Abstract
A series of alterations in the cellular genome affecting the expression or function of genes controlling cell growth and differentiation is considered to be the main cause of cancer. These mutational events include activation of oncogenes and inactivation of tumor suppressor genes. The elucidation of human cancer at the molecular level allows the design of rational, mechanism-based therapeutic agents that antagonize the specific activity of biochemical processes that are essential to the malignant phenotype of cancer cells. Because the frequency of RAS mutations is among the highest for any gene in human cancers, development of inhibitors of the Ras–mitogen-activated protein kinase pathway as potential anticancer agents is a very promising pharmacologic strategy. Inhibitors of Ras signaling have been shown to revert Ras-dependent transformation and cause regression of Ras-dependent tumors in animal models. The most promising new class of these potential cancer therapeutics are the farnesyltransferase inhibitors. The development of these compounds has been driven by the observation that oncogenic Ras function is dependent upon posttranslational modification, which enables membrane binding. In contrast to many conventional chemotherapeutics, farnesyltransferase inhibitors are remarkably specific and have been demonstrated to cause no gross systemic toxicity in animals. Some orally bioavailable inhibitors are presently being evaluated in phase II clinical trials. This review presents an overview on some inhibitors of the Ras signaling pathway, including their specificity and effectiveness in vivo. Because Ras signaling plays a crucial role in the pathogenesis of some hematologic malignancies, the potential therapeutic usefulness of these inhibitors is discussed.
The RAS gene family
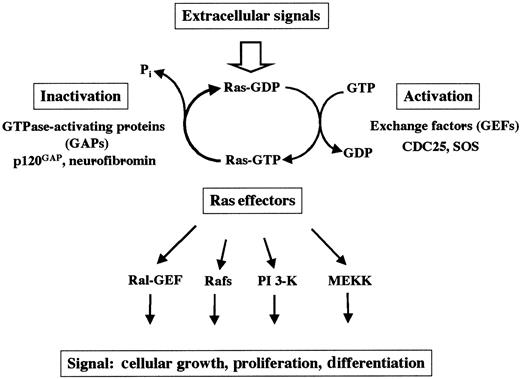
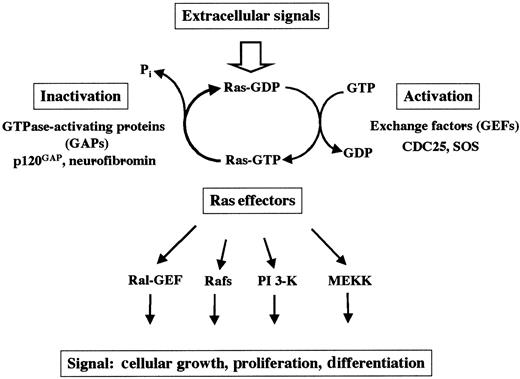
At the cellular surface, many different receptors are expressed that allow cellular response to extracellular signals provided by the environment. After ligand binding, receptor activation leads to a large variety of biochemical events in which small guanosine triphosphate hydrolases (GTPases; eg, Ras) are crucial. Ras proteins are prototypical G-proteins that have been shown to play a key role in signal transduction, proliferation, and malignant transformation. G-proteins are a superfamily of regulatory GTP hydrolases that cycle between 2 conformations induced by the binding of either guanosine diphosphate (GDP) or GTP1-3 (Figure1). The Ras-like small GTPases are a superfamily of proteins that include Ras, Rap1, Rap2, R-Ras, TC21, Ral, Rheb, and M-Ras. The RAS gene family consists of 3 functional genes, H-RAS, N-RAS, and K-RAS. The RAS genes encode 21-kd proteins, which are associated with the inner leaflet of the plasma membrane (H-Ras, N-Ras, and the alternatively spliced K-RasA and K-RasB). Whereas H-Ras, N-Ras, and K-RasB are ubiquitously expressed, K-RasA is induced during differentiation of pluripotent embryonal stem cells in vitro.4
The switch function of Ras.
Ras cycles between the active GTP-bound and the inactive GDP-bound state. Mitogenic signals activate guanine GEFs such as SOS and CDC25. GEFs increase the rate of dissociation of GDP and stabilize the nucleotide-free form of Ras, leading to binding of GTP to Ras proteins. Ras can also be activated by the inhibition of the GAPs.
The switch function of Ras.
Ras cycles between the active GTP-bound and the inactive GDP-bound state. Mitogenic signals activate guanine GEFs such as SOS and CDC25. GEFs increase the rate of dissociation of GDP and stabilize the nucleotide-free form of Ras, leading to binding of GTP to Ras proteins. Ras can also be activated by the inhibition of the GAPs.
Regulatory proteins that control the GTP/GDP cycling rate of Ras include GTPase-activating proteins (GAPs), which accelerate the rate of GTP hydrolysis to GDP, and guanine nucleotide exchange factors (GEFs; eg, SOS and CDC25), which induce the dissociation of GDP to allow association of GTP.3 In the GTP-bound state, Ras couples the signals of activated growth factor receptors to downstream mitogenic effectors. By definition, proteins that interact with the active GTP-bound form of Ras (and thus become GTP-dependently activated) to transmit signals are called Ras effectors.5-8 Mechanisms by which GTP-Ras influences the activity of its effectors include direct activation (eg, B-Raf, PI-3 kinase), recruitment to the plasma membrane (eg, c-Raf-1), and association with substrates (eg, Ral-GDS). Other candidates for Ras effectors include protein kinases, lipid kinases, and GEFs.3 5-8
Posttranslational modification of Ras
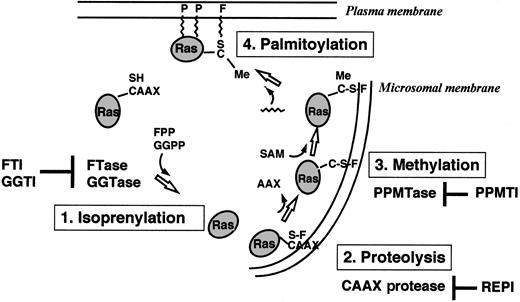
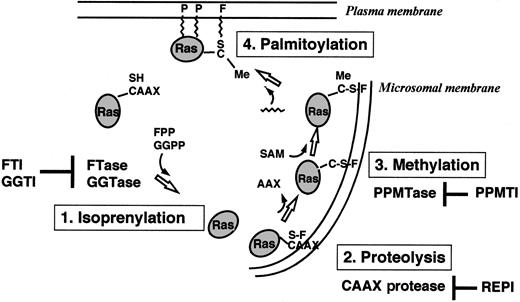
Ras proteins are produced as cytoplasmatic precursor proteins and require several posttranslational modifications to acquire full biologic activity. These modifications include prenylation, proteolysis, carboxymethylation, and palmitoylation9-13(Figure 2).
Overview of the posttranslational modifications of Ras proteins in cells.
FTase or GGTase I transfers a farnesyl (F) group or a geranylgeranyl group from FPP or GGPP to the thiol group of the cysteine residue in the CAAX motif. The C-terminal tripeptide is removed by a CAAX-specific endoprotease in the endoplasmatic reticulum. A PPMTase attaches the methyl group from S-adenosylmethionine (SAM) to the C-terminal cysteine. Finally, a prenyl protein–specific palmitoyltransferase (PPPTase) attaches palmitoyl groups (P) to cysteines near the farnesylated C-terminus. PPMTI indicates prenyl protein–specific methyltransferase inhibitor.
Overview of the posttranslational modifications of Ras proteins in cells.
FTase or GGTase I transfers a farnesyl (F) group or a geranylgeranyl group from FPP or GGPP to the thiol group of the cysteine residue in the CAAX motif. The C-terminal tripeptide is removed by a CAAX-specific endoprotease in the endoplasmatic reticulum. A PPMTase attaches the methyl group from S-adenosylmethionine (SAM) to the C-terminal cysteine. Finally, a prenyl protein–specific palmitoyltransferase (PPPTase) attaches palmitoyl groups (P) to cysteines near the farnesylated C-terminus. PPMTI indicates prenyl protein–specific methyltransferase inhibitor.
Prenylation of proteins by intermediates of the isoprenoid biosynthetic pathway represents a newly discovered form of posttranslational modification and is catalyzed by 3 different enzymes: protein farnesyltransferase (FTase), protein geranylgeranyltransferase type I (GGTase I), and geranylgeranyltransferase type II (GGTase II).9-13 Prenylated proteins share characteristic carboxy-terminal consensus sequences and can be separated into the proteins with a CAAX (C, cysteine; A, aliphatic amino acid; X, any amino acid) motif and proteins containing a CC or CXC sequence.14-17 FTase I transfers a farnesyl group from farnesyldiphosphate (FPP), and GGTase I transfers a geranylgeranyl group from geranylgeranyldiphosphate (GGPP) to the cysteine residue of the CAAX motif.18 GGTase II transfers the geranylgeranyl groups from GGPPs to both cysteine residues of CC or CXC motifs.
Farnesylation is the first step in the posttranslational modification of Ras. This modification occurs by covalent attachment of a 15-carbon farnesyl moiety in a thioether linkage to the carboxy-terminal cysteine of proteins that contain the CAAX motif. The reaction is catalyzed by FTase, a heterodimer consisting of a 48-kd and a 45-kd subunit (αF/GGI and βF). Binding sites for the substrates, FPP and the CAAX motif, are located on the αF- and βF-subunits.19-21Substrates for FTase include all known Ras proteins, nuclear lamins A and B, the γ-subunit of the retinal trimeric G-protein transducin, rhodopsin kinase, and a peroxisomal protein termed PxF.9-13
Farnesylation of Ras proteins is followed by endoproteolytic removal of the 3 carboxy-terminal amino acids (AAX) by a cellular thiol-dependent zinc metallopeptidase.22 This endoproteolytic activity (RACE, or Ras and a-factor converting enzyme) is a composite of 2 different CAAX proteases: a zinc-dependent activity encoded by AFC1 and the type IIb signal peptidase-like RCE1 (Ras converting enzyme 1).23 The final step in the carboxy-terminal modification of proteins with a CAAX motif (eg, Ras) is the methylation of the carboxyl group of the prenylated cysteine residue by an as yet uncharacterized methyltransferase.
Some Ras proteins (H-Ras, N-Ras, Ras2) are further lipidated by palmitoylation at 1 or 2 cysteines near the farnesylated carboxy-terminus.9-13,24-27 Like farnesylation, H-Ras palmitoylation plays an important role for signaling functions in vivo.27 Microinjection experiments in Xenopusoocytes revealed that palmitoylation of H-Ras dramatically enhances its affinity for membranes as well as its ability to activate mitogen-activated protein kinase (MAPK) and initiate meiotic maturation.11,27 Both a Ras-specific protein (palmitoyltransferase) and a palmitoyl-protein (thioesterase) have been characterized.28,29 In contrast to farnesylation and proteolysis, palmitoylation and methylation of Ras are thought to be reversible and may have a regulatory role.11 12
The Ras-to-MAPK signal transduction pathway
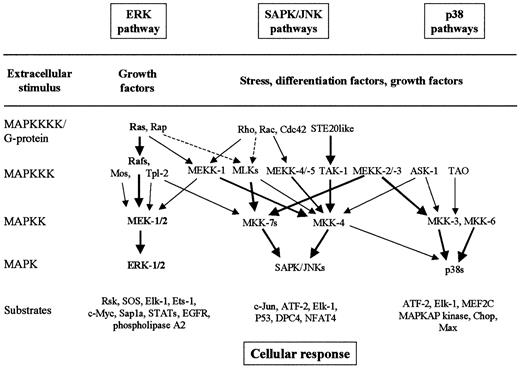
The MAPK signaling cascades
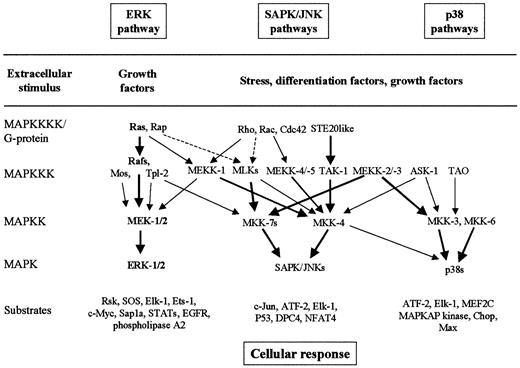
MAPK pathways are well-conserved major signaling systems involved in the transduction of extracellular signals into cellular responses in a variety of organisms, including mammals.30-35 The core components of the MAPK signaling cascades are 3 sequential kinases, including MAP kinase (MAPK, or extracellular signal-regulated kinase, ERK), MAPK kinase (MAPKK, or MAPK/ERK kinase, MEK), and MAPKK kinase (MAPKKK, or MEK kinase, MEKK) (Figure3). The MAPKs are activated by dual phosphorylation on tyrosine and threonine residues by upstream dual-specificity MAPKKs. MAPKKs are also phosphorylated and activated by serine- and threonine-specific MAPKKKs. At least 6 MAPK cascades have been clearly identified in mammalian cells.30-35 The best characterized MAPK signaling pathways are (1) the Ras-to-MAPK signal transduction pathway (or ERK pathway), which is responsive to signals from receptor tyrosine kinase, hematopoietic growth factor receptors, and some heterotrimeric G-protein–coupled receptors, which promote cell proliferation or differentiation; (2) the stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) pathway, which is activated in response to stresses such as heat, high osmolarity, UV irradiation, and proinflammatory cytokines such as tumor necrosis factor–α and interleukin-1 (IL-1); and (3) the p38 pathway, which is responsive to osmotic stress, heat shock, lipopolysaccharide, tumor necrosis factor–α, and IL-1 (Figure 3).30-35Scaffolding/adapter proteins such as MP-1, JSAP-1, and JIP-1 route MAPK modules in mammals by binding ERK-1 and MEK-1, JNK-3 and SEK-1 and MEKK-1, or JNK and MKK-7 and MLKs, respectively.34 35
The best-characterized MAPK modules are the ERK pathway, the SAPK/JNK pathway, and the p38 pathway.
The classical Ras-to-MAPK cascade is shown in bold. The MAPK cascades consist of a MAPKKK, a MAPKK, and a MAPK. MAPKKKs are activated through a large variety of extracellular signals such as growth factors, differentiation factors, and stress. The activated MAPKKK can phosphorylate and activate 1 or several MAPKKs, which, in turn, phosphorylate and activate a specific MAPK. Activated MAPK phosphorylates and activates various substrates in the cytoplasma and the nucleus of the cell, including transcription factors. These downstream targets control cellular responses (eg, apoptosis, proliferation, and differentiation). Thick arrows connect the signaling proteins with their preferred substrates (effectors). Note the complexity and the potential for crosstalk between the pathways.
The best-characterized MAPK modules are the ERK pathway, the SAPK/JNK pathway, and the p38 pathway.
The classical Ras-to-MAPK cascade is shown in bold. The MAPK cascades consist of a MAPKKK, a MAPKK, and a MAPK. MAPKKKs are activated through a large variety of extracellular signals such as growth factors, differentiation factors, and stress. The activated MAPKKK can phosphorylate and activate 1 or several MAPKKs, which, in turn, phosphorylate and activate a specific MAPK. Activated MAPK phosphorylates and activates various substrates in the cytoplasma and the nucleus of the cell, including transcription factors. These downstream targets control cellular responses (eg, apoptosis, proliferation, and differentiation). Thick arrows connect the signaling proteins with their preferred substrates (effectors). Note the complexity and the potential for crosstalk between the pathways.
Ras-to-MAPK signaling via receptor tyrosine kinases and cytokine receptors
The Ras-to-MAPK pathway appears to be an essential shared element of mitogenic signaling. The MAPKs ERK-1 and ERK-2 are activated by various mitogens in all cells. Ras functions as a membrane-associated biologic switch that relays signals from ligand-stimulated receptors to cytoplasmatic MAPK cascades. These receptors include G-protein–coupled serpentine receptors, tyrosine kinase receptors (eg, platelet-derived growth factor receptor [PDGFR], epidermal growth factor [EGF] receptor) and cytokine receptors that cause stimulation of associated nonreceptor tyrosine kinases (NRTKs; eg, Src, Lyn, Fes). Ligand binding to the extracellular domain of receptor tyrosine kinases (RTKs) causes receptor dimerization, stimulation of protein tyrosine kinase activity, and autophosphorylation.36-40 Tyrosine autophosphorylation sites in growth factor receptors (eg, EGF receptor) function as high-affinity binding sites for SH-2 (src homology) domains of signaling molecules such as PI-3 kinase (PI-3K), phospholipase C (PLC)-γ, p120-GAP, Shc, and SHP-2 tyrosine phosphatase.39
In contrast to receptor tyrosine kinases, cytokine receptors (such as the prototypical IL-3, IL-5, GM-CSF receptors) do not contain a kinase domain. These receptors are heterodimers of a ligand-specific α-subunit and a β-subunit that is common to IL-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) receptors.41,42 The NRTKs Lyn and Fes and the Janus kinase JAK2 are physically associated with the β-subunit. The conserved proline-rich motifs in the α- and β-subunits (eg, IL-3, IL-5, GM-CSF-R, IL-2-R, G-CSF-R, and erythropoietin-R) are critical for JAK2 binding and activation. (Figure 4). After ligand binding and receptor dimerization, the receptor-bound tyrosine kinases become activated and cause a cascade of tyrosine phosphorylations. As in the receptor tyrosine kinases, these phosphotyrosines represent docking sites for many signaling molecules, including adapter proteins (eg, PI-3K, Shc, SHP-2, Grb-2).41 42
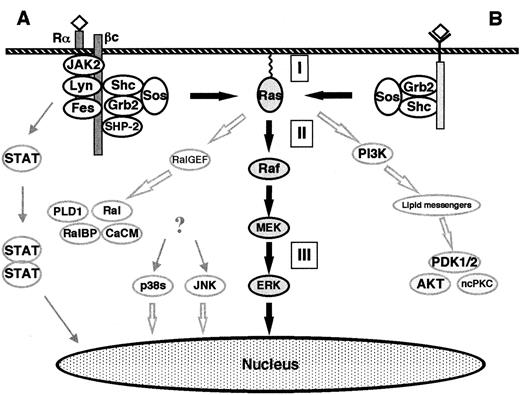
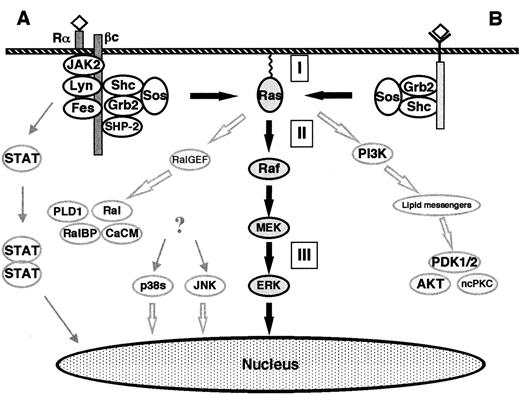
The classical Ras-to-MAPK cascade.
(A) Signaling by cytokine receptors. The IL-3, IL-5, and GM-CSF receptors consist of a ligand-specific α-subunit and a common β-subunit. The β-subunit binds the NRTKs Lyn, Fes, and JAK2. After ligand binding, the α- and β-subunits are thought to dimerize, thus activating the receptor-bound NRTKs and subsequently causing a cascade of tyrosine phosphorylations. The phosphotyrosine residues represent docking sites for various signaling molecules (eg, Shc, SHP-2). ERKs are activated via the classical Ras-to-MAPK pathway. In addition, the MAPKs p38 and JNK become activated. The activation pathway is not completely understood, but some lines of evidence support the involvement of Ras or HPK-1 (hematopoietic progenitor kinase, a mammalian Ste20-related protein). Activated JAK2 phosphorylates the STAT (signal transducers and activators of transcription) family of nuclear factors which form heterodimers and homodimers, thus causing their translocation to the nucleus and subsequent binding to γ-activating sequences of the promoter region of various genes.41,42 (B) Signaling by receptor tyrosine kinases. Extracellular stimuli such as mitogens or stress result in the intracellular activation of different MAPK cascades. The ERK-1/2 pathway is activated by mitogens in all cells and is an essential part of mitogenic signaling. Translocation of a fraction of activated ERKs to the nucleus subsequently leads to activation of transcription factors such as Elk-1, CREB, SRF, and Fos.40 The Raf kinases connect upstream tyrosine kinases and Ras with downstream serine/threonine kinases. When Ras becomes GTP-loaded, Rafs bind to Ras. It is unclear if Ras-Raf binding is itself always sufficient to activate the Raf kinases, which subsequently phosphorylate and activate the downstream MEKs. GTP-Ras also binds and activates PI-3K and Ral-GEF. PI-3K produces lipid second messengers, which activate AKT (Akt kinase) and ncPKC. Ral-GEF activates Ral-GTPases by promoting the GTP-bound state of Ral. Ral-GTP binds to Ral-BP1 (a GAP for CDC42 and Rac), phospholipase D (PLD1), and Ca2+ calmodulin (CaCM). (I) Inhibitors of Ras membrane association (eg, FTI, GGTI, PPMTI, and REPI); (II) sulindac; (III) MEK inhibitors (eg, PD098059, U0126, and Ro 09-2110). The thick, black arrows show the classical Ras-to-MAPK cascade. The thick, open arrows represent the Ras-to-Ral and the Ras–to–PI-3K signaling pathways. The STAT pathway is shown on the left.
The classical Ras-to-MAPK cascade.
(A) Signaling by cytokine receptors. The IL-3, IL-5, and GM-CSF receptors consist of a ligand-specific α-subunit and a common β-subunit. The β-subunit binds the NRTKs Lyn, Fes, and JAK2. After ligand binding, the α- and β-subunits are thought to dimerize, thus activating the receptor-bound NRTKs and subsequently causing a cascade of tyrosine phosphorylations. The phosphotyrosine residues represent docking sites for various signaling molecules (eg, Shc, SHP-2). ERKs are activated via the classical Ras-to-MAPK pathway. In addition, the MAPKs p38 and JNK become activated. The activation pathway is not completely understood, but some lines of evidence support the involvement of Ras or HPK-1 (hematopoietic progenitor kinase, a mammalian Ste20-related protein). Activated JAK2 phosphorylates the STAT (signal transducers and activators of transcription) family of nuclear factors which form heterodimers and homodimers, thus causing their translocation to the nucleus and subsequent binding to γ-activating sequences of the promoter region of various genes.41,42 (B) Signaling by receptor tyrosine kinases. Extracellular stimuli such as mitogens or stress result in the intracellular activation of different MAPK cascades. The ERK-1/2 pathway is activated by mitogens in all cells and is an essential part of mitogenic signaling. Translocation of a fraction of activated ERKs to the nucleus subsequently leads to activation of transcription factors such as Elk-1, CREB, SRF, and Fos.40 The Raf kinases connect upstream tyrosine kinases and Ras with downstream serine/threonine kinases. When Ras becomes GTP-loaded, Rafs bind to Ras. It is unclear if Ras-Raf binding is itself always sufficient to activate the Raf kinases, which subsequently phosphorylate and activate the downstream MEKs. GTP-Ras also binds and activates PI-3K and Ral-GEF. PI-3K produces lipid second messengers, which activate AKT (Akt kinase) and ncPKC. Ral-GEF activates Ral-GTPases by promoting the GTP-bound state of Ral. Ral-GTP binds to Ral-BP1 (a GAP for CDC42 and Rac), phospholipase D (PLD1), and Ca2+ calmodulin (CaCM). (I) Inhibitors of Ras membrane association (eg, FTI, GGTI, PPMTI, and REPI); (II) sulindac; (III) MEK inhibitors (eg, PD098059, U0126, and Ro 09-2110). The thick, black arrows show the classical Ras-to-MAPK cascade. The thick, open arrows represent the Ras-to-Ral and the Ras–to–PI-3K signaling pathways. The STAT pathway is shown on the left.
The SH3 domain of Grb-2 binds to SOS, which is a GEF for Ras and facilitates the replacement of GDP with GTP.3-8,36-40 When Ras becomes GTP-loaded, Ras effectors (such as Rafs, MEKK, PI-3K, and Ral) bind to Ras and become activated. The Raf kinases (A-Raf, B-Raf, c-Raf-1) are important Ras effectors and have been demonstrated to act as MAPKKKs/MEKKs in the Ras-to-MAPK (or ERK) pathway.36-40,43-45 Raf kinases have been shown to selectively phosphorylate and activate MAPKKs MEK-1 and MEK-2.36-40,43-45 Other MEK-1/MEK-2 activators include TPL-2, MEKK-1, and c-Mos.46-48 MEK-1 and MEK-2 are dual-specificity kinases that activate the MAPKs of the ERK subgroup (ERK-1 and ERK-2).30-35,49-52 ERK-1 and ERK-2 are proline-directed protein kinases that phosphorylate Ser/Thr-Pro motifs in the consensus sequence Pro-Xaan-Ser/Thr-Pro, where Xaa is any amino acid and n = 1 or 2. Several cytoplasmatic and nuclear substrates of the ERKs have been identified. The best-characterized ERK substrates are cytoplasmatic phospholipase A2(cPLA2), the ribosomal protein S6 kinases (RSKs), and the transcription factor Elk-1.30,32,53 54
The Ras-to-Ral and the Ras–to–PI-3K signaling pathways
Since the discovery of Raf as a direct Ras effector, numerous other putative Ras effectors have been identified.3-8Among these, evidence to date best supports “effector” roles for the Ral-GEFs (Ral-GDS, RGL, and RGF) and the p110 subunit of PI-3K3-8,55 56 (Figure 4).
Ral-GEFs are activated via binding to GTP-Ras. Ral-GEFs in turn activate Ral-GTPases by promoting the GTP-bound state of Ral. As members of the Ras subfamily of Ras-related GTPases, Ral proteins (RalA and RalB) also cycle between the active GTP-bound states and inactive GDP-bound states. Ral-GTP binds Ral-BP1 (Ral-binding protein-1 or Rlip1 = Rip1 [Ral-interacting protein-1]), which is a GAP for CDC42 and Rac. These 2 GTPases are involved in the regulation of the actin cytoskeleton, the SAPK/JNK pathway, and the p38 pathway (Figure3).
Ras-GTP also binds to and activates the catalytic domain of PI-3K. The lipid second-messenger molecules produced (eg, phosphatidylinositol phosphates PtdIns 3,4-P2 and PtdIns 3,4,5-P3) activate the phosphoinositide-dependent kinases PDK-1 and PDK-2, which then activate Akt kinase and nonconventional isoforms of protein kinase C (ncPKC). PI-3K has been implicated in 4 apparently distinct cellular functions, including mitogenic signaling (DNA synthesis), inhibition of apoptosis, intracellular vesicle trafficking and secretion, and regulation of actin and integrin functions. These functions are most likely mediated by distinct phosphoinositide products of PI-3K56 (Figure 4).
Role of Ras activation in hematologic malignancies
The constitutive activation of Ras appears to be an important factor for the malignant growth of human cancer cells. Recently, the Ras-related proteins R-Ras, M-Ras, and TC21 have also been shown to possess transforming activities similar to those of Ras.57-59 However, their role in human malignancies is unclear. Mutations of the RAS proto-oncogenes (H-RAS, N-RAS, K-RAS) are frequent genetic aberrations found in 20% to 30% of all human tumors, although the incidences in tumor type vary greatly.60,61 The highest rate of RAS mutations was detected in adenocarcinomas of the pancreas (90%), the colon (50%), and the lung (30%). In follicular and undifferentiated carcinomas of the thyroid, the incidence of RAS mutations is also considerable (50%). The most commonly observed RAS mutations arise at sites critical for Ras regulation—namely, codons 12, 13, and 61. Each of these mutations results in the abrogation of the normal GTPase activity of Ras. While all the Ras mutants still form complexes with GAP, the GTPase reaction of Ras cannot be stimulated by GAP, thus causing an increase in the half-lives of Ras-GTP mutants.1,5Transformation results, at least in part, from unregulated stimulation of the mitogenic signal transduction pathway.60 61
Ras activation is frequently observed in hematologic malignancies such as myeloid leukemias and multiple myelomas. In about one-third of the myelodysplastic syndromes (MDS) and acute myeloid leukemias (AML),RAS genes are mutationally activated62-73 (Table1). N-RAS is mutated and activated in most of the cases, and the presence of the mutation is not associated with any particular FAB type, cytogenetic abnormality, or clinical feature, including prognosis.71RASmutations occur in about 40% of newly diagnosed multiple myeloma patients, and the frequency increases with disease progression.74 Mutations in N-RAS—especially codon 61 mutations—are more frequent than K-RASmutations.74-78
In addition to activation by mutation, Ras is thought to be deregulated by constitutive activation of proto-oncogenes and inactivation of tumor suppressor genes.79,80 Several types of human cancers show oncogenic activation of RTKs or NRTKs. Constitutively activated versions of normal receptor tyrosine kinases contain single point mutations (eg, CSF-1 receptor, the Neu/Erb-B2 receptor, and the c-Kit receptor), duplications of juxtamembrane domain-coding sequences (eg, FLT3 receptor), or deletions of the negative regulatory regions in the ligand binding or the transmembrane domains (eg, Erb-B receptor). Point mutations of the CSF-1 receptor (c-FMS) at codons 301 and 969 were found in 10% to 20% of AML or MDS.81,82 Point mutations in the catalytic domain of the c-Kit receptor are found in some cases of myeloproliferative disorders and in 10% of the patients with mastocytosis.83-85 Furthermore, activating tandem internal duplication of the FLT3 receptor has been reported in 20% of AML.86 The members of the c-Kit/c-FMS receptor kinase family (eg, c-Kit, c-FMS, FLT3) are linked with components of the Ras-to-MAPK signaling pathway (eg, Grb-2 and Shc), suggesting that activating mutations of c-FMS and FLT3 may induce activation of Ras.87 88
In addition, translocations involving receptor tyrosine kinases produce chimeric proteins in which varying N-terminal portions of either the ligand-binding or the transmembrane domain are replaced with novel protein sequences.79,80 Several of these chimeric proteins have been found in human hematologic malignancies. The Npm-Alk fusion protein, a fusion of the N-terminal portion of Npm with the entire cytoplasmatic domain of the receptor tyrosine kinase Alk, is generated by the t(2;5) chromosomal translocation in anaplastic large cell lymphoma.89,90 Tel-PDGFRβ is a fusion protein consisting of the transcription factor Tel (translocation, Ets, leukemia) and PDGFRβ, a well-known receptor tyrosine kinase.91,92 It is generated by the t(5;12) translocation in a subset of chronic myelomonocytic leukemias that results in receptor dimerization and activation and thus leads to the constitutive activation of the Ras-MAPK pathway.3 Another Tel fusion protein, Tel-Abl, is generated by the t(12;9) translocation in AML.93,94 Abl is an NRTK that is also mutated and activated in chronic myelogenous leukemia.95-97 In Bcr-Abl, the product of the t(9;22) translocation, the N-terminal Bcr portion serves as an oligomerization domain. Bcr-Abl is a constitutively activated cytosolic tyrosine kinase that causes abrogation of growth factor dependence, blockade of differentiation, and direct inhibition of apoptosis. Although Ras mutations are extremely rare in chronic myelogenous leukemia, the involvement of Ras has been demonstrated in Bcr-Abl+ cells by the presence of increased levels of GTP-Ras, which leads to the activation of the Raf kinases and other Ras effectors.95-97 Thus, the deregulation of Ras function appears to be a common theme in the transformation by activated receptor and NRTKs. Ras activation may cause elevated cell cycle progression and inhibition of apoptosis.71,79,80 95-97
In addition to oncogenes, tumor suppressor genes have also been found to be involved in the deregulation of Ras. Neurofibromin, the product of the NF1 gene, encodes a Ras-GAP and is mutated in the autosomal dominant type 1 neurofibromatosis.98 Interestingly, neurofibromatosis type 1 is associated with an increased tendency to develop myeloid leukemias, especially juvenile myelomonocytic myeloid leukemia (JMML).99-107 About 15% of children with JMML cases have clinical neurofibromatosis.99 Additionally, inactivating mutations of the NF1 gene have been found in 15% of JMML without clinical diagnosis of neurofibromatosis, suggesting the existence of NF1 mutations in approximately 30% of all JMML cases.100,102 The involvement of Ras is demonstrated by the finding that leukemic cells from children with neurofibromatosis type 1 show a moderate elevation in the percentage of GTP-Ras.103-106 Furthermore, 15% to 30% of JMML cases lacking the NF1 mutation have activating RASmutations.107 The observation that human JMML cells exhibit hypersensitivity to GM-CSF suggests a common pathophysiologic mechanism involving downstream Ras signaling.106-108
The pathophysiologic importance of the Ras-MAPK signaling pathway is underscored by the positioning of several oncogene and tumor suppressor gene products on this pathway (Figure 4). Furthermore, it has recently been demonstrated that mutant N-RAS induces myeloproliferative disorders resembling human chronic myelogenous leukemia, AML, and apoptotic syndromes similar to human MDS in bone marrow–repopulated mice.109 These observations make Ras and the Ras-MAPK pathway an attractive target for the development of new anticancer agents.
Inhibitors of the Ras-MAPK pathway
Inhibitors of Ras farnesyltransferase
Elimination of Ras function by homologous gene recombination or antisense RNA has demonstrated that expression of activated Ras is necessary for maintaining the transformed phenotype of tumor cells.110-113 Inhibitors of oncogenic Ras activity may therefore prove useful as anticancer agents against Ras-induced tumors. One strategy to impede oncogenic Ras function in vivo is the inhibition of Ras posttranslational modification. It has been demonstrated that mutation of the evolutionarily conserved CAAX motif in Ras abolishes plasma membrane binding as well as transforming activity.114-121 Although Ras undergoes several steps of posttranslational modification, only farnesylation is necessary for its membrane localization and cell-transforming activity.121Therefore, it has been proposed that the activity of oncogenic Ras could be blocked by inhibiting the FTase responsible for this modification. However, many CAAX-containing proteins need additional palmitoylation for stable membrane association.
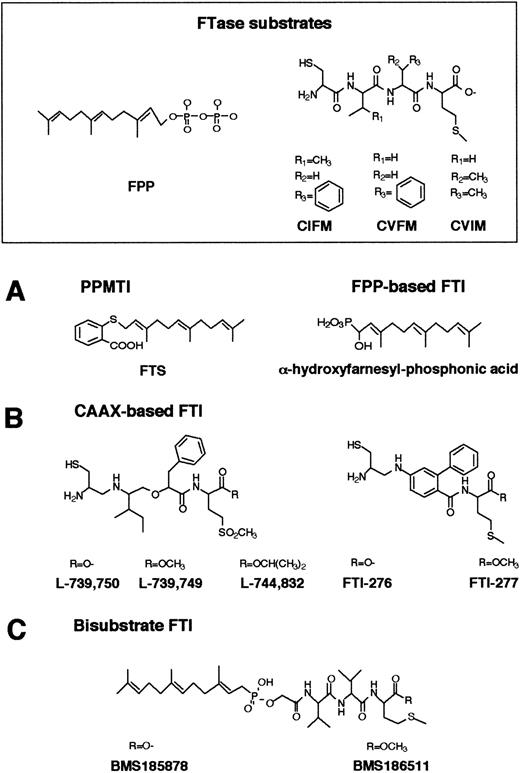
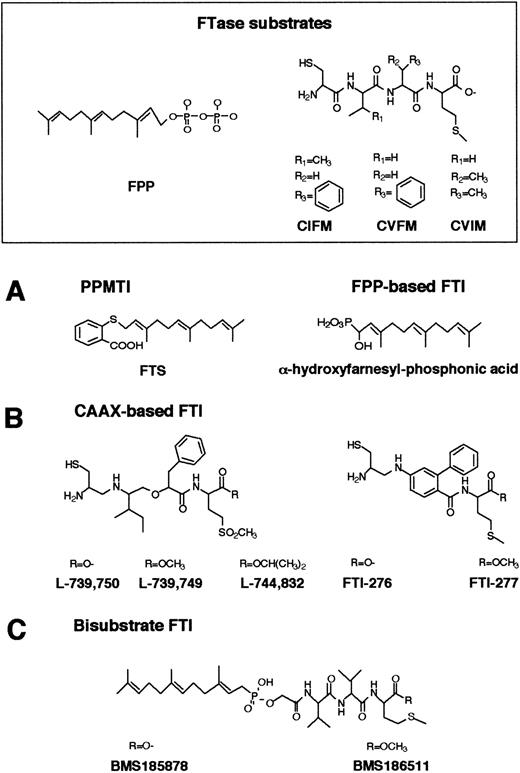
FTase has become a very attractive target for the development of anticancer agents because control of Ras farnesylation can control the function of oncogenic Ras.114-121 Numerous inhibitors of Ras FTase have been synthesized or identified. These Ras FTase inhibitors can be grouped into 3 classes: (1) FPP analogues such as (α-hydroxyfarnesyl) phosphonic acid, β-ketophosphonic and β-hydroxyphosphonic acid derivatives, and J-104871122-124(Figure 5A); (2) CAAX peptide analogues such as BZA-5B, BZA-2B,125-127 L-731,734, L-731,735, L-739,749,128-132 L-739,787,133L-739,750, L-744,832,129,134-137 B581,138Cys-4-ABA-Met and Cys-AMBA-Met,139 FTI-276, FTI-277,140-143 B956, and its methyl ester B1096144 (Figure 5B); in addition, nonpeptidic, tricyclic FTase inhibitors have been developed such as SCH44342, SCH54429, SCH59228, and SCH66336145-149 (Figure6); and (3) bisubstrate inhibitors such as phosphonic acid analogues, the phosphinate inhibitors BMS-185878 and BMS-186511, the phosphonate inhibitor BMS-184467, phosphinyl acid–based derivatives, and the hydroxamine acid analogues150-152 (Figure 5C).
Structures of FPP-, CAAX-based, and bisubstrate inhibitors of FTase.
(A) Chemical structures of FPP and FPP-based inhibitors of FTase and PPMTase. FPP is composed of a hydrophobic farnesyl group and a highly charged pyrophosphate moiety. The basic structural element in the FTase inhibitors is a farnesyl group, a pyrophosphate isostere, and a linker. (B) CAAX-based FTase inhibitors. Structural comparison between CAAX-based FTase inhibitors of the pseudopeptide class and the CAAX tetrapeptides CIFM and CVFM. The potent, nonsubstrate FTase inhibitors CIFM and CVFM were identified by systematic amino acid replacements within the CAAX sequence. In FTI-276 and FTI-277, the AA residues of the CAAX motif have been replaced by a hydrophobic linker. (C) In bisubstrate FTase inhibitors, the farnesyl group of FPP and the tripeptide group of the CAAX motif are connected via a linker.
Structures of FPP-, CAAX-based, and bisubstrate inhibitors of FTase.
(A) Chemical structures of FPP and FPP-based inhibitors of FTase and PPMTase. FPP is composed of a hydrophobic farnesyl group and a highly charged pyrophosphate moiety. The basic structural element in the FTase inhibitors is a farnesyl group, a pyrophosphate isostere, and a linker. (B) CAAX-based FTase inhibitors. Structural comparison between CAAX-based FTase inhibitors of the pseudopeptide class and the CAAX tetrapeptides CIFM and CVFM. The potent, nonsubstrate FTase inhibitors CIFM and CVFM were identified by systematic amino acid replacements within the CAAX sequence. In FTI-276 and FTI-277, the AA residues of the CAAX motif have been replaced by a hydrophobic linker. (C) In bisubstrate FTase inhibitors, the farnesyl group of FPP and the tripeptide group of the CAAX motif are connected via a linker.
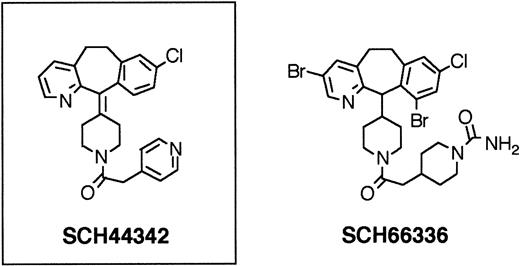
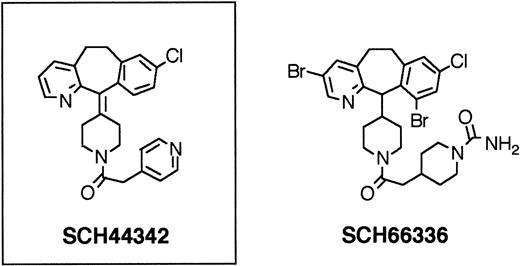
Nonpeptidic, tricyclic FTase inhibitors.
FTase inhibitor SCH44342 had no in vivo efficacy. Further substitutions led to SCH66336, a highly potent FTase inhibitor, which was found to have therapeutically useful serum levels and half-lives when given orally to rodents and primates. SCH66336 is being tested in human clinical phase II trials.
Nonpeptidic, tricyclic FTase inhibitors.
FTase inhibitor SCH44342 had no in vivo efficacy. Further substitutions led to SCH66336, a highly potent FTase inhibitor, which was found to have therapeutically useful serum levels and half-lives when given orally to rodents and primates. SCH66336 is being tested in human clinical phase II trials.
In addition to chemically synthesized compounds, several natural products have been identified as FTase inhibitors. These include limonene,153 manumycin (UCF1-C) and its related compounds UCF1-A and UCF1–B,154-156 chaetomellic acid A and B, zaragozic acids, pepticinnamins, gliotoxin,115 barceloneic acid A,157 RPR113228,158 actinoplanic acids A and B,159 oreganic acid,160 lupane derivatives,161 saquayamycins,162 valinoctin A and its analogues,163 and ganoderic acid A and C.164
Effects of FTase inhibitors in intact tumor cells.
Several FTase inhibitors were demonstrated to be active in intact cells (Table 2). These compounds have been shown to modulate several critical aspects of Ras transformation in whole cells, including selective inhibition of anchorage-independent growth of Ras-transformed fibroblasts in soft agar, morphologic reversion of the Ras-induced phenotype, and inhibition of oocyte maturation induced by oncogenic Ras without gross cytotoxic effects on normal cells. One of the first FTase inhibitors found to be active in intact tumor cells, the FPP analogue (α-hydroxyfarnesyl) phosphonic acid, only partially inhibited the farnesylation of Ras in H-Ras–transformed NIH3T3 fibroblasts.165 Subsequently, more potent FTase inhibitors have been developed. L-739,749 inhibited growth of Ras-transformed rat fibroblasts and caused rapid morphologic reversion of the transformed phenotype.130 The compound B581 inhibited colony formation of Ras-transformed cells in soft agar.138 BZA-5B and BZA-2B, both benzodiazepine peptidomimetic FTase inhibitors, have been shown to slow the growth of H-Ras–transformed cells at concentrations that do not affect the growth of nontransformed cells.125-127,166,167 The peptidomimetic FTase inhibitor B956 and its methyl ester B1086 inhibited the formation of soft agar colonies of 14 human tumor cell lines expressing oncogenic forms of H-Ras, N-Ras, and K-Ras.144 Five human tumor cell lines expressing wild-type Ras required higher concentrations of the drug to inhibit colony formation. About 50% of K-Ras–transformed cell lines were observed to be as resistant as non–Ras-transformed cell lines. It has been suggested that nontransformed cells may produce a form of Ras that is isoprenylated even in the presence of FTase inhibitors.144Additionally, this phenomenon may be due to functional redundancy within the RAS family. The tricyclic inhibitor SCH44342 specifically blocks morphologic transformation induced by Val12-Ha-Ras-CVLS but not Val12-Ha-Ras-CVLL, a form of Ras engineered to bind to GGTase I, indicating that the compound is a specific inhibitor of H-Ras modification by FTase rather than K-Ras modification by GGTase.145
Similarly, several bisubstrate inhibitors of FTase were shown to inhibit oncogenic Ras-induced growth in vivo. The phosphinyl acid–based bisubstrate analogue FTase inhibitors Nos. 17 to 19 were found to inhibit the anchorage-independent colony growth of Ha-RAS–transformed NIH3T3 cells.150-152 The bisubstrate FTase inhibitor BMS-186511 inhibited FTase activity in whole cells and produced strong inhibition of Ras-transformed growth. Although both H-Ras– and K-Ras–transformed cells were affected by BMS-186511, K-Ras cells appeared to be less sensitive.151BMS-186511 did not produce any signs of gross, unspecific cytotoxicity in untransformed normal cells.
The FTase inhibitor L-744,832 blocked the anchorage-dependent and -independent growth of 31 of 42 human tumor cell lines.136The origin of the tumor cell and the presence or absence of mutationally activated Ras did not correlate with the response to the FTase inhibitor. Interestingly, cell lines with wild-type Ras and activated receptor tyrosine kinases were also sensitive to L-744,832. In contrast, nontransformed epithelial cell lines were far less sensitive. Recently, L-739,749 and L-744,832 have also been reported to inhibit the colony growth of juvenile myelomonocytic leukemia cells, which are known to exhibit deregulated cytokine signal transduction involving the Ras pathway.132
Biologic mechanisms of FTase inhibitors in intact cells.
Recent investigations into the biologic mechanism of the growth inhibition of Ras-transformed cells have shown that farnesylation of K-Ras and N-Ras is more resistant to FTase inhibitors than farnesylation of H-Ras.126,144,167,168 In part, this phenomenon is a result of a 10- to 50-fold higher affinity of FTase for K-Ras4B than for other Ras isoforms.126,170 In the absence of FTase inhibitors, all Ras proteins are present only in the farnesylated form. However, K-Ras and N-Ras (but not H-Ras) become geranylgeranylated by GGTase I in vivo in a dose-dependent manner when intracellular farnesylation is inhibited by an FTase inhibitor.126,169-171 Subsequently, both FTase and GGTase I inhibitors are required for inhibition of K-Ras processing.168,172 The lack of growth inhibition and gross cytotoxic effects of FTase inhibitors on normal cells is thought to be a result of the resistance of K-Ras processing to FTase inhibitors.167
Treatment of Ras-transformed cells with FTase inhibitors results in selective suppression of Ras-dependent oncogenic signaling. This includes the inhibition of Ras processing, which results in a decrease in the relative amount of fully processed Ras; the progressive, dose-dependent cytoplasmatic accumulation of unprocessed Ras and inactive Ras-Raf complexes; inhibition of the Ras-induced constitutive activation of MAPK138,140,141,146,173; and decreased transcriptional activity of both c-Jun and Elk-1.138Transformation by mutationally activated Raf, MEK, Mos, or Fos (all of which are downstream effectors of Ras) is not blocked by FTase inhibitors.129 136
Although FTase inhibitors block Ras farnesylation and the Ras-induced transformed phenotype, proteins other than Ras may be targets of these compounds.174,175 FTase inhibitors block anchorage-independent growth of many human tumor cell lines in soft agar culture, but there is no correlation between biologic susceptibility and the presence of Ras mutations.136,144In addition, anchorage-independent growth of K-Ras–transformed cells is abrogated by FTase inhibitors even though K-Ras processing is not affected.172 Although it is unclear whether soluble species of oncogenic Ras exert any biologically significant effect in drug-treated cells, it has recently been shown that nonfarnesylated H-Ras proteins can be palmitoylated and thus are biologically active. These proteins bound modestly to the plasma membranes (40%) but were still able to trigger exaggerated differentiation of PC12 cells and potent transformation of NIH3T3 fibroblasts.176
Recently, it has been suggested that the antitransforming effects of FTase inhibitors are mediated at least in part by alteration of farnesylated Rho proteins, including RhoB.174,175,177,178In contrast to Ras proteins, RhoB exists normally in vivo in a farnesylated (RhoB-FF) and a geranylgeranylated version (RhoB-GG).179 RhoB-GG is essential for the degradation of p27KIP1 and facilitates the progression of cells from G1 to S phase. Treatment with FTase inhibitors results in a loss of RhoB-FF and a gain of RhoB-GG.178 Expression of a mutant RhoB-GG protein induces phenotypic reversion, cell growth inhibition, and activation of the cell cycle kinase inhibitor p21WAF1 in cells sensitive to FTase inhibitors, including Ras-transformed cells.178,180P21WAF1 mediates the inhibition of cyclinE-associated protein kinase activity, pRB hypophosphorylation, and inhibition of DNA replication, which results in G1 arrest.180 In addition to the induction of the G1 block, treatment of tumor cells with FTase inhibitors induces apoptosis by upregulating Bax and Bcl-xs expression and by activating caspases.131 181-183
Synergy of FTase inhibitors with established anticancer treatments such as radiation and chemotherapeutic treatment was recently reported. Agents that prevent microtubule depolymerization, such as taxol and epothilones, act synergistically with FTase inhibitors to block cell growth. FTase inhibitors cause increased sensitivity to induction of the metaphase block by taxol and epothilones.184 In addition, FTase inhibitors have been shown to increase the radiosensitivity of human tumor cells with activating mutations ofRAS oncogenes.143
Effects of FTase inhibitors in animal models.
FTase inhibitors have also been shown to inhibit the growth of Ras-induced tumors in mouse xenograft models and, more dramatically, in transgenic mouse models (Table 3). Manumycin was reported to inhibit the growth of K-Ras–transformed fibrosarcoma transplanted into nude mice by approximately 70% compared with untreated controls.154 The CAAX peptide analogue L-739,749 specifically suppressed the tumor growth of H-Ras–, N-Ras–, and K-Ras–induced Rat-1 cell tumors in nude mice by 51% to 66%.129 Interestingly, L-739749 exhibited no evidence of systemic toxicity. The peptidomimetic FTase inhibitors B956 and B1086 were shown to inhibit tumor growth of EJ-1 human bladder carcinoma, HT 1080 human fibrosarcoma and, to a lesser extent, HCT116 human colon carcinoma xenografts in nude mice. Inhibition of Ras processing correlated with the inhibition of the tumor growth by B956.144 Analogues of the tetrapeptide CVFM,189 the compound Nos. 46 and 51, showed inhibition of anchorage-independent growth of stably H-Ras–transformed NIH3T3 fibroblasts as well as antitumor activity in an athymic mouse model implanted with H-Ras–transformed Rat-1 cells.187J-104871, an FPP-competitive FTase inhibitor, suppressed tumor growth in nude mice transplanted with activated H-RAS–transformed NIH3T3 cells.124 In contrast to these results, however, treatment of irradiated mice engrafted with NF-1 deficient hematopoietic cells (−/−) with the FTase inhibitor (FTI) L-744,832 failed to revert a myeloproliferative disorder similar to JMML.190 Although L-744,832 abrogated the GM-CSF–induced growth, H-Ras processing and MAPK activation of NF-1 (−/−) hematopoietic cells, this FTI did not reduce the constitutively elevated MAPK activity levels in these cells. This may be due to the resistance of N-Ras and K-Ras processing to inhibition by the FTI.190
In addition to the mouse xenograft models, FTase inhibitors have been tested in transgenic mouse models. The CAAX-based FTase inhibitor L-744,832 induced regression of mammary and salivary carcinomas in MMTV-v-Ha-RAS mice. These mice harbor the viral Ha-RAS oncogene under the control of the mouse mammary tumor virus (MMTV) long terminal repeat and develop spontaneous mammary and salivary carcinomas.135 In agreement with earlier observations, no systemic toxicity was observed in these mice. Furthermore, L-744,832 was also effective in mammary and lymphoid tumors overexpressing N-RAS in MMTV transgenic mice.137 In contrast to H-Ras, N-Ras remained mostly processed. Consistent with these findings, the antineoplastic effect was less intense in the N-RAS model than the H-RAS model.135,137 This observation suggests that proteins in addition to Ras may be targets of the compound. More recently, L-744,832 was shown to induce regression of mammary tumors in MMTV–TGF-α and MMTV–TGF-α/neu transgenic mice.183 Because the mammary tumor cells harbor an activated receptor tyrosine kinase but wild-type Ras, a feature common in breast cancer, these mice provide a useful model system for breast cancer research. Tumor regression by L-744,832 was demonstrated biochemically by inhibition of MAPK activity and biologically by an increase in G1-phase, a decrease in S-phase fractions, and induction of apoptosis.183
In both cell culture and mouse models, there is essentially no cytotoxicity or apparent systemic toxicity at doses capable of reverting Ras-induced transformation or of causing tumor regression. FTase inhibitors seem to selectively target a unique aspect of the transformed cell physiology.
Mechanisms of resistance to FTase inhibitors.
As with any drug, the development of tumor resistance to FTase inhibitors is an important issue. To date, the relative frequency, the mechanisms, and the development of tumor resistance to FTI are unclear.K-RAS–transformed cell lines have been shown to be more resistant to FTase inhibitors than H-RAS– orN-RAS–transformed cells.126,144,167 This phenomenon is thought to be a result of a higher affinity of FTase for K-Ras than for other Ras isoforms.126,167 In addition, K-Ras and N-Ras become geranylgeranylated in the presence of FTI.169,171 Subsequently, both FTI and geranylgeranyltransferase inhibitor (GGTI) are required for inhibition of K-Ras processing.168,172 Recently, a variantRAS-transformed cell line was identified that was resistant to phenotypic reversion by FTI.193 This phenomenon was not due to mutation of the FTase subunits, changes in intracellular drug accumulation, or amplification of the multiple-drug resistance gene. The precise mechanism of resistance in these cells remained unclear. However, mutational alteration of FTase might also lead to resistance toward FTI. The Y361L mutant of FTase has been shown to exhibit increased resistance to FTI while maintaining FTase activity toward substrates possessing CIIS carboxy-termini.194 Withdrawal of FTI from successfully treated tumor-bearing mice led to subsequent tumor growth in the absence of the drug. A second FTI treatment resulted in a second response in some mice, but some tumors were found to become resistant to FTI.135 Therefore, chronic, uninterrupted treatment with FTI might be required.
Inhibitors of geranylgeranyl transferase I
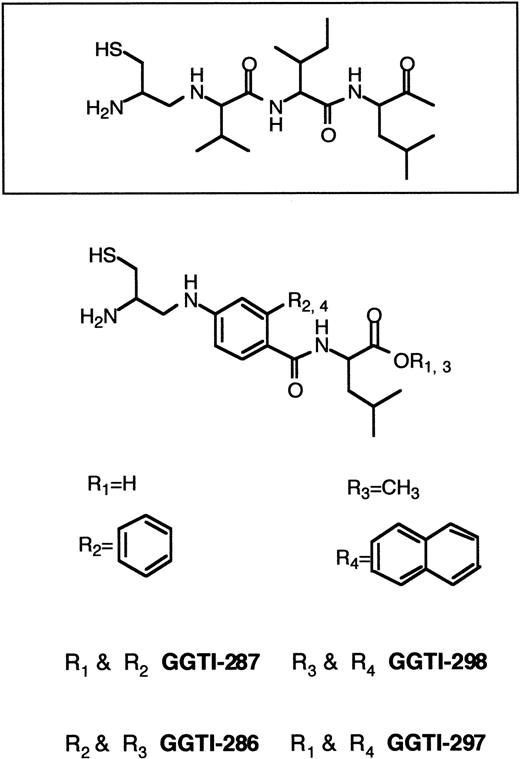
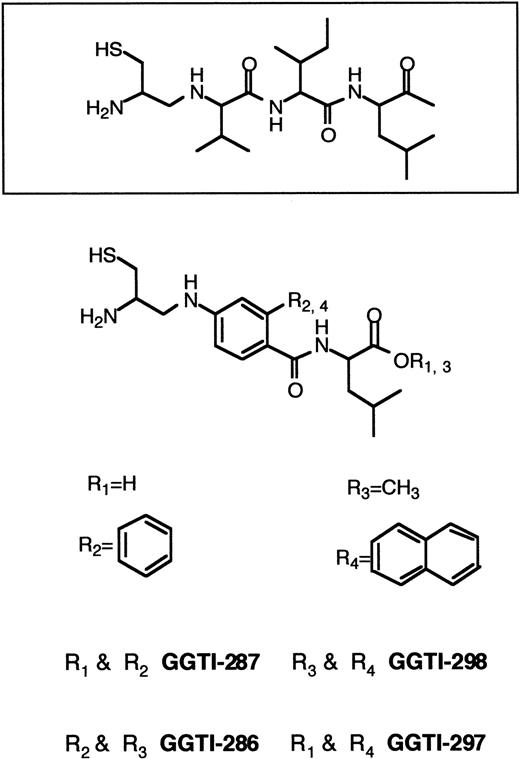
Until recently, the emphasis has been on designing specific FTase inhibitors to block Ras processing. This strategy was employed to avoid possible toxic effects originating from inhibition of GGTase I. Because K-RAS mutations are most common in human cancers,60,61 a critical goal is the development of inhibitors that block the growth of human tumors that harbor K-Ras. The resistance of K-Ras to FTase inhibitors,167 the lack of potency of FTase inhibitors against K-Ras–transformed cells,144 and the observation that K-Ras becomes geranylgeranylated in the presence of FTase inhibitors126,169-172 led to the development of GGTase I inhibitors (Figure 7). GGTI-279, GGTI-287, GGTI-297, and GGTI-298 are CAAL-based peptidomimetics that are selective for GGTase I over FTase.173,195-199 In contrast, FTI-276 and FTI-277 are CAAM-based peptidomimetics that are potent and selective inhibitors of FTase over GGTase I.173H-Ras processing in human tumor cell lines was highly sensitive to FTI-277 and resistant to GGTI-286, whereas K-Ras4B processing was more sensitive to GGTI-286 than FTI-277.173 Processing of H-Ras and N-Ras was inhibited by FTI-277, but inhibition of K-Ras processing required both FTase and GGTase I inhibitors. Whereas FTI-277 preferentially blocks activation of MAPK by oncogenic H-Ras, GGTase inhibitors selectively inhibit the activation of MAPK by oncogenic K-Ras4B.173 Although GGTI-298 had very little effect on soft agar growth of several human tumor cell lines harboring H-RAS, N-RAS, or K-RAS mutations, the combination of FTI-277 and GGTI-298 resulted in significant soft agar growth inhibition.172 Both FTase inhibitors and GGTase inhibitors have been reported to arrest Ras-transformed cells in G0/G1 phase of the cell cycle and to induce apoptosis.142,180,196,198,199 In nude mouse xenografts, the GGTase inhibitor GGTI-297 suppressed human lung A-549 and Calu-1 carcinoma tumor growth by 60%. However, both FTase and GGTase inhibitors were required to inhibit K-Ras processing.168Treatment of cells with GGTI-298 blocks PDGF- and EGF-dependent tyrosine phosphorylation of their respective receptors and induces G0/G1-phase arrest and apoptosis.196-198 GGTI-298 has also been shown to induce the cyclin-dependent kinase inhibitor p21WAF but not p27KIP.199
CAAL-based inhibitors of GGTase I.
GGTase I catalyzes the geranylgeranylation of proteins terminating with CAAX sequences where X is restricted to leucine, isoleucine or, to a lesser extent, phenylalanine. In cells, geranylgeranylation of proteins is far more common than farnesylation. Proteins modified by GGTase I include Rap1A, Rap1B, Rac1, Rac2, G25K, and RhoA.
CAAL-based inhibitors of GGTase I.
GGTase I catalyzes the geranylgeranylation of proteins terminating with CAAX sequences where X is restricted to leucine, isoleucine or, to a lesser extent, phenylalanine. In cells, geranylgeranylation of proteins is far more common than farnesylation. Proteins modified by GGTase I include Rap1A, Rap1B, Rac1, Rac2, G25K, and RhoA.
Inhibitors of the prenylated protein methyltransferase
The C-terminal prenylated protein methyltransferase (PPMTase) is another potential therapeutically relevant target in the development of inhibitors against the posttranslational processing of Ras. N-acetyl-trans,trans-farnesyl-l-cysteine (AFC) is a substrate for PPMTase and acts as a competitive inhibitor.201Although AFC has been shown to inhibit Ras methylation in Ras-transformed NIH3T3 fibroblasts, it does not inhibit the growth of these cells.201 New farnesyl derivatives of rigid carboxylic acid, eg, S-trans,trans-farnesylthiosalicylic acid (FTS), were found to inhibit the growth of H-Ras–transformed cells and to reverse their transformed morphology by a mechanism unrelated to the inhibition of Ras methylation by PPMTase202,203 (Figure 5). It is thought that FTS specifically interacts with Ras farnesylcysteine binding domains and affects membrane anchorage of Ras.202,203 In addition, it has been reported that FTS dislodges Ras from H-Ras–transformed cell membranes and renders the Ras protein susceptible to proteolytic degradation.188 At the same concentration, growth and morphology of non–Ras-transformed or nontransformed cells were not affected by FTS.203 Despite the lack of FTS-induced cytotoxicity in nontransformed cells, FTS reduced Ras levels in cell membranes and inhibited Ras-dependent cell growth.203 In contrast to FTase inhibitors (eg, BZA-5B), FTS also inhibited the growth signaling of receptor tyrosine kinases.203 FTS was shown to decrease total cellular Ras levels, MAPK activity, Raf-1 activity, and DNA synthesis in Ras-transformed EJ-1 cells. This inhibition was also demonstrated in serum-, EGF-, and thrombin-stimulated, untransformed Rat-1 cells.204,205 S-farnesyl-thioacetic acid (FTA), another competitive inhibitor of PPMTase, has been shown to suppress growth and induce apoptosis in HL-60 cells.206 Five-chloro– and 4- or 5-fluoro–derivatives of FTS and a C20 S-geranylgeranyl derivative of thiosalicyclic acid also cause inhibition of Ras-dependent MAPK activity, DNA synthesis, and EJ-1 cell growth. However, several other derivatives were inactive, suggesting stringent structural requirements for the anti-Ras activity of S-prenyl analogues.207Recently, FTS was shown (1) to reduce the amount of activated N-Ras and wild-type Ras isoforms in human melanoma cells and Rat-1 fibroblasts, (2) to disrupt ERK signaling, (3) to revert their transformed phenotype, and (4) to cause a significant reduction in the growth of human melanoma in SCID mice.188 205
The dorrigocins are novel antifungal antibiotics that were found to reverse the morphology of Ras-transformed NIH3T3 fibroblasts. Dorrigocin A did not inhibit protein prenylation or protein synthesis but was instead found to inhibit the C-terminal methylation in K-Ras–transformed cells.208
Selective inhibitors of Ras C-terminal sequence-specific endoprotease
UM96001, TPCK, and BFCCMK are Ras C-terminal sequence-specific endoprotease inhibitors (REPI) and potently inhibit ras-transformed rat kidney cell growth as well as growth of human cancer cells.209 These compounds have been reported to almost completely block the anchorage-independent clonogenic growth of these cancer cells. REPIs may selectively induce apoptosis in these cells.209
Selective inhibitors of MAPKKs, or MEK
PD098059 is a synthetic inhibitor of the Ras-MAPK pathway that selectively blocks the activation of MEK-1 and, to a lesser extent, the activation of MEK-2.210,211 The inhibition of MEK-1 activation was demonstrated to prevent activation of MAPKs ERK-1/2 and subsequent phosphorylation of MAPK substrates both in vitro and in intact cells. In contrast to FTase inhibitors, PD098059 inhibited stimulation of cell growth by several growth factors.210,211 Furthermore, PD098059 reversed the transformed phenotype of Ras-transformed BALB3T3 mouse fibroblasts and rat kidney cells.211 PD098059 failed to inhibit the stress, and IL-1 stimulated JNK/SAPK and the p38 pathways,210 demonstrating its specificity for the ERK pathway. PD098059 has subsequently been used as a tool to study MAPK signaling in various cell types and in carcinogenesis.
Recently, 2 novel inhibitors of MEK-1 and MEK-2 have been identified: U0126212,213 and Ro 09-2210.214 Ro 09-2210, which was identified by screening microbial broths, exhibits potent antiproliferative effects on activated T cells.214Similarly, U0126 was found to inhibit T-cell proliferation in response to both antigenic stimulation and cross-linked anti-CD3 plus anti-CD28 antibodies.212 U0126 and PD098059 are noncompetitive inhibitors with respect to both MEK substrates (ATP and ERK) and bind to free MEK as well as MEK*ERK and MEK*ATP complexes. U0126 displays significantly higher affinity for all forms of MEK (44- to 357-fold) than does PD098059. U0126 and Ro 09-2210 have an inhibitory concentration of 50% (IC50) of 50 to 70 nmol/L, whereas PD098059 has an IC50 of 5 μmol/L.212-214PD098059 and U0126 impede the growth of Ras-transformed cells in soft agar but show minimal effects on cell growth under normal culture conditions.210,213 In contrast to U0126 and PD098059, Ro 09-2210 is also able to inhibit other dual-specificity kinases such as MKK-4, MKK-6, and MKK-7, albeit at 4- to 10-fold higher IC50 concentrations compared with its effect on MEK-1.214
Inhibitors of Ras transformation with unknown mechanisms of action
Screening tests for drugs that revert RAS-transformed cells to a normal phenotype led to the identification of a number of compounds, such as azatyrosine, oxanosine, and antipain.215-217 The mechanism by which these compounds revert the RAS-induced phenotype is not understood. The pyrazolo-quinoline compound SCH51344 was identified based on its ability to depress human smooth muscle α-actin promoter activity inRAS-transformed cells. Treatment of v-abl-,v-mos-, v-raf-, RAS-, and mutant active MEK-transformed NIH3T3 cells resulted in growth inhibition of these cells in soft agar.218 SCH51344 had very little effect on the activities of proteins in the ERK pathway. The ability of SCH51344 to inhibit the anchorage-independent growth of RAC-V12–transformed Rat-1 cells suggests that the point of inhibition is downstream from RAC.219
The nonsteriodal, anti-inflammatory drug sulindac has been demonstrated to attenuate the growth and progression of colonic neoplasms in animal models and in patients with familial adenomatous polyposis.220,221 Recently, it has been shown that sulindac sulfide (the active metabolite of sulindac) inhibits Ras signaling and transformation by noncovalent binding to the Ras protein. Furthermore, it has been demonstrated that sulindac sulfide impairs Ras-Raf binding, Raf activation, and nucleotide exchange on Ras and that it accelerates the Ras-GTPase reaction.222 Sulindac is being investigated in a randomized study for the prevention of colon cancer (protocol RUH-SSH-190-0698, NCI-V98-1425).
Disruption of the Ras-to-MAPK signaling pathway has also been shown for the benzoquinone ansamycin geldanamycin. Geldanamycin binds to HSP90 and disrupts the HSP90–Raf-1 multimolecular complex, which causes destabilization of Raf-1 through enhanced degradation of Raf-1.223 However, the geldanamycin-HSP90 complex also causes depletion of other HSP90 substrates such as protein kinases and nuclear hormone receptors (including mutant p53 and ErbB2).224 Several National Cancer Institute–sponsored clinical phase I trials are currently studying the effects of geldanamycin analogues in patients with advanced malignancies.
Conclusions and future directions
FTase and GGTase inhibitors have strong growth inhibitory and antitumor activity in cell culture and animal tumor models without showing nonspecific gross toxicity in animals. The specificity and the lack of nonspecific toxicity contrasts dramatically with the nonspecificity and high toxicity of currently available chemotherapeutic drugs. The recent development of orally bioavailable FTase inhibitors with potent and selective in vivo antitumor activity underscores their potential usefulness in the future treatment of human malignancies. The observation that FTase and GGTase inhibitors induce apoptosis in treated tumor cells as well as a G0-G1 arrest suggests that they are not merely cytostatic but cytotoxic for tumor cells. However, the absence of toxicity due to FTase inhibitors in normal cells and tissues in mice at doses that inhibit tumor growth is poorly understood. Ras knockout experiments have demonstrated that H-RAS– and N-RAS–deficient mice are born and grow normally, whereas K-RAS–deficient embryos die between embryonic day 12.5 and term. This finding suggests a partial functional overlap within the RAS gene family.225-228However, H-RAS and N-RAS cannot compensate for the loss of K-RAS function in K-RAS–deficient mice. Functionally redundant pathways might allow normal cells to tolerate treatment with FTase inhibitors.
Because mutated RAS genes have a high prevalence in human cancers (eg, pancreatic, lung, and colon cancers), inhibitors specific for FTase, GGTase, and MEK were initially designed to block the Ras-to-MAPK signaling in solid tumor cells. More than 90% ofRAS mutations found in human tumors occur in N-RAS or K-RAS. Whereas the reversion of the H-RAS–induced transformation by FTase inhibitors correlates well with the intracellular inhibition of H-Ras processing, N-Ras and K-Ras are cross-prenylated by GGTase I in cells treated with FTase inhibitors. However, many of these N-RAS– or K-RAS–transformed cell lines (and even tumor cell lines that do not harbor RAS mutations) are sensitive to FTase inhibitors. Cell biology studies suggest that FTase and GGTase inhibitors may act at additional levels beyond the inhibition of Ras processing. The exact mechanism of action has emerged as a question of major interest, especially because transformed tumor cells respond to treatment with these inhibitors while normal cells remain largely unaffected. Non-Ras targets of FTase and GGTase inhibitors may include other cellular proteins (eg, Rho) that are farnesylated or geranylgeranylated.174,175,178 229-231
FTase inhibitors (eg, R115777, L-778,123, and SCH66336) have entered several phase I/II clinical trials (Table4). These trials are still ongoing, and preliminary results have not been published. Because favorable synergistic effects have been described for combinations of FTase inhibitors with traditional anticancer treatments such as radiation and chemotherapy,143 184 it will be interesting to see if these results translate into improved patient outcome in clinical trials. The high prevalence of mutationally activated Ras in solid tumors has been the driving force of Ras inhibitor research. However, recent studies in cell culture and animal models suggest that transformed cells with an activated Ras pathway (eg, via mutations upstream of Ras) are also highly sensitive for FTase inhibitors. The involvement of N-RAS in the molecular pathophysiology of myeloid leukemias and multiple myeloma suggests that these malignancies may also represent promising targets for inhibitors of Ras signaling. While it is impossible to predict the outcome of the clinical trials, the biologic properties of these inhibitors are potentially informative because transformation-specific mechanisms are targeted.
Acknowledgment
We thank Dr Kristine A. Henningfeld for help with the figures and for critical reading of the manuscript.
Supported by a grant to C.W.M.R. from the German Research Council (Deutsche Forschungsgemeinschaft Re 864/4-1) and a grant from the University of Ulm (P.541).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
C. Reuter, Dept of Internal Medicine III, University of Ulm, Robert-Koch-Str 8, D-89081 Ulm, Germany; e-mail: christoph.reuter@medizin.uni-ulm.de.