Abstract
Hematopoiesis is a remarkable cell-renewal process that leads to the continuous generation of large numbers of multiple mature cell types, starting from a relatively small stem cell compartment. A highly complex but efficient regulatory network is necessary to tightly control this production and to maintain the hematopoietic tissue in homeostasis. During the last 3 decades, constantly growing numbers of molecules involved in this regulation have been identified. They include soluble cytokines and growth factors, cell–cell interaction molecules, and extracellular matrix components, which provide a multifunctional scaffolding specific for each tissue. The cloning of numerous growth factors and their mass production have led to their possible use for both fundamental research and clinical application.
Introduction
The regulation of hematopoiesis is a complex process that has received much attention (for reviews see Moore et al,1 1990; Metcalf,2 1993; Ogawa,3 1993). Research continues to identify the various components involved in this regulation (for review see Levesque et al,4 1991). Many of the growth factors can now be cloned for research and clinical purposes (for review see Simmons and Haylock,5 1995).
Type β transforming growth factors (TGF-βs) were discovered by De Larco and Todaro6 in 1978. Originally called “sarcoma growth factors,” they were first isolated from the supernatant fluids of Moloney MuSV-transformed mouse 3T3 fibroblasts and described as a family of growth-stimulating polypeptides. The further nomenclature “transforming growth factor” was adopted because of the ability of these molecules to confer on untransformed indicator fibroblasts functional properties associated with neoplastic transformation.6 7
At present, TGF-βs are considered pleiotropic factors because they have been shown to play a regulatory role in most processes linked to the control of somatic tissue development and renewal. As pointed out by Sporn and Roberts,8 TGF-βs may be considered as “prototypic multifunctional signaling molecules.” Indeed, these factors can exert either a positive or a negative effect on proliferation, differentiation, or cell death, depending on the developmental stage of the target cell, its in vivo environment, or the medium used for in vitro studies. As will be described in this review, this is particularly true in the hematopoietic system, where TGF-βs play a pivotal role.
Structure of TGF-βs
Latent and active forms
Three highly similar isoforms of TGF-β, called TGF-β1, -β2, and -β3, were identified and cloned from mammals between 1985 and 1988.9-11 Although the regulatory role of these 3 isoforms may differ, it has been established that all 3 are involved in the regulation of hematopoiesis. Two other isoforms, called TGF-β4 and -β5, have been cloned, respectively, in the chicken12 and in xenopus.13 More recently, an mRNA encoding a new member of the TGF-β family, called endometrial bleeding associated factor (ebaf), has been identified in mammals.14
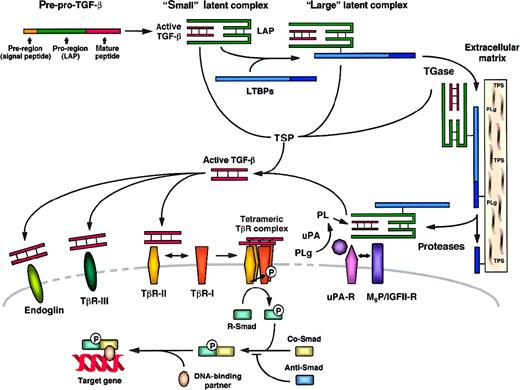
TGF-βs are synthesized as precursor proteins, which are biologically inactive. They consist of pre–pro-peptides, which require a 2-step process to give rise to active TGF-βs15 (for reviews see Lawrence,16 1991; Gleizes et al,17 1997). A first proteolytic cleavage leads to the elimination of a hydrophobic signal peptide, in the N-terminal region of the precursor protein, yielding pro–TGF-β. A second cleavage leads to the separation of the pro-region of the protein from the TGF-β mature peptide (Figure 1). In the case of TGF-β1, the entire precursor (pre–pro-peptide) is a 390–amino acid chain. The signal peptide corresponds to amino acids 1 to 29, the pro-region of the precursor to amino acids 30 to 278, and the mature peptide to amino acids 279 to 390.15 The bioactive forms of TGF-βs (25 kd) are composed of 2 mature peptide chains linked by disulfide bonds. TGF-βs are usually produced as homodimers (TGF-β1.1, -β2.2, -β3.3), but natural heterodimeric molecules have also been identified (TGF-β1.2 and -β2.3).18
TGF-β: structure, latency, activation, and receptors.17 67
LAP indicates latency-associated peptide; LTBP, latent TGF-β binding protein; M6P/IGFII-R, mannose-6-phosphate/type II insulin-like growth factor receptor; PLg, plasminogen; PL, plasmin; Smad, TGF-β signal transduction proteins; Anti-Smad, antagonistic Smad; Co-Smad, common-partner Smad; R-Smad, receptor-regulated Smad; TGase, transglutaminase; TβR-I, -II, -III, TGF-β receptor type I, II, III; TSP, thrombospondin; uPA, urokinase plasminogen activator; and uPA-R, uPA receptor.
TGF-β: structure, latency, activation, and receptors.17 67
LAP indicates latency-associated peptide; LTBP, latent TGF-β binding protein; M6P/IGFII-R, mannose-6-phosphate/type II insulin-like growth factor receptor; PLg, plasminogen; PL, plasmin; Smad, TGF-β signal transduction proteins; Anti-Smad, antagonistic Smad; Co-Smad, common-partner Smad; R-Smad, receptor-regulated Smad; TGase, transglutaminase; TβR-I, -II, -III, TGF-β receptor type I, II, III; TSP, thrombospondin; uPA, urokinase plasminogen activator; and uPA-R, uPA receptor.
Once synthesized and processed, TGF-βs are released by cells as latent complexes, which are biologically inactive. Two forms of latent complexes have been described, the “small” and “large” latent complexes, as shown in Figure 1. In the small latent complex, one molecule of mature, active TGF-β is noncovalently associated with one disulfide-bonded pro-peptide dimer, called latency-associated protein or LAP (74 kd in the case of TGF-β1). In the large latent complex, LAP is linked by disulfide bonds to one member of a family of high-molecular-weight proteins (125-160 kd), called latent TGF-β–binding proteins or LTBPs.19,20 The cDNAs of various related LTBPs have been cloned.21-23 In the erythroleukemic cell line HEL, the synthesis of LTBPs has been found to be coordinated with that of TGF-β small latent complex to form the large latent complex, which is then secreted by cells.24The LTBPs confer to this complex the ability to associate with the extracellular matrix, permitting the storage of TGF-β (for reviews see Munger et al,25 1997; Taipale and Keski-Oja,26 1997). Because LTBPs exist in several isoforms, the bioavailability of TGF-β and its specific targeting to different organs may be regulated in part by the formation of different types of large latent complexes. It has also been suggested that LTBPs participate in bone formation as structural matrix proteins.27 The release of latent TGF-β from the extracellular matrix is triggered by proteolytic enzymes such as chymase, elastase, and plasmin, which are able to cleave LTBPs.28-30
Activation of latent TGF-βs
Extracellular activation of the TGF-β latent complexes is a critical process in the regulation of TGF-β functions in vivo. The interaction between TGF-β and LAP is not covalent and can be disrupted in vitro by heat treatment or acidification.31Although physicochemical variables such as local acidification32 or exposure to active oxygen species33 may participate in the regulation of TGF-β activation, mechanisms involving proteolytic cleavage or conformational modification of LAP are more likely to operate in vivo.
Different mechanisms of activation are presented in Figure 1. Plasmin has been shown to promote the activation of latent TGF-β by proteolytic nicking within the N-terminal region of the LAP.34,35 This disrupts noncovalent bonds and results in the release of active TGF-β.35 In monocytes, macrophages, and endothelial cells, cellular activation of latent TGF-β has been reported to involve the mannose-6-phosphate/type II insulinlike growth factor receptor (M6P/IGFII-R) and the urokinase plasminogen activator receptor (uPA-R).36-38 One proposed mechanism is that M6P/IGFII-R, which binds latent TGF-β, complexes with uPA-R. Plasmin would be generated locally from plasminogen through the action of uPA and would allow the production of active TGF-β. Another enzyme, transglutaminase, has been identified as an effector controlling both the deposition rate of LTBPs in the matrix39 and the cell-surface activation of latent TGF-β.37,40 Transglutaminase-mediated activation of latent TGF-β depends on interactions with specific residues of LTBP.41 Thrombospondin (TSP), a platelet α-granule and extracellular matrix protein, has also been shown to promote activation of latent forms of TGF-β. In contrast to what has been described for plasmin and transglutaminase, TSP-mediated activation of latent TGF-β occurs through a cell- and protease-independent mechanism, as demonstrated by in vitro studies. This effector induces a conformational change of LAP, which then results in the release of active TGF-β.42,43 The role of TSP in the activation of latent TGF-β in vivo has been demonstrated by the generation ofTSP-null mice.44 In this model, major histologic abnormalities have been observed and correlated with a lack of active TGF-β. These defects could be reversed by a treatment that activates TGF-β. Regulation of the glycosylation of LAP has also been proposed to participate in the control of TGF-β latency.45 Recently, activation of latent TGF-β via an interaction with the integrin α5 β6 has been reported, providing a novel possible mechanism regulating the function of TGF-β.46
TGF-β signal transduction pathway
Two families of serine/threonine kinase receptors form heteromeric complexes
Among the several transmembrane or membrane-bound proteins known to interact with TGF-βs, the type I and type II TGF-β receptors (TβR-I or ALK, and TβR-II) are directly involved in signal transduction. TβR-I and TβR-II represent 2 families of transmembrane serine/threonine kinase receptors of 53 to 65 kd47-49 and 80 to 95 kd,50 respectively, that interact and form heterotetrameric complexes. The mechanism by which signaling by these 2 receptors occurs is now well established.51 TGF-β first binds to TβR-II, which is a constitutively active kinase. TβR-I is then recognized and recruited into the TGF-β/TβR-II complex and phosphorylated by TβR-II. Phosphorylation allows TβR-I to propagate the signal to downstream intracellular substrates.
Because TβR-I and TβR-II exist in multiple forms, it has been proposed that homodimeric and heterodimeric forms of TGF-β may induce a specific response by interacting with different heterotetrameric receptor complexes of specific signaling capacities.52
Accessory receptors
In addition to TβR-I and TβR-II, accessory TGF-β receptors, not necessarily required for signal transduction, can be expressed at the surface of cells responsive to TGF-β (for review see Piek et al,52 1999). The type III TGF-β receptor (TβR-III or βglycan), a 300- to 400-kd membrane-anchored proteoglycan,53,54 and the 180-kd glycoprotein endoglin could function as regulators of ligand access to the signaling receptors. Although the precise roles of endoglin and βglycan are not fully understood, some of their properties suggest distinct functions for these 2 TGF-β receptors. First, βglycan is able to interact with TGF-β1, -β2, and -β3,55 whereas endoglin interacts with TGF-β1 and -β3 but not efficiently with TGF-β2.56 Second, the role of βglycan could be to present TGF-βs to TβR-II and facilitate their binding,57,58 whereas endoglin appears to diminish rather than enhance TGF-β responses in certain cell types.59Third, endoglin and βglycan possess a specific cell-distribution pattern, which may confer the ability of different cell types to respond differentially to TGF-β1, -β2, and -β3. For example, endoglin is coexpressed with TβR-I and TβR-II on vascular endothelial cells60,61 and on hematopoietic cells including macrophages,62 erythroid cell subsets,63 and B-cell precursors,64 whereas these cells express little or no βglycan. Marrow stromal cells may express both endoglin and βglycan,65 whereas none of these TGF-β receptors appear to be present on the cell surface of hematopoietic progenitors including early colony-forming units (CFU)–granulocyte/erythrocyte/monocyte/megakaryocyte (GEMM), CFU–granulocyte/monocyte (GM), and burst-forming units-erythrocyte (BFU-E).63
Other cell-surface receptors have been identified for their ability to bind TGF-β and are classified as TβR-IV to TβR-VI (for review see Massagué,66 1992). However, the function of these other receptor families in TGF-β signaling remains to be clarified.
The Smad intracellular proteins
The intracellular TGF-β signaling pathway involves the Smad protein family as substrates for the signaling receptors (for review see Massagué,67 1998; Piek et al,521999). This network involves the cooperation among 3 subclasses of Smad proteins, which can be distinguished by distinct functions in TGF-β signal transduction. Briefly, a first group of Smads called “receptor-activated”67 or “receptor-regulated”52 Smads (R-Smads) are directly phosphorylated by activated TβR-I. Upon phosphorylation, R-Smads interact with members of a second subclass of Smads called “common-partner Smads” or Co-Smads, with which they form heterodimeric complexes. R-Smad/Co-Smad complexes are translocated to the nucleus, where they associate with DNA-binding partners and then regulate the transcriptional response of the target genes. A third subclass of Smads called “antagonistic”67 or “inhibitory”52 Smads (Anti-Smads) prevents the interaction between R-Smads and Co-Smads and participates in negative feedback to repress TGF-β responses. In the case of signal transduction by TGF-β in mammalian cells, R-Smads include Smad2 and Smad3,68,69 Co-Smads include Smad4,69,70 and Anti-Smads include Smad6 and Smad7.71-73 Smad5, otherwise described as an R-Smad and involved in signal transduction by bone morphogenetic proteins (BMPs),74 has been demonstrated to mediate the inhibitory effect of TGF-β on human hematopoietic stem/progenitor cells.75 The specificity of the cellular response depends on interactions between these different possible partners.
Studies of human malignancies involving TGF-β reveal its essential role in the control of hematopoiesis
Studies of hematopoietic pathologies involving TGF-β have provided important evidence of its key role in the regulation of human hematopoietic stem/progenitor cell quiescence, proliferation, and differentiation. These human pathologies are often more informative than knockout mice as regards the role of this pleiotropic factor. Indeed, knockout mice often exhibit secondary disorders subsequent to the accumulation of early defects during embryonic development. This situation is also often observed in the case of genetic diseases, in which more than one function or organ can be affected during development by a single mutation. In contrast, the pathologic situation resulting from a single gene mutation occurring in the adult will produce a clonal defect, allowing the precise evaluation of the role of the mutated gene in a specific function. This is particularly true in the case of various cancers.
Inactivation of the TGF-β signaling cascade leads to malignant transformation of early human hematopoietic cells
The inactivation of one of the various genes involved in the TGF-β signal transduction pathway may represent a possible mechanism by which some early hematopoietic progenitors, which are normally quiescent, escape from cell-cycling inhibition. Abnormalities in the expression of TGF-β receptors have been described in proliferative syndromes including both early myeloid76,77 and lymphocytic leukemia.78,79 In these pathologies, a selective advantage is given to the tumor cells by the loss of TβR-I or TβR-II expression and by the fact that these cells continue to produce TGF-β1 to inhibit normal cell proliferation. Active TGF-β present in the bone marrow microenvironment and autocrine/paracrine TGF-β1 secreted by normal and leukemic hematopoietic cells are able to exert a negative control on the growth of normal progenitors, but not on leukemic cells, which have overcome TGF-β regulatory signals. A loss of sensitivity to the growth-inhibitory effect of TGF-β due to an inactivation of TβR-II has also been described in the case of human cutaneous T-cell lymphoma cells.80-82
A mutational analysis of the gene coding for the TGF-β signal transducer Smad2 has been performed on 50 primary lymphoid and myeloid leukemia cells, but no genetic defects were found in this gene.83 However, a larger panel of hematologic disorders should be analyzed before excluding the possibility of various mutations in Smad genes in some of these pathologies. Blocking of TGF-β signaling by repression of Smad3 activity has been reported in chronic myeloid leukemia. In these cases, the dysregulation was not due to mutations in the Smad3 gene, but was correlated with an abnormal expression of Evi-1, a zinc-finger oncoprotein that interacts with Smad3 and suppresses its transcriptional activity.84 In hematopoietic cells, Evi-1 expression is normally restricted to a transient stage of myeloid differentiation.85 Its constitutive expression can result from chromosomal rearrangements and may contribute to leukemogenesis by specifically blocking the growth-inhibitory signaling of TGF-β.86
Pathologic overproduction of TGF-β induces bone marrow fibrosis and decreases stem/progenitor frequency
In physiologic conditions, the amount of TGF-β produced by bone marrow stromal cells and hematopoietic cells should be adequate to maintain homeostasis of the stem/progenitor cell compartment. In pathologic situations, excessive production of TGF-β by stromal cells has been correlated with a failure of early hematopoietic progenitors in the marrow. This situation has been described in the case of human chronic idiopathic neutropenia, in which a drastic reduction in CD34+ progenitor cell frequency is observed.87The same phenomenon has been described for B-cell lymphocytic leukemia. In this case, the pathogenesis is due to the proliferation of leukemic cells, but also to an increased inhibition of normal progenitor cell growth, in response to excessive amounts of TGF-β1 secreted by bone marrow stromal cells.88
An abnormally elevated production of TGF-β has also been shown to contribute to the pathogenesis of leukemia by promoting the progression of bone marrow fibrosis (for review see Le Bousse-Kerdiles and Martyre,89 1999). In these pathologies, TGF-β is often secreted in excess by leukemic cells, monocytes, and megakaryocytes, which results in stimulation of collagen synthesis in bone marrow fibroblasts and deposition in the marrow.90-93 This observation is in agreement with the fact that TGF-β is able to activate the promoter of the human type VII collagen gene through the action of Smad3 and Smad4.94
Vascular pathologies involving TGF-β
Defects in genes coding for a TGF-β receptor have also been correlated with the genetic diseases called hereditary hemorrhagic telangiectasia (HHT). These syndromes concern mainly endothelial cells and are characterized by arteriovenous malformations and recurrent hemorrhage. HHT syndromes were first found to be caused by mutations in the endoglin gene95 but have also been correlated with mutations in the TβR-Igene,96 suggesting that endoglin and TβR-I act through a common pathway to control blood vessel development and repair.
TGF-β has also been reported to be involved in another vascular disease, atherosclerosis, which has a multifactorial pathology implicating many other interacting phenomena. Atherosclerosis has been associated with the presence of lipoprotein Lp(a), a glycoprotein that has a structure similar to that of plasminogen. Lp(a) binds to the membranes of endothelial cells and monocytes and thereby inhibits plasminogen binding and the subsequent generation of plasmin by these cells. Because plasmin is a potent activator of latent TGF-β in vivo, this results in an insufficient rate of activation of TGF-β and, as a consequence, in the migration and proliferation of smooth muscle cells in the arterial intima (for review see Angles-Cano,971997).
TGF-β and the control of hematopoietic stem/progenitor cell proliferation: a model for other somatic cells?
We have reviewed above various studies showing that the inactivation of the TGF-β signaling cascade participates in malignant transformation of early hematopoietic cells, which then escape from negative cell-cycle controls. It is interesting to note that the same phenomenon has been described in cancers affecting various types of nonhematopoietic somatic cells, which suggests that TGF-β may act as a cell-cycle inhibitor in several nonhematopoietic somatic tissues in vivo. Indeed, although they probably do not represent the entire cause of the pathology, mutations or genetic defects resulting in a lack of TβR-I or TβR-II function are associated with the acquisition of a transformed phenotype in several types of murine and human cancers, including colon cancers,98,99 gastric cancers,100,101 prostate cancers,102pancreatic cancers,103 thyroid tumors,104hepatic tumors,105,106 retinoblastoma,107,108and lung adenocarcinoma.109 Moreover, the importance of TGF-β signaling for the control of normal somatic cell proliferation has been demonstrated in skin keratinocytes,110,111 cells of the mammary gland, lung,112 and exocrine pancreas113 with the use of transgenic mice expressing a dominant-negative mutant TβR-II (for review see Letterio and Bottinger,114 1998).
In addition, elements of the TGF-β signal transduction pathway downstream to the TGF-β receptors have been identified as potential targets for oncogenic transformation. Indeed, mutations or somatic alterations resulting in a disruption of the Smad signaling cascade have been observed in several tumor cells resistant to the growth-inhibitory effect of TGF-β1 (for review see Hata et al,115 1998). Briefly, in these cells, insensitivity to TGF-β1 was reported to be due either to an inactivation of the TGF-β signal transducers Smad2 and Smad4116-118 or to an enhanced expression of the TGF-β1 signaling inhibitor Smad6.119 These observations should promote a search forSmad gene mutations in the hematopoietic system, especially in leukemia.
TGF-β and the regulation of murine hematopoiesis in vivo
The role of TGF-β in the regulation of hematopoiesis has also been analyzed in vivo using different murine models (for review see Bottinger et al,120 1997). However, the involvement of this pleiotropic factor in the regulation of various nonhematopoietic tissue functions renders these studies sometimes difficult to interpret because of possible indirect regulatory effects.
TGF-β protects stem/progenitor cells from agents that selectively kill cycling cells
Hematopoietic stem/progenitor cells are able to reconstitute the pool of mature blood cells in the case of severe hematopoietic failure. This implies that these cells can rapidly pass from a quiescent or slow cycling state to active proliferation. This phenomenon can be observed in mice treated with chemotherapeutic drugs, such as 5-fluorouracil (5-FU), that selectively kill the cycling cells. Following the administration of 5-FU, a dramatic decrease in hematopoietic progenitor cell frequency in the bone marrow is observed, as only the most primitive and quiescent cells remain unaffected by this treatment. A few days later, the quiescent stem/progenitor cells enter a hyperproliferative state, promoting hematopoietic reconstitution. This experimental system has been used to evaluate the ability of TGF-β to affect hematopoietic stem/progenitor cell cycling. TGF-β1 has been shown to delay hematologic recovery after a sublethal injection of 5-FU.121 Moreover, both the TGF-β1 and -β2 isoforms were able to protect hematopoietic stem/progenitor cells from a treatment by a high dose of 5-FU,122 which demonstrates the ability of these molecules to exert a negative control on the cell cycle of primitive murine hematopoietic cells in vivo. It is important to note that this effect was reversible,121 122 which suggests that TGF-β is not an inducer of cell death for primitive stem/progenitor cells in vivo. This point will be discussed more extensively later.
In vivo administration of TGF-β in mice modulates hematopoietic development in a lineage-specific manner
In vivo administration of TGF-β in mice has also been performed to study its specific effects on early and late progenitors and on the different hematopoietic lineages. One approach, which was to test in vitro the clonogenic capacity of hematopoietic progenitors after the local administration of TGF-β1 into the femur of mice, revealed a preferential growth-inhibitory effect of this factor on the earlier progenitors.123 A second approach was to perform histologic analyses of hematopoietic tissues from mice treated with TGF-β1. Such studies revealed an inhibition of erythropoiesis and thrombopoiesis in TGF-β1–treated mice,124,125 whereas granulopoiesis was stimulated.124-126 Although some of these effects may be directly mediated, the possibility that administration of exogenous TGF-β in vivo may deregulate the production of other factors involved in the control of hematopoiesis should be taken into account. For example, an increased production of tumor necrosis factor (TNF)-α has been observed after the administration of TGF-β in mice.127
Hematopoiesis in knockout mice
An opposing strategy has been to study in vivo the development of the hematopoietic tissue in the absence of endogenous TGF-β, or in a context in which the cell responsiveness to TGF-β is abrogated. For this purpose, a variety of knockout mice have been generated, in which a targeted disruption of genes encoding a TGF-β isoform or another element of the TGF-β signaling cascade has been performed. HomozygousTGF-β1 knockout mice have a 50% intrauterine death rate because of severe developmental retardation. Defective hematopoiesis, resulting in a reduced number of erythroid cells128 as well as a lack of Langerhans dendritic cells,129 has been correlated with the absence of TGF-β1. However, TGF-β1knockout mice also present defects in liver development130and bone formation,131 as well as many other dysregulations including autoimmune manifestations.132,133The phenotype of TβR-IIknockout mice has been reported to be indistinguishable from that ofTGF-β1 knockout mice.134 Mice lacking endoglin show defective angiogenesis,135 providing a good animal model of HHT.136TGF-β3knockout mice show abnormal lung and craniofacial development due to altered epidermal–mesenchymal interactions,137 but these mice do not provide clear information concerning the role of this gene in the development of hematopoietic tissue. Knockout mice for theTGF-β2 gene exhibit a wide range of developmental defects that do not overlap with those of the other TGF-β knockout phenotypes.138 Concerning the Smad genes,Smad3 knockout mice demonstrate defects in immune function,139 whereas Smad4 and Smad5knockout mice have multiple embryonic and extraembryonic defects.140 141
Control of human and murine stem/progenitor cell proliferation by TGF-β: in vitro studies
Human and murine hematopoietic stem/progenitor cells are usually in a quiescent or slow cycling state in adults.142 143 As suggested by in vivo studies, TGF-β is a good candidate for controlling this quiescence. This possible function of TGF-β has been studied extensively in vitro using clonal semisolid colony-forming assays, stroma-supported culture systems, and single-cell liquid cultures in both the murine and human hematopoietic systems.
TGF-β exerts a preferential growth-inhibitory effect on the most primitive stem/progenitor cells
A first approach has been to study the effects of exogenous TGF-β added to clonal cultures of hematopoietic progenitors. The first TGF-β isoform, TGF-β1, has been shown to inhibit colony formation by early murine144-146 and human hematopoietic progenitors in semisolid media,147-149 but not that of late progenitors.145 147-149
In these studies, the effects of TGF-β on colony formation have often been tested in combination with only 1 or 2 other exogenous growth factors, added to serum-containing or conditioned culture media that contain undefined combinations of other growth factors. The cytokines used were mainly interleukin (IL)-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), and/or erythropoietin (Epo). In such culture conditions, TGF-β1 was found to efficiently inhibit colony formation by early human multipotential progenitors (CFU-Mix) at concentrations between 10 and 100 pg/mL, whereas later progenitors were less affected or were, in contrast, stimulated by these low TGF-β1 concentrations.149
An alternative approach has been to use blocking antibodies or antisense oligonucleotides to neutralize TGF-β secreted by cells and present in the culture media. A study performed at a clonal level in semisolid or in single-cell liquid assays has revealed that the quiescence of human stem/progenitor cells is controlled in part through an autocrine loop involving TGF-β1 with the retinoblastoma susceptibility gene Rb as a downstream effector.150 In this study, blocking of autocrine TGF-β1 was sufficient to release from quiescence primitive erythro-myeloid (CFU-Mix), myeloid (CFU-GM), and erythroid progenitors (BFU-E), which then gave rise to macroscopic colonies, in the presence of IL-3, IL-6, granulocyte colony-stimulating factor (G-CSF), and Epo. Using the same antisense oligonucleotide, it was observed that autocrine TGF-β1 inhibits colony formation by early murine and human hematopoietic stem/progenitor cells stimulated with KIT ligand (SF).151 152
Since the first studies on TGF-β, many others have subsequently addressed its effects on progenitor cell growth when present in association with various other cytokines. For example, in a single-cell liquid assay, it has been demonstrated that this factor directly inhibits early human hematopoietic progenitor cell proliferation in the presence of Epo, SF, GM-CSF, and IL-3.153 Moreover, in a semisolid culture system containing a combination of 7 stimulatory growth factors (IL-3, IL-6, IL-11, Epo, SF, GM-CSF, and G-CSF), it has been shown that whereas 10 to 30 pg/mL of TGF-β1 is sufficient to inhibit 90% of primitive high proliferative potential (HPP)-Mix, 100 to 300 pg/mL of TGF-β1 is required to inhibit 70% of the bipotent HPP-GM and the HPP–BFU-E. Concentrations of up to 1000 to 3000 pg/mL of TGF-β1 had little or no effect on the development of late CFU-G and CFU-M.154
Preferential growth inhibition of the most primitive human hematopoietic cells has also been reported in studies in which the effects of anti–TGF-β1 antisense oligonucleotides, which neutralize autocrine TGF-β1 production155 or exogenous TGF-β1,156 were investigated on CD34+CD38− cells and on CD34+CD38+ cells. It was indeed observed that primitive CD34+CD38− cells show a high sensitivity to cell-cycle inhibition by TGF-β1, whereas more mature CD34+CD38+ cells are poorly affected or are even stimulated by TGF-β1. Interestingly, the concentrations of TGF-β1 for which these inhibitory effects were observed on primitive cells correspond to those detected in human plasma in an active form (usually less than 300 pg/mL).157
The second isoform, TGF-β2, has also been shown to inhibit early progenitor cell proliferation in both the human148,149,158and murine hematopoietic systems,144 but with a lower efficiency.149,158 The third isoform, TGF-β3, also inhibits colony formation by early human hematopoietic progenitors149 or slows their rate of proliferation159 at least as efficiently as does TGF-β1. However, only TGF-β1 and -β2 were shown to exert bidirectional effects on proliferation of early and late hematopoietic progenitors, whereas the effects of TGF-β3 were only inhibitory.149
TGF-β in stroma-supported cultures of hematopoietic cells
Stroma-supported cultures containing both hematopoietic progenitors and nonhematopoietic accessory cells allow the proliferation and differentiation of primitive stem/progenitor cells for several weeks without the addition of exogenous factors (for reviews see Dexter,160 1979; Eaves et al,1611991). In these systems, progenitor cell development is regulated through a complex interaction between positive and negative factors that are secreted by both stromal and hematopoietic cells.
Studies performed on adherent bone marrow primary stromal cells or cell lines have shown that these cells can produce a variety of growth factors, including cytokines such as G-CSF; GM-CSF162,163; IL-1β, IL-6, and IL-7162,164; SF and M-CSF165; and thrombopoietin (TPO).166They also produce chemokines such as the monocyte chemoattractant protein-1 and the interferon-inducible protein-10,165 as well as amounts of active TGF-β sufficient to control the proliferation of hematopoietic progenitors.162,167 Two aspects render the presence of TGF-β in stroma-supported cultures critical for the development of hematopoietic cells. First, TGF-β acts directly on these cells. Second, TGF-β is able to modulate the growth factor production by stromal cells,163-166 a process that indirectly controls their development.
The term “long-term culture initiating cell” (LTC-IC) has been assigned to a subpopulation of primitive human hematopoietic stem/progenitor cells that possess the potential to sustain continuous production of progenitors for at least 8 weeks in the presence of stroma.161 Anti–TGF-β added to stroma-supported cultures of human hematopoietic stem/progenitor cells was able to prolong or reactivate the proliferation of LTC-ICs,162implicating TGF-β as an endogenous inhibitor of primitive hematopoietic cells. The proliferation of these cells was also selectively inhibited when exogenous TGF-β was added.168
Similar observations have been reported for murine hematopoietic stem/progenitor cells cultured according to the Dexter method. Dexter-type culture systems consist of total marrow cultures in which an expansion of primitive hematopoietic stem/progenitor cells is maintained for several months because of the presence of a bone marrow–derived adherent layer consisting of different types of nonhematopoietic accessory cells including adipocytes.169The addition of antibodies neutralizing the biologic activity of TGF-β1, -β2, and -β3 in such cultures resulted in a significant increase in early hematopoietic cells, demonstrating the ability of these factors to inhibit their cell cycling.170
These stroma-supported in vitro culture systems have provided important information concerning the role of TGF-β in the control of the cell-cycle status of primitive murine and human hematopoietic stem/progenitor cells. However, they do not permit discrimination between the effect of TGF-β secreted by stromal cells (paracrine TGF-β) and that of TGF-β produced by the hematopoietic progenitors themselves (autocrine and paracrine TGF-β).
Remarks
All of these in vitro studies converge to suggest that TGF-β inhibits preferentially the cell cycling of the most primitive hematopoietic cells. These results have also been reproduced in an in vivo model. Indeed, nonobese diabetic/severe combined immunodeficient mice have been transplanted with human bone marrow and umbilical cord blood cells and injected with TGF-β1 at 6 weeks after transplantation. Analysis of the specific responses of different progenitor cell types has revealed that TGF-β1 is active on primitive progenitor cell populations, including LTC-IC and HPP–colony-forming cells, but not on the more mature cells.171
It should be pointed out that differences in the responsiveness to TGF-β have been observed between human stem/progenitor cells of distinct ontogenic origins. Indeed, fetal liver stem/progenitor cells, which are generally in a more active cycling status than cells from adult bone marrow, appear to be less sensitive to growth inhibition by TGF-β.172
Mechanisms by which TGF-β controls primitive stem/progenitor cell cycling
TGF-β modulates growth factor receptor expression
The ability of TGF-β to modulate the expression of cytokine receptors has been observed both in transformed cell lines and in normal hematopoietic progenitors. In murine progenitor cell lines, TGF-β has been shown to down-modulate the expression of the receptors for IL-1,173 IL-3, G-CSF, and GM-CSF.174 In normal hematopoietic bone marrow cells, similar observations were made in humans for the IL-1 receptor173 and in mice for the IL-1, IL-3, and the M-CSF receptors, but not for the GM-CSF receptor which, on the contrary, is up-modulated by TGF-β on murine progenitor cells.175,176 G-CSF receptor expression was not affected.176 These effects appear to be isoform dependent. For example, TGF-β3 may be a more potent inhibitor of IL-3 receptor expression than TGF-β1, whereas TGF-β3 is less efficient than TGF-β1 in stimulating the expression of the GM-CSF receptor.176
More recently, TGF-β1 has been reported to down-modulate the c-KIT receptor on murine177,178 and human hematopoietic progenitors,179 explaining its ability to inhibit the action of SF.152,180 A similar modulation of the FLT3 receptor has been described on early human hematopoietic progenitors,181 which is consistent with previous data showing an inhibition of FL-stimulated cell cycling of murine progenitors by TGF-β1.182,183 TGF-β1 has also been shown to down-modulate the IL-6 receptor (IL-6R) on human hematopoietic progenitors.181 Finally, the cell-surface expression of another important receptor involved in the control of primitive hematopoietic progenitor cell proliferation, the TPO receptor c-MPL, has been found to be down-modulated by TGF-β1 (Fortunel et al, unpublished results). This explains the ability of TGF-β1 to abrogate TPO-induced progenitor cell growth.184
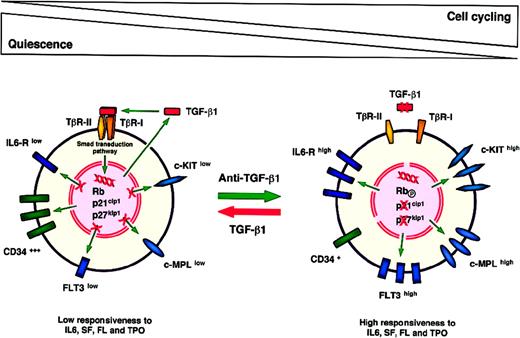
The HPP-Q working model
The concept of the high-proliferative-potential quiescent cell (HPP-Q) has been introduced to refer to a primitive subpopulation of human hematopoietic stem/progenitor cells that are highly sensitive to growth inhibition by TGF-β1.181 TGF-β1 maintains these cells in a quiescent or slow cycling state by down-modulating various cytokine receptors, preventing them from responding rapidly to mitogenic stimulation (Figure 2). In the in vitro HPP-Q assay, which can be performed both in semisolid conditions and in single-cell liquid cultures,150 181the use of antisense oligonucleotides or anti–TGF-β1 blocking antibodies allows these primitive cells to escape from cell-cycle inhibition. A short treatment with anti–TGF-β1 is sufficient to activate HPP-Q cells and render them competent and responsive to cytokines (Figure 2). Indeed, similar effects were obtained when cells were pretreated for only 6 to 12 hours before plating and when anti–TGF-β1 was maintained or added repeatedly throughout the culture period (Hatzfeld A et al, unpublished results).
A working model to explain the inhibition of primitive hematopoietic stem/progenitor cell cycling by TGF-β1.
A working model to explain the inhibition of primitive hematopoietic stem/progenitor cell cycling by TGF-β1.
The HPP-Q population presents at least 3 characteristics of a primitive stem/progenitor cell compartment: (1) HPP-Q cells possess a high proliferative potential, as they are able to generate, within 18 days, clones containing more than 105 cells when cultured in a liquid medium in single-cell conditions; (2) they give rise to multilineage hematopoietic clones or colonies when cultured in the presence of the appropriate cytokine combination; and (3) they are in a quiescent or slow cycling state in spite of their high proliferative potential. A fourth characteristic of the HPP-Q cell population is under study. It concerns the ability of HPP-Q cells to promote long-term engraftment.
One main interest of this working model is the possibility of further phenotyping HPP-Q stem/progenitor cells on the basis of cytokine receptor expression. These cells express low levels of various cytokine receptors such as KIT, FLT3, IL-6R, and MPL. This renders possible the sorting of cell subpopulations enriched in HPP-Q stem/progenitor cells. The long-term engraftment capacity of CD34+/ KITlow/ FLT3low/ IL-6Rlow/ MPLlow cells should be studied in xenograft animal models such as preimmune sheep fetuses.185 Up to now, in this sheep model, the CD34+/ KITlow population, which includes HPP-Q cells, has been reported to contain the marrow-engrafting stem/progenitor cells. This was not the case for KIThigh and KITneg cells.186
Is TGF-β a reversible cell-cycle inhibitor or an inducer of hematopoietic cell apoptosis?
The identification of growth inhibitors able to control the proliferation of leukemic cells is of particular importance in the development of therapeutic approaches. As stated earlier, the acquisition of resistance to growth inhibition by TGF-β is often associated with malignant transformation. As discussed later, the selective advantage of cancer cells that are insensitive to TGF-β may be due not only to the inactivation of mechanisms involved in cell-cycle control, but also could result from resistance to apoptotic cell death. Indeed, TGF-β1 has been reported to induce cell death in several leukemic cell types, including myeloid leukemia–derived cell lines187 and B-lymphoma cell lines,188,189 as well as in primary leukemia cells such as acute myelogenous cells190 and lymphoblastic leukemia cells.191This suggests that the growth-inhibitory effect exerted by TGF-β on some leukemic blasts could occur in part through programmed cell death and, as a consequence of this, that abrogation of TGF-β1–mediated apoptosis could contribute to the pathogenesis of leukemia in the case of cells that have lost their sensitivity to TGF-β by mutation ofTβR-I or TβR-II. On the contrary, TGF-β1 has been reported to protect other types of leukemia-derived cells, such as HL-60 cells, from apoptosis.192 193
The relations between TGF-β and the control of apoptosis have also been studied in various types of normal hematopoietic cells and appear to be strictly dependent on the differentiation status of the cells and on the culture variables such as the concentrations of cytokines. For example, TGF-β has been found to induce apoptosis in human eosinophils,194 whereas this factor has been found to sustain viability of rat195 and human T cells196 and also of human dendritic cell precursors.197 Whether TGF-β is an inducer of apoptosis for hematopoietic stem/progenitor cells is a question frequently raised. In a previous paragraph, we stressed the fact that TGF-β injected in mice only transiently inhibited the proliferation of primitive hematopoietic stem/progenitor cells. This suggests a reversible mechanism, which is not compatible with induction of cell death. Reversibility also has been suggested by in vitro studies in which TGF-β1 was found to delay proliferation of primitive murine198 and human hematopoietic stem/progenitor cells199 in batch cultures. However, some properties of TGF-β are consistent with both apoptotic and antiapoptotic effects exerted on stem/progenitor cells, which may lead to confusion. Indeed, TGF-β1 has been found to abrogate Fas-induced apoptosis of murine bone marrow progenitors,200 while counteracting the ability of growth factors such as SF201 and FL202 to sustain their viability. The reversibility of the growth-inhibitory effect exerted by TGF-β1 on hematopoietic stem/progenitor cells in vitro has been verified recently at a clonal level.203 In this study, the proliferative potential of single human umbilical cord blood CD34+ cells was analyzed after a 3-day culture in the presence of TGF-β1. A reversible cell-cycle inhibition was observed on primitive stem/progenitors, without detectable cell-death induction. In another study, it was demonstrated that the autocrine production of TGF-β1 by human peripheral blood cycling stem/progenitor cells contributed to sustaining their survival throughout successive cell divisions, in part by maintaining a high expression of the antiapoptotic protein bcl-2.204
Lineage-specific control by TGF-β in hematopoiesis
TGF-β controls multiple phases of erythropoiesis
TGF-β is one of the key regulators of erythropoiesis because it is involved in the control of both early and later stages of erythroid progenitor cell development (Figure 3). The first isoform, TGF-β1, has been identified as a cell-cycle inhibitor for early human and mouse BFU-E, but not for later erythroid progenitors, which are poorly affected by its growth-inhibitory effect.150,154,155,205 This negative regulation occurs in part through an autocrine negative-control loop.150 It has been reported that TGF-β2 and TGF-β3 could also inhibit the development of human BFU-E,149 but the effects of these 2 isoforms on erythropoiesis have not been investigated in detail.
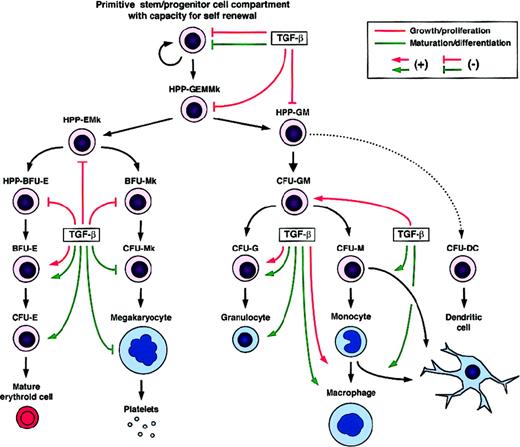
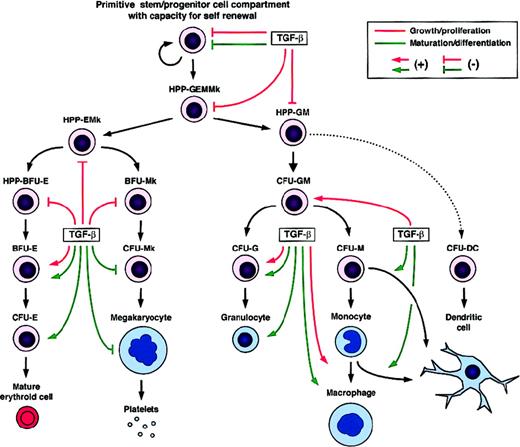
Regulatory effects of TGF-β on the growth/proliferation and maturation/differentiation of developmentally distinct hematopoietic cells.
BFU indicates burst-forming unit; CFU, colony-forming unit; HPP, high proliferative potential; DC, dendritic cell; E, erythroid cell; G, granulocyte; M, monocyte/macrophage; and Mk, megakaryocyte.
Regulatory effects of TGF-β on the growth/proliferation and maturation/differentiation of developmentally distinct hematopoietic cells.
BFU indicates burst-forming unit; CFU, colony-forming unit; HPP, high proliferative potential; DC, dendritic cell; E, erythroid cell; G, granulocyte; M, monocyte/macrophage; and Mk, megakaryocyte.
In contrast, TGF-β1 has been reported to exert a positive effect on the growth of a subset of later human erythroid progenitors. Interestingly, this effect has been observed in the presence of very low concentrations of TGF-β1 (less than 30 pg/mL), which otherwise selectively inhibit the growth of the most primitive progenitors.154 A growth-inducing effect of TGF-β1 has also been observed in an avian model system, in which TGF-β1 sustains erythroid progenitor cell proliferation and self-renewal. In the case of these avian cells, this effect occurs through a cooperation between the TGF-β and the TGF-α receptors via the Mek-Map kinase pathway,206 but this mechanism has not yet been investigated in a mammalian erythroid system.
In addition to its role in the control of erythroid progenitor cell proliferation, TGF-β1 is involved in the regulation of erythroid differentiation. Indeed, studies performed using the erythroleukemic cell lines K-562 and UT-7 as models have shown that TGF-β1 promotes late erythroid maturation processes such as the synthesis and accumulation of hemoglobin.207,208 A differentiating effect of TGF-β1 on erythroid cells has also been reported in nontransformed human cells by Krystal et al,209 who demonstrated that TGF-β1 triggers the conversion of early BFU-E into later BFU-E or CFU-E. These authors also showed that TGF-β1 up-modulates the expression of the differentiation marker glycophorin A and induces the synthesis of hemoglobin in mature cells.209
As already described, TGF-β1 inhibits the effects of SF on early human and mouse hematopoietic progenitors by a functional down-modulation of its cell-surface receptor c-KIT.177-180This is theoretically sufficient to explain the inhibitory effect of TGF-β1 on early events of erythrocytic progenitor development. Indeed, among the various cytokines involved in controlling this process, SF may be one of the most potent factors triggering proliferation of early BFU-E. In the human hematopoietic system, the burst-promoting activity of SF is required for the growth of at least some early BFU-E.210 If this interaction does not occur, further development is prevented and the colony formation by BFU-E in semisolid culture medium is inhibited. Furthermore, it has been shown that retroviral-mediated gene transfection of c-kitinto human umbilical cord blood progenitors enhances erythroid colony formation and decreases their sensitivity to growth inhibition by TGF-β1.211 The ability of TGF-β1 to counteract the activity of SF also explains its positive effect on the maturation and differentiation of both K-562 cells and normal human erythrocytic progenitors. Indeed, in addition to its mitogenic activity, SF has been shown to retard the erythroid differentiation program.212 213
Another key regulator of erythropoiesis is Epo. In addition to being the principal factor promoting the terminal differentiation of erythrocytes, this cytokine is able to stimulate earlier stages of erythropoiesis. Indeed, Epo is well known to stimulate the growth and development of CFU-E, and has been shown to sustain the proliferation of some BFU-E, but only if anti–TGF-β is added to the culture medium.214 Studies performed on normal mouse and rat erythroid progenitors have revealed that the potent growth-inducing activity of Epo on BFU-E is counteracted by TGF-β1,214,215 the effect of which could be explained by a down-modulation of the Epo receptor by TGF-β1. This possibility has been verified in the human leukemic cell line UT-7,215 but not yet for normal erythroid progenitors.
Other reports provide convincing evidence that TGF-β1 and the retinoblastoma gene product pRb could function through a common pathway to inhibit the cell cycling of early BFU-E and to promote erythroid differentiation. Indeed, TGF-β1–induced cell-cycle arrest has been found to be mediated through the control of pRb function in human hematopoietic stem/progenitor cells, including primitive CFU-Mix and BFU-E.150 Moreover, several studies performed on human and murine erythroleukemic cell lines,216-218 as well as on normal human erythroid progenitors,219 have clearly demonstrated the involvement of pRb in the regulation of later events in erythroid differentiation. Furthermore, analyses of hematopoietic tissues derived from Rb−/− knockout mice showed lineage-specific abnormalities in the development and maturation of erythroid cells.220-222 This confirms the role of pRb in the regulation of cellular events associated with the maturation and differentiation of normal erythroid cells, processes that are also controlled by TGF-β1.
Bidirectional effects of TGF-β1 on granulocytic and monocytic/macrophagic cell development
Mature granulocytic (G) and monocytic/macrophagic (M) cells develop from a common bipotent myeloid progenitor (CFU-GM). Both positive and negative effects of TGF-β on myelopoiesis have been described in vitro (Figure 3). Inhibitory effects were mainly observed on the earlier bipotent myeloid progenitors, whereas cells in later stages were either slightly inhibited or even stimulated, depending on the other factors present. TGF-β1 added to a semisolid medium containing IL-3, IL-6, IL-11, Epo, SF, GM-CSF, and G-CSF has been found to inhibit the growth of early human HPP-GM, but not that of late CFU-G and CFU-M.154 In contrast, when the TGF-β1 regulatory effects were studied in the presence of individual cytokines, clear stimulating effects were observed on the middle and late stages of myeloid cell development. As an example, in culture media containing only GM-CSF and TGF-β1, these 2 factors were found to synergize and then promote the development of a population of human mid-stage CFU-GM (day-7 CFU-GM), whereas earlier CFU-GM (day-14 CFU-GM) were not stimulated.149
The effects of TGF-β1 on the development of the G and M cell lineages have also been analyzed separately. TGF-β1 was found to synergize with GM-CSF to stimulate the growth and terminal differentiation of murine granulocytic progenitors.175 Both positive and negative effects of TGF-β1 on the proliferation of macrophagic cells have been described. Indeed, TGF-β1 has been shown to inhibit GM-CSF– and M-CSF–dependent proliferation of early murine marrow-derived macrophages, whereas proliferation of later ones was enhanced.223 Another study performed on murine cells showed a positive effect of TGF-β1 on GM-CSF–induced macrophage proliferation but an inhibitory effect on the proliferation of macrophages stimulated by M-CSF.224 As shown for granulocytes, TGF-β1 can also promote the maturation and differentiation of monocytic cells.225 The roles of TGF-β2 and -β3 in the control of myelopoiesis have been less well studied than that of TGF-β1. However, it appears that TGF-β2 can exert bidirectional proliferative effects on myeloid cells as does TGF-β1, whereas the effect of TGF-β3 is only inhibitory.149
In contrast to what has been observed for many cytokine receptors controlling the cell cycling of hematopoietic stem/progenitor cells, TGF-β1 has been shown to up-modulate the GM-CSF receptor on murine myeloid progenitors.175,176 This is in accordance with its ability to enhance GM-CSF–induced growth and differentiation of granulocytic and monocytic/macrophagic cells. TGF-β3 was less potent than TGF-β1 in up-modulating this receptor, which is in agreement with its inability to stimulate late myelopoiesis efficiently.176 TGF-β1 has also been shown to increase the cell-surface expression of the M-CSF receptor in human fetal liver monocytic progenitors,225 suggesting a possible positive cooperation between these factors. Thus, because TGF-β1 is secreted by myeloid cells at various stages of their development, this factor could regulate myelopoiesis through both negative and positive autocrine and paracrine loops.
Control of early and late megakaryopoiesis by TGF-β
Megakaryopoiesis includes 2 major steps that lead to the production of platelets. The first step of this process consists of progenitor expansion, which produces large numbers of megakaryocytic precursors. These cells subsequently differentiate into mature megakaryocytes (Mks), which produce platelets.
Early megakaryocytic progenitors first enter into a proliferating state when stimulated by appropriate growth factors. For instance, IL-3, IL-6, IL-11, SF, and TPO have been identified as potent “early-acting” Mk-stimulating factors.226-230 In contrast, several studies have shown TGF-β to inhibit early stages of megakaryopoiesis,227,231,232 as reported for other hematopoietic cell lineages (Figure 3). Addition of exogenous TGF-β1 to clonal semisolid cultures of murine bone marrow cells has been shown to inhibit the growth of primitive HPP-Mk stimulated by IL-3, IL-6, and IL-11.227 A similar effect of TGF-β1 was reported for early human Mk progenitors cultured in the presence of IL-3 and IL-6.232 In contrast, when anti–TGF-β blocking antibodies were added to the cultures, an increased proliferation of rat megakaryocytes was observed.231
TGF-β has been reported to promote maturation and late differentiation processes in several hematopoietic cell lineages. However, this is not the case for the megakaryocytic lineage (Figure3). The second step of megakaryocyte development consists of endomitosis, leading to the generation of mature polyploid cells, which then differentiate into platelets. This process is promoted by “late-acting” Mk potentiators including IL-6, IL-11, and TPO.229,233,234 TGF-β has also been shown to exert an inhibitory effect on these late events of megakaryopoiesis. Inhibition of endomitosis by TGF-β1 was first observed using the human megakaryocytic cell line Dami as a model,235,236 and this effect has since been reproduced using rat megakaryocytes.231
TGF-β1 is produced in large amounts by megakaryocytes and platelets. Once synthesized, it is sequestered within the intracytoplasmic α-granules237,238 and is released by cells spontaneously or in response to stimulation by growth factors such as IL-3 and, to a lesser extent, IL-1 or IL-11.239 Because TGF-β1 inhibits efficiently both early and late stages of megakaryocytic development, this factor could be considered as a possible feedback regulator of megakaryopoiesis. The physiologic relevance of this regulatory mechanism has been clearly demonstrated by studies of human thrombocythemia, although TGF-β is obviously not the only factor responsible for this complex process. In these pathologies, abnormally increased platelet production has been reported to be due to a decreased sensitivity of Mk progenitors to TGF-β1.240 241
We have described the ability of TGF-β1 to down-modulate the expression of several cytokine receptors, including the MPL receptor, on primitive hematopoietic stem/progenitor cells (Fortunel et al, unpublished results). Because TPO is probably one of the main factors promoting both megakaryocyte proliferation and differentiation,233,242 such an effect could explain at least in part the inhibitory effects of TGF-β1 on megakaryocytic cell development. Receptor modulation studies performed at distinct developmental stages of megakaryopoiesis would help to elucidate this point. Other studies have provided evidence that the control of late megakaryocytic differentiation by TGF-β1 could involve the transcription factors c-jun and c-fos as downstream effectors.243 244
TGF-β and the development of dendritic antigen-presenting cells
To discuss the effects of TGF-β on lymphoid cell lineages would require a specific review. Here we will just mention the role of TGF-β in the regulation of the development of cells of the immune system by introducing the case of dendritic cells (DCs) (Figure 3). TGF-β contributes to the generation of DCs, which correspond to a particular population of leukocytes specialized in antigen presentation for T-cell responses. DCs can be obtained in vitro from human CD34+ cells,197,245 murine lineage-negative (Lin−)KIT+ cells,246 human monocytes,247-249 and from their precursors.250
In vitro studies have demonstrated that the growth-factor requirement for the development of DCs includes TGF-β1, which is in agreement with the in vivo observation that TGF-β1 knockout mice lack DCs.129 This factor has been shown to promote efficiently the generation of DCs from CD34+ progenitors, in combination with GM-CSF, SF, TNF-α,245 and FL,251 in part by protecting progenitors from apoptosis.197
Generation of human and mouse DCs in response to TGF-β1 may occur via a monocyte/macrophage differentiation pathway.246-250 Indeed, TGF-β1 has been found to induce differentiation of human peripheral blood monocytes into DCs, in cooperation with cytokines such as GM-CSF and IL-4,247 and to prevent their noncognate maturation.248 Similarly, TGF-β1 has been reported to promote the generation of DCs from human CD14+ precursor cells, when present in association with GM-CSF and TNF-α.250 This was also the case for murine monocytic/macrophagic cells cultured with the same cytokine combination.246 In contrast, generation of DCs independently of the monocytic/macrophagic cell lineage does not require the presence of TGF-β1.250
Potential clinical applications of TGF-β research: 2 examples
Efficient retroviral-mediated gene transfer into stem/progenitor cells released from quiescence by anti–TGF-β
Progress in the identification and cloning of genes involved in human genetic diseases has led to important efforts to improve gene-transfer technology. Various types of viral vectors have been developed, each presenting different advantages. Among these, retroviral vectors ensure the insertion of a transgene into the host genome and its transmission to cell progeny. However, efficient retroviral-mediated gene transfection implies that the host cells are in a cycling state, which is not normally the case for primitive hematopoietic cells.252,253 Because autocrine and paracrine TGF-β1 produced by hematopoietic progenitors is partly responsible for their maintenance in a quiescent or slow cycling state,150,151 the use of anti–TGF-β1 blocking antibodies to neutralize the bioactivity of endogenous TGF-β1 during the retroviral transfection procedure was attempted to increase gene transfer into primitive stem/progenitor cells. This strategy has been tested with success on hematopoietic cells of several origins, including human umbilical cord blood,254 bone marrow,255 peripheral blood,256 and murine bone marrow.257 The efficiency of this method was shown to be improved by the coupled use of anti–TGF-β1 blocking antibodies with antisense oligonucleotides against the cyclin-dependent kinase inhibitor (CDKI) p27kip1.255 This approach could be extended to other effectors involved in the negative regulation of hematopoietic stem/progenitor cell cycling by TGF-β1, such as pRb150 or the CDKI p21cip1.258
Moreover, it has been observed that CD34+ cells produce TGF-β1 during retroviral transfection protocols, promoting a return to quiescence of the most primitive stem/progenitor cells after gene transfer. The HPP-Q assay, which allows activation of these quiescent progenitors in vitro, permits an optimal evaluation of gene-transfer efficiency in these primitive hematopoietic stem/progenitor cells (Ducos et al, unpublished results).
In vitro amplification of stem/progenitor cells: key role of TGF-β?
The development of methods for in vitro expansion of hematopoietic stem/progenitor cells is an important goal for clinical research, given the therapeutic potential of these cells. The challenge is to obtain defined culture systems in which the cell cycling of primitive quiescent stem/progenitor cells is stimulated, while their maturation and senescence are prevented.
TGF-β1 present in a culture medium at concentrations from 30 to 300 pg/mL has been reported to up-modulate the expression of the immaturity marker CD34 on slowly cycling human progenitors,202promoting the maintenance of an immature stem/progenitor cell compartment (Figure 2). In this study, the expression of the mucinlike protein CD34 was rapidly lost on umbilical cord blood progenitors grown in a differentiating medium containing IL-3, IL-6, IL-11, Epo, SF, GM-CSF, and G-CSF. This was particularly the case when anti–TGF-β1 was added. In contrast, low TGF-β1 concentrations decreased cell proliferation but promoted the maintenance of a stem/progenitor cell subpopulation that still expressed a high level of CD34 after 4 divisions. These results suggest the ability of TGF-β1 to up-modulate the expression of a membrane antigen associated with the hematopoietic stem/progenitor cell undifferentiated phenotype. This stresses the importance of research into the potential use of TGF-β for the in vitro maintenance and production of stem cells for therapeutic purposes.
Conclusions and future challenges
The pleiotropic effects of TGF-βs already observed in various tissues are particularly well illustrated in the hematopoietic system. Depending on the degree of maturation of the hematopoietic cells, these factors are able to control either positively or negatively cell proliferation, differentiation, or apoptosis. The importance of TGF-βs in the development of multicellular organisms is stressed by their high degree of conservation during evolution and by the fact that they are present in most tissues where they are stored in a latent form, ready for rapid local activation. Moreover, TGF-βs are often involved in autocrine control loops, which allow precise tuning at the cell level.
Despite the considerable numbers of studies performed to understand the complex regulatory role of TGF-βs in hematopoiesis—many of which could not be cited here because of space limitations—many questions remain. For example, the physiologic concentrations of active TGF-β that control each particular function in vivo remain unknown. This is of particular importance because different specific responses can be observed in vitro depending on the concentration of TGF-β used, which in the literature ranges from 10 pg/mL to more than 10 ng/mL. Moreover, it remains unclear whether TGF-βs, which block the cell cycle in G1,259 can promote a return to G0. A progressive disappearance of G1 mRNAs could explain the reversion to quiescence induced by TGF-β. Different degrees of cell-cycle inhibition by TGF-βs may exist, ranging from profound quiescence to a slow cycling state. Whether hematopoietic stem/progenitor cells maintained in a slow cycling state by TGF-βs could be amplified in vitro more efficiently, without differentiation and senescence, must be studied further to define optimal in vitro culture systems for cell therapy.
In this review, we have focused on studies performed on TGF-β1, -β2, and -β3. Other members of the TGF-β superfamily, such as the BMPs, should also be considered as important regulators of hematopoiesis. Indeed, in addition to being involved in the control of bone formation, BMPs could regulate the developmental program of human hematopoietic stem cells, as do TGF-βs, including cell proliferation, differentiation, and stem cell survival.260
Signal transduction by the members of the TGF-β family involves interactions among multiple possible receptors and intracellular signaling proteins, allowing the diversity of specific biologic responses. Many postgenomic studies will certainly be required to unravel the complexity of this key regulatory system. These studies will probably lead to the understanding of many different pathologies. In a few cases, gene therapy will perhaps enable the correction of a single gene mutation responsible for a disease. In most cases, it will not be easy to develop a drug sufficiently specific for one single combination of regulatory proteins in a specific cell type, without affecting other cells. Antisense oligonucleotides have been used successfully in vitro to neutralize the activity of genes involved in TGF-β signal transduction. This strategy has been efficient for short time effects, such as a rapid activation of quiescent stem/progenitor cells. However, these experiments were performed using purified cells, which is far from being representative of the in vivo situation, where it is difficult to target the treatment to a specific cell population. Promoters controlled by TGF-β, such as the promoter of collagen VII, which is involved in the pathogenesis of myelofibrosis, or oncogenes involved in leukemogenesis, such as Evi-1, could be potential targets to devise more specific drugs to treat diseases caused by dysregulation of TGF-β production or activity.
In conclusion, the development of therapeutic agents to correct, in vivo, dysfunctions of the TGF-β signaling pathway represents an important challenge for the future. However, a main difficulty will come from the pleiotropism of its regulatory role.
Acknowledgment
We are indebted to Dr Mary Osborne for her critical reading of the manuscript.
Supported by European Contract no. BIO4-CT96-0646, the Centre National de la Recherche Scientifique (CNRS), and the Association pour la Recherche sur le Cancer (ARC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jacques A. Hatzfeld, Laboratoire de Biologie des Cellules Souches Somatiques Humaines, UPR 1983, Centre National de la Recherche Scientifique, IFC1, 7, rue Guy Môquet, 94800, Villejuif, France; e-mail: hatzfeld@infobiogen.fr.