Abstract
Allogeneic bone marrow transplantation is an effective postremission strategy for patients with acute myelogenous leukemia (AML) in first complete remission (CR). The value of administering consolidation chemotherapy before human leukocyte antigen (HLA)–identical sibling transplantation is not established. Outcomes of patients with AML in first CR receiving no consolidation therapy, standard-dose cytarabine consolidation therapy, and high-dose cytarabine consolidation therapy before HLA–identical sibling transplantation were compared. Five-year treatment-related mortality rates were 30% (95% confidence interval [CI], 18% to 42%) in patients receiving no consolidation chemotherapy, 22% (95% CI, 17% to 28%) in those receiving standard-dose cytarabine consolidation, and 24% (95% CI, 17% to 31%) in those receiving high-dose cytarabine (P = NS). Five-year cumulative incidences of relapse were 19% (10% to 30%), 21% (16% to 27%), and 17% (11% to 24%), respectively (P = NS). Five-year probabilities of leukemia-free survival were 50% (36% to 63%), 56% (49% to 63%), and 59% (50% to 66%), respectively (P = NS). Five-year probabilities of overall survival were 60% (46% to 71%), 56% (49% to 63%), and 60% (51% to 67%), respectively (P = NS). The data indicate that postremission consolidation with cytarabine before allogeneic transplantation for AML in first CR is not associated with improved outcome compared to proceeding directly to transplantation after successful induction.
Introduction
The need for intensive postremission therapy after successful remission induction in patients with acute myelogenous leukemia (AML) is well established.1-3 Postremission consolidation therapy decreases leukemia recurrence and improves survival. High-dose chemotherapy, with or without radiation with human leukocyte antigen (HLA)–identical sibling bone marrow transplantation, is an effective postremission therapy. However, one or more cycles of less intensive postremission chemotherapy are also commonly given to patients before HLA–identical sibling transplantation in first complete remission (CR). The value of this strategy is not established. HLA–identical sibling transplantation for AML in first CR results in long-term, disease-free survival in approximately 50% of patients.4-8 It is possible that postremission therapy with high-dose cytarabine (in this study, defined as doses of at least 1 gm/m2) before transplantation may reduce the leukemic burden and thereby improve transplantation outcome. Alternatively, intensive postremission therapy may result in toxicities severe enough to preclude subsequent transplantation or might yield complications that increase the risk for transplant-related death. Additionally, consolidation chemotherapy increases the total cost of treatment. To address the value of pretransplantation consolidation, a large cohort of patients receiving HLA–identical sibling transplants worldwide and reported to the International Bone Marrow Transplant Registry (IBMTR) was studied. The outcomes of those receiving no consolidation, standard-dose cytarabine consolidation, and high-dose cytarabine consolidation were compared.
Patients, materials, and methods
Patients
We studied 431 patients who received HLA–identical sibling bone marrow transplants for AML in first CR between 1989 and 1995 and who were reported to the IBMTR by 108 transplant centers. Only patients receiving 1 or 2 cycles of standard-dose cytarabine for remission induction (100-200 mg/m2) were included. Patients who received high-dose cytarabine during induction or who required more than 2 cycles of induction to achieve CR were excluded. Sixty-two patients received no postremission therapy before transplantation, 222 received standard-dose cytarabine with additional drugs for consolidation, and 147 received high-dose cytarabine. One hundred thirteen of 147 patients received high-dose cytarabine with additional drugs, and 34 received high-dose cytarabine alone. Additional drugs included anthracycline, etoposide, mitoxantrone, methotrexate, amsacrine, vincristine, prednisone, 6-mercaptopurine, and 6-thioguanine. For purposes of this analysis, all patients receiving high-dose cytarabine were considered as a single group. Cytogenetic abnormalities were divided into those with good, intermediate, or poor prognoses. Cytogenetic abnormalities with good prognoses included t(8;21) with or without other abnormalities, t(15;17) with or without other abnormalities, or inv or del(16) with or without other abnormalities. Cytogenetic abnormalities with intermediate prognoses included trisomy 8 with or without other abnormalities, trisomy 21 with or without other abnormalities, t(6;9) with or without other abnormalities, other translocations, other numerical abnormalities, or other structural abnormalities. Cytogenetic abnormalities with poor prognoses included t(9;22) with or without other abnormalities, −7 or del(7) with or without other abnormalities, or del(11) with or without other abnormalities and complex karyotypes.9 Patients with cytogenetic abnormalities of good and poor prognoses were considered in the poor prognosis group.
Endpoints
Endpoints were transplant-related mortality (TRM), relapse rate, leukemia-free survival (LFS), and overall survival. For analyses of TRM, failure was defined as death during a continuous CR; data were censored at the time of relapse or, among patients with continuous remissions, at the time of last follow-up. For analyses of leukemia-free survival, treatment was considered to have failed at the time of clinical or hematologic relapse at any site or at the time of death from any cause; data on patients who were alive and in CR were censored at the time of last follow-up visit.
Statistical methods
Baseline patient-, disease-, and treatment-related characteristics of patients receiving no postremission therapy, standard-dose cytarabine postremission therapy, and high-dose cytarabine therapy were compared using the χ2 statistic and Wilcoxon test for categoric and continuous variables, respectively. Estimates of TRM and relapse rates were calculated using cumulative incidence rates to accommodate competing risks10; estimates of LFS and survival were calculated using the Kaplan-Meier estimator.11 Rates of TRM, leukemia relapse, LFS, and survival were compared using the log-rank test.12 Forward stepwise Cox proportional hazards regression was used to examine the effect of postremission therapy on TRM, relapse, and treatment failure (inverse of LFS) in multivariate analyses, adjusting for other significant covariates.13 Variables considered in stepwise regression analysis were age, sex, performance score, cytomegalovirus serology status, French–American–British (FAB) type, leukocyte count at diagnosis, extramedullary disease, cytogenetics, number of induction cycles, time from diagnosis of CR1, time from CR1 to transplantation, conditioning regimen, graft-versus-host disease (GVHD) prophylaxis, and year of transplantation. Because the primary variable of interest was postremission chemotherapy, each model contained at least 2 covariates, one for standard-dose cytarabine and one for high-dose cytarabine. The most significant additional factor was added to the model in each step. Model building stopped when there were no additional significant factors (at 5% significance level). The proportionality assumptions of the Cox model were checked by adding a time-dependent covariate. Interactions between significant covariates and consolidation effects were tested. A score test was used to determine whether there were center-specific effects.14
Results
Patients
Patient-, disease-, and treatment-related characteristics are summarized in Table 1. Several characteristics differed significantly among the 3 treatment groups, including FAB classification, leukocyte count at diagnosis, cytogenetic abnormalities, number of cycles to achieve CR, median time from CR to transplantation, percentage of patients receiving total body irradiation for conditioning, GVHD prophylaxis, and year of transplantation. There were no differences in other parameters including gender, performance score, recipient cytomegalovirus status, the presence of extramedullary disease at diagnosis, median time from diagnosis to achievement of CR, or median follow-up time.
Patient-, disease- and treatment-related characteristics of patients with AML receiving HLA–identical sibling bone marrow transplantation in first complete remission
| . | (1) No postremission therapy . | (2) Standard-dose cytarabine . | (3) High-dose cytarabine . | P12 . | P13 . | P23 . |
|---|---|---|---|---|---|---|
| Number of patients | 62 | 222 | 147 | |||
| Median age, years | 32 (2-55) | 29 (<1-56) | 28 (1-52) | 0.34 | 0.24 | 0.37 |
| Male sex, n (%) | 33 (53) | 112 (50) | 79 (54) | 0.70 | 0.95 | 0.54 |
| Performance score ≥ 90%, n (%) | 54 (87) | 196 (88) | 132 (90) | 0.80 | 0.57 | 0.65 |
| Cytomegalovirus seropositive, n (%) | 37 (60) | 128 (59) | 100 (68) | 0.95 | 0.22 | 0.07 |
| FAB subtype, n (%) | 0.02 | <0.001 | 0.53 | |||
| Unclassified | 7 (11) | 9 (4) | 2 (1) | |||
| M1, M2 | 35 (57) | 98 (44) | 61 (42) | |||
| M3 | 7 (11) | 26 (12) | 18 (12) | |||
| M4 | 6 (10) | 53 (24) | 36 (25) | |||
| M5-M7 | 7 (11) | 36 (16) | 30 (20) | |||
| Median leukocytes at diagnosis, ×109/L | 7 (1-400) | 17 (1-409) | 14 (1-510) | 0.02 | 0.02 | 0.94 |
| Cytogenetics, n (%) | 0.47 | 0.90 | 0.02 | |||
| Normal | 16 (33) | 51 (27) | 43 (35) | |||
| Good prognosis | 9 (19) | 28 (15) | 18 (14) | |||
| Intermediate prognosis | 7 (15) | 21 (11) | 22 (18) | |||
| Poor prognosis | 1 (2) | 3 (1) | 5 (4) | |||
| Unknown | 15 (31) | 89 (46) | 36 (29) | |||
| Extramedullary disease at DX, n (%) | 3 (5) | 30 (14) | 20 (14) | 0.06 | 0.07 | 0.99 |
| Additional drugs for consolidation | — | 218 (98) | 113 (77) | — | — | <0.001 |
| No. cycles to achieve CR, n (%) | 0.01 | 0.24 | 0.09 | |||
| 1 | 33 (53) | 156 (70) | 91 (62) | |||
| 2 | 29 (47) | 66 (30) | 56 (38) | |||
| Median time from DX to CR, mo | 1.3 (0.2-13.7) | 1.0 (0.2-12.8) | 1.3 (0.4-5.6) | 0.72 | 0.70 | 0.99 |
| Median time from CR to transplantation, mo | 2.2 (0.5-15.1) | 4.2 (0.2-26.3) | 5.1 (0.4-16.9) | <0.001 | <0.001 | 0.004 |
| TBI for conditioning, n (%) | 22 (35) | 120 (54) | 88 (60) | 0.01 | <0.001 | 0.27 |
| GVHD prophylaxis, n (%) | <0.001 | 0.05 | 0.05 | |||
| CsA + MTX | 29 (47) | 142 (64) | 80 (54) | |||
| CsA or MTX | 24 (39) | 32 (14) | 35 (24) | |||
| Other | 3 (5) | 1 (<1) | 3 (2) | |||
| T-depletion | 6 (9) | 47 (21) | 29 (20) | |||
| Year of transplantation | 0.72 | 0.08 | 0.04 | |||
| 1989-1991 | 39 (63) | 134 (60) | 73 (50) | |||
| 1992-1995 | 23 (37) | 88 (40) | 74 (50) | |||
| Median follow-up time, mo | 60 | 61 | 61 | 0.22* | 0.10* | 0.44* |
| . | (1) No postremission therapy . | (2) Standard-dose cytarabine . | (3) High-dose cytarabine . | P12 . | P13 . | P23 . |
|---|---|---|---|---|---|---|
| Number of patients | 62 | 222 | 147 | |||
| Median age, years | 32 (2-55) | 29 (<1-56) | 28 (1-52) | 0.34 | 0.24 | 0.37 |
| Male sex, n (%) | 33 (53) | 112 (50) | 79 (54) | 0.70 | 0.95 | 0.54 |
| Performance score ≥ 90%, n (%) | 54 (87) | 196 (88) | 132 (90) | 0.80 | 0.57 | 0.65 |
| Cytomegalovirus seropositive, n (%) | 37 (60) | 128 (59) | 100 (68) | 0.95 | 0.22 | 0.07 |
| FAB subtype, n (%) | 0.02 | <0.001 | 0.53 | |||
| Unclassified | 7 (11) | 9 (4) | 2 (1) | |||
| M1, M2 | 35 (57) | 98 (44) | 61 (42) | |||
| M3 | 7 (11) | 26 (12) | 18 (12) | |||
| M4 | 6 (10) | 53 (24) | 36 (25) | |||
| M5-M7 | 7 (11) | 36 (16) | 30 (20) | |||
| Median leukocytes at diagnosis, ×109/L | 7 (1-400) | 17 (1-409) | 14 (1-510) | 0.02 | 0.02 | 0.94 |
| Cytogenetics, n (%) | 0.47 | 0.90 | 0.02 | |||
| Normal | 16 (33) | 51 (27) | 43 (35) | |||
| Good prognosis | 9 (19) | 28 (15) | 18 (14) | |||
| Intermediate prognosis | 7 (15) | 21 (11) | 22 (18) | |||
| Poor prognosis | 1 (2) | 3 (1) | 5 (4) | |||
| Unknown | 15 (31) | 89 (46) | 36 (29) | |||
| Extramedullary disease at DX, n (%) | 3 (5) | 30 (14) | 20 (14) | 0.06 | 0.07 | 0.99 |
| Additional drugs for consolidation | — | 218 (98) | 113 (77) | — | — | <0.001 |
| No. cycles to achieve CR, n (%) | 0.01 | 0.24 | 0.09 | |||
| 1 | 33 (53) | 156 (70) | 91 (62) | |||
| 2 | 29 (47) | 66 (30) | 56 (38) | |||
| Median time from DX to CR, mo | 1.3 (0.2-13.7) | 1.0 (0.2-12.8) | 1.3 (0.4-5.6) | 0.72 | 0.70 | 0.99 |
| Median time from CR to transplantation, mo | 2.2 (0.5-15.1) | 4.2 (0.2-26.3) | 5.1 (0.4-16.9) | <0.001 | <0.001 | 0.004 |
| TBI for conditioning, n (%) | 22 (35) | 120 (54) | 88 (60) | 0.01 | <0.001 | 0.27 |
| GVHD prophylaxis, n (%) | <0.001 | 0.05 | 0.05 | |||
| CsA + MTX | 29 (47) | 142 (64) | 80 (54) | |||
| CsA or MTX | 24 (39) | 32 (14) | 35 (24) | |||
| Other | 3 (5) | 1 (<1) | 3 (2) | |||
| T-depletion | 6 (9) | 47 (21) | 29 (20) | |||
| Year of transplantation | 0.72 | 0.08 | 0.04 | |||
| 1989-1991 | 39 (63) | 134 (60) | 73 (50) | |||
| 1992-1995 | 23 (37) | 88 (40) | 74 (50) | |||
| Median follow-up time, mo | 60 | 61 | 61 | 0.22* | 0.10* | 0.44* |
CMV, cytomegalovirus; FAB, French-American-British; DX, diagnosis; CR, complete remission; TBI, total body irradiation; GVHD, graft-versus-host disease; CsA, cyclosporine; MTX, methotrexate.
P12, probability of testing (1) = (2).
P13, probability of testing (1) = (3).
P23, probability of testing (2) = (3).
Log-rank test.
Outcomes
The median follow-up time was 61 months among 431 patients (range, 6-103 months among surviving patients). Eighty-one patients had relapses, and 174 patients died. A score test (P = .14) indicated no statistically significant intercenter differences.
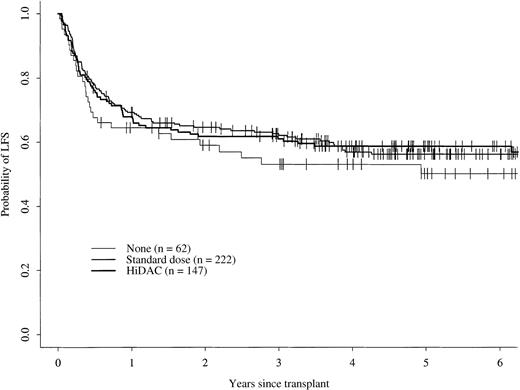
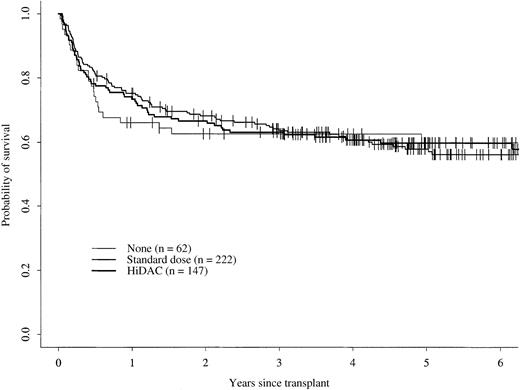
The 5-year cumulative incidence of TRM for patients receiving no postremission therapy was 30% (95% confidence interval [CI], 18%-42%); this was not statistically different from 22% (95% CI, 17%-28%) for patients receiving either standard-dose cytarabine or 24% (95% CI, 17%-31%) for those receiving high-dose cytarabine (Figure 1). There were no statistically significant differences in the 5-year cumulative incidences of relapse for patients in the 3 groups, 19% (95% CI, 10%-30%) for patients receiving no postremission therapy, 21% (95% CI, 16%-27%) for those receiving standard-dose cytarabine, and 17% (95% CI, 11%-24%) for those receiving high-dose cytarabine (Figure2). Five-year probabilities of LFS were 50% (95% CI, 36%-63%) for patients receiving no postremission therapy, 56% (95% CI, 49%-63%) for those receiving standard-dose cytarabine, and 59% (95% CI, 50%-66%) for patients receiving high-dose cytarabine; these probabilities were not significantly different (Figure 3). The 5-year probabilities of survival were 60% (95% CI, 46%-71%) for patients receiving no postremission therapy, 56% (95% CI, 49%-63%) for patients receiving low-dose cytarabine, and 60% (95% CI, 51%-67%) for patients receiving high-dose cytarabine (Figure4); there were no statistically significantly differences among the groups.
Cumulative incidence of transplant-related mortality.
Cumulative incidence of transplant-related mortality for patients receiving no postremission therapy, standard-dose cytarabine therapy, or high-dose cytarabine (HiDAC) therapy.
Cumulative incidence of transplant-related mortality.
Cumulative incidence of transplant-related mortality for patients receiving no postremission therapy, standard-dose cytarabine therapy, or high-dose cytarabine (HiDAC) therapy.
Cumulative incidence of relapse.
Cumulative incidence of relapse for patients receiving no postremission therapy, standard-dose cytarabine therapy, high-dose cytarabine (HiDAC) therapy.
Cumulative incidence of relapse.
Cumulative incidence of relapse for patients receiving no postremission therapy, standard-dose cytarabine therapy, high-dose cytarabine (HiDAC) therapy.
Probability of leukemia-free survival.
Probability of leukemia-free survival for patients receiving no postremission therapy, for those receiving standard-dose cytarabine therapy, and for those receiving high-dose cytarabine (HiDAC) therapy.
Probability of leukemia-free survival.
Probability of leukemia-free survival for patients receiving no postremission therapy, for those receiving standard-dose cytarabine therapy, and for those receiving high-dose cytarabine (HiDAC) therapy.
Probability of overall survival.
Probability of overall survival for patients receiving no postremission therapy, standard-dose cytarabine therapy, or high-dose cytarabine (HiDAC) therapy.
Probability of overall survival.
Probability of overall survival for patients receiving no postremission therapy, standard-dose cytarabine therapy, or high-dose cytarabine (HiDAC) therapy.
Stepwise forward Cox proportional hazards regression models were used to compare the risks for TRM, relapse, treatment failure (death or relapse), and overall mortality in multivariate analyses, adjusting for the effects of other significant covariates (Table2). There were no significant differences. Factors significantly associated with one or more outcomes were age, sex, year of transplantation, and performance score (Table2).
Relative risks of transplant outcomes in multivariate analyses adjusting for the effects of other prognostic variables
| Outcome/treatment group . | RR (95% CI) . | P . |
|---|---|---|
| TRM* | Poverall = 0.382-155 | |
| (1) No postremission therapy | 1.00 | P12 = 0.22 |
| (2) Standard-dose cytarabine | 0.71 (0.41-1.22) | P13 = 0.69 |
| (3) High-dose cytarabine | 0.89 (0.51-1.58) | P23 = 0.31 |
| Relapse† | Poverall = 0.782-155 | |
| (1) No postremission therapy | 1.00 | P12 = 0.97 |
| (2) Standard-dose cytarabine | 1.01 (0.52-1.96) | P13 = 0.66 |
| (3) High-dose cytarabine | 0.85 (0.42-1.74) | P23 = 0.49 |
| Death or relapse‡ | Poverall = 0.682-155 | |
| (1) No postremission therapy | 1.00 | P12 = 0.38 |
| (2) Standard-dose cytarabine | 0.83 (0.55-1.26) | P13 = 0.54 |
| (3) High-dose cytarabine | 0.87 (0.56-1.36) | P23 = 0.78 |
| Death2-153 | Poverall = 0.912-155 | |
| (1) No postremission therapy | 1.00 | P12 = 0.79 |
| (2) Standard-dose cytarabine | 0.94 (0.60-1.47) | P13 = 0.98 |
| (3) High-dose cytarabine | 1.01 (0.63-1.61) | P23 = 0.69 |
| Outcome/treatment group . | RR (95% CI) . | P . |
|---|---|---|
| TRM* | Poverall = 0.382-155 | |
| (1) No postremission therapy | 1.00 | P12 = 0.22 |
| (2) Standard-dose cytarabine | 0.71 (0.41-1.22) | P13 = 0.69 |
| (3) High-dose cytarabine | 0.89 (0.51-1.58) | P23 = 0.31 |
| Relapse† | Poverall = 0.782-155 | |
| (1) No postremission therapy | 1.00 | P12 = 0.97 |
| (2) Standard-dose cytarabine | 1.01 (0.52-1.96) | P13 = 0.66 |
| (3) High-dose cytarabine | 0.85 (0.42-1.74) | P23 = 0.49 |
| Death or relapse‡ | Poverall = 0.682-155 | |
| (1) No postremission therapy | 1.00 | P12 = 0.38 |
| (2) Standard-dose cytarabine | 0.83 (0.55-1.26) | P13 = 0.54 |
| (3) High-dose cytarabine | 0.87 (0.56-1.36) | P23 = 0.78 |
| Death2-153 | Poverall = 0.912-155 | |
| (1) No postremission therapy | 1.00 | P12 = 0.79 |
| (2) Standard-dose cytarabine | 0.94 (0.60-1.47) | P13 = 0.98 |
| (3) High-dose cytarabine | 1.01 (0.63-1.61) | P23 = 0.69 |
RR, relative risk; CI, confidence interval.
Other significant covariates were age older than 30 years at transplantation (RR = 1.90, 95% CI, 1.28-2.85, P= .002); year of transplantation 1991 or later (RR = 0.56, 95% CI, 0.36-0.85, P = .007); and Karnofsky performance score ≥90 (RR = 0.52, 95% CI, 0.30-0.90, P = .020).
Another significant covariate for relapse was female sex (RR = 0.59, 95% CI, 0.38-0.93, P = .024).
Other significant covariates for death or relapse were age older than 30 years at transplantation (RR = 1.55, 95% CI, 1.15-2.08,P = .004); year of transplantation 1991 or later (RR = 0.68, 95% CI, 0.50-0.92, P = .013); Karnofsky performance score ≥ 90 (RR = 0.63, 95% CI, 0.41-0.97, P = .037); and female sex (RR = 0.68, 95% CI, 0.51-0.92, P = .011).
Other significant covariates for death were age older than 30 years at transplantation (RR = 1.67, 95% CI, 1.24-2.27,P = .001); year of transplantation 1991 or later (RR = 0.65, 95% CI, 0.47-0.89, P = .008); and female sex (RR = 0.73, 95% CI, 0.54-0.99, P = .045).
Two degrees of freedom.
Discussion
Allogeneic bone marrow transplantation is a highly effective postremission treatment for patients with AML in first CR. Multiple studies indicate that this approach results in 5-year LFS rates of 45% to 50%.4-8 However, treatment-related morbidity and mortality rates remain significant, the latter accounting for failure in 20% to 30% of patients.4-8,15,16 Furthermore, although relapse rates appear to be lower than those observed among patients receiving intensive nontransplantation consolidation or autologous transplantation, relapse remains problematic in approximately 25% of patients. The impact of postremission chemotherapy administered before allogeneic bone marrow transplantation in first CR has not been adequately addressed. This issue is important because toxicities resulting from consolidation may preclude subsequent allogeneic transplantation or increase the risks for transplant-related mortality.8 17
In the current study, LFS and overall survival rates are not higher among patients receiving postremission therapy with either high-dose cytarabine or standard-dose cytarabine than they are in patients receiving no postremission therapy before an HLA–identical sibling transplant. These data suggest that patients preparing to undergo HLA–identical sibling transplantation in first CR do not benefit from consolidation chemotherapy with respect to TRM, relapse, or survival.
As expected, there was a significantly longer interval between CR and transplantation among patients receiving postremission chemotherapy. This delay in transplantation might be expected to bias the comparison in favor of consolidation therapy because patients with early relapse would be excluded from the comparison, which considered only patients who underwent transplantation in first CR. However, no such bias was observed.
There were other differences among the 3 groups because this was a nonrandomized study. For example, the distribution of FAB subtypes differed in that patients in the 2 consolidation groups were more likely to have M4, M5, M6, and M7 morphology subtypes, with less favorable prognoses, than those in the group receiving no postremission therapy.3 Patients in the high-dose consolidation group had higher median leukocyte counts, which might have conferred less favorable outcomes. More patients in the groups receiving consolidation had total body irradiation as part of the conditioning regimen, and more received methotrexate in combination with cyclosporine as GVHD prophylaxis. More patients in the consolidation groups had extramedullary disease, though the latter difference was not statistically significant. It is possible that patients with high-risk features—such as FAB M4-7 morphology, higher white blood cell counts, or less favorable karyotypes—were selected to receive high-dose cytarabine consolidation and that this therapy had a beneficial effect such that their outcome was similar to that of other groups.18 However, multivariate analyses adjusting for potentially confounding effects of other prognostic factors gave the same results as univariate analyses. The most likely explanation for similarities in outcome among the 3 groups may be the strength of graft–versus–leukemia effects provided by allogeneic transplantation in the setting of minimal residual disease after induction, which may overcome or be less affected by differences in pretreatment patient- or disease-related characteristics.
Several limitations of this analysis warrant further comment. In this retrospective study, all patients did not receive precisely equivalent doses of high-dose cytarabine (data not shown). Furthermore, the additional drugs received by patients in the cytarabine cohorts varied. This analysis cannot account for toxicities and mortality from consolidation chemotherapy that might have precluded transplantation. An inherent selection bias related to the timing of transplantation may exist. Finally, the factors that influenced the decision to administer low-dose or high-dose cytarabine, or no consolidation, are unknown.
Cahn et al,19 on behalf of the European Group for Blood and Marrow Transplantation, observed a difference in LFS or TRM according to the dose of cytarabine given as consolidation before allogeneic transplantation in a multivariate analysis. Intermediate-dose cytarabine (between 200 mg/m2 per day for 5-10 days and 1.5 g/m2per 12 hours for 4-6 days) had an unfavorable influence on LFS and TRM compared to standard-dose (100-200 mg/m2 per day) and high-dose cytarabine (1.5 g/m2 every 12 hours for 4-6 days).
It is possible that with further advances in the techniques of allogeneic bone marrow transplantation, such as the use of allogeneic peripheral blood progenitor cells as the source of stem cells for reconstitution20-22 or T-cell depletion,23 24as yet unknown benefits or hazards of consolidation therapy may emerge.
The data presented here suggest that postremission consolidation therapy with cytarabine in patients with AML in first CR who undergo allogeneic transplantation does not improve outcome compared to proceeding directly to transplantation after successful induction. However, the role of postremission therapy before allogeneic transplantation should be examined prospectively, during which intention-to-treat analysis will be particularly important.
Supported by Public Health Service grants P01-CA-40053 and U24-76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute, of the United States Department of Health and Human Services; and grants from Alpha Therapeutic Corporation; Amgen, Inc; Anonymous; Baxter Fenwal; Berlex Laboratories; BioWhitakker, Inc; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Bristol–Myers Squibb Company; Cell Therapeutics, Inc; Centeon; Center for Advanced Studies in Leukemia; Chimeric Therapies; Chiron Therapeutics; Charles E. Culpeper Foundation; Eleanor Naylor Dana Charitable Trust; Eppley Foundation for Research; Genentech, Inc; Human Genome Sciences; Immunex Corporation; Kettering Family Foundation; Kirin Brewery Company; Robert J. Kleberg Jr and Helen C. Kleberg Foundation; Herbert H. Kohl Charities, Inc; Nada and Herbert P. Mahler Charities; Milstein Family Foundation; Milwaukee Foundation/Elsa Schoeneich Research Fund; NeXstar Pharmaceuticals, Inc; Samuel Roberts Noble Foundation; Novartis Pharmaceuticals; Orphan Medical; Ortho Biotech, Inc; John Oster Family Foundation; Jane and Lloyd Pettit Foundation; Alirio Pfiffer Bone Marrow Transplant Support Association; Pfizer, Inc; RGK Foundation; Rockwell Automation Allen Bradley Company; Roche Laboratories; SangStat Medical Corporation; Schering AG; Schering–Plough Oncology; Searle; SEQUUS Pharmaceuticals; SmithKline Beecham Pharmaceutical; Stackner Family Foundation; Starr Foundation; Joan and Jack Stein Foundation; SyStemix; United Resource Networks; and Wyeth–Ayerst Laboratories.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary M. Horowitz, International Bone Marrow Transplant Registry, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: marymh@mcw.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal