Abstract
CD20-targeted radioimmunotherapy is a promising new treatment for B-cell non-Hodgkin lymphoma (NHL). We now provide updated and long-term data on 59 chemotherapy-relapsed/refractory patients treated with iodine 131I tositumomab in a phase I/II single-center study. Fifty-three patients received individualized therapeutic doses, delivering a specified total-body radiation dose (TBD) based on the clearance rate of a preceding dosimetric dose. Six patients received dosimetric doses only. Dose-escalations of TBD were conducted separately in patients who had or had not undergone a prior autologous stem cell transplant (ASCT) until a nonmyeloablative maximally tolerated TBD was established (non-ASCT = 75 cGy, post-ASCT = 45 cGy). Fourteen additional non-ASCT patients were treated with 75 cGy. Unlabeled antibody was given prior to labeled dosimetric and therapeutic doses to improve biodistribution. Forty-two (71%) of 59 patients responded; 20 (34%) had complete responses (CR). Thirty-five (83%) of 42 with low-grade or transformed NHL responded versus 7 (41%) of 17 with de novo intermediate-grade NHL (P = .005). For all 42 responders, the median progression-free survival was 12 months, 20.3 for those with CR. Seven patients remain in CR 3 to 5.7 years. Sixteen patients were re-treated after progression; 9 responded and 5 had a CR. Reversible hematologic toxicity was dose limiting. Only 10 patients (17%) had human anti-mouse antibodies detected. Long-term, 5 patients developed elevated thyroid-stimulating hormone levels, 5 were diagnosed with myelodysplasia and 3 with solid tumors. A single, well-tolerated treatment with iodine 131I tositumomab can, therefore, produce frequent and durable responses in NHL, especially low-grade or transformed NHL.
Introduction
Although the majority of patients with non-Hodgkin lymphoma (NHL) achieve remissions after initial treatment with chemotherapy with or without radiation therapy, only about 25% of patients are cured.1 In general, only patients diagnosed with intermediate- or high-grade NHL are cured, whereas patients with low-grade NHL are rarely cured.1,2Indeed, the natural history of low-grade NHL has not been altered since the 1960s.2 Although the median survival of patients with low-grade NHL is 7 to 8 years, patients typically experience multiple relapses of the disease. Response rates and duration of response diminish with re-treatment, and many patients experience transformation to a higher-grade histology, termed transformed NHL.2,3Likewise, for patients with relapsed intermediate- or high-grade NHL, the prognosis is poor. High-dose chemotherapy with or without external-beam total-body irradiation with bone marrow or peripheral blood autologous stem cell transplantation (ASCT) has been reported to cure 20% to 50% of such patients.4 Many patients, however, are inappropriate candidates for this treatment because of advanced age, comorbid conditions, or primary resistance to chemotherapy.5 6 Thus, new treatments based on novel therapeutic principles are needed for NHL.
Among new treatments being developed for NHL, radioimmunotherapy (RIT) has shown promise. RIT involves the administration of radionuclide-labeled monoclonal antibodies reactive with a tumor cell-surface antigen. Provided the tumor-associated antigen is expressed by the tumor cells and minimally or not expressed by other tissues, selective targeting of radiation to tumor sites can be theoretically accomplished with relative sparing of radiation exposure of normal tissues. Moreover, the antibody moiety carrying the radionuclide can potentially recruit host cytolytic immune mechanisms or produce direct antiproliferative effects against targeted tumor cells.
NHL is a particularly appropriate choice for this treatment approach for several reasons. First, lymphomas are highly radiosensitive. Second, a variety of lymphoid lineage-specific antigens have been identified as potential targets for antibody-based therapies. Third, unlabeled monoclonal antibodies directed against some of these antigens have demonstrated antitumor activity in patients with NHL.7-12
The CD20 antigen fulfills many of the desired features of a target antigen for RIT of B-cell NHL, the most common type of NHL. This 35-kd transmembrane glycoprotein is abundantly expressed by more than 95% of B-cell NHL.13,14 Although some normal B cells express CD20, it is not expressed on the cell surface by early progenitor B cells or by mature plasma cells.13 The antigen is not shed into the circulation nor is it internalized on antibody binding,15,16 providing a prolonged antibody residence on the cell surface and, consequently, an extended exposure of the tumor to radiation. In addition, CD20 is involved in the differentiation and proliferation of B cells.17 18
Tositumomab (previously referred to as anti-B1 antibody) is a mouse immunoglobulin G2a (IgG2a) monoclonal antibody specific for CD20.13 Our group has demonstrated that tositumomab can mediate cytolysis of human B-cell tumor cells in vitro in conjunction with human peripheral blood mononuclear cells, as well as inhibit the growth of human lymphoma xenografts in nude mice.19 More recently, tositumomab and other anti-CD20 antibodies have been found to be able to induce apoptosis of human B-cell tumor cell lines.20-22
Given the favorable features of CD20 as a target for RIT and the preclinical data on tositumomab, we initiated a phase I/II study of tositumomab labeled with iodine-131 (iodine 131I tositumomab) in 1990 for patients with relapsed or refractory B-cell NHL. Iodine-131 (131I) was selected for radiolabeling because of its short path-length beta particle emission, its gamma emission, its well-established labeling chemistry, its appropriately long half-life, and its common use in clinical practice. The beta emission allows the irradiation of small and large foci of targeted tumor tissue with relatively little exposure of surrounding normal tissues. The gamma emission permits noninvasive tumor imaging, quantitative tumor and organ dosimetry, as well as patient-specific dosing based on calculation of an individual patient's clearance of total-body radioactivity. Early reports of results obtained in the initial patients entered in this study suggested high response rates and excellent tolerability.23-25 We now report results on the entire cohort of this unique set of patients, including long-term safety and survival data up to 8 years after treatment.
Patients, materials, and methods
Patient selection
The study enrolled 59 patients from April 24, 1990, to January 17, 1996. Eligible patients were required to have a histologically confirmed diagnosis of CD20-positive B-cell NHL, to be at least 18 years old, to have measurable disease, to have relapsed or failed to respond to at least one prior chemotherapy regimen, and to have not received treatment for at least 4 weeks before study entry. In addition, patients were required to have less than 25% of their marrow space affected by lymphoma (as assessed by bilateral iliac crest bone marrow biopsy), an absolute neutrophil count greater than 1500/μL, a platelet count greater than 100 000/μL, normal hepatic and renal function, a Karnofsky performance score of at least 60, a life expectancy of at least 3 months, no serum human antimouse antibodies (HAMA), and no serious coexisting illness. The study was approved by the Institutional Review Board of the University of Michigan; written informed consent was required of all patients.
Preparation of iodine 131I tositumomab
Tositumomab was provided by Coulter Pharmaceutical, Inc (South San Francisco, CA). The antibody was radiolabeled on-site with131I, and the product was purified and tested for immunoreactivity and pyrogen contamination as previously described.23,26,27 Antibody concentration was originally estimated on the basis of an optical density extinction coefficient of 1.0. Subsequently, the correct extinction coefficient was found to be 1.45 at 280 nm. All doses in this report are based on the corrected extinction coefficient, which has the net effect of apparently “lowering” the injected mass of tositumomab from previously published reports.23 24 For example, doses of 95 mg and 475 mg in this report were previously presented as 135 mg and 685 mg, respectively.
Dosimetric doses
Because of individual patient variability in clearance of iodine131I tositumomab, a dosimetric dose consisting of 10 mg of the antibody labeled with approximately 5 mCi 131I was first given intravenously over 30 minutes. Total-body clearance kinetics were then measured by serial quantitative total-body radioactivity counts obtained 1 hour after injection and daily for at least 5 days with the use of an NaI scintillation probe and a gamma camera. This information was then used to calculate the number of millicuries needed in the labeling of a therapeutic dose so that a specified total-body-radiation dose (TBD) could be delivered by a therapeutic dose. The methods for these calculations have been previously described.23,25,28 29 Serial quantitative gamma camera images were also obtained to determine tumor and normal organ doses.
All patients were admitted to the hospital and were premedicated before antibody infusion with oral doses of 50 mg of diphenhydramine and 650 mg of acetaminophen. Saturated solution of potassium iodide (SSKI) was administered as 2 drops orally, 3 times daily, beginning 1 day before the initial antibody infusion and continuing for at least 14 days following the last infusion, to inhibit potential thyroid uptake of free 131I.
The first 25 patients underwent an evaluation to determine whether an infusion of unlabeled tositumomab prior to the infusion of radiolabeled antibody could optimize biodistribution and tumor targeting by preblocking normal CD20 antigenic sinks, such as B cells in the peripheral blood, bone marrow, and spleen. Dosimetric doses of iodine131I tositumomab were immediately preceded by no predose or a 60-minute infusion of 95 mg or 475 mg of unlabeled tositumomab on successive weeks in the first 25 patients. The last 34 patients received a single 475-mg infusion prior to the dosimetric dose when it was determined that this dose of unlabeled antibody decreased splenic targeting, increased the terminal half-life of the radiolabeled antibody, and appeared to cause tumor regression in some patients.23 24
Therapeutic doses
A therapeutic dose of iodine 131I tositumomab was administered at least 1 week after a dosimetric dose. There were no specific requirements with regard to biodistribution of the dosimetric dose that precluded patients from receiving a therapeutic dose. Each therapeutic dose consisted of a patient-specific millicurie amount of iodine 131I tositumomab adjusted to deliver a specified TBD. As previously described, a phase I dose escalation was initially performed until a maximally tolerated dose (MTD) was established.23 24 Subsequently, additional patients were treated at the MTD (75 cGy) in the phase II part of the study. A separate determination of MTD was conducted for patients who had undergone prior ASCT. This determination was initiated at a 65-cGy TBD and escalated or deescalated in 10-cGy increments.
In the initial 25-patient cohort, the radiolabeled therapeutic dose was preceded by an infusion of unlabeled tositumomab (0, 95, or 475 mg) that resulted in the highest tumor-to-TBD ratio. All subsequent therapeutic doses were preceded by a 475-mg infusion of unlabeled tositumomab. Patients were again premedicated with diphenhydramine and acetaminophen, and SSKI was continued for 14 days. In addition, oral potassium perchlorate (200 mg, 3 times daily) was given for 7 days, beginning the day of infusion.
Patients who had tumor regression were considered for re-dosing in an attempt to upgrade the response after it was determined that there was no further regression of disease after the preceding dose. Patients were not allowed to be re-dosed any sooner than 6 weeks following a therapeutic dose. For patients who had achieved a partial or complete response and had not developed HAMA, re-treatment was considered following progression of disease. Re-dosing or re-treatment was given at the original TBD unless grade 2 or greater toxicity had been encountered, in which case an attenuated dose was administered (generally 10 cGy less than the original dose).
Response criteria and evaluation
Complete response (CR) was defined as the complete disappearance of all detectable disease (including bone marrow involvement) for at least 1 month or a lack of change in a minimal residual radiographic abnormality for at least 6 months. A partial response (PR) was defined as a reduction by at least 50% in the sum of the products of the largest perpendicular diameters of all measurable lesions for at least 1 month. Responses not meeting the above criteria were categorized as stable disease. Progressive disease was defined as the appearance of new lesions or a more than 25% increase in the sum of the products of the longest perpendicular diameters of all measurable lesions. Patients withdrawing from the study prior to a response assessment were classified as having progressive disease.
Disease status was evaluated by physical examination; computed tomography (CT) scans of the chest, abdomen, and pelvis; and bone marrow biopsies if the bone marrow was positive for lymphoma at baseline. Evaluations were performed 6 weeks and 12 weeks after therapy and every 3 months until 2 years after treatment at which time evaluations were scheduled every 6 months.
Evaluation of toxicity
The National Cancer Institute common toxicity criteria were used. Complete blood cell and platelet counts were obtained weekly for at least 6 weeks and twice weekly whenever any hematologic toxicity was encountered until recovery or stabilization of counts. Hepatic, renal, and electrolyte studies were performed at 2, 6, and 12 weeks and every 3 to 6 months after RIT. Serum thyroid-stimulating hormone (TSH) was measured every 3 to 6 months.
All adverse events were monitored for 12 weeks after treatment, and all possibly related adverse events were recorded after 12 weeks. Hematologic toxicity was recorded as an adverse event only if a therapeutic intervention was administered.
Peripheral blood B and T cells were quantitated by flow cytometry as previously described23 at study entry, 6 and 12 weeks after RIT, and every 3 months thereafter until the number of B cells returned to normal. Serum immunoglobulin concentrations were measured each time peripheral blood B and T cells were quantitated.
HAMA
Serum was tested for HAMA before each dosimetric and therapeutic dose and at 2, 6, and 12 weeks after a therapeutic dose, and prior to consideration of a re-treatment dose. A previously described enzyme-linked immunosorbent assay was used,24 as well as a commercial test kit (ImmunoSTRIP, Immunomedics, Morris Plains, NJ).
Statistical methods
The response rates were summarized for all patients who received any study drug (ie, intent-to-treat) and for all patients who received the therapeutic dose (ie, evaluable patients). Kaplan-Meier censored data techniques were used to estimate duration of response, progression-free survival (PFS), and survival.30 Log-rank tests were used to perform subgroup comparisons of the duration of response.31 The Cox proportional hazards model was used to perform multivariate analyses of PFS, and the logistic regression model was used to perform multivariate analyses of response.32The McNemar test for proportions using exact binomial probabilities was used to compare the response to iodine 131I tositumomab to the response to the last chemotherapy.33 A matched pairs test generalized to censored duration data was used to compare the PFS after iodine 131I tositumomab to the PFS after the last chemotherapy.34 In the latter test, paired durations of response within 1 month of each other were considered equivalent.
Results
Patient characteristics
The characteristics of the 59 enrolled patients are detailed in Table 1. The median age was 50 years (range 23-75). Eighty-eight percent had Ann Arbor stage III or IV disease at study entry. Seventy-one percent had either low-grade or transformed NHL at entry, and the rest had de novo intermediate- or high-grade NHL at time of diagnosis. The median time from diagnosis to study entry was 45 months. In general, patients had been heavily pretreated with a median of 3 (mean, 3.9) prior chemotherapy regimens and a median of 4 prior therapeutic interventions (range, 1-13) that included chemotherapy, radiation, and biologics. Forty-eight percent had not responded to their most recent chemotherapy. Fourteen patients had undergone ASCT. The percentages of patients with a tumor burden more than 500 grams (as determined by volumetric measurements from CT scans23 24) or an elevated serum lactate dehydrogenase (LDH) level were 36% and 51%, respectively.
Clinical and demographic characteristics of 59 patients with B-cell lymphoma
| Characteristics . | Number . | Percent . |
|---|---|---|
| Age (yr) | ||
| ≤60 | 49 | 83 |
| >60 | 10 | 17 |
| Gender | ||
| Male | 37 | 63 |
| Female | 22 | 37 |
| Stage of disease | ||
| I/II | 7 | 12 |
| III/IV | 52 | 88 |
| Histology* | ||
| Low-grade | 28 | 47 |
| Transformed low-grade | 14 | 24 |
| De novo intermediate/high-grade | 17 | 29 |
| Tumor burden >500 grams | ||
| Yes | 21 | 36 |
| No | 38 | 64 |
| Bone marrow involvement | ||
| None | 38 | 68 |
| 1%-25% | 17 | 29 |
| >25% | 1 | 2 |
| Lactate dehydrogenase (LDH) | ||
| Elevated | 30 | 51 |
| Normal/low | 29 | 49 |
| Prior bone marrow transplant | ||
| Yes | 14 | 24 |
| No | 45 | 76 |
| Number of prior therapies | ||
| 1-3 | 20 | 34 |
| ≥4 | 39 | 66 |
| Response to last therapy | ||
| No response | 28 | 48 |
| Response (PR + CR) | 30 | 52 |
| Characteristics . | Number . | Percent . |
|---|---|---|
| Age (yr) | ||
| ≤60 | 49 | 83 |
| >60 | 10 | 17 |
| Gender | ||
| Male | 37 | 63 |
| Female | 22 | 37 |
| Stage of disease | ||
| I/II | 7 | 12 |
| III/IV | 52 | 88 |
| Histology* | ||
| Low-grade | 28 | 47 |
| Transformed low-grade | 14 | 24 |
| De novo intermediate/high-grade | 17 | 29 |
| Tumor burden >500 grams | ||
| Yes | 21 | 36 |
| No | 38 | 64 |
| Bone marrow involvement | ||
| None | 38 | 68 |
| 1%-25% | 17 | 29 |
| >25% | 1 | 2 |
| Lactate dehydrogenase (LDH) | ||
| Elevated | 30 | 51 |
| Normal/low | 29 | 49 |
| Prior bone marrow transplant | ||
| Yes | 14 | 24 |
| No | 45 | 76 |
| Number of prior therapies | ||
| 1-3 | 20 | 34 |
| ≥4 | 39 | 66 |
| Response to last therapy | ||
| No response | 28 | 48 |
| Response (PR + CR) | 30 | 52 |
According to the International Working Formulation,35 of the 28 patients with low-grade non-Hodgkin lymphoma (NHL), 16 had follicular small-cleaved, 11 had follicular mixed small-cleaved and large cell, and 1 had small lymphocytic lymphoma. Of the 14 patients with transformed low-grade NHL, 8 had diffuse large cell, 2 had immunoblastic large cell, and 4 had follicular large cell. Of the 17 patients with intermediate- or high-grade lymphoma at diagnosis, 9 had diffuse large cell, 4 had mantle cell, 2 had diffuse mixed large and small cell, and 2 had immunoblastic large cell lymphoma. PR indicates partial response; CR, complete response.
Dosimetric studies
The mean effective half-life of iodine 131I tositumomab was 59.3 hours (range, 24.6-88.6). Because patients had markedly different rates of clearance of the radiolabeled antibody, there was considerable variation in the millicurie dose needed to achieve the desired TBD. The 20 patients treated at the 75-cGy dose level were administered a mean (± SD) of 97.3 ± 26.0 mCi, but the range extended from 56.8 to 153 mCi. Tumor-specific targeting was observed with gamma camera scanning; tumor absorbed radiation dose with a 475-mg predose was 1010 cGy ± 696 per 75 cGy whole-body dose. This dose was higher than that to any normal tissue, including the spleen, which received 399 cGy ± 215. A detailed account of dosimetric and pharmacokinetic data will be published separately.
Tumor responses and survival
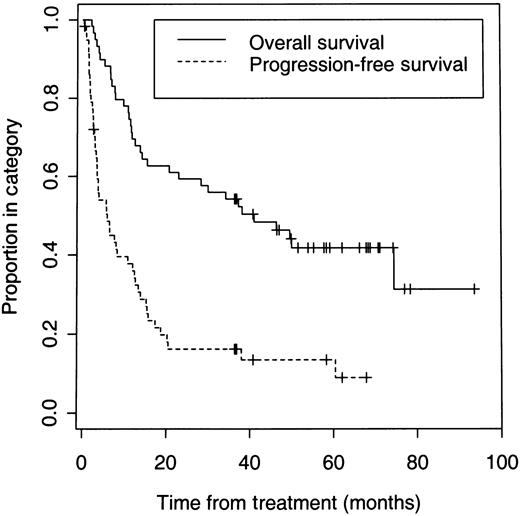
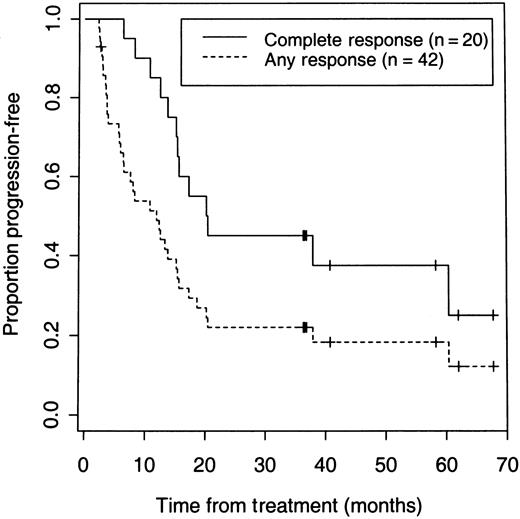
On an intent-to-treat basis, a CR or PR was achieved in 42 (71%; 95% confidence interval [CI]: 58%-82%) of the total 59 patients, and 20 of 59 (34%; 95% CI: 22%-47%) patients achieved a CR. Six patients underwent dosimetric studies but did not receive a therapeutic dose because of early disease progression in 2 patients, development of HAMA after multiple dosimetric studies in 3 patients, or an adverse event in 1 patient. Forty (75%) of the 53 patients who received a therapeutic dose responded. With a median follow-up of 3.1 years, the 5-year Kaplan-Meier estimates for all 59 patients are 42% for overall survival and 14% for PFS (Figure 1). Twenty-five patients are alive and 7 remain disease- and progression-free after their initial treatment for 3 to 5.7 years. The median PFS was 12 months for all responders and 20.3 months for patients who achieved a CR (Figure 2).
Overall and PFS of 59 patients with relapsed or refractory B-cell lymphoma who received dosimetric and/or therapeutic doses of iodine 131I tositumomab.
Overall and PFS of 59 patients with relapsed or refractory B-cell lymphoma who received dosimetric and/or therapeutic doses of iodine 131I tositumomab.
PFS of 59 patients according to response after receiving iodine 131I tositumomab.
PFS of 59 patients according to response after receiving iodine 131I tositumomab.
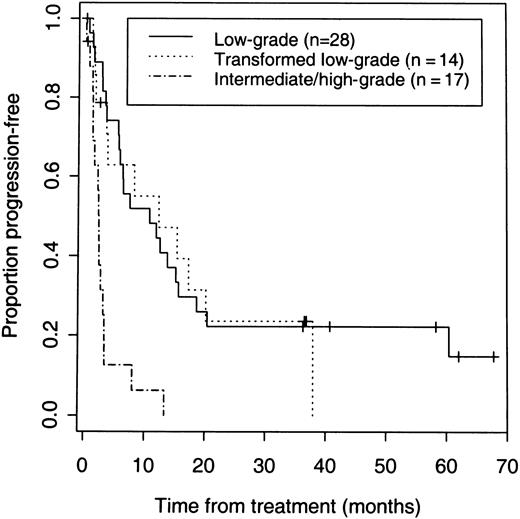
The 42 patients with low-grade or transformed low-grade NHL had a higher response rate (83%) than the 17 patients with de novo intermediate or high-grade disease (41%) (P = .005) (Table 2). Response rates and PFS were similar for low-grade and transformed low-grade NHL. Response rates of 86% and 79%, CR rates of 46% and 50%, and median PFS for responders of 12.3 and 13.9 months were observed for low-grade and transformed low-grade NHL, respectively (Table 2, Figure3). The median PFS and response rate with iodine 131I tositumomab for the 31 patients with response durations of less than 6 months after their last chemotherapy was 7 months and 77%. The median PFS and response rate for the 11 patients with response durations of more than 6 months after their last chemotherapy was 17.2 months and 100%.
Response rates by patient subgroup
| . | No. of patients . | Response . | Complete response . | ||
|---|---|---|---|---|---|
| % (N) . | P value . | % (N) . | P value . | ||
| Age | |||||
| >60 | 10 | 60 (6) | 20 (2) | ||
| ≤60 | 49 | 73 (36) | .391 | 37 (18) | .308 |
| Histology | |||||
| Low grade | 28 | 86 (24) | 46 (13) | ||
| Low transformed | 14 | 79 (11) | 50 (7) | ||
| De novo intermediate/high | 17 | 41 (7) | .005 | 0 (0) | .002 |
| Lactate dehydrogenase (LDH) | |||||
| Elevated | 30 | 57 (17) | 17 (5) | ||
| Normal/low | 29 | 86 (25) | .012 | 52 (15) | 0.005 |
| Bulky disease | |||||
| No | 38 | 82 (31) | 37 (14) | ||
| Yes | 21 | 52 (11) | .018 | 29 (6) | .521 |
| Prior bone marrow transplant | |||||
| No | 45 | 78 (35) | 33 (15) | ||
| Yes | 14 | 50 (7) | .045 | 36 (5) | .869 |
| Stage of disease | |||||
| I/II | 7 | 57 (4) | 29 (2) | ||
| III/IV | 52 | 73 (38) | .382 | 35 (18) | .751 |
| Prior chemotherapies (No.) | |||||
| 1-2 | 16 | 69 (11) | 31 (5) | ||
| ≥3 | 43 | 72 (31) | .801 | 35 (15) | .793 |
| Last chemotherapy | |||||
| No response | 28 | 75 (21) | 29 (8) | ||
| Response | 30 | 70 (21) | .670 | 40 (12) | .360 |
| . | No. of patients . | Response . | Complete response . | ||
|---|---|---|---|---|---|
| % (N) . | P value . | % (N) . | P value . | ||
| Age | |||||
| >60 | 10 | 60 (6) | 20 (2) | ||
| ≤60 | 49 | 73 (36) | .391 | 37 (18) | .308 |
| Histology | |||||
| Low grade | 28 | 86 (24) | 46 (13) | ||
| Low transformed | 14 | 79 (11) | 50 (7) | ||
| De novo intermediate/high | 17 | 41 (7) | .005 | 0 (0) | .002 |
| Lactate dehydrogenase (LDH) | |||||
| Elevated | 30 | 57 (17) | 17 (5) | ||
| Normal/low | 29 | 86 (25) | .012 | 52 (15) | 0.005 |
| Bulky disease | |||||
| No | 38 | 82 (31) | 37 (14) | ||
| Yes | 21 | 52 (11) | .018 | 29 (6) | .521 |
| Prior bone marrow transplant | |||||
| No | 45 | 78 (35) | 33 (15) | ||
| Yes | 14 | 50 (7) | .045 | 36 (5) | .869 |
| Stage of disease | |||||
| I/II | 7 | 57 (4) | 29 (2) | ||
| III/IV | 52 | 73 (38) | .382 | 35 (18) | .751 |
| Prior chemotherapies (No.) | |||||
| 1-2 | 16 | 69 (11) | 31 (5) | ||
| ≥3 | 43 | 72 (31) | .801 | 35 (15) | .793 |
| Last chemotherapy | |||||
| No response | 28 | 75 (21) | 29 (8) | ||
| Response | 30 | 70 (21) | .670 | 40 (12) | .360 |
PFS of 59 patients according to the histologic grade of B-cell lymphoma.
Seven (41%) of 17 patients with de novo intermediate or high-grade NHL had a response, but none of these patients achieved a CR and responses were short-lived (Figure 3).
Age, stage at entry, the number of prior treatment regimens, and the last response to chemotherapy did not appear to significantly affect response rates and PFS (Table 2). A normal serum LDH level was predictive for a response; patients with normal levels responded more frequently (86%) than those with elevated levels (57%) (P = .012). Most patients with high tumor burdens responded (52%) but not as frequently as those with low tumor burdens (82%) (P = .018). Higher response rates and more durable CRs were seen with increasing TBD. The response rate to a TBD under 65 cGy was 57% compared with 86% to doses of 65 cGy or higher (P = .012). PFS among patients who received at least 55 cGy was significantly longer than that of patients who received lower doses. All patients with a response longer than 1.5 years received at least 55 cGy. Patients who had relapsed after ASCT also responded, although at a lower rate than patients who had not undergone ASCT (50% versus 78%, P = .045). Among the post-ASCT patients, the median PFS of responders was not reached after 4.7 years, and 4 patients remain disease-free more than 3 years after therapy.
In a multivariate analysis, only de novo intermediate grade histology (odds ratio [OR] = 7.9; P = .009), the presence of high tumor burden (OR = 9.3; P = .008), and TBD below 55 cGy (OR = 6.2; P = .02) were associated with reduced response rates. Only de novo intermediate-grade histology was associated with a reduced PFS (relative risk [RR] = 4.8;P < .0001).
Seven patients were re-dosed in an attempt to upgrade or to prolong their response before disease progression after achieving stable disease (1 patient), a PR (5 patients), or a CR (1 patient) after the first dose. The patient who had stable disease converted to a PR, but no upgrade occurred in the response of the other 6 patients. Sixteen patients who responded to the initial therapeutic dose were re-treated following disease progression. Nine (56%) of 16 patients achieved a second response with a median PFS of 11.4 months. Five (31%) patients achieved a CR of which 3 continue to be progression-free up to 37 months after re-treatment.
Each patient's response to his or her most recent chemotherapy regimen was used as a paired control for the response to iodine131I tositumomab therapy. The 71% response rate to iodine131I tositumomab was significantly higher than the 52% response rate to the last prior chemotherapy (P = .021). The results for patients with low-grade or transformed low-grade NHL were more striking. In these 42 patients, 83% responded to iodine131I tositumomab compared with a 50% response rate to the last prior chemotherapy (P = .0013). Furthermore, the duration of response was 11.7 months after iodine 131I tositumomab compared with 8.0 months after the last chemotherapy regimen (P = .032, McNemar test). Indeed, 28 patients had a longer remission by at least 1 month with iodine 131I tositumomab than the last chemotherapy, whereas only 10 had a longer remission with the last chemotherapy.
Early adverse experiences
Infusions were well tolerated, and only 2 (1.1%) of 177 infusions were interrupted or rate-adjusted. The most common nonhematologic adverse events were fever, asthenia, nausea, and chills (Table3). Fevers and chills generally occurred around the time of the dosimetric dose, and asthenia and nausea occurred more often after the therapeutic dose. The great majority of events were grade I in severity, and grade II events were rare. There were no grade IV events. The incidence and severity of adverse events among the re-treated patients were similar to those after initial treatment. No substantive changes were observed in serum electrolytes, liver enzymes, renal function tests, or immunoglobulin levels.
Nonhematologic adverse events
| Event . | All patients (n = 59) . | Unknown . | Grade I . | Grade II . | Grade III . | Grade IV . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | |
| Fever | 31 | 53 | 0 | 0 | 11 | 19 | 20 | 34 | 0 | 0 | 0 | 0 |
| Asthenia | 20 | 34 | 0 | 0 | 19 | 32 | 1 | 2 | 0 | 0 | 0 | 0 |
| Nausea | 20 | 34 | 0 | 0 | 18 | 31 | 1 | 2 | 1 | 2 | 0 | 0 |
| Chills | 17 | 29 | 0 | 0 | 10 | 17 | 3 | 5 | 4 | 7 | 0 | 0 |
| Infection | 13 | 22 | 2 | 3 | 9 | 15 | 2 | 3 | 0 | 0 | 0 | 0 |
| Arthralgia | 13 | 22 | 1 | 2 | 11 | 19 | 1 | 2 | 0 | 0 | 0 | 0 |
| Rash | 11 | 19 | 1 | 2 | 7 | 12 | 3 | 5 | 0 | 0 | 0 | 0 |
| Pain | 9 | 15 | 0 | 0 | 9 | 15 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 8 | 14 | 0 | 0 | 6 | 10 | 1 | 2 | 1 | 2 | 0 | 0 |
| Urticaria | 8 | 14 | 0 | 0 | 3 | 5 | 5 | 8 | 0 | 0 | 0 | 0 |
| Headache | 7 | 12 | 0 | 0 | 6 | 10 | 1 | 2 | 0 | 0 | 0 | 0 |
| Myalgia | 7 | 12 | 1 | 2 | 6 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 6 | 10 | 0 | 0 | 5 | 8 | 0 | 0 | 1 | 2 | 0 | 0 |
| Pharyngitis | 6 | 10 | 0 | 0 | 6 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pruritus | 6 | 10 | 0 | 0 | 5 | 8 | 1 | 2 | 0 | 0 | 0 | 0 |
| Dizziness | 5 | 8 | 1 | 2 | 3 | 5 | 0 | 0 | 1 | 2 | 0 | 0 |
| Urinary tract infection | 5 | 8 | 0 | 0 | 3 | 5 | 2 | 3 | 0 | 0 | 0 | 0 |
| Abdominal pain | 4 | 7 | 0 | 0 | 4 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Serum sickness | 4 | 7 | 1 | 2 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 |
| Anorexia | 4 | 7 | 1 | 2 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Injection site reaction | 3 | 5 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 0 | 0 | 0 |
| Constipation | 3 | 5 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastritis | 3 | 5 | 0 | 0 | 2 | 3 | 1 | 2 | 0 | 0 | 0 | 0 |
| Hypothyroidism | 3 | 5 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 |
| Paresthesia | 3 | 5 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cough increased | 3 | 5 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhinitis | 3 | 5 | 0 | 0 | 2 | 3 | 1 | 2 | 0 | 0 | 0 | 0 |
| Sweating | 3 | 5 | 0 | 0 | 1 | 2 | 2 | 3 | 0 | 0 | 0 | 0 |
| Conjunctivitis | 3 | 5 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Event . | All patients (n = 59) . | Unknown . | Grade I . | Grade II . | Grade III . | Grade IV . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | N . | % . | |
| Fever | 31 | 53 | 0 | 0 | 11 | 19 | 20 | 34 | 0 | 0 | 0 | 0 |
| Asthenia | 20 | 34 | 0 | 0 | 19 | 32 | 1 | 2 | 0 | 0 | 0 | 0 |
| Nausea | 20 | 34 | 0 | 0 | 18 | 31 | 1 | 2 | 1 | 2 | 0 | 0 |
| Chills | 17 | 29 | 0 | 0 | 10 | 17 | 3 | 5 | 4 | 7 | 0 | 0 |
| Infection | 13 | 22 | 2 | 3 | 9 | 15 | 2 | 3 | 0 | 0 | 0 | 0 |
| Arthralgia | 13 | 22 | 1 | 2 | 11 | 19 | 1 | 2 | 0 | 0 | 0 | 0 |
| Rash | 11 | 19 | 1 | 2 | 7 | 12 | 3 | 5 | 0 | 0 | 0 | 0 |
| Pain | 9 | 15 | 0 | 0 | 9 | 15 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 8 | 14 | 0 | 0 | 6 | 10 | 1 | 2 | 1 | 2 | 0 | 0 |
| Urticaria | 8 | 14 | 0 | 0 | 3 | 5 | 5 | 8 | 0 | 0 | 0 | 0 |
| Headache | 7 | 12 | 0 | 0 | 6 | 10 | 1 | 2 | 0 | 0 | 0 | 0 |
| Myalgia | 7 | 12 | 1 | 2 | 6 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 6 | 10 | 0 | 0 | 5 | 8 | 0 | 0 | 1 | 2 | 0 | 0 |
| Pharyngitis | 6 | 10 | 0 | 0 | 6 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pruritus | 6 | 10 | 0 | 0 | 5 | 8 | 1 | 2 | 0 | 0 | 0 | 0 |
| Dizziness | 5 | 8 | 1 | 2 | 3 | 5 | 0 | 0 | 1 | 2 | 0 | 0 |
| Urinary tract infection | 5 | 8 | 0 | 0 | 3 | 5 | 2 | 3 | 0 | 0 | 0 | 0 |
| Abdominal pain | 4 | 7 | 0 | 0 | 4 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Serum sickness | 4 | 7 | 1 | 2 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 |
| Anorexia | 4 | 7 | 1 | 2 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Injection site reaction | 3 | 5 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 0 | 0 | 0 |
| Constipation | 3 | 5 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastritis | 3 | 5 | 0 | 0 | 2 | 3 | 1 | 2 | 0 | 0 | 0 | 0 |
| Hypothyroidism | 3 | 5 | 0 | 0 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 |
| Paresthesia | 3 | 5 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cough increased | 3 | 5 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhinitis | 3 | 5 | 0 | 0 | 2 | 3 | 1 | 2 | 0 | 0 | 0 | 0 |
| Sweating | 3 | 5 | 0 | 0 | 1 | 2 | 2 | 3 | 0 | 0 | 0 | 0 |
| Conjunctivitis | 3 | 5 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
All adverse events that occurred in at least 5% of patients.
As expected, the dose-limiting toxicity was hematologic. In the phase I dose-escalation part of the study, the MTD was established as 75 cGy in 25 patients who had not undergone a prior ASCT.24 Fourteen additional patients were then treated with this MTD in the phase II segment of the study. The MTD for patients who had a prior ASCT was determined in 13 patients to be 45 cGy. For 20 patients who had not had a prior ASCT and received the 75-cGy MTD, the median time to nadir for platelet and neutrophil counts was 35 days and 43 days, respectively (Table 4). The median time from treatment to recovery to baseline hematologic grade for these blood components was 51 and 64 days, respectively. Four (20%) patients experienced grade IV platelet or neutrophil nadirs lasting a median of 19 and 11.5 days, respectively. Two of the 20 patients were given granulocyte colony-stimulating factor, and 3 patients received a platelet and/or a red blood cell transfusion. The hematologic toxicity profile for patients who were re-dosed or re-treated was similar to that observed after initial treatment.
Hematologic toxicity in 20 patients receiving a 75-cGy whole-body dose
| . | Absolute neutrophil count . | Platelets . | Hemoglobin . |
|---|---|---|---|
| Time to nadir (days) | |||
| Median | 43 | 35 | 41 |
| 95% confidence interval | 41-55 | 33-37 | 33-48 |
| Range | 22-106 | 29-45 | 6-78 |
| Time to recovery to baseline (days) | |||
| Median | 64 | 51 | 50 |
| 95% confidence interval | 58-84 | 48-55 | 37-62 |
| Range | 29-159 | 37-405 | 9-9 |
| Maximum toxicity | |||
| Grade III | 35% | 20% | 5% |
| Grade IV | 20% | 20% | 5% |
| Median duration of grade IV (days) | 11.5 | 19 | 2 |
| Range | 10-22 | 2-85+ | 2-2 |
| Supportive care | |||
| Any supportive care | 4 (20%) | ||
| G-CSF | 2 (10%) | ||
| GM-CSF | 0 (0%) | ||
| Erythropoietin | 0 (0%) | ||
| Platelet transfusion | 3 (15%) | ||
| RBC transfusion | 3 (15%) | ||
| . | Absolute neutrophil count . | Platelets . | Hemoglobin . |
|---|---|---|---|
| Time to nadir (days) | |||
| Median | 43 | 35 | 41 |
| 95% confidence interval | 41-55 | 33-37 | 33-48 |
| Range | 22-106 | 29-45 | 6-78 |
| Time to recovery to baseline (days) | |||
| Median | 64 | 51 | 50 |
| 95% confidence interval | 58-84 | 48-55 | 37-62 |
| Range | 29-159 | 37-405 | 9-9 |
| Maximum toxicity | |||
| Grade III | 35% | 20% | 5% |
| Grade IV | 20% | 20% | 5% |
| Median duration of grade IV (days) | 11.5 | 19 | 2 |
| Range | 10-22 | 2-85+ | 2-2 |
| Supportive care | |||
| Any supportive care | 4 (20%) | ||
| G-CSF | 2 (10%) | ||
| GM-CSF | 0 (0%) | ||
| Erythropoietin | 0 (0%) | ||
| Platelet transfusion | 3 (15%) | ||
| RBC transfusion | 3 (15%) | ||
G-CSF indicates granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; RBC, red blood cell.
A depletion of or substantial drop in CD20-positive peripheral blood cells was observed in 47 of the 59 patients shortly after treatment. The median time to B-cell recovery (defined as a return to the normal range of the absolute number of CD20-expressing cells) was 3.6 months (95% CI: 3.2-6.6). No significant effect on T-cell counts was observed.
Late adverse experiences
An elevation of TSH following the therapeutic dose was noted in 5 patients at 3 to 29 months following initial therapy or re-treatment. None of these patients were symptomatic and 2 patients were placed on thyroid hormone supplementation.
Five patients were diagnosed with myelodysplasia (MDS) 1.2 to 7.5 years after iodine 131I tositumomab treatment; 1 patient subsequently developed acute myeloid leukemia. These patients had previously received a median of 4 prior chemotherapies (range 2-8) and all had previously received alkylating agents. Bone marrow cytogenetic analysis at the time of diagnosis of MDS was performed in 4 cases, and, in each case, clonal abnormalities of chromosomes 5 and/or 7 were observed.
Three patients were diagnosed with solid tumors after RIT; 2 with superficial transitional-cell bladder cancer and 1 with squamous cell carcinoma of the rectum. Both patients with bladder cancer had been previously exposed to cyclophosphamide, a risk factor for the development of bladder cancer.36 One of these patients had findings suggestive of bladder cancer prior to RIT and the other was diagnosed with bladder cancer 6 months after RIT.
HAMA
Ten (17%) patients developed HAMA following either initial treatment9 or re-treatment.1 Five patients were part of the initial cohort of patients who received more than one dosimetric dose with their first treatment. No patient who received only one dosimetric dose was prevented from receiving a therapeutic dose because of HAMA. The median time to first HAMA detection was 15 days after first exposure to antibody (range, 12-194).
Discussion
This report summarizes the updated results of the first phase I/II study of anti-CD20 radioimmunotherapy for NHL using nonmyeloablative doses. Initial reports of this study, as it was accruing patients, strongly suggested promising results.23-25 A larger patient cohort with extended follow-up has now allowed us (1) to better define clinical response rates and the durability of remissions; (2) to analyze the impact of various factors on response, response duration, and survival; (3) to establish the MTD for patients who have undergone ASCT; (4) to evaluate the feasibility and utility of re-treatment; and (5) to better characterize short-term and long-term safety.
This study demonstrates that a single course of iodine 131I tositumomab resulted in a high rate of tumor responses of considerable duration. On an intent-to-treat basis, responses were observed in 42 (71%) of 59 patients and CRs in 20 (34%) of 59 patients. The PFS was 12 months for all responders and 20.3 months for those who achieved a CR. In long-term follow-up, 7 of the 20 CRs have remained disease-free from 3 to almost 6 years. Four of these 7 patients had relapsed after ASCT.
These results are particularly noteworthy, given the generally poor prognosis of the patients entered in the study. These patients had received a median of 4 prior therapeutic interventions for their NHL, and about half had no response to their most recent chemotherapy treatment. Many patients had high tumor burdens and elevated LDH values. Fourteen patients had relapsed after ASCT. Indeed, patients with the poor prognostic factors of high tumor burdens, elevated LDH, relapse after ASCT, or no response to the last administered chemotherapy had response rates of more than 50%.
A multivariate analysis demonstrated that only tumor histology was an independent predictor of PFS after iodine 131I tositumomab therapy. Patients with low-grade histologies and transformed histologies had significantly higher response rates and a longer PFS than patients with de novo intermediate- or high-grade histologies. Indeed, the 4-year overall survival and PFS for patients with low-grade or transformed NHL was 62% and 20%, respectively. The response rate was as high as 92% for the 24 patients with low-grade NHL and 79% for the 14 with transformed NHL who actually received a therapeutic dose of iodine 131I tositumomab. Twenty (53%) of these 38 patients achieved a CR. In the 15 de novo higher-grade patients who received a therapeutic dose, 7 PRs were observed. It should be noted, however, that patients with various histologies and clinical characteristics were represented in the group with de novo higher-grade NHL, including 4 patients with mantle-cell NHL, 5 patients who relapsed after ASCT, and 8 who received a whole-body dose of 55 cGy or less. Additional studies of this treatment are thus warranted in the higher-grade histologies.
In contrast to an expected decline in response rate and duration with each subsequent treatment for patients with low-grade and transformed low-grade NHL, iodine 131I tositumomab produced a significantly higher response rate (83%) than the immediately preceding chemotherapy (50%) in these patients. Indeed, 17 (81%) of 21 patients responded to RIT but not to their most recent chemotherapy. Also, the number of patients who had a longer response with iodine131I tositumomab was nearly triple the number who had a longer response to chemotherapy. This finding suggests an independent mechanism of action from chemotherapy and the potential role of this radiolabeled antibody in chemotherapy-relapsed or refractory disease.
Another encouraging finding was that patients who relapsed after achieving a response to iodine 131I tositumomab could achieve another remission with re-treatment in 56% of the cases. The opportunity to re-induce remissions was afforded by several factors. First, this treatment was very well tolerated. Interruption or slowing of infusions for adverse reactions was rarely required. Although myelosuppression was dose-limiting, this toxicity was generally moderate, predictable, reversible, and seldom required supportive intervention, even in post-ASCT patients. This factor may be a result of the individualized dosing scheme based on dosimetric dose clearance rates and the restriction on the level of bone marrow involvement by NHL for study entry. Second, relapsing tumors continued to express CD20. All 6 patients who underwent a tumor biopsy prior to re-treatment had immunohistochemical evidence of CD20 expression by the tumor, and all re-treated patients had clearly detectable tumor targeting by gamma camera imaging. Third, the rate of HAMA development was low after the first treatment. Although the usual time of follow-up for the development of HAMA was 12 weeks (thus possibly missing the detection of later development of HAMA in some patients), HAMA developed in only 1 of 16 patients after re-treatment. These data support the notion that the incidence of HAMA is low in this patient population and that it may be possible to re-treat patients multiple times.
In terms of late adverse events, the incidence of elevated TSH levels was low and no patient had symptoms of hypothyroidism. This finding indicates that the protection of the thyroid from free 131I by the administration of SSKI is adequate for the millicurie dose range given in this study.
In long-term follow-up, 5 cases of MDS were diagnosed. Each of the 5 patients had received multiple prior chemotherapy regimens (median of 4), at least 1 of which included an alkylating agent. Chromosomal analysis was performed on 4 cases, and all 4 cases had abnormalities involving chromosomes 5 and/or 7. These specific abnormalities are commonly associated with exposure to alkylators, but they have also been reported following radiotherapy.37 With the limited number of cases in our study, it is not presently possible to determine the contribution of the RIT to the development of MDS.
A number of other studies of RIT in NHL—including those using antibodies directed against antigens other than CD20 as well as CD20, labeled with either 131I or other radioisotopes using nonmyeloablative doses or myeloablative doses with stem cell support—have shown promising results.38-51 Differing patient characteristics and selection criteria make comparisons of these studies difficult, and thus questions about the relative merits of different isotopes for use in RIT in NHL remain. The short pathlength of 131I beta particles may provide an advantage over those from other isotopes in targeting smaller tumors and micrometastases while minimizing exposure of surrounding normal tissue. In addition, because of gamma emissions, 131I can be used for imaging and dosimetric purposes to noninvasively assess radiation clearance on an individual basis and thus permit patient-specific dosing. Moreover, under new U.S. Nuclear Regulatory Commission guidelines, the risk of caregiver exposure to gamma emissions is sufficiently low to allow outpatient 131I treatment.52 53
Another issue is the incremental value of the radionuclide. Part of the therapeutic advantage of the radionuclide is believed derived from the fact that the emitted beta particles can cause irradiation of many tumor cells in the vicinity of the decaying atom. This creates a situation in which cells within a tumor are caught in a crossfire of beta particles, including cells to which antibody has not penetrated or bound and cells lacking expression of the target antigen. The higher response rates we achieved following a higher TBD support this hypothesis. However, unlabeled tositumomab, especially at the 475-mg dose, appeared to have antitumor activity in some patients after a dosimetric dose in our study.23 24 Although, this study was not designed to separately assess the response rate of the unlabeled antibody, an ongoing, multicenter, randomized trial comparing iodine 131I tositumomab with unlabeled tositumomab should help resolve this issue.
As a result of the promising data obtained during the progress of our study, several multicenter trials of iodine 131I tositumomab have either recently been completed or are in progress. Phase II and III studies in patients with relapsed/refractory low-grade and transformed NHL appear to have confirmed the response rates we obtained.54,55 An ongoing phase II trial is being conducted by our group to assess efficacy and safety of iodine131I tositumomab in previously untreated patients with advanced-stage low-grade NHL.56 These studies, along with future clinical trials, will help clarify the role of this new therapeutic approach in the treatment of NHL.
Acknowledgments
We are indebted to all the patients who participated in the study, to their physicians who referred them to us, to the nursing staff of the General Clinical Research Center at the University of Michigan Hospital for their outstanding care of the patients, and to Susan Blaisdell for her assistance in the preparation of the manuscript.
Supported in part by grants R01-CA56794 and PO1-CA-42678 from the National Cancer Institute, Bethesda, MD, and grant M01-RR-00042 from the National Institutes of Health, Bethesda, MD.
M.S.K. and R.L.W. have received grant support from and are paid consultants for Coulter Pharmaceutical, Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark S. Kaminski, Division of Hematology/Oncology, Department of Internal Medicine, University of Michigan Cancer and Geriatrics Center, Rm 4316, 1500 East Medical Center Dr, Ann Arbor, MI 48109-0936; e-mail: mkaminsk@umich.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal