given threshold level. If a cell is expressing sufficiently high numbers of the relevant receptors and is exposed to sufficiently high concentrations of the cognate ligands, so that the number of ligand-receptor signaling complexes is above a certain threshold, the probability that the cell will then undergo a self-renewal division will be high. Conversely, if a cell expresses too few receptors, or if its receptors have been sufficiently down-regulated, or if the cognate ligand concentrations are sufficiently depleted, the number of ligand/receptor signaling complexes is likely to fall below this threshold, with the consequence that the proportion of cells in the population that then undergo a self-renewal division will be significantly reduced. In either situation, the threshold comparison must be to some time-integration of the ligand-receptor complex numbers and resulting signal transduction. One especially interesting prediction from this model, with great potential for technologic applications, is that presentation of the ligand in a mode that minimizes ligand depletion or receptor down-regulation can potentially enhance the likelihood that suprathreshold complex levels will be maintained, hence the probability of self-renewal cell divisions occurring will be maximized. This prediction has, as implied previously, been borne out in the cases of EGF and IL-2 in the regulation of proliferation responses of fibroblasts and T lymphocytes, respectively.54,69 71
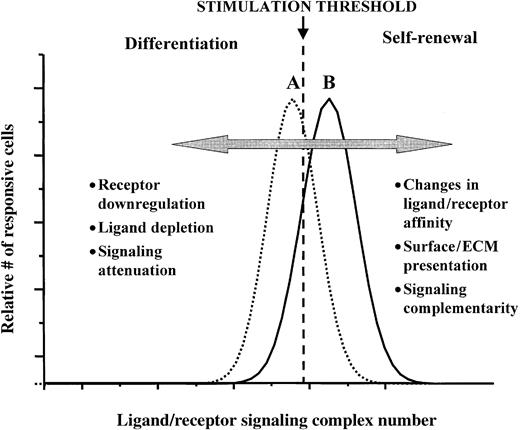
A ligand-receptor signaling threshold (LIST) model of stem cell differentiation control.
Examples of different mechanisms by which proliferating cells can move from a net loss in the numbers of undifferentiated cells (scenario A), to a net gain of undifferentiated cells (scenario B), as well as the reverse of this process, are listed. See text for further discussion.
A ligand-receptor signaling threshold (LIST) model of stem cell differentiation control.
Examples of different mechanisms by which proliferating cells can move from a net loss in the numbers of undifferentiated cells (scenario A), to a net gain of undifferentiated cells (scenario B), as well as the reverse of this process, are listed. See text for further discussion.
Measurements of low (but present) numbers of cytokine receptors on hematopoietic progenitors79 further substantiates this model by allowing spatially or temporally controlled changes in ligand densities to result in greater relative changes in the fraction of occupied receptors.80 (The temporal variance in the number of receptors bound at a constant ligand concentration is inversely proportional to receptor number, ie, if the cell has only one receptor, it fluctuates between the bound and unbound state at a binding constant-dependent rate.68) This model may also go a long way to explaining the heterogeneity of responses of seemingly identical cell populations to the same culture conditions. For example, given the order of magnitude differences in gp130 receptor numbers on ES cells, it is likely that, even at the highest LIF concentration, a proportion of cells may not be able to form sufficient numbers of ligand-receptor complexes to elicit a self-renewal division. This hypothesis is consistent with observations by our group,81 as well as others67 in various ES cell lines.
The existence of overlapping domains in many cytokine receptors may also be consistent with this model. Here we view self-renewal versus differentiation as a simple yes/no response, despite the fact that the events leading up to this decision must be at least as complex as the numbers of cytokines to which the cell may be sensitive. Because the signaling threshold is determined by receptor expression or availability, the effective ligand concentration, and the particular binding properties of the ligand/receptor pair, multiple scenarios may result in an intensity of signaling required to promote a “self-renewal” cell division. An experimental example of this can be found once again in the ES cell model, where the concentration of LIF, oncostatin M, and ciliary neurotrophic factor required to prevent differentiation differ significantly, even though these cytokines use the same gp130 transmembrane molecule for signaling.66Because each cytokine in this family has its own binding properties, but uses a limited number of common receptor subunits, the balance between competition for these different receptor subunits and ligand concentration may provide a mechanism for the differential control of cell responses to microenvironmental changes. Results showing that this family of cytokines may be organized as exchangeable modules,82 and the hypothesis that even for a particular cytokine, concentration-dependent changes in receptor complex stochiometry83 (each with their own signaling capacity) may exist, implicates additional levels of control that may influence how thresholds are (or are not) reached.
Of course, this model cannot be the whole story. First of all, the fact that very high concentrations of cytokines are required to differentially stimulate cells with low numbers of receptors suggests that the formation of active ligand-receptor complexes may be limited by the diffusion of low affinity partners in the plasma membrane.80 Other factors, such as receptor clustering or asymmetrical localization of other critical components are also likely to be important in allowing cells to respond to changes in the concentrations of protein signals over several orders of magnitude.84
In vivo mechanisms for controlling stimulation levels
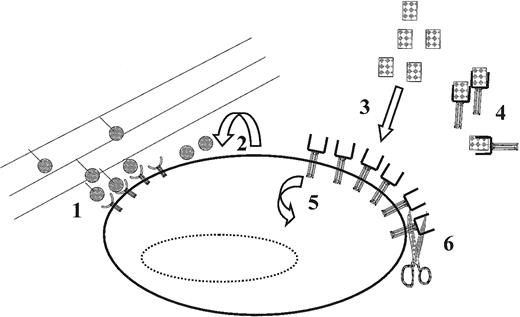
Although many of the previously discussed studies show that multipotential cells have the capacity to differentially respond to a wide range of concentrations of soluble ligands, multiple mechanisms, including autocrine,85-87 juxtacrine47,88 and exocrine89 stimulation, may be responsible for achieving the same level of control in vivo (Figure3). In fact, recent studies of autocrine cell signaling support the role of this mechanism in the robust control of ligand concentrations in the cellular microenvironment.90 As suggested earlier for SF, high levels of receptor activation can be obtained when responsive cells react with ligands that are expressed as transmembrane elements on the surface of other cells, or attached to extracellular matrix components.47 The prevalence transmembrane growth factors on primitive hematopoietic cells that can be converted to soluble factors by proteolytic processing or membrane shedding, without losing their biologic activity, provides yet another mechanism for the local control of cellular stimulation.91 Taken together, this suggests a model whereby transmembrane, soluble and extracellular matrix-bound cytokines provide a dynamically variable array of stimuli that can be stored in the cellular microenvironment.92Understanding the response patterns of cells to dynamic changes in such arrays could be key to developing an ability to predict the kinetics of cell behavior in different culture systems.
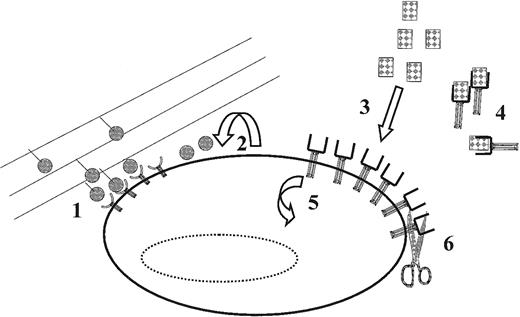
Examples of mechanisms that cells can use for the in vivo for the control of effective ligand concentrations and receptor expression (and thus the level of receptor-ligand complex activation).
(1) Preventing or diminishing ligand/receptor complex internalization through interactions between extracellular matrix (shown) or cell-surface bound ligands; (2) autocrine ligand secretion; (3) interactions with proteins secreted by other cells (either locally or systemically); (4) ligand interactions with agonistic or antagonistic soluble receptors (shown) or nonreceptor cytokine binding proteins (eg, uromodulin); (5) receptor internalization/synthesis; and (6) proteolytic cleavage of surface-bound receptors (shown) and/or ligands. Each of these mechanisms may determine whether a particular (threshold) level of receptor ligand activation is achieved. See text for further discussion.
Examples of mechanisms that cells can use for the in vivo for the control of effective ligand concentrations and receptor expression (and thus the level of receptor-ligand complex activation).
(1) Preventing or diminishing ligand/receptor complex internalization through interactions between extracellular matrix (shown) or cell-surface bound ligands; (2) autocrine ligand secretion; (3) interactions with proteins secreted by other cells (either locally or systemically); (4) ligand interactions with agonistic or antagonistic soluble receptors (shown) or nonreceptor cytokine binding proteins (eg, uromodulin); (5) receptor internalization/synthesis; and (6) proteolytic cleavage of surface-bound receptors (shown) and/or ligands. Each of these mechanisms may determine whether a particular (threshold) level of receptor ligand activation is achieved. See text for further discussion.
The fact that serum levels of Flt3L are disregulated during leukemogenesis and transplantation,93,94 along with the documented ability of high concentrations of this cytokine to promote stem cell self-renewal,8 provides further evidence that changes in exogenous levels of particular cytokines may be important in regulating HSC differentiation in vivo. Similarly, the physiologic relevance of changes in the relative concentrations of other cytokines in regulating hematopoietic progenitors in vivo can be inferred from the altered serum cytokine levels that correlate with conditions of HSC recovery. For example, 2- to 3-fold changes in serum LIF and IL-3 concentrations in the autologous transplantation setting have been associated with an induction of HSC cycling and differentiation caused by myeloablation.95 Conversely, increased serum SF and Flt3L concentrations correlate with increased numbers of hematopoietic progenitors in some patients with aplastic anemia,89,93 and administration of these cytokines has been reported to have a therapeutic effect in this disease.96 It is important to note that the changes in progenitor numbers (both relative and absolute) in these and other studies are due to changes in the serum concentrations (not their presence or absence) of cytokines relative to untreated controls.
The differential expression of soluble and surface bound isoforms of many cytokines and their receptors is another way by which stimulatory levels of critical factors may be controlled. Indeed, the modulatory effects of cytokines that have been shown to be important in the regulation of embryonic and HSC responses, including FGF,97 LIF,98 SF,43 and Flt3L,99 can be regulated in this manner. In some cases at least, such changes are found to influence the ability of cytokines to bind to membrane receptors and subsequently generate a response.100 Although many examples of this regulation exist, the fact that soluble receptors generally retain their ligand binding ability and can act as either competitive inhibitors (eg, IL-1, IL-2, G-CSF101) or as positive effectors (sIL-6R102 103), gives an insight into the complex processes that have evolved to regulate ligand-stimulated thresholds.
Molecular mechanisms for differentially transducing activation thresholds
Although evidence strongly suggests that threshold levels of receptor activation induced by soluble and bound factors can modulate the in vitro and in vivo responses of unspecialized cells, what has not been clarified are the downstream mechanisms by which such cells perceive and differentially respond to different levels of receptor activation. The idea that certain intracellular signaling thresholds stimulated by exogenous cytokines are important to cell fate decisions is not new.74 Receptors with intrinsic or associated tyrosine kinase activity are known to be capable of alternatively eliciting proliferative or differentiation responses in factor-dependent cell lines104-106 and it is likely that levels of signaling intermediates represent key determinants in these decisions. For example, both the duration and magnitude of extracellular signal-regulated kinase (ERK) activation by nerve growth factor (NGF) and EGF in the PC12 neuronal cell line influence whether these cells will proliferate or differentiate.107 The fact that a given cytokine can elicit different outcomes in the context of different expression levels of the corresponding receptor supports the view that a quantitative metric for signaling (eg, ERK activation) helps govern the biologic outcome in this system.108
Recent studies of the regulation of ES cell self-renewal and differentiation suggest this system holds much promise for further analysis of this process. A critical step in connecting receptor occupancy to the genetic programs involved in ES differentiation is signaling though the JAK/STAT (janus kinase/signal transducer and activator of transcription) pathway. Evidence that intracellular STAT3 activation is involved in LIF-mediated changes in ES cell self-renewal is provided by the observation that a threshold level of STAT3 activation is essential for this response.67 Dimerization of gp130 by LIF induces both the Ras-mitogen activated protein kinase (MAPK) and JAK/STAT pathways in ES cells.109 The results of Raz et al67 suggest that it is the level of activated STAT3 that is critical to maintaining a block of ES cell differentiation, MAPK activity being predominantly associated with mitogenesis.
Importantly, this type of signaling regulation also has an in vivo parallel. In the preimplantation embryo, asymmetrical localization of STAT3 correlates with LIF concentration gradients during the morula stage of development.110 These concentration gradients may be achieved by incorporation of locally produced LIF into the surrounding extracellular matrix100 and may allow temporal and spatial control of differentiation decisions during this early stage of development. A prevalence of gp130-mediated responses in other stem cell systems, including HSC47,48,102,111-113 along with the documented importance of the JAK/STAT signaling pathway in hematopoiesis114 suggest that regulation of stem cell differentiation decisions by this family of cytokines may be widely conserved. Genetic evidence of common mechanisms regulating mammalian embryonic development, tissue patterning, and adult HSC differentiation115 provides further support for the generality of a model in which the numbers of effective ligand/receptor interactions control differentiation decisions.
Conclusion
Several fundamental questions must be answered before it will be feasible to usefully predict and control HSC responses to exogenous cytokines on other than empirical grounds. In particular, a better understanding of how specific cytokines may alter the fate of mitogenically activated HSC is needed. This is likely to require knowledge of both the dynamics of changes in stem cellpopulations occurring over prolonged periods, as well as the cellular fate outcomes of individual stem cell divisions.
These issues will be key to the design of bioreactors in which cytokine-mediated expansion of HSC populations would be achieved. It has been proposed that the successful use of cytokines in bioprocessing applications will require the control of 2 attenuation mechanisms, cytokine depletion and receptor down-regulation,71,116,117Measurements of cytokine depletion rates by hematopoietic cells48,17,119 underscore the importance of monitoring and appropriately regulating the concentrations of cytokines considered necessary for optimizing the growth of HSC in culture. Strict monitoring of cytokine concentrations is likely to be most important where a decrease in the cytokine concentration is known to affect the desired cellular response, or if the rate of cytokine depletion is high. This may be particularly important in mixed cell systems typical of hematopoietic expansion cultures, where cell type–specific rates of cytokine depletion (and secretion) have been demonstrated.118
Additional studies are required to determine if modulation of the strength of receptor-induced signaling, possibly caused by differences in the timing, amount, or duration of receptor stimulation, can be linked to the activation of distinct programs of gene activity. A critical aspect of this analysis will be to measure temporal changes in the nuclear and cytoplasmic concentrations of intracellular intermediates such as the phosphorylated STAT proteins. Such information would not only facilitate the development of more controlled HSC expansion processes, it will undoubtedly also offer new insights into the fascinating biology of HCS self-renewal mechanisms.
Supported by a National Science Foundation Engineering Research Center (ERC) grant to the Biotechnology Process Engineering Center at the Massachusetts Institute of Technology and the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Run and P01HL 55435 from the National Institutes of Health (USA). P.W.Z. held a Natural Sciences and Engineering Research Council of Canada postdoctoral fellowship; C.J.E. is a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter W. Zandstra, Institute of Biomaterials and Biomedical Engineering, Rm 407, Roseburgh Bldg, 4 Taddle Creek Rd, Toronto, Ontario M5S 3G9, Canada; e-mail.zandstra@ibme.utoronto.ca.