Abstract
Chemokines are proinflammatory cytokines that play a role in leukocyte migration and activation. Recent reports showed that RANTES (regulated on activation normal T-cell expressed and secreted chemokine), eotaxin, macrophage-derived chemokine (MDC), and stromal cell–derived factor-1 (SDF-1) are NH2-terminally truncated by the lymphocyte surface glycoprotein and protease CD26/dipeptidyl peptidase IV (CD26/DPP IV). Removal of the NH2-terminal dipeptide resulted in impaired inflammatory properties of RANTES, eotaxin, MDC, and SDF-1. The potential CD26/DPP IV substrate macrophage inflammatory protein–1β (MIP-1β) and the related chemokine, LD78α (ie, one of the MIP-1α isoforms), were not affected by this protease. However, CD26/DPP IV cleaved LD78β, a most potent CCR5 binding chemokine and inhibitor of macrophage tropic human immunodeficiency virus–1 (HIV-1) infection, into LD78β(3-70). Naturally truncated LD78β(3-70), but not truncated MIP-1β, was recovered as an abundant chemokine form from peripheral blood mononuclear cells. In contrast to all other chemokines processed by CD26/DPP IV, LD78β(3-70) had increased chemotactic activity in comparison to intact LD78β. With a minimal effective concentration of 30 pmol/L, LD78β(3-70) became the most efficient monocyte chemoattractant. LD78β(3-70) retained its high capacity to induce an intracellular calcium increase in CCR5-transfected cells. Moreover, on CCR1 transfectants, truncated LD78β(3-70) was 30-fold more potent than intact LD78β. Thus, CD26/DPP IV can exert not only a negative but also a positive feedback during inflammation by increasing the specific activity of LD78β. CD26/DPP IV–cleaved LD78β(3-70) is the most potent CCR1 and CCR5 agonist that retains strong anti–HIV-1 activity, indicating the importance of the chemokine-protease interaction in normal and pathologic conditions.
Introduction
Leukocyte migration is regulated by a complex network of molecules including proteases, chemotactic cytokines or chemokines, and adhesion molecules such as selectins and integrins.1 Most chemokines are classified in 2 subfamilies, CXC and CC, depending on whether or not an extra unspecified amino acid separates the NH2-terminal cysteines.2-4 The target cell specificity of chemokines depends on the cellular expression of the different CC or CXC chemokine receptors (CCRs or CXCRs, respectively).5 The murine CC chemokine macrophage inflammatory protein–1α (MIP-1α) was originally isolated from macrophages as an inflammatory mediator and inhibitor of stem cell proliferation.6,7 Human MIP-1α is encoded by 2 highly related nonallelic genes,LD78α and LD78β.8-10 The LD78α and LD78β proteins activate and chemoattract mononuclear cells by interaction with and signaling through CCR1 and CCR5. Although LD78α and LD78β only differ in 3 of 70 amino acids (including a substitution of serine for proline at position 2), recent studies have proven that both molecules interact differently with CCR5, resulting in a distinguished activation of leukocyte subsets.11 12 CCR5 has been shown to be the main coreceptor for macrophage (or R5) tropic human immunodeficiency virus–1 (HIV-1) strains. LD78β, the chemokine with the highest affinity for CCR5, is the most potent chemokine inhibiting HIV-1 infection.
The membrane-associated serine protease dipeptidyl peptidase IV (DPP IV), which is identical to the lymphocyte surface glycoprotein CD26, cleaves dipeptides from the NH2-terminus of proteins with a proline or alanine residue in the penultimate position.13 CD26/DPP IV is highly expressed on fibroblasts, epithelial and endothelial cells, and specific leukocyte subsets. The extracellular protease domain of CD26/DPP IV, which retains full protease activity, also exists in a soluble form in plasma and in cerebrospinal and seminal fluids. In addition to neuropeptides, growth factors, and hormones, recently a number of chemokines, but not cytokines, have been identified as CD26/DPP IV substrates. The limited NH2-terminal truncation of these chemokines by this serine protease results in drastic alterations in receptor specificities and subsequently in reduced inflammatory and modified antiviral potencies.
CD26/DPP IV has been shown to process the CC chemokines RANTES, macrophage-derived chemokine (MDC), and eotaxin.14-17 Although monocyte chemotactic protein–2 (MCP-2) contains a potential cleavage site for CD26/DPP IV, this chemokine is protected from proteolytic processing by an NH2-terminal pyroglutamic acid.18 In contrast, RANTES, MDC, and eotaxin are efficiently processed by CD26/DPP IV, resulting in partial loss of their receptor binding capacity and in impaired inflammatory properties. Nevertheless, the antiviral activities of truncated MDC and eotaxin remain essentially the same as those of the intact molecules.16,19 After removal of the NH2-terminal dipeptide by CD26/DPP IV, RANTES, which interacts with CCR1, CCR3, and CCR5, becomes specific for CCR5.15,17 Truncated RANTES has improved anti–HIV-1 activity and remains chemotactic for lymphocytes, but it lacks monocyte and eosinophil chemotactic activities.14,15 20
Because LD78β and MIP-1β, but not LD78α, contain a penultimate proline, they are both potential substrates for CD26/DPP IV. Although the complementary DNA (cDNA) sequence of LD78β has been known for more than a decade,8-10 the protein became available only recently in natural or recombinant forms.11,12 To evaluate the effect of CD26/DPP IV on LD78β, the intact chemokine was chemically synthesized and folded into biologically active LD78β. Because MIP-1β and LD78β preferentially bind CCR5,11which is coexpressed with CD26/DPP IV on activated T lymphocytes,21 we investigated the biochemical and biological effects of this protease on these CCR5 ligands. Against all expectations, MIP-1β was not truncated by CD26/DPP IV, whereas cleavage of LD78β resulted in improved rather than impaired monocyte chemotactic activity and hence enhanced inflammatory properties by increased affinity for CCR1. This is the first example of chemokine processing by CD26/DPP IV resulting in a proinflammatory response by generating a most active CCR1 and CCR5 agonist.
Materials and methods
Cell lines, chemokines, and immunoassays
Murine lymphocytic ESb/MP cells (kindly provided by Dr J.M. Wang, National Cancer Institute, Frederick, MD) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Biowhittaker Europe, Verviers, Belgium) supplemented with 10% fetal calf serum (FCS) (Life Technologies, Paisley, Scotland) and β-mercaptoethanol. Human THP-1 cells were grown in Roswell Park Memorial Institute medium (RPMI 1640) (Biowhittaker Europe) with 10% FCS. Human osteosarcoma (HOS) cells transfected with CD4 and either CCR1 or CCR522 were grown in DMEM supplemented with 10% FCS and 1 μg/mL puromycin (Sigma Chemical, St Louis, MO). Human peripheral blood mononuclear cells (PBMCs) were purified from fresh human buffy coats (from 132 blood donations, Blood Transfusion Centers of Leuven and Antwerp, Belgium) by density gradient centrifugation on a Ficoll/sodium diatrizoate solution (Lymphoprep; Life Technologies).17 Enriched monocyte cultures were obtained after adhesion of PBMCs to plastic for 2 hours. Lymphocytes were isolated from the PBMC fraction by magnetic cell sorting. Briefly, lymphocytes were labeled with paramagnetic microbeads coated with anti-CD3 monoclonal antibodies (mAbs) and passed over a column in a magnetic field (VarioMacs; Miltenyi Biotec, Auburn, CA). The obtained lymphocyte population was more than 80% pure, as determined on a fluorescence-activated cell sorter (FACS) (FACScan; Becton Dickinson, San Jose, CA).
Recombinant RANTES and LD78α (MIP-1α) (PeproTech; Rocky Hill, NJ) and recombinant MCP-1 (gift of Dr J. J. Oppenheim) were used. MCP-3 was prepared by solid-phase synthesis, and recombinant MCP-2 was produced in Escherichia coli as described.17,18 Natural chemokines were purified from conditioned medium from PBMCs or purified monocytes. Briefly, PBMCs or enriched monocyte monolayers were stimulated with 2 μg/mL lipopolysaccharide and 2 μg/mL concanavalin A, and the supernatant was harvested after 48 hours. Chemokines were purified to homogeneity through a 4-step purification schedule as previously described.11 17 After adsorption to silicic acid, the chemokines were bound to a heparin-Sepharose column (Amersham Pharmacia Biotech, Uppsala, Sweden) and eluted in a 0.05-2 mol/L sodium chloride (NaCl) gradient (pH 7.4).
Fractions from the heparin-Sepharose column that contained chemotactic activity or chemokine immunoreactivity were dialyzed against 50 mmol/L formate buffer (pH 4.0), loaded on a fast protein liquid chromatography (FPLC) Mono S cation exchange column (Amersham Pharmacia Biotech), and eluted with a 0-1 mol/L NaCl gradient. The final purification step consisted in C8 reversed phase high-pressure liquid chromatography (RP-HPLC) on a Brownlee Aquapore RP-300 column (2.1 by 220 mm) (Perkin Elmer, Norwalk, CT). The proteins were eluted from the column in an acetonitrile gradient (0%-80% acetonitrile in 0.1% trifluoroacetic acid). The purity was verified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and the NH2-terminal sequences were determined by automated Edman degradation on a pulsed liquid phase 477A/120A protein sequencer (PE Biosystems, Foster City, CA).14 Chemokine concentrations were measured with specific immunoassays, such as enzyme-linked immunoabsorbent assay (ELISA), for interleukin-8 (IL-8), MCP-1, MCP-2, and MCP-3.23 The MIP-1α ELISA (Biosource Europe, Nivelles, Belgium) detected both intact and truncated LD78α and LD78β equally well.
Chemical synthesis and folding of intact LD78β
LD78β was synthesized on a 0.1-mmol scale using amino acids with 9-fluorenylmethyloxycarbonyl (Fmoc)–protected α-amino groups on a model 433A solid-phase peptide synthesizer using the standard FastMoc programs (PE Biosystems). The following side chain protecting groups (Advanced ChemTech, Louisville, KY) were used: tert-butyl for serine, threonine, and tyrosine; tert-butyl ester for aspartic and glutamic acids; trityl for asparagine, cysteine, and glutamine; t-butyloxycarbonyl for lysine; and 2,2,5,7,8,-pentamethylchroman-6-sulfonyl for arginine. The COOH-terminal Fmoc-protected alanine was linked to a p-hydroxymethyl-phenoxymethyl-polystyrene (HMP) resin (PE Biosystems) by a symmetrical anhydride binding. The Fmoc group was cleaved from the peptide resin, and subsequent HBTU/HOBt-activated Fmoc-protected amino acids were attached. After each coupling step, incomplete peptide chains were capped with acetic anhydride. The final deprotection and cleavage of the peptide from the resin was performed by incubating the synthesis product for 100 minutes at room temperature in the following cleavage mixture: 10 mL trifluoroacetic acid, 0.5 mL water, 0.5 mL thioanisole, 0.25 mL ethanedithiol, and 0.75 g crystalline phenol. The synthetic chemokine was separated from the resin on a medium-porosity glass filter, precipitated into cold methyl t-butyl ether, washed, dissolved in water, and subsequently lyophilized.
Crude synthetic LD78β was separated from incomplete fragments by RP-HPLC on a Resource RPC column (Amersham Pharmacia Biotech). Intact LD78β was folded into the biologically active protein by incubating the chemokine at room temperature for 1.5 hour in 150 mmol/L Tris (tris[hydroxymethyl] aminomethane) (pH 8.6), 2 mol/L ureum, 3 mmol/L EDTA (ethylenediamine tetraacetic acid), 0.3 mmol/L oxidized glutathion, and 3 mmol/L reduced glutathion. The folded chemokine was repurified by C8 RP-HPLC on an Aquapore C8 RP-300 column (PE Biosystems). The purity and identity of the unfolded and folded LD78β were confirmed by Edman degradation and mass spectrometry on an ESQUIRE ion trap mass spectrometer (Bruker Daltonik, Bremen, Germany).
Cleavage of LD78β by CD26/DPP IV
Natural soluble CD26/DPP IV (specific activity, 22 U/mg) was purified to homogeneity from total seminal plasma by anion exchange chromatography and affinity chromatography on immobilized adenosine deaminase.24 Synthetic or natural LD78β or natural MIP-1β were incubated with soluble CD26/DPP IV (0.2 U/10 μg chemokine) in 100 mmol/L Tris (pH 8.5) at 37°C. After CD26/DPP IV processing, the chemokines were either blotted on polyvinylidene difluoride (PVDF) membranes (Prosorb, PE Biosystems) for identification by automated Edman degradation or were purified by C8 RP-HPLC on an Aquapore RP-300 column for biological testing. The relative amounts of the different NH2-terminal forms were calculated from the initial yields from the protein sequencer. Control incubations without enzymes did not influence the integrity or the biological activity of the chemokine.
Chemotaxis and calcium release assays
The chemokines were tested in the Boyden microchamber for their monocyte chemotactic potencies on human THP-1 cells (0.5 × 106 cells per mL for 2 hours at 37°C) and PBMCs (2 × 106 cells per mL for 2 hours at 37°C). The lymphocyte chemotactic activity was evaluated on murine ESb/MP cells (2 × 106 cells per mL for 2 hours at 37°C) and on fresh human lymphocytes purified from PBMCs (107 cells/mL for 4 hours at 37°C). The cells that migrated through the 5-μm pore size polycarbonate membranes (coated with fibronectin for PBMC-derived lymphocytes) were fixed, stained, and counted microscopically in 10 oil immersion fields. The chemotactic index (the mean of triplicates in each chamber) was calculated as the number of cells that migrated to the test sample divided by the number of cells that migrated to the dilution medium.
Alterations in the intracellular calcium concentration ([Ca++]i) were monitored by fluorescence spectrometry. Briefly, PBMCs or HOS cells transfected with either CCR1 or CCR5 were loaded with the fluorescent dye fura-2. Upon excitation at 340 and 380 nm, fura-2 fluorescence was measured at 510 nm in an LS50B luminescence spectrophotometer (PerkinElmer). The [Ca++]i was calculated from the Grynkiewicz equation with a Kd of 224 nmol/L.17
Results
Isolation of natural human MIP-1α isoforms and synthesis of intact LD78β
Purified monocyte-conditioned medium (from 132 blood donations) was screened for its chemokine content by measuring MCP-1, MCP-2, MCP-3, IL-8, and MIP-1α immunoreactivity. In total, approximately 1200 μg IL-8, 20 μg MCP-1, 0.9 μg MCP-2, 0.3 μg MCP-3, and 1000 μg MIP-1α immunoreactivity were recovered after concentration and purification by adsorption to silicic acid, heparin affinity, cation exchange chromatography, and C8 RP-HPLC (see “Materials and methods”). Approximately 50% of the chemokine immunoreactivity that was detected in the crude conditioned medium was recovered in the final RP-HPLC fractionation. The isoforms of natural MIP-1α could only be partially separated from each other. In most of the RP-HPLC fractions, more than one LD78 isoform was detected upon extended amino acid sequence analysis (beyond amino acid 39) and/or mass spectrometry. With the yields that were calculated after protein sequence analysis of the LD78 forms present in the RP-HPLC fractions containing MIP-1α immunoreactivity, an estimation of the relative amounts of the different natural LD78 isoforms was made. In total, about 50% of the proteins that were detected with the MIP-1α ELISA consisted of the LD78β protein.
The only difference between LD78α and LD78β is located at the penultimate NH2-terminal residue (serine or proline) and at positions 39 (glycine or serine) and 47 (serine or glycine). Approximately 20% of the sequenced LD78β protein (ie, 10% of the total MIP-1α immunoreactivity) had a truncated NH2-terminus missing the first 2 amino acids (alanine and proline). The second half of the MIP-1α immunoreactive proteins had an NH2-terminal sequence that corresponded to either LD78α or LD78β lacking the first 4 amino acids. Both truncated LD78α(5-70) and LD78β(5-70) were clearly present because both serine and glycine were detected at position 39 (position 35 in the truncated molecule). Moreover, approximately 100 μg natural MIP-1β was purified from the monocyte-derived conditioned medium. Sequence analysis of the HPLC fractions containing the MIP-1β protein peak always revealed a single NH2-terminal sequence corresponding to intact MIP-1β.
Because only limited amounts of the natural LD78β could be purified to homogeneity from stimulated leukocytes11 and because recombinant LD78β was not commercially available, LD78β was chemically synthesized by Fmoc solid-phase chemistry. After synthesis and deprotection (see “Materials and methods”), determination of the molecular mass of the RP-HPLC–purified synthetic protein by mass spectrometry revealed an average relative molecular mass (Mr) of 7797.8 ± 0.7, which corresponded to the theoretical average Mr of reduced LD78β (7797.7). Folded synthetic and natural LD78β had an averageMr of 7793.7 ± 0.5 and 7793.9 ± 0.8, respectively. NH2-terminal sequence analysis confirmed the intact primary structure of synthetic LD78β. Furthermore, the chemotactic potencies of natural and synthetic LD78β for monocytes and lymphocytes were comparable (data not shown).
CD26/DPP IV removes the NH2-terminal dipeptide from LD78β but not from MIP-1β
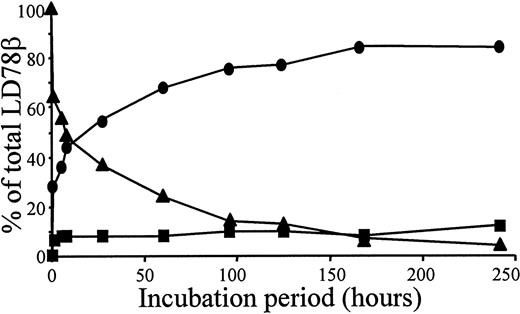
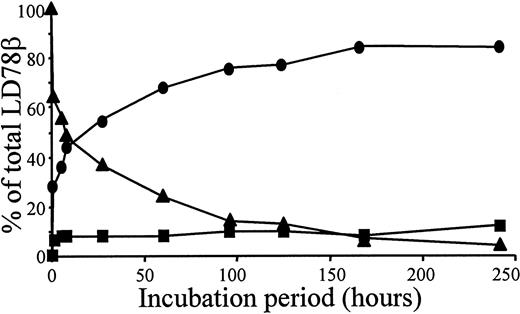
Overnight incubation of natural or synthetic LD78β with CD26/DPP IV at 37°C resulted in the removal of the NH2-terminal alanine-proline dipeptide yielding LD78β(3-70) (Table1). In contrast, even after a 48-hour incubation, this protease did not process natural MIP-1β. Recombinant intact LD78α, with a penultimate NH2-terminal serine, was not cleaved upon exposure to CD26/DPP IV for 24 hours. Upon exposure of 5 μg LD78β to 0.1 unit CD26/DPP IV, the half-life of the chemokine was 5 hours (Figure 1). Because CD26/DPP IV is also known to process peptides with a penultimate alanine, the enzyme was expected to cleave LD78β further. However, only 12% of intact synthetic LD78β(1-70) was converted into LD78β(5-70) after prolonged incubation with CD26/DPP IV for 10 days (Figure 1).
Kinetics of LD78β processing by CD26/DPP IV.
We incubated 5 μg intact synthetic LD78β at 37°C with 0.1 unit pure soluble CD26/DPP IV in 200 μL of 100 mmol/L Tris (pH 8.5); 20 μL samples were taken at the indicated time-points. The samples were blotted on a PVDF membrane, washed with 0.1% trifluoroacetic acid, and subjected to automated Edman degradation. The molar amounts of intact LD78β (▴), LD78β(3-70) (●), and LD-78β(5-70) (■) present in the samples were calculated from the initial yields of the protein sequencer and are expressed as the percent of the total amount of LD78β.
Kinetics of LD78β processing by CD26/DPP IV.
We incubated 5 μg intact synthetic LD78β at 37°C with 0.1 unit pure soluble CD26/DPP IV in 200 μL of 100 mmol/L Tris (pH 8.5); 20 μL samples were taken at the indicated time-points. The samples were blotted on a PVDF membrane, washed with 0.1% trifluoroacetic acid, and subjected to automated Edman degradation. The molar amounts of intact LD78β (▴), LD78β(3-70) (●), and LD-78β(5-70) (■) present in the samples were calculated from the initial yields of the protein sequencer and are expressed as the percent of the total amount of LD78β.
Enhanced monocyte chemotactic activity of LD78β(3-70)
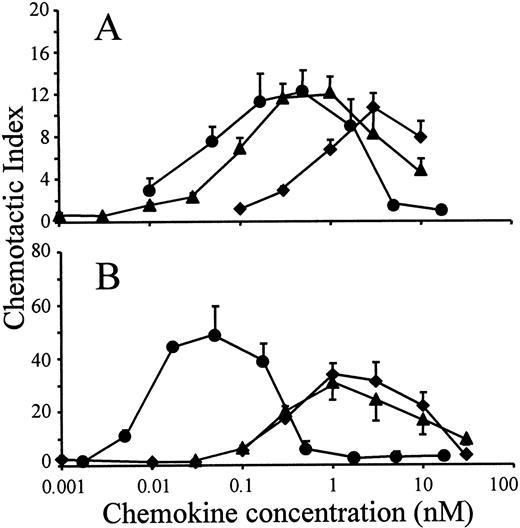
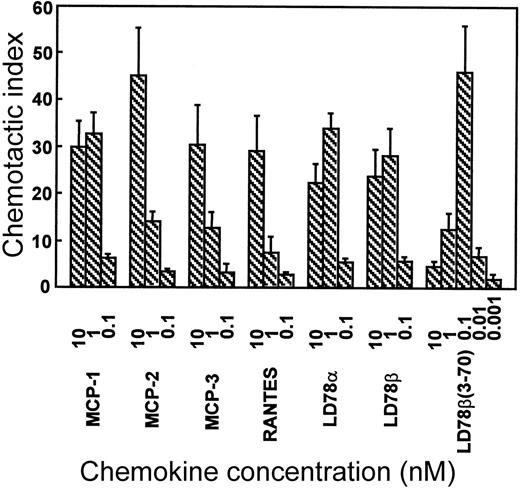
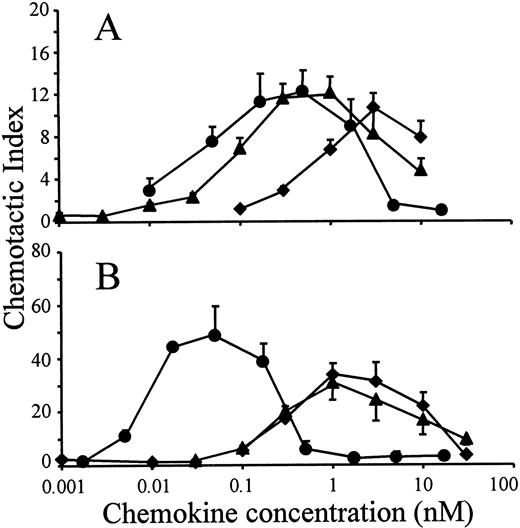
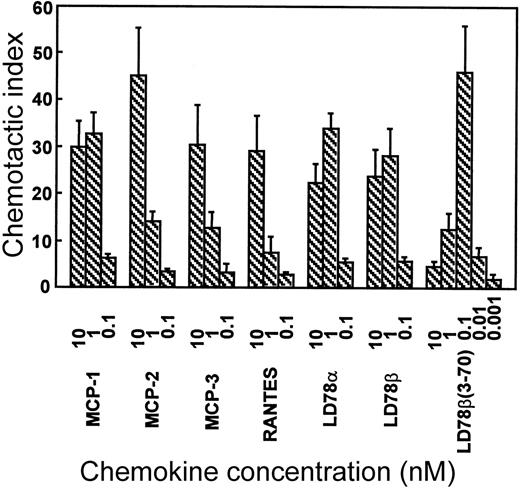
The in vitro chemotactic activities of intact LD78α, LD78β, and LD78β(3-70) truncated by CD26/DPP IV were compared on lymphocytes and monocytes. Typical bell-shaped dose-response curves were obtained for the different LD78 forms. The potent lymphocyte (on murine ESb/MP cells) chemotactic activity of LD78β (EC50 of 0.08 nmol/L) was preserved and even slightly augmented after cleavage with CD26/DPP IV (Figure 2A). As a consequence, with a minimal effective concentration of 0.01 nmol/L (EC50 of 0.04 nmol/L), LD78β(3-70) became 20-fold to 30-fold more potent than LD78α (minimal effective concentration of 0.3 nmol/L and EC50 of 0.7 nmol/L). The effect of CD26/DPP IV cleavage on the chemotactic activity of LD78β was much more pronounced on monocytic THP-1 cells (Figure 2B). The monocyte chemotactic activities of intact LD78α and LD78β were comparable (EC50 of 0.3 nmol/L), but removal of the NH2-terminal dipeptide from LD78β by CD26/DPP IV resulted in a 30-fold increase of activity, thereby yielding a minimal effective concentration of 0.005 nmol/L (EC50 of 0.009 nmol/L). Moreover, LD78β(3-70) was the most potent chemokine when the chemotactic activity of this CD26/DPP IV–processed molecule was compared to that of other monocyte chemotactic proteins including MCP-1, MCP-2, MCP-3, and RANTES (Figure3).
Chemotactic activity of LD78α, intact LD78β, and LD78β(3-70) on lymphocytic and monocytic cell lines.
LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were tested at different concentrations in the in vitro Boyden microchamber chemotaxis test on (A) murine lymphocytic ESb/MP cells and (B) monocytic THP-1 cells. The results are expressed as the mean chemotactic index plus or minus SEM of 5 or more independent experiments.
Chemotactic activity of LD78α, intact LD78β, and LD78β(3-70) on lymphocytic and monocytic cell lines.
LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were tested at different concentrations in the in vitro Boyden microchamber chemotaxis test on (A) murine lymphocytic ESb/MP cells and (B) monocytic THP-1 cells. The results are expressed as the mean chemotactic index plus or minus SEM of 5 or more independent experiments.
LD78β(3-70) is the most potent monocyte chemoattractant.
MCP-1, MCP-2, MCP-3, RANTES, LD78α, LD78β, and LD78β(3-70) were tested in parallel at different concentrations in the in vitro Boyden chamber chemotaxis test on monocytic THP-1 cells. The results are expressed as the mean chemotactic index plus or minus SEM of 3 or more independent experiments.
LD78β(3-70) is the most potent monocyte chemoattractant.
MCP-1, MCP-2, MCP-3, RANTES, LD78α, LD78β, and LD78β(3-70) were tested in parallel at different concentrations in the in vitro Boyden chamber chemotaxis test on monocytic THP-1 cells. The results are expressed as the mean chemotactic index plus or minus SEM of 3 or more independent experiments.
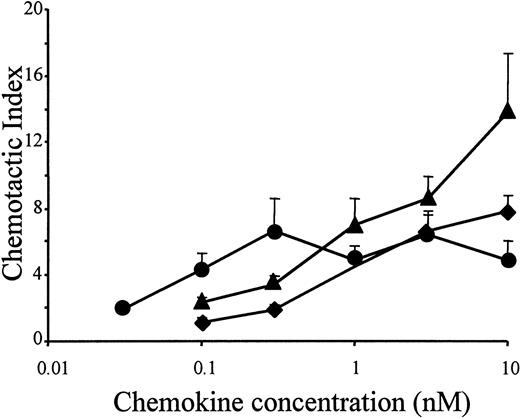
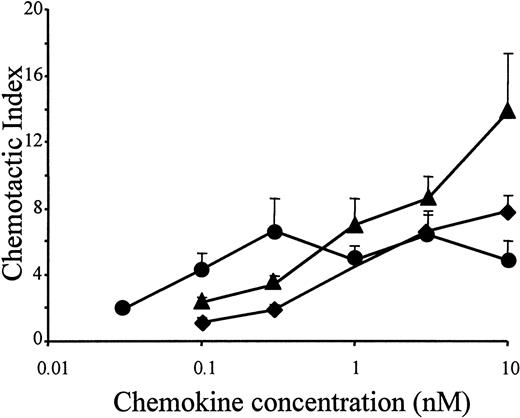
In accordance with the results of the murine lymphocytic ESb/MP cell line, the potent chemotactic activity of LD78β also moderately increased on freshly isolated peripheral blood–derived human lymphocytes, when the chemokine was truncated by CD26/DPP IV (Figure4). As an analogy, the drastic increase in chemotactic potency of LD78β by truncation with CD26/DPP IV on THP-1 cells was confirmed on normal human monocytes (Figure5A). Chemotactic activity for PBMCs was already observed with 0.03 nmol/L LD78β(3-70) (EC50 of 0.05 nmol/L), and the concentration for maximal in vitro chemotaxis was 0.3 nmol/L. In contrast, on blood monocytes the minimal effective concentration of intact LD78α and LD78β and the EC50 values (0.7 nmol/L and 0.3 nmol/L, respectively) were 10-fold higher than for LD78β(3-70).
Chemotactic activity of LD78α, intact LD78β, and LD78β(3-70) on human lymphocytes.
LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were tested at different concentrations in the in vitro Boyden microchamber chemotaxis test on freshly isolated peripheral blood–derived human lymphocytes. The results are expressed as the mean chemotactic index plus or minus SEM of 4 or more independent experiments.
Chemotactic activity of LD78α, intact LD78β, and LD78β(3-70) on human lymphocytes.
LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were tested at different concentrations in the in vitro Boyden microchamber chemotaxis test on freshly isolated peripheral blood–derived human lymphocytes. The results are expressed as the mean chemotactic index plus or minus SEM of 4 or more independent experiments.
Increased peripheral blood monocyte chemotaxis and receptor signaling by CD26/DPP IV–processed LD78β.
LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were tested (A) at different concentrations in the Boyden chamber chemotaxis test and (B) for their ability to increase the [Ca++]i on freshly isolated PBMCs. Chemotaxis results are expressed as the mean chemotactic index plus or minus SEM of 3 or more independent experiments. The detection limit for a significant increase in [Ca++]i is indicated by the dashed line. One representative experiment of 3 is shown in panel B.
Increased peripheral blood monocyte chemotaxis and receptor signaling by CD26/DPP IV–processed LD78β.
LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were tested (A) at different concentrations in the Boyden chamber chemotaxis test and (B) for their ability to increase the [Ca++]i on freshly isolated PBMCs. Chemotaxis results are expressed as the mean chemotactic index plus or minus SEM of 3 or more independent experiments. The detection limit for a significant increase in [Ca++]i is indicated by the dashed line. One representative experiment of 3 is shown in panel B.
Receptor signaling properties of LD78β(3-70)
The capacities of intact LD78β, LD78α, and CD26/DPP IV–truncated LD78β(3-70) to induce an intracellular calcium rise in PBMCs were compared. A significant increase of the [Ca++]i in PBMCs was obtained upon stimulation with at least 1 nmol/L LD78β or LD78α (Figure 5B). For LD78β(3-70), the concentration of 0.1 nmol/L for a half-maximal increase of [Ca++]i was 10-fold lower. To explain the increased chemotactic potency and signaling capacity of LD78β(3-70), intact and truncated LD78β were compared in the calcium assay using CCR1- and CCR5-transfected HOS cells. Compared to intact LD78α and LD78β, LD78β(3-70) was 10-fold to 30-fold more potent on CCR1-transfected cells. The minimal effective concentrations that induce an increase of the [Ca++]i were 0.1, 1, and 4 nmol/L for LD78β(3-70), intact LD78α, and LD78β, respectively (Figure 6A). Although intact LD78β was already 10 times more potent than LD78α on CCR5-transfected cells, CD26/DPP IV treatment moderately increased the signaling activity of LD78β, thereby resulting in a minimal effective concentration of 0.1 nmol/L (Figure 6B). As a consequence, LD78β(3-70) is the most potent agonist for both CCR1 and CCR5.
Enhanced CCR1 receptor signaling capacity of CD26/DPP IV–processed LD78β.
LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were compared for their ability to induce an increase in [Ca++]i in HOS cells transfected with (A) CCR1 or (B) CCR5. The results represent the mean increase plus or minus SEM in [Ca++]i in 3 or more independent experiments. The detection limit for a significant increase in [Ca++]i is indicated by the dashed lines.
Enhanced CCR1 receptor signaling capacity of CD26/DPP IV–processed LD78β.
LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were compared for their ability to induce an increase in [Ca++]i in HOS cells transfected with (A) CCR1 or (B) CCR5. The results represent the mean increase plus or minus SEM in [Ca++]i in 3 or more independent experiments. The detection limit for a significant increase in [Ca++]i is indicated by the dashed lines.
Discussion
The protease CD26/DPP IV posttranslationally cleaves proteins with a proline or alanine at the second position. CD26/DPP IV is expressed on fibroblasts, endothelial and epithelial cells, and lymphocytes, but it also occurs in a soluble form in plasma.13 Due to the presence of a proline at the penultimate NH2-terminal position, the chemokines LD78β and MIP-1β are also potential substrates for CD26/DPP IV. However, upon treatment of the intact chemokines with pure soluble CD26/DPP IV, we have now found that LD78β, but not MIP-1β, was efficiently processed. This shows that in addition to the penultimate proline, the surrounding residues and the accessibility of the NH2-terminus are important for enzyme-substrate recognition. In this respect, MIP-1β is a second exception because MCP-2 has previously been reported to be resistant to CD26/DPP IV due to its NH2-terminal pyroglutamate.18 The observed cleavage of LD78β, but not MIP-1β, by CD26/DPP IV is consistent with our finding that naturally truncated MIP-1β could not be purified (data not shown) and that significant amounts of LD78β(3-70) were detected in monocyte-derived conditioned medium.
Outside the chemokine family, a number of natural peptides, including some with a penultimate alanine (eg, glucagon-like peptide-1), are described as good CD26/DPP IV substrates both in vitro and in vivo.13 In an attempt to stabilize these peptides (some of them have therapeutical applicability) by increasing their resistance toward CD26/DPP IV–mediated truncation, a number of analogues were synthesized. In some cases, substitution of proline by glycine or serine at the second position did not completely protect the peptides against CD26/DPP IV.13 Such an unexpected NH2-terminal truncation was also observed for MDC. After proline but also after glycine, CD26/DPP IV cleaved MDC, resulting in the removal of 2 NH2-terminal dipeptides.16In contrast, under identical conditions, we did not observe truncation of RANTES(3-68)14 or LD78α (this study) that both contain a penultimate serine. Moreover, only a limited further truncation of LD78β(3-70) (with a penultimate alanine) could be detected after prolonged incubation with CD26/DPP IV (Figure 1). This also suggests that other aminopeptidases may be involved in the further degradation of this chemokine to LD78β(5-70).
Processing by CD26/DPP IV has differential effects on chemokine activity and receptor interaction. Although 2 NH2-terminal amino acids were removed by CD26/DPP IV from granulocyte chemotactic protein-2, no alterations of its inflammatory properties were detected.14 Due to its decreased CXCR4 affinity and loss of receptor signaling capacity, SDF-1(3-68) had impaired chemotactic and anti–HIV-1 activity after truncation by CD26/DPP IV.25,26 Truncated RANTES(3-68) had reduced monocyte and eosinophil chemotactic activity due to the loss of CCR1 and CCR3 recognition, but it became a potent antiviral chemokine with increased receptor affinity for CCR5.14,15,17 LD78β has recently been reported to be a potent CCR5 ligand and to be the most effective chemokine against M-tropic (or R5) HIV-1 strains when directly compared to RANTES and LD78α.11,12 Moreover, according to oral communication with Dominique Schols (February 2000), the potent antiviral activity of LD78β (inhibition of PBMC infection with the M-tropic HIV-1 BaL strain) still moderately increased (by a factor of 2-fold to 3-fold) when the chemokine was truncated by CD26/DPP IV. Furthermore, in contrast to all other reported chemokine substrates,13 in this study, cleavage of LD78β by CD26/DPP IV resulted in enhanced lymphocyte and monocyte chemotactic activity. In lymphocytes, the chemotactic activity of LD78β(3-70) was increased 2-fold to 3-fold, which might be explained by the moderately improved signaling through CCR5.
The latter finding has been indirectly confirmed by others using crude culture supernatant of a CD26+ cell line expressing recombinant LD78β that has retained its anti–HIV-1 activity.27 As shown here, the effect of CD26/DPP IV on LD78β was most pronounced on monocytes, ie, a 10-fold to 30-fold increase in chemotactic and calcium signaling activities that coincided with a similar increase in signaling through CCR1. So far, LD78β is the only chemokine reported to acquire stronger inflammatory properties upon processing by CD26/DPP IV. Hereby, LD78β(3-70) supersedes LD78α, previously the most potent reported CCR1 ligand,28 in inducing increases in [Ca++]i in CCR1-transfected cells. In vitro monocyte chemotaxis assays have shown that LD78β(3-70) is even 10-fold to 100-fold more potent than LD78α; RANTES19; and the monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3.29 This was confirmed in parallel monocyte chemotaxis tests (Figure 3) with the chemokines MCP-1, MCP-2, MCP-3, RANTES, LD78α, LD78β, and LD78β(3-70).
Our results show that the processing of LD78β by CD26/DPP IV into LD78β(3-70) results in enhanced chemotactic activity. Nibbs et al12 showed that LD78α and the more truncated LD78β(5-70) have a comparable binding affinity to CCR1 and that LD78β(5-70) has significantly reduced antiviral activity compared to intact LD78β. Moreover, compared to intact LD78β, LD78β(5-70) showed low-binding affinity and signaling properties through CCR5.12 These authors concluded that the penultimate proline is responsible for the enhanced signaling properties of LD78β through CCR5. Because the calcium signaling activity of LD78β(3-70) on CCR5-transfected cells is even enhanced in this study, probably the whole configuration of the NH2-terminus, not the penultimate proline, is important for CCR5 signaling.
Comparative receptor binding and calcium signaling experiments with murine MIP-1α, human LD78α, and human LD78β showed that LD78β is possibly the functional human homologue of murine MIP-1α.12 Studies with mice deficient in CCR1 or MIP-1α demonstrate that CCR1 and murine MIP-1α play a crucial role in in vivo models of inflammation.30-32 Although the cDNA sequence of LD78β has been known for more than a decade,8-10 initial reports on the biological activity of this chemokine have been published only very recently.11,12 This study shows that a minor NH2-terminal truncation of LD78β by the protease CD26/DPP IV significantly increases its inflammatory properties by rendering it the most potent agonist for both CCR1 and CCR5. It must be concluded that CD26/DPP IV plays an important role in the posttranslational regulation of chemokine activity by enhancing the potency of one chemokine but reducing that of most other chemokines. Such opposite changes in the qualitative properties of individual chemoattractants contribute to the robustness of the chemokine network.33
Acknowledgments
The authors thank René Conings and Jean-Pierre Lenaerts for technical assistance, and Dr Ghislain Opdenakker for critically reading the manuscript. The chemokine receptor–transfected HOS cells were obtained from Nathaniel Landau through the AIDS (autoimmunodeficiency syndrome) Research and Reference Program (Division of AIDS, National Institute of Allergy and Infectious Diseases, the National Institutes of Health, Bethesda, MD).
Supported by the Fund for Scientific Research of Belgium (FWO-Vlaanderen, where P.P., P.M., S.S., and I.D.M hold fellowships), Belgium; the Concerted Research Actions of the Regional Government of Flanders, Belgium; the InterUniversity Attraction Pole (IUAP) of the Federal Government, Belgium; and the Biotech program of the European Union.
Submitted December 20, 1999; accepted May 2, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul Proost, Laboratory of Molecular Immunology, Rega Institute for Medical Research, University of Leuven, Minderbroedersstraat 10, B-3000 Leuven, Belgium; e-mail:paul.proost@rega.kuleuven.ac.be.

![Fig. 5. Increased peripheral blood monocyte chemotaxis and receptor signaling by CD26/DPP IV–processed LD78β. / LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were tested (A) at different concentrations in the Boyden chamber chemotaxis test and (B) for their ability to increase the [Ca++]i on freshly isolated PBMCs. Chemotaxis results are expressed as the mean chemotactic index plus or minus SEM of 3 or more independent experiments. The detection limit for a significant increase in [Ca++]i is indicated by the dashed line. One representative experiment of 3 is shown in panel B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/5/10.1182_blood.v96.5.1674/5/m_h81700069005.jpeg?Expires=1769084115&Signature=TdQJW5L6vZWVnbe4FirTvURJerkRrfpkQSQIupYpghBvc7e~zINLbpc2HTeLLRQKfoJZN0z2v1pIFR17UhbhxG8Hho10dkANpA1frb5yUHvA9mnZ-7tb69AZG7Hn8KoYe5xdXZx8Vsw3-SOzGQYrX1g9hiSrIIYaMdZusN18PnP0F6FGDm9glbnErlRjqtSoPo7Fg7lgZVpZwvTQ5zfU~J651yRqFnJR8cLZENEBEOsLpqsyPkDLDAxprjHAZ8Q7zpNJvaGE4m8EyPezZsNlg1sG87QL801yHV89lPGggZcAjpj7IfGqwysSwe6z6DkO07q6RaWOA0PicwCBbCAjKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Enhanced CCR1 receptor signaling capacity of CD26/DPP IV–processed LD78β. / LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were compared for their ability to induce an increase in [Ca++]i in HOS cells transfected with (A) CCR1 or (B) CCR5. The results represent the mean increase plus or minus SEM in [Ca++]i in 3 or more independent experiments. The detection limit for a significant increase in [Ca++]i is indicated by the dashed lines.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/5/10.1182_blood.v96.5.1674/5/m_h81700069006.jpeg?Expires=1769084115&Signature=tEb8aJR7scaV1LhO97f-8pRPm6Dx3LCJfFZDXMjp9rPWnuKmf4H3rYHii5lOcE88kAS2Ii0HGjbrDaC6r9lw0U~nwxVnsaZzwbvjhkwMo1fZJ2CTgvYI4G18pWntTaEIbzp-UOnc7XaJ77-tcbjq8vmX4nqw-x7-fDfQBeyMhOm0xY~QFfPl7qOq15a6GlfziBru9bLKYcnhOOczTQzqvh393hbpQYiNtVpFTS4K77uzOBK6DrfjpLIbNuD~wsg2jYq54sdIj~tDmkMSIy0NjiecFM1nAFJiwXQRH~FT3Tpv773w~DlryEu19O2buDwDerqNfuM2ydQsiKokQC--KA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Increased peripheral blood monocyte chemotaxis and receptor signaling by CD26/DPP IV–processed LD78β. / LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were tested (A) at different concentrations in the Boyden chamber chemotaxis test and (B) for their ability to increase the [Ca++]i on freshly isolated PBMCs. Chemotaxis results are expressed as the mean chemotactic index plus or minus SEM of 3 or more independent experiments. The detection limit for a significant increase in [Ca++]i is indicated by the dashed line. One representative experiment of 3 is shown in panel B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/5/10.1182_blood.v96.5.1674/5/m_h81700069005.jpeg?Expires=1769084116&Signature=S720lPatyC3n-qpmXscP1Z~uG8cGvUsu9Dy~8xuHGcCIlp9NdK9Yjy1GFWzvBw79TR-M1~UpiLpH-GfBSpj2r5tuK6nlsziXG0sHgl4ZF7HjVlyKls-pOe6I~c7SJEohF9FcFHpcOXd03oXqn-XrLbg4MsAJMk98YYSabYTkgoAw1vCZ9aMBqeFd8dBoaYE6s9Gvgg-FNE-p8er93Srn~-6hgMv9L08VZvzMCtQ3faZbE64J~XiB-KaudLikCuC6zVF8buLnWCaldO-q-C4d~U0iHFlWABG09TrZmZ8fhxyGYs1988x~UbobJd6Dr4PwlIktRL2yyq9WqTg4-ourIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Enhanced CCR1 receptor signaling capacity of CD26/DPP IV–processed LD78β. / LD78α (♦), intact LD78β (▴), and truncated LD78β(3-70) (●) were compared for their ability to induce an increase in [Ca++]i in HOS cells transfected with (A) CCR1 or (B) CCR5. The results represent the mean increase plus or minus SEM in [Ca++]i in 3 or more independent experiments. The detection limit for a significant increase in [Ca++]i is indicated by the dashed lines.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/5/10.1182_blood.v96.5.1674/5/m_h81700069006.jpeg?Expires=1769084116&Signature=sCe27~r9jwe4ZLN-P5Q3cdBEC5GbomOeobYgtGjyp8ajpBWu7pPvV8TgYM5zO9a6XH0RRVRtcGiqEbMGfo14lAJsUIE3WKs8qK5Ky1PNlfFcNySomkeHj3vOmW5W0GNuy-YH22hSKGdYJpbkRlGVp4qXITCf5SQh7dMZV1-IMNc1mXPGel5z7wvGLc6pODYl2KAiIHdlkPCo6ZDa13tKqTPPFPS06iHRrP0hANbZcb9GH95zKs4wycZYbE9WnrOakav~vYTfP5nFHMJE2EyfuIbW60RFWPm0rJ8ed~uePiG5Q-ItxhDxrS0ilsHzgM8JYQyCSAoVVCbwIMIP05jh2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)