Abstract
Preliminary independent reports of the Italian GIMEMA and the Spanish PETHEMA trials for newly diagnosed acute promyelocytic leukemia (APL) indicated a similarly high antileukemic efficacy in terms of complete remission and disease-free survival rates. To better investigate these studies and the prognostic factors influencing relapse risk, this study analyzed the updated results of 217 patients with PML/RARα-positive APL enrolled in GIMEMA (n = 108) and PETHEMA (n = 109). All patients received identical induction (AIDA schedule) and maintenance. For consolidation, GIMEMA patients received 3 courses including idarubicin/cytarabine, mitoxantrone/etoposide, and idarubicin/cytarabine/thioguanine, whereas PETHEMA patients received the same drugs and dose schedule of idarubicin and mitoxantrone with the omission of nonintercalating agents. Depending on whether molecular relapses were classified as censored or uncensored events, the 3-year Kaplan-Meier estimates of relapse-free survival (RFS) for the combined series were 90 ± 2% and 86 ± 2%, respectively. Minor differences observed between the 2 patient cohorts were negligible. Multivariate regression analysis of RFS showed that initial leukocyte (WBC) and platelet counts were the only variables with independent prognostic value. The resulting predictive model for RFS demonstrated its capability of segregating patients into low-risk (WBC count ≤ 10 × 109/L, platelet count > 40 × 109/L), intermediate-risk (WBC count ≤ 10 × 109/L, platelets ≤ 40 × 109/L), and high-risk (WBC count > 10 × 109/L) groups, with distinctive RFS curves (P < .0001). The conclusions are that omission of nonanthracycline drugs from the AIDA regimen is not associated with reduced antileukemic efficacy and a simple predictive model may be used for risk-adapted therapy in this disease.
Introduction
During the past decade, clinical and laboratory research has contributed important advances in the management of acute promyelocytic leukemia (APL). The 2 main determinants of such progress have been the inclusion of all-trans retinoic acid (ATRA) in front-line therapy and the cloning of the disease-specific t(15;17) karyotypic aberration. Data from recently reported large multicenter trials1-7 indicate that up-front ATRA combined with chemotherapy (CHT) results in long-lasting remission and a potential cure in up to 70% of newly diagnosed cases. In particular, best results have been obtained in patients with a genetically proven diagnosis who received a simultaneous ATRA plus CHT combination.3,5-7 Furthermore, 2 randomized studies have demonstrated a substantial benefit by including an ATRA-containing maintenance in the treatment program for APL.2 6 Despite this progress, however, treatment failure still occurs in approximately 30% of patients receiving state-of-the-art therapy due to early death or, more frequently, disease relapse.
Meanwhile, identification in the early 1990s of thePML/RARα gene fusion underlying the t(15;17) chromosomal translocation has permitted the development of reverse transcriptase–polymerase chain reaction (RT-PCR) assays for the rapid detection of the specific genetic lesion and for minimal residual disease monitoring.8,9 The inclusion of such a test in prospective clinical studies3,5,7,10 has proven invaluable for diagnostic refinement and sensitive assessment of the patient's response to treatment. Because the achievement of a sustained PCR-negative status for PML/RARα has been correlated with an improved outcome,8,9 several investigators have suggested that molecular remission should be defined as the best therapeutic goal presently available for this disease. In addition, given its high predictive value for hematologic relapse, conversion from PCR-negative to PCR-positive status during hematologic remission has been established in some studies as a criterion for anticipating salvage therapy.7,10 11
Although there is general agreement on the inclusion of ATRA for remission induction in front-line therapy for APL, no consensus presently exists as to the type and intensity of CHT to be used during induction and consolidation. Based on the long-established sensitivity of APL to anthracyclines, some investigators have used idarubicin alone for induction,3,7,12 whereas others have used conventional acute myeloid leukemia-like protocols either for induction2,4-6 or consolidation.6 Using ATRA plus IdArubicin (AIDA) for remission induction and 3 CHT consolidation courses, the Italian multicenter group GIMEMA reported, in a preliminary analysis of their study,3 a 95% response rate and a 2-year event-free survival (EFS) of 79%. The efficacy of such a regimen was recently confirmed by the Spanish PETHEMA group7 where an identical induction schedule was adopted. However, different from GIMEMA, the PETHEMA treatment omitted cytarabine and other non-intercalating drugs also from the consolidation phase. Using RT-PCR tests with similar sensitivity (10−4), both studies reported the achievement of molecular remission after the end of consolidation in more than 90% of patients.3,7 In addition to an efficacy comparable to that obtained by others with more intensive regimens, the PETHEMA group also reported a significant reduction of treatment-related toxicity during the consolidation phase. These results provide additional support to previous evidence suggesting that drugs other than anthracyclines, such as cytarabine and etoposide, do not play a critical role in the treatment of APL.12
Based on the above considerations, we have carried out a combined analysis of the updated results of the GIMEMA and PETHEMA APL trials. Our aims were to perform a better adjusted comparison between the 2 APL regimens, which differ only in the inclusion or not of drugs other than anthracyclines during consolidation; to identify prognostic factors for relapse risk; and to build a predictive model for relapse to be used in the design of improved risk-adapted protocols.
Patients and methods
Patients
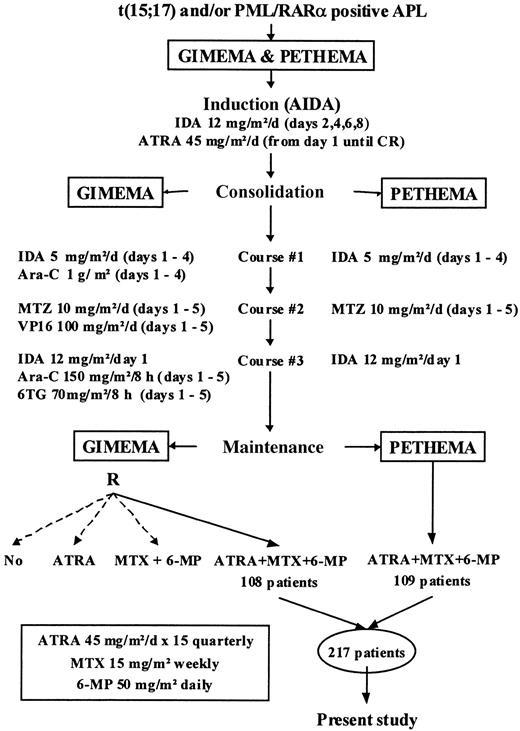
The present analysis was performed on 217 patients with newly diagnosed PML/RARα-positive APL who, after completion of induction and consolidation treatment, were assigned consecutively to receive maintenance with ATRA and CHT in the GIMEMA 0493 AIDA and PETHEMA LAP96 protocols (108 and 109 patients, respectively). Patients randomly allocated to other maintenance options in the GIMEMA trial3 were not included in the present study.
Laboratory studies
In addition to using the morphologic and cytochemical criteria defined by the French-American-British (FAB) classification, as well as routine immunophenotyping, the diagnosis of APL was confirmed genetically in all cases by demonstration of thePML/RARα hybrid gene or the chromosomal translocation t(15;17) or both. Immunophenotypic and cytogenetic analyses were performed systematically at presentation only. For the purpose of rapid diagnosis, we occasionally used immunohistochemical analysis of the PML protein distribution, using the monoclonal antibody PG-M3 (kindly provided by B. Falini) using a procedure reported elsewhere.13
RT-PCR monitoring
Details of the processing of bone marrow samples for RNA extraction and RT-PCR protocols for PML/RAR amplification have been given in previous reports.3,7 For the PETHEMA patients, RT-PCR tests were carried out by 12 different Spanish laboratories, involved in an external quality control program, which included interlaboratory exchange of samples, as reported elsewhere.19 In addition, PCR-positivity at the end of consolidation or during clinical remission was additionally checked in a reference laboratory (P.B., Valencia, Italy). For the GIMEMA patients, RT-PCR tests were performed by sample centralization in 2 reference laboratories as previously reported.11An identical schedule of pre-established time intervals for PCR monitoring was planned for each of the 2 studies. In brief, samples were collected at diagnosis, after induction, after consolidation, every 3 months during the first 2 years, and every 4 to 6 months thereafter. In cases of doubtful or positive PCR during hematologic complete remission (HCR) after the end of consolidation, an extra bone marrow sample was taken 2 to 4 weeks later to confirm the result.
Treatment
As shown in Figure 1, the treatment schedule of the GIMEMA and PETHEMA protocols was based on a common CHT backbone, including identical induction (ATRA plus idarubicin) and maintenance therapies (ATRA plus mercaptopurine and methotrexate), as well as the same dose schedule of intercalating drugs (idarubicin and mitoxantrone) for consolidation. The only difference was the omission of nonintercalating drugs from consolidation in the PETHEMA protocol. Details of the treatment schedules have been provided elsewhere.3 7
Definitions and study end points
Hematologic complete remission and hematologic relapse were defined according to the criteria of the National Cancer Institute.14 Molecular remission was defined as the disappearance on an ethidium bromide gel of the PML/RARα-specific band visualized at diagnosis, using an RT-PCR assay with a sensitivity level of 10−4. Molecular relapse was defined as the reappearance of PCR positivity in 2 consecutive bone marrow samples at any time after consolidation therapy. For Kaplan-Meier actuarial estimates, in which the event “relapse” was considered as an end point, the relapse-free survival (RFS) was calculated in 2 ways. Firstly, hematologic relapse was the only uncensored event considered; in such cases, patients who were treated intensively because of a molecular relapse were censored for survival analysis at the time of salvage treatment. Secondly, hematologic and molecular relapses were considered equally as uncensored events.
Statistical methods
Chi-square and Fisher exact tests were used to analyze differences in the distribution of variables among patient subsets. For univariate comparison, unadjusted time-to-event analyses were performed using the Kaplan-Meier estimate,15 as well as log-rank tests and their generalizations.16-18 All survival estimates are reported ± 1 SE. The median duration of follow-up was 27 months (range, 4-68 months). To identify the most significant independent prognostic factors, additional multivariate analysis was performed using the Cox model.19 The variables for analysis are listed in Table 1. Patient follow-up was updated in January 2000. Computations were performed using 4F, 1L, and 2L programs from the BMDP statistical library (BMDP Statistical Software, Los Angeles, CA).
Results
Patient characteristics
The distributions of the main clinical and biologic presenting features for each series are summarized in Table 1. There were no significant differences between the 2 groups with respect to gender, white blood cell (WBC) and platelet counts, FAB subtype, or PML breakpoint. With respect to age, more pediatric (younger than 16 years) and fewer elderly patients (older than 60 years) were observed in the GIMEMA cohort; however, differences in age distribution were not statistically significant (P = .07). A slightly significant difference was observed between the 2 cohorts with respect to hemoglobin level, with more anemic patients (hemoglobin ≤ 10 g/dL) in the GIMEMA series (P = .02).
Outcome
As of January 2000, a total of 27 relapses (12.4%) were recorded, taking into account all types of disease recurrence (ie, hematologic, extramedullary, and molecular relapses) in both series. Eleven patients in the PETHEMA and 9 in the GIMEMA group had clinical relapse at a median interval of 11 and 16 months from the achievement of HCR, respectively. Four of these clinical relapses occurred primarily in the central nervous system, in 2 patients in the PETHEMA trial, and 2 in the GIMEMA. Seven additional patients (4 in PETHEMA and 3 in GIMEMA) had a molecular relapse in the bone marrow at intervals of 6 to 22 and 9 to 16 months, respectively.
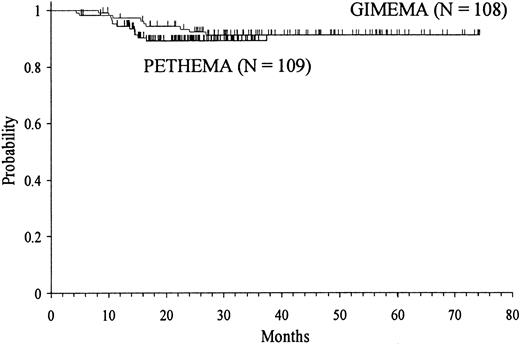
Depending on the assignation of molecular relapses as censored or uncensored events, the 3-year Kaplan-Meier estimates of RFS for the total series were 90 ± 2% and 86 ± 2%, respectively. Minor differences observed between GIMEMA and PETHEMA were not statistically significant (Figure 2).
Kaplan-Meier product-limit estimate of RFS in the GIMEMA and PETHEMA series.
Prognostic factors for remission duration
Univariate analysis.
Table 2 summarizes the results of univariate analysis for each separate group, as well as for the combined GIMEMA and PETHEMA series. For the total series, as well as in each study group, the only factor that had a significant prognostic influence on remission duration (considering either hematologic or hematologic plus molecular remission) was the WBC count at presentation. Platelet count also showed a significant influence or a trend in the total series and in each study group. Finally, the BCR subtype showed a trend in the GIMEMA group only.
Multivariate analysis.
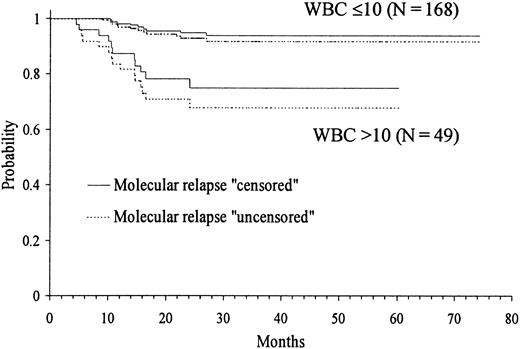
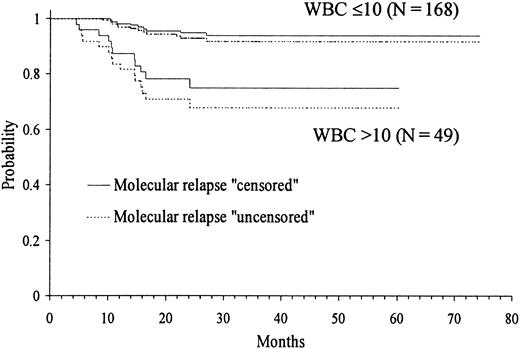
Because several cut-off points of platelet and WBC count had shown discriminant value in the univariate analysis, we created by transformation several categorized variables of platelet and WBC count. Then, in addition to the remaining presenting features, all these variables were simultaneously introduced in the multivariate analysis (proportional hazards regression model). After selecting WBC count with the cut-off point of 10 × 109/L in step 1 as the first variable to enter into regression, the categorized platelet count with the cut-off point of 40 × 109/L was selected in step 2. Table 3 shows the principal results of the multivariate analysis, where the variables are listed in the order entered by the forward stepwise modeling procedure. The only 2 characteristics selected were presenting WBC and platelet counts. Figures 3 and4 show the actuarial RFS according to the different discriminating cut-offs for WBC and platelet counts, respectively. The classification of molecular relapse as a censored or uncensored event did not change these results, except for theP value limit when entering platelet count into the model (.05 and .07, respectively).
Kaplan-Meier product-limit estimate of RFS according to WBC count at presentation.
Kaplan-Meier product-limit estimate of RFS according to WBC count at presentation.
Kaplan-Meier product-limit estimate of RFS according to platelet count at presentation.
Kaplan-Meier product-limit estimate of RFS according to platelet count at presentation.
Development of a simplified predictive model for RFS.
Based on the above results, a simple predictive model was constructed for defining the diagnosis risk groups of RFS. This permitted the identification of the following patient categories: (1) low-risk group: presenting WBC count below or equal to 10 × 109/L and platelet count above 40 × 109/L; (2) intermediate-risk group: presenting WBC and platelet counts below or equal to 10 × 109/L and 40 × 109/L, respectively; and (3) high-risk group: presenting WBC greater than 10 × 109/L. The numerical distribution of patients in the 3 above groups was as follows: low-risk 53 (24%), intermediate 115 (53%), and high-risk 49 (23%). The differences in RFS curves of the 3 risk groups (Figure 5) were highly significant (P < .0001).
Kaplan-Meier product-limit estimate of RFS according to the risk groups defined by the predictive model.
Kaplan-Meier product-limit estimate of RFS according to the risk groups defined by the predictive model.
Discussion
This study shows that, in patients with newly diagnosed APL receiving up-front AIDA, the use of an anthracycline-based consolidation omitting cytarabine and other nonintercalating agents seems to be equally effective as a more intensive regimen including these drugs. In addition, in this study we have defined a simple model that permits the easy identification at diagnosis of 3 distinct prognostic groups among patients receiving AIDA-derived treatments. This, in turn, provides a rationale for the design of risk-adapted protocols aimed at further improving treatment outcome for this type of leukemia.
With regard to the first issue, our findings extend and strengthen a recently reported preliminary observation from the PETHEMA group.7 The comparison of updated results of that study with those obtained from the GIMEMA trial for patients receiving more intensive consolidation indicates that similarly high RFS rates are obtained with either regimen. Our results are also in line with the study of Estey and colleagues12 who reported a disease-free survival (DFS) at 1 year of 87% using an AIDA-like regimen for induction, followed by a postremission therapy including idarubicin and alternating cycles of mercaptopurine, vincristine, methotrexate, and prednisone. Taken together, these findings open new important perspectives in the treatment of APL; with respect to the risk of relapse, they strongly suggest that omission of cytarabine from consolidation might permit better adaptation to the ATRA/anthracycline combination.
The remarkable similarity in patient characteristics, protocol design, and response to therapy between the PETHEMA and GIMEMA studies prompted us to analyze prognostic factors influencing RFS in the 2 groups combined. Minor differences in the 2 series, such as age and hemoglobin levels, appeared irrelevant for the purpose of our study. In fact, these 2 variables were not associated with an increased relapse risk in the present study, or in previous ones.4-7
Although prognostic factors have been analyzed in only a marginal way in the majority of recent studies on APL, there is general agreement on the prognostic impact of WBC count in remission response,5-7,20 EFS,3,5,7 DFS, and relapse risk.3,7 To the best of our knowledge, no other clinical presenting factors, except for platelet count, correlate consistently with relapse risk. In fact, a long-term follow-up report of the APL 1991 trial from the European APL Group found a significantly higher incidence of relapse for patients who had fewer than 50 × 109/L platelets at presentation.21Importantly, in our study, this factor retained its prognostic value in the multivariate analysis. Although the significance of such a finding is presently unclear, it may be speculated that platelet number at diagnosis somehow reflects a level of residual polyclonal hematopoiesis spared by the leukemic process. PCR positivity after consolidation deserves a separate mention as an index with predictive value for relapse; however, this biologic parameter is not available at the time of diagnosis.5 11
Interestingly, our prognostic model that includes only WBC and platelet counts permits the identification of a patient subset with an extremely low relapse risk, in which the use of the less intensive postinduction regimen adopted by the PETHEMA trial seems most appropriate. On the other hand, patients assigned to intermediate-risk and especially to high-risk categories would potentially benefit from intensification of postremission therapy aimed at obtaining greater efficacy in eradicating minimal residual leukemia.
The use of combined ATRA and CHT has led to striking improvements in the outlook for APL, as reported by a number of recently published studies conducted at the multi-institutional level.1-7Therefore, it is important that our proposed prognostic model be validated in an independent patient series, in particular for those patients where the ATRA plus CHT combination indicates a greater therapeutic efficacy. In the meantime, we firmly believe that such a model should be reproducible for PML/RARα-positive APL patients receiving approaches similar to ours, that is, simultaneous ATRA and anthracycline-based induction followed by intensive consolidation and maintenance containing ATRA. In this respect, we note that randomized studies have recommended both the up-front combination of simultaneous ATRA and CHT and the inclusion of ATRA maintenance (as used in the present study), as a standard therapy for the management of APL.
The authors thank Luis Benlloch (from PETHEMA) and Paola Fazi, Maria Luce Vegna, and Marco Vignetti (from GIMEMA) for data collection and management. The following institutions and personnel participated in PETHEMA trial: Hospital Universitario La Fe, Valencia (M.A. Sanz, G. Martı́n, P. Bolufer, E. Barragán); Hospital Central de Asturias, Oviedo (C. Rayón); Hospital Clı́nico San Carlos, Madrid (J. Dı́az-Mediavilla, A. Villegas); Hospital Clı́nico Universitario, Valencia (M.J. Terol, I. Marugán); Hospital Insular de Las Palmas, Las Palmas (J.D. González); Hospital Clinic, Barcelona (J. Esteve, D. Colomer); Hospital General, Alicante (C. Rivas); H.U. Germans Trias i Pujol, Badalona (J.M. Ribera); Complexo H. Xeral-Calde, Lugo (J. Arias); Hospital Universitario, Salamanca (M. González, C. Chillón); Hospital de Cruces, Baracaldo (M.C. Alvarez); Complejo Hospitalario, León (F. Ramos); Hospital Juan Canalejo, A Coruña (G. Debén); H. Ntra Sra del Pino/Sabinal, Las Palmas (R. Mataix, T. Gómez); Hospital Reina Sofia, Córdoba (S. Tabares, J. Román); Hospital Clı́nico Universitario, Valladolid (F. Fernández); H. Universitario Vall D'Hebron, Barcelona (J. Bueno); Hospital Son Dureta, Palma de Mallorca (A. Novo); Hospital Xeral de Galicia, Santiago de Compostela (M. Pérez); Hospital Ramón y Cajal, Madrid (J. Odriozola, C. Ferro); Hospital do Meixoeiro, Vigo (C. Loureiro); Hospital Severo Ochoa, Leganés (P. Sánchez); Hospital Dr. Peset, Valencia (M.J. Sayas); Hospital 12 de Octubre, Madrid (J. De la Serna, R. Bornstein); Hospital General de Murcia, Murcia (J.M. Moraleda); H.U. Virgen de la Victoria, Málaga (I. Pérez); H.U. Puerta del Mar, Cádiz (F.J. Capote); H. San Pedro de Alcántara, Cáceres (J.M. Bergua); Basurtuko Ospitalea, Basurto (J.M. Beltrán de Heredia); Hospital Rio Hortega, Valladolid (M.J. Peñarrubia); H. General Jerez de la Frontera, Jerez de la Frontera (A. León); Hospital General, Albacete (J.R. Romero); Hospital Xeral Cı́es, Vitoria (C. Poderós); Hospital Txagorritxu, Vitoria (J.M. Guinea); Hospital San Pau, Barcelona (S. Brunet); H. General Oncologı́a Pediátrica, Alicante (C. Esquembre); Hospital Rio Carrión, Palencia (F. Ortega); H.U. Marqués de Valdecilla, Santander (E. Conde, C. Richard); H.U. La Fe (Hospital Infantil), Valencia (V. Castell); Universidad de Navarra, Pamplona (M.J. Calasanz).
The following institutions participated in the AIDA 0493 trial: Ematologia, Universita' “La Sapienza”, Roma (F. Mandelli, G. Avvisati, D. Diverio, F. Lo Coco, M.C. Petti, M.L. Vegna); Divisione di Ematologia, Ospedale S. Martino, Genova (E. Damasio, R. Cerri); Istituto di Ematologia L. e A. Seragnoli, Universita', Bologna (S. Tura, G. Visani, G. Martinelli); Divisione di Ematologia, Ospedale S. Bortolo, Vicenza (F. Rodeghiero, E. Di Bona); Divisione di Ematologia, Policlinico S. Matteo, Pavia (C. Bernasconi, M. Lazzarino); Divisione di Medicina E, Opedale S. Giovanni, Torino (L. Resegotti, M. Falda); Divisione di Ematologia, Policlinico Careggi, Firenze (P. Rossi Ferrini, F. Leoni); Divisione di Ematologia, Ospedali Riuniti, Bergamo (T. Barbui, A. Rambaldi); Divisione di Ematologia, Ospedale Civile, Pescara (G. Fioritoni, A. Recchia); Servizio di Ematologia, Policlinico, Bari (V. Liso, G. Specchia); Divisione di Ematologia, Ospedale A. Businco, Cagliari (G. Broccia, W. Deplano); Servizio di Ematologia, Ospedale Civile, Avellino (E. Volpe, N. Cantore); Divisione di Ematologia, Ospedale A. Pugliese, Catanzaro (A. Peta, F. Iuliano); Divisione di Ematologia, Ospedale S. Gerado, Monza (E. Pogliani, G. Corneo); Ematologia, Ospedale Generale e Regionale, Bolzano (P. Coser, P. Fabris); Sezione di Ematologia Spedali Civili, Brescia (T. Izzi, G. Rossi); Cattedra di Ematologia, Universita', Catania (E. Cacciola, F. Di Raimondo); Cattedra di Ematologia, Universita', Parma (V. Rizzoli, C. Almici); Cattedra di Ematologia, Universita', Verona (G. Perona, D. Veneri); Cattedra di Ematologia, Universita', Genova (M. Gobbi, M. Clavio); Divisione di Ematologia, Ospedale Cardarelli, Napoli (R. Cimino, F. Ferrara); Divisione di Ematologia, Osp. Nuovo Pellegrini, Napoli (R. De Biasi, E. Miraglia); Divisione di Ematologia, T.E.R.E., Napoli (L. De Rosa, V. Mettivier); Cattedra di Ematologia, Universita' Tor Vergata, Roma (S. Amadori, G. Aronica); Clinica Pediatrica, Ospedale S. Gerardo, Monza (G. Masera, A. Biondi, A. Luciano); Divisione di Ematologia, Universita' Cattolica, Roma (G. Leone, S. Sica); Divisione di Ematolgia, Ospedali Riuniti, Reggio Calabria (F. Nobile, B. Martino); Sezione di Ematolgia, Ospedale S. Croce, Cuneo (E. Gallo, A. Gallamini); Divisione di Ematologia, Ospedale S.Maria Goretti, Latina (L. Deriu, A. Cherichini); Sezione di Ematologia, CTMO, Cremona (A. Porcellini, S. Morandi); Divisione di Ematologia, Nuovo Policlinico, Napoli (B. Rotoli, C. Selleri); Cattedra di Ematologia, Università, Perugia (M.F. Martelli, A. Tabilio); Clinica Medica, Universita', Palermo (A. Cajozzo, M. Musso); Divisione di Ematologia, Ospedale V.Cervello, Palermo (F. Caronia, S. Mirto, A. Santoro); Divisione di Ematologia, Ospedale B.Gesù, Roma (G. De Rossi, M. Caniggia); Istituto di Ematologia, Nuovo Ospedale Torrette, Ancona (P. Leoni, M. Montillo); Centro di Riferimento Oncologico, Aviano (S. Monfardini, V. Zagonel); Patologia Medica, Universita', Genova (R. Ghio, E. Balleari); Clinica Medica, Policlinico S. Matteo, Pavia (E. Ascari, R. Invernizzi); Divisione di Ematologia, Universita', Pisa (B. Grassi, M. Petrini); Ematologia, Ospedale S.S. Annunziata, Taranto (P. Mazza, G. Lazzari); Cattedra di Ematologia, Universita', Udine (M. Baccarani, A. Candoni); Ematologia Pediatrica, Universita', Catania (G. Schiliro', A.M. Ippolito); Ematologia, IV Divisione Pediatrica, Genova (L. Massimo, C. Micalizzi); Cinica Pediatrica, Universita', Pavia (F. Severi, F. Locatelli); Ematologia, Ospedale Regionale A. Di Summa, Brindisi (G. Quarta, A. Melpignano); Cattedra di Ematologia, Universita', Ferrara (G. Castoldi, F. Lanza); Semeiotica Medica, Universita', Genova (F. Patrone, M. Sessarego); Divisione di Ematologia, Ospedale Niguarda, Milano (E. Morra, A.M. Nosari); Ematologia, Ospedale S. Raffaele, Milano (C. Bordignon, L. Camba); Ematologia ed Autotrapianto Ospedale S.Martino, Genova (A.M. Carella, F. Frassoni); Sezione di Ematologia, Ospedale S. Francesco, Nuoro (A. Gabbas, G. Latte); Cattedra di Ematologia, Policlinico, Palermo (P. Citarella, S. Grisanti); Divisione di Ematologia, Ospedale S. Salvatore, Pesaro (G. Lucarelli, G. Sparaventi); Sezione di Ematologia, Ospedale S. Carlo, Potenza (F. Ricciuti, M. Pizzuti); Divisione di Ematologia, Ospedale S. Camillo, Roma (A. De Laurenzi, L. Pacilli); Div. di Ematologia, Casa Sollievo della Sofferenza, S.G. Rotondo (M. Carotenuto, L. Melillo); Divisione di Ematologia, Ospedale A.Sclavo, Siena (E. Dispensa, A. Bucalossi); Clinica Pediatrica, Ospedale G. Salesi, Ancona (P. Giorgi, L. Felici); Clinica Pediatrica I, Policlinico, Bari (F. Schettini, N. Santoro); Onco-Ematologia Pediatrica, Ospedale Regionale, Cagliari (P. Biddau); II Divisione Pediatrica, Ospedale Pausilipon, Napoli (V. Poggi, M.F. Pintà); Clinica Pediatrica I, Universita', Napoli (M.T. Di Tullio, M. Giuliano); Clinica Pediatrica II, Universita', Padova (L. Zanesco, M. Pilon); Clinica Pediatrica III, Universita', Pisa (P. Macchia, C. Favre); Clinica Pediatrica, Universita', Torino (E. Madon, R. Miniero); Department of Hematology, University, Nijmegen (NL) (T. de Witte, P. Muus); Medizinische Klinik III, University, Munich (D) (U. Jehn); Department of Hematology, University, Leiden (NL) (R. Willemze); Department of Hematology, University, Ankara (TK) (M. Beksac); Az Middelheim, AfdelingHemato-Oncologie, Antwerpen (B) (R. De Bock).
Supported in part by grants no. 96/1734 and 99/0806 from the Fondo de Investigación Sanitaria (FIS), Ministerio de Sanidad of Spain; grant FIJC PETH-99 from the International José Carreras Leukemia Foundation; grant no. HI1998-0147 from Ministerio de Educación y Cultura of Spain; MURST Azioni Integrate Italia-Spagna, AIRC (Associazione Italiana per la Ricerca sul Cancro); and ROMAIL (Associazione Italiana contro le Leucemie, Sezione di Roma).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Miguel A. Sanz, Servicio de Hematologı́a, Hospital Universitario La Fe, Av. Campanar 21, 46009 Valencia, Spain; e-mail: msanz@uv.es.