Abstract
A prospective multicenter trial of 119 children 1 to 18 years of age with newly diagnosed aplastic anemia (AA) was conducted, comparing treatment using antithymocyte globulin (ATG), cyclosporine (CyA), and danazol (DAN) with or without rhG-CSF (400 μg/m2, day on days 1-90). All children with very severe AA received rhG-CSF (VSAA group, n = 50). The other children were randomized to receive ATG, CyA, DAN, and rhG-CSF (G-CSF+ group, n = 35) or ATG, CyA, and DAN without rhG-CSF (G-CSF− group, n = 34). After 6 months, the hematologic response rate was 71%, 55%, and 77% in the VSAA group, G-CSF+ group, and G-CSF− group, respectively. There was no difference in the incidence of febrile episodes and documented infections between the G-CSF+ and G-CSF− groups. Bone marrow transplantation (BMT) was attempted in 22 patients in whom initial immunosuppressive therapy (IST; n = 18) failed or in whom a relapse occurred after an initial response (n = 4). Nineteen of the 22 patients are alive and well after a median follow-up of 18 months (range, 3 to 66 months) since BMT. The probability of survival at 4 years was 83% ± 7% in the VSAA group, 91% ± 5% in the G-CSF+ group, and 93% ± 6% in the G-CSF− group. Myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) developed in one patient in each of the three groups; the overall risk for MDS/AML was 3% ± 2% at 4 years. Because the results of IST were encouraging, it is suggested that children with AA receive IST as first-line therapy if there is no human leukocyte antigen-matched sibling donor.
Introduction
Acquired aplastic anemia (AA) is an uncommon but serious disorder characterized by pancytopenia resulting from nonfunction of the bone marrow. Although bone marrow transplantation (BMT) from a human leukocyte antigen (HLA)-identical sibling donor is the treatment of choice, this approach is limited by the availability of such donors. Immunosuppressive therapy (IST) has been an alternative treatment for patients who do not have suitable donors. Several recent studies have shown encouraging results with a combination of antithymocyte globulin (ATG) and cyclosporine (CyA),1,2but the patients treated in these studies were primarily adults. There are several differences in disease characteristics, treatment methods, and therapeutic response between children and adults that have received little attention.3
Several hematopoietic growth factors have been used as single agents in the treatment of AA and in combination with IST.4 Among them, recombinant human granulocyte colony-stimulating factor (rhG-CSF) and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) have been the most extensively evaluated.5,6Both rhG-CSF and rhGM-CSF can effect a transient increase of the neutrophil count in most patients with AA. Death from infectious complications is a risk associated with IST for AA, but the use of hematopoietic growth factors may lower this risk and improve the chance for a good response. In fact, a European pilot study of ATG, CyA, and rhG-CSF therapy showed encouraging results in 40 patients with severe AA.7
Recently, several studies have suggested that long-term survivors of AA who have been treated with IST are at risk for myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).8,9 Our retrospective analysis showed that MDS/AML with cytogenetic abnormalities developed in 11 of 62 children treated with immunosuppressive agents and rhG-CSF.10 Because hematopoietic growth factors can stimulate leukemic clones, long-term administration may facilitate the progression of AA to MDS or AML.11 These findings formed the basis for our prospective, randomized, controlled study comparing ATG and CyA therapy with or without rhG-CSF in children with AA. This is the largest prospective multicenter study of childhood AA.
Patients, materials, and methods
Patients
This multicenter trial was designed by the Japan Childhood Aplastic Anemia Study Group and involved 49 hospitals. Patients with acquired AA were eligible if they met the following criteria: age younger than 18 years, recently diagnosed disease (within 180 days) without specific prior treatment, and moderate to very severe AA. The disease was considered severe if at least two of the following were noted: a neutrophil count of less than 0.5 × 109/L, a platelet count of less than 20 × 109/L, and a reticulocyte count of less than 20 × 109/L with hypocellular bone marrow.12 AA was considered very severe if the criteria for severe disease were fulfilled and the neutrophil count was below 0.2 × 109/L. Moderate disease was defined by at least 2 of the following hematologic values: a neutrophil count of less than 1.0 × 109/L, a platelet count of less than 50 × 109/L, and a reticulocyte count of less than 60 × 109/L with hypocellular bone marrow.
Patients were excluded if they had congenital AA or paroxysmal nocturnal hemoglobinuria with positive findings on the Ham test/sucrose test. Cytogenetic studies were performed for all patients. Patients with clonal cytogenetic abnormalities were included in the study if their bone marrow aspirate findings did not meet the criteria for MDS.
Treatment protocol
All patients with very severe AA were administered ATG, CyA, DAN, and rhG-CSF. Patients who had severe AA and neutrophil counts of more than 0.2 × 109/L and patients who had moderate AA were randomized to receive either ATG, CyA, DAN, and rhG-CSF or ATG, CyA, and DAN. Horse ATG (Lymphoglobuline; Merieux, Lyon, France) was administered at a dose of 1.5 vials/10 kg per day for 5 days as a slow intravenous infusion over 12 hours. For the prevention of serum sickness, methylprednisolone (2 mg/kg per day) was administered intravenously on days 1 to 7. Then methylprednisolone was given at 1 mg/kg per day orally on days 8 to 14, and the dose was tapered to end on day 30. Cyclosporine (6 mg/kg per day orally) was started on day 1 and continued at least until day 180. The dose was adjusted to achieve a whole blood trough level of 100 to 200 ng/mL. Blood levels of cyclosporine were measured by radioimmunoassay with monoclonal antibody. Danazol (5 mg/kg per day orally) was started on day 1 and continued until day 180. rhG-CSF (Filgrastim; Kirin-Sankyo, Tokyo, Japan) was administered intravenously or subcutaneously at a dose of 400 μg/m2 per day from day 1 to day 90. After the neutrophil count reached more than 5 × 109/L, it was administered 3 times a week.
Evaluation of response and toxicity
Complete response (CR) was defined as a neutrophil count >1.5 × 109/L, a platelet count >100 × 109/L, and a hemoglobin level of >11.0 g/dL. Partial response (PR) was defined as a neutrophil count >0.5 × 109/L, a platelet count 20 × 109/L, and a hemoglobin level of >8.0 g/dL in patients with severe or very severe AA. It was defined as a neutrophil count 1.0 × 109/L, a platelet count >30 × 109/L, and a hemoglobin level of >8.0 g/dL in patients with moderate AA. Relapse was indicated by the return of the peripheral blood cell counts to levels meeting the definition of severe or moderate AA and the requirement for blood transfusion. Toxicity of treatment was evaluated for the first 3 months and was graded according to the criteria of the World Health Organization.13
All participating hospitals were required to complete case report forms. Survival and relapse were analyzed using the Kaplan-Meier method.14 Differences between treatment groups were evaluated by the log-rank test and the generalized Wilcoxon test. The Fisher Exact test was used to compare response rates. The Mann-WhitneyU test or the Student t-test was used to compare continuous variables. All comparisons were made with two-tailed tests. Informed written consent was obtained from all patients or their parents, and the study was approved by the ethics committee of each participating hospital.
Results
Patient characteristics
From November 1992 to September 1997, 119 children with newly diagnosed AA were entered in the study. Interim analysis was performed in April 1999. Fifty patients fulfilled the criteria for very severe AA (VSAA group) and were treated with ATG, CyA, DAN, and rhG-CSF. In addition, 35 patients were randomized to receive ATG, CyA, DAN, and rhG-CSF (G-CSF+ group), and 34 were randomized to receive ATG, CyA and DAN (G-CSF− group). Nine patients were excluded from analysis because of a diagnosis of MDS after randomization (1 patient), treatment without ATG (6 patients), or bone marrow transplantation within 3 months of diagnosis (2 patients). Clinical characteristics of the G-CSF+ and G-CSF− groups were comparable (Table1). AA was associated with hepatitis in 21 patients; it was drug-induced in 1 patient and of unknown etiology in the others. Among the patients randomized to the G-CSF− arm, 6 patients with infections received rhG-CSF at a dose of 400 μg/m2 per day for a median of 6 days (range, 3 to 40 days). Based on the intent-to-treat principle, these 6 patients were analyzed as part of the G-CSF− group.
Neutrophil response
Neutrophil recovery occurred earlier in the G-CSF+ group than in the G-CSF− group. After 2 weeks, the mean absolute neutrophil count increased to 5.5 ± 5.5 × 109/L, and a plateau was observed subsequently in the G-CSF+ group. In the G-CSF− group, the mean absolute neutrophil count increased to 2.1 ± 1.5 × 109/L after 2 weeks and decreased to 0.84 ± 0.46 × 109/L after 4 weeks; a plateau was observed thereafter. There was a significant difference in the mean absolute neutrophil count between the G-CSF+ and the G-CSF− groups during the first 3 months of therapy. In the VSAA group, the mean absolute neutrophil count increased to 3.1 ± 4.7 × 109/L after 2 weeks, and a plateau was reached thereafter.
Trilineage response
After 3 months, CR was observed in 4 (9%) and PR in 17 (38%) patients, for an overall response rate of 47% in the VSAA group (Table2). In the G-CSF+ group, 5 (15%) patients achieved CR and 8 (24%) achieved PR, for an overall response rate of 39%. In the G-CSF− group, 1 (3%) patient achieved CR and 16 (50%) achieved PR, for an overall response rate of 53%. There were no statistically significant differences in overall response rates between the G-CSF+ and the G-CSF− groups (P = .21). After 6 months, the overall response rate increased from 47% to 71% in the VSAA group, from 39% to 55% in the G-CSF+ group, and from 53% to 77% in the G-CSF− group. The difference in response rates between the G-CSF+ and the G-CSF− groups was still not statistically significant (P = .18). Overall response rates showed little change between 6 months and 12 months in each group. Results were similar when the 36 patients with severe AA were analyzed separately. After 6 months, 11 of 18 (61%) patients randomized to the G-CSF+ group responded compared with 15 of 18 (83%) patients randomized to the G-CSF− group. The difference was not significant (P = .26).
Infectious complications
During the first 3 months of therapy, documented infections developed in 11 of 46 patients from the VSAA group (Table3), including severe infections such as bacteremia (4 patients), pneumonia (2 patients), and splenic abscess (1 patient). Two patients whose neutrophil counts remained critically low died of bacteremia at 35 days and 102 days after the start of treatment. Pneumonia developed in only one patient in the G-CSF+ group, and no severe infections developed in the G-CSF− group. Including mild to moderate infections, there was no difference in the incidence of documented infections between the G-CSF+ group and the G-CSF− groups. The median number of febrile days (>38°C) was also the same in the two groups. Three patients contracted typical interstitial pneumonitis at 38, 60, and 68 days after the start of IST, and 2 of them died. Cytomegalovirus was detected in the peripheral blood cells of 2 of them by the antigenemia assay or the polymerase chain reaction assay. It is noteworthy that all 3 patients had hepatitis-associated AA and that none of the patients with idiopathic AA had this complication.
Survival
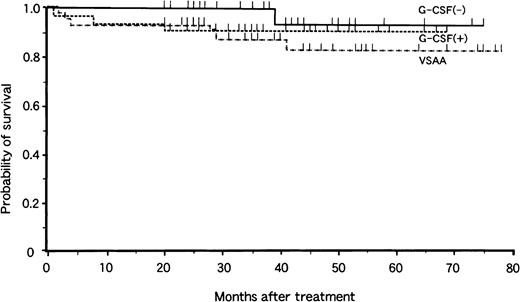
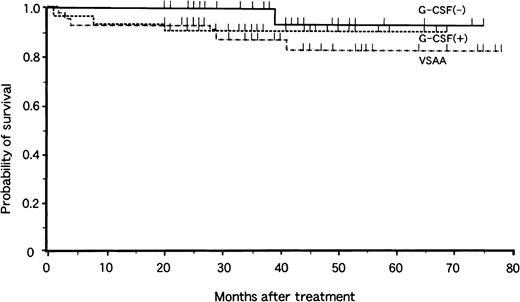
Analysis of survival at 4 years revealed no significant difference related the mode of treatment (Fig. 1). The overall probability of survival was 83% ± 7% in the VSAA group, 91% ± 5% in the G-CSF+ group, and 93% ± 6% in the G-CSF− group, with a median follow-up period of 40 months (range, 20 to 78 months), 36 months (range, 20 to 75 months), and 37 months (range, 20 to 78 months), respectively. There were 6 deaths in the VSAA group, 3 in the G-CSF+ group, and 1 in the G-CSF− group. Causes of death were bacteremia (2), interstitial pneumonitis (2), BMT-related toxicity (3), MDS (1), intracranial hemorrhage (1), and car accident (1).
Actuarial survival of children with aplastic anemia in the VSAA group (n = 46), the G-CSF+ group (n = 33), and the G-CSF− group (n = 31).
Tick marks denote surviving patients.
Actuarial survival of children with aplastic anemia in the VSAA group (n = 46), the G-CSF+ group (n = 33), and the G-CSF− group (n = 31).
Tick marks denote surviving patients.
Cytogenetic analysis and clonal disease
At the time of diagnosis, an adequate number of mitoses for cytogenetic studies were available in 101 of 119 patients. A clonal cytogenetic abnormality was detected in 3 of 101 (2.9%) patients, who otherwise had morphologically typical AA. None of these 3 patients had MDS/AML, and the disappearance of clonal abnormalities was observed during follow-up in 2 of them.
New clonal abnormalities appeared in 7 patients after IST: trisomy 8 (3 patients), monosomy 7 (2 patients), trisomy 11 (1 patient), and del (13) (1 patient). Three of the 7 patients with cytogenetic abnormalities (2 with monosomy 7 and 1 with trisomy 11) had a dysplastic morphology, and MDS developed at 9, 11, and 17 months after the diagnosis of AA. In these 3 patients, a normal karyotype was confirmed and no morphologic abnormalities were detected at the time of diagnosis of AA. One patient with very severe AA did not respond to initial therapy and remained dependent on blood transfusions and rhG-CSF. He was treated with rhG-CSF for 510 days and died of leukemic transformation. Nine months after the diagnosis of AA, 1 patient randomized to the G-CSF− arm and not given rhG-CSF contracted refractory anemia with excess of blasts (RAEB) with monosomy 7. Another patient randomized to the G-CSF+ arm was given rhG-CSF for 85 days and contracted RAEB with trisomy 11. Both patients underwent bone marrow transplantation from alternative donors and are still alive. Because distinctive morphologic features of MDS were not found in 3 patients with trisomy 8 and 1 patient with del (13), MDS was not diagnosed. Malignant disease did not develop in either of them during the follow-up period, nor were the symptoms of paroxysmal nocturnal hemoglobinuria observed in any patient.
Relapse
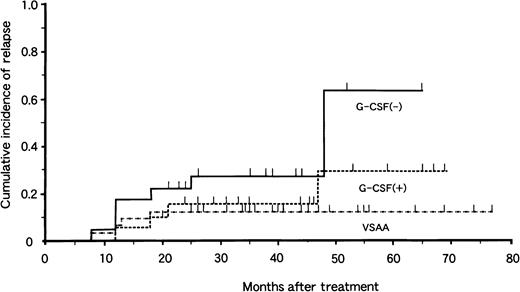
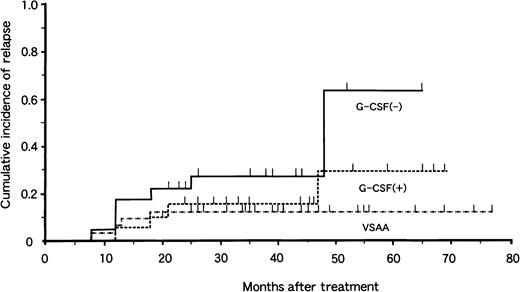
Of the 77 patients with responses, 17 had relapses 7 to 48 months after treatment. The risk for relapse at 4 years was 13% ± 6% in the VSAA group, 29% ± 15% in the G-CSF+ group, and 64% ± 19% in the G-CSF− group (Fig. 2). There was no significant difference in relapse rate between the G-CSF+ and G-CSF− groups (P = .10). Among the 17 patients who had relapses, 9 received a second course of IST with ATG and CyA. Three of 9 patients responded to the second therapy. BMT from an HLA-matched unrelated donor was attempted in 4 patients, and 1 died of complications related to BMT. Thus, 16 patients are alive at 3 to 52 months after relapse.
Cumulative incidence of relapse in children with aplastic anemia in the VSAA group (n = 46), the G-CSF+ group (n = 33), and the G-CSF− group (n = 31).
Tick marks denote surviving patients.
Cumulative incidence of relapse in children with aplastic anemia in the VSAA group (n = 46), the G-CSF+ group (n = 33), and the G-CSF− group (n = 31).
Tick marks denote surviving patients.
Bone marrow transplantation
BMT was attempted in 22 patients in whom the initial IST failed (n = 8) or who had relapses after initial responses (n = 4). Marrow donors included HLA-identical siblings (n = 3), HLA-mismatched family members (n = 4), and HLA-matched unrelated donors (n = 15). The median time between initial IST and BMT was 17 months (range, 3 to 66 months). Two patients with unrelated donors and one with an HLA-mismatched family donor died at 1, 8, and 16 months after transplantation. Causes of death were cardiac failure, graft rejection, and chronic graft-versus-host disease (GVHD).
Toxicity
Overall, treatment was very well tolerated. Only one patient could not complete treatment, and this patient experienced an anaphylactic reaction to ATG. The incidence of side effects associated with ATG and CyA therapy was similar in the G-CSF+ and G-CSF− groups. Typical side effects of CyA, such as renal toxicity and tremor, never exceeded grade 1. We did not observe any toxicity attributable to rhG-CSF, such as fever, myalgia, or bone pain.
Discussion
The results of this prospective multicenter study showed an improved prognosis for children with acquired AA when intensive immunosuppressive therapy is given. Overall, 71% of the patients responded within 6 months, and the probability of survival at 4 years was more than 90%. Recently, a German pediatric group also reported the results of combined immunosuppressive therapy with rhG-CSF in newly diagnosed AA in children.15 Ninety-three children with very severe, severe, and moderate AA (n = 54, 31, and 8, respectively) were entered in the trial between 1994 and 1998. At first, rhG-CSF was administered for patients with very severe and severe AA. Since 1996, it was administered only for patients with very severe AA. The response rate and actuarial survival rate were compatible with those obtained in our study. Complete or partial response was achieved in 74% of the patients, and the probability of survival at 4 years was 85%. In patients treated with a single immunosuppressive agent, low neutrophil count and young age have been reported16 to be poor prognostic factors. An earlier European study17 found only one long-term survivor among 11 children younger than 5 years who had a neutrophil count of less than 0.2 × 109/L after ATG therapy. In the current study, all 12 children with these same characteristics are still alive. Although the number of patients who died in the current study was small, making the analysis of prognostic factors difficult, neither young age nor low neutrophil count seems to be associated with poor outcome.
Several hematopoietic growth factors have been used in the treatment of AA, both as single agents and in combination with IST. Among them, rhG-CSF has been the most extensively evaluated and has been shown to cause a transient increase in neutrophil count in most patients.5,18 In the European pilot study, 40 patients received a combination of ATG, CyA, and rhG-CSF, with 82% achieving trilineage hematopoietic reconstitution and an actuarial survival rate of 92% at 2 years.7 These encouraging data formed the basis of later prospective randomized trials. Although we found that rhG-CSF was well tolerated and that it accelerated neutrophil recovery, there was no difference in the trilineage response, incidence of documented infections, and overall survival between the G-CSF+ group and the G-CSF− group. At the same time, the European group conducted a randomized trial aimed at evaluating the effect of rhG-CSF in combination with ATG and CyA in 102 patients with severe and very severe AA19; it included children and adults. Results showed that there was no difference between the G-CSF+ and the G-CSF− groups in the rate of a trilineage response and the 2-year survival rate. It was concluded that rhG-CSF therapy did not modify long-term hematopoietic recovery and survival in patients with severe and very severe AA.
Expert panelists of the American Society of Clinical Oncology have proposed evidence-based guidelines for the use of hematopoietic growth factors.20 Of the endpoints they considered when evaluating the benefit of hematopoietic growth factors, they placed great weight on overall survival rates, reduction of documented infections, decreased hospitalization, and reduced costs, whereas they assigned less weight to alterations in the absolute neutrophil count. Our results suggested that there is no good reason to use rhG-CSF in combination with immunosuppressive agents as the initial therapy for patients with neutrophil counts greater than 0.2 × 109/L. The incidence of severe infections was so low that it was difficult for meaningful comparison of the G-CSF+ and G-CSF− groups. On the other hand, life-threatening infections were more common in patients with very severe AA who received rhG-CSF, because their infections occurred early during treatment before neutrophil recovery had begun. Hence, the potential role of rhG-CSF in treating patients with very severe AA warrants further study.
Unexpectedly, typical interstitial pneumonitis developed in 3 of 21 (14%) patients with hepatitis-associated AA after treatment. Cytomegalovirus was detected in peripheral blood cells from 2 of them. We believe that this represents the first report of cytomegalovirus pneumonitis after IST in patients with AA. Cytomegalovirus pneumonitis is a common cause of significant sickness and of death in severely immunocompromised patients. Abnormal cellular or humoral immunity is noted in patients with hepatitis-associated AA.21,22 We previously reported a marked decrease of CD4+ lymphocytes in these patients.23 Therefore, it seems that intensive immunosuppression with ATG and CyA may cause cytomegalovirus pneumonitis in immunocompromised hosts. B-cell lymphoproliferative disorders related to Epstein-Barr virus are also known to develop in severely immunocompromised hosts, as is cytomegalovirus pneumonitis. Such a disorder also arose in a patient with hepatitis-associated AA who was treated with ATG and CyA.24 Thus, prophylactic therapy and virologic surveillance for cytomegalovirus are recommended after IST in patients with hepatitis-associated AA.
The most difficult to treat patients with severe AA are those who do not respond to intensive immunosuppression, and they continue to have poor prognoses and die of infection or bleeding. Salvage therapy is limited to BMT from alternative donors, such as HLA-partially matched family members or HLA-matched unrelated donors. To date, the results of BMT using alternative donors have generally been unsatisfactory in patients with AA. There is a high incidence of graft rejection and severe or fatal GVHD. In the National Marrow Donor Program Study,25 only 29% of 31 patients with AA who underwent transplantation from unrelated donors survived for 2 years or longer. Higher engraftment and survival rates were reported by the Milwaukee group,26 who studied 28 young patients treated with intensive conditioning and partial T-cell depletion. In their study, the probability of survival at 4 years was 54%. We offered BMT from alternative donors to 19 patients in whom IST failed or who had relapses. Sixteen of 19 (84%) patients are alive and well after a median follow-up of 21 months since BMT (range, 3 to 66 months). The inclusion of irradiation and ATG in the preparations for transplantation may reduce graft rejection.27 In addition, the smaller diversity of histocompatibility antigens among Japanese might be associated with a low incidence of severe acute GVHD.28 Considering the very encouraging results, alternative donor BMT is recommended as salvage therapy in patients with severe AA who have not responded to initial IST or who relapse after the initial response.
One of the objectives of this study was to investigate whether the therapeutic modality influenced the development of MDS/AML in patients with AA. MDS/AML developed in 1 patient in each of the 3 groups, and the overall incidence of MDS/AML was 3% ± 2% at 6 years. Our previous study showed that the probability of developing MDS/AML was 47% ± 17% in patients who received IST and rhG-CSF.10However, cytogenetic analysis of bone marrow cells could not be performed at the time of AA diagnosis in a considerable percentage of the patients in the study. Among the 85 patients treated with ATG, CyA, DAN, and rhG-CSF, the cumulative incidence of MDS/AML was 2 ± 3% in the current study. In the previous study, the median duration of rhG-CSF administration was 25 months (range, 11 to 73 months) in the 11 patients in whom MDS/AML developed. Six of these 11 patients received rhG-CSF at doses exceeding 10 μg/kg per day. Administration of rhG-CSF for more than 1 year was the most important risk factor for MDS/AML in adult patients with AA.29 Based on these observations, patients treated for longer periods or given higher cumulative doses of rhG-CSF seem to be at increased risk for MDS/AML. In the current study, rhG-CSF was only administered for the first 3 months of treatment. In an exceptional nonresponder treated with rhG-CSF for 510 days, MDS developed with monosomy 7. The different durations of rhG-CSF administration seemed to be the cause of the difference in the incidence of MDS/AML between our two studies.
Cytogenetic studies on marrow samples are not commonly performed in patients with AA, primarily because of the difficulty in obtaining sufficient cells for analysis, especially at the onset of the disease. Thus, there are few prospective studies on cytogenetic abnormalities in acquired AA. In the current study, an adequate number of mitoses was available for 101 of 119 patients before the start of therapy, and a clonal cytogenetic abnormality was detected in 3 of 101 patients with otherwise morphologically typical AA. Between 4% and 11% of patients with AA are reported to have chromosomal abnormalities at diagnosis.30,31 Interestingly, 2 of 3 patients with cytogenetic abnormalities responded to initial IST, and their karyotypic abnormalities disappeared. None of them evolved to MDS/AML. Geary et al32 also reported that 8 of 13 patients with AA who had an abnormal cytogenetic clone responded to IST with or without oxymetholone. These results suggest that IST should be given to patients with the morphologic diagnosis of AA but with an abnormal karyotype.
New abnormal cytogenetic clones were detected in 7 patients during follow-up, 2 of whom were part of the G-CSF− group. Cytogenetic abnormalities developed after treatment at comparable rates in the G-CSF+ and G-CSF− groups, but the follow-up period is still too short to evaluate the true risk. We believe it is important to continue to observe this cohort of patients for a long time to access the risk for late clonal disease.
In conclusion, although rhG-CSF is safe for short-term administration, the addition of rhG-CSF to IST has no benefit in terms of the hematologic response, incidence of documented infection, and overall survival rate in children with AA whose neutrophil counts are greater than 0.2 × 109/L. Therefore, there is no good reason to use rhG-CSF as an adjunct to initial IST therapy. Results of IST therapy were very encouraging in the current study. Children with AA should receive IST as first-line therapy if there is no HLA-identical sibling donor. Alternative donor BMT offers a good chance of survival for those who do not respond to intensive IST. The excellent overall survival rate in this study reflects the results of salvage treatments and of initial therapy.
The following centers and persons participated in the Japan Childhood Aplastic Anemia Study Group: Japanese Red Cross Nagoya First Hospital (T. Matsuyama, K. Kato); Kyoto Prefectural University of Medicine (S. Hibi); Kobe University School of Medicine (Y. Kosaka); Hyogo College of Medicine (M. Yamamoto); Ibaragi Children's Hospital (M. Tsuchida); Nihon University (H. Mugishima); Yamanashi Medical University (K. Sugita); Tokai University (H. Yabe); Toho University School of Medicine (A. Ohara, I. Tsukimoto); Shizuoka Children's Hospital (J. Mimaya); Shinshu University School of Medicine (K. Koike); Fukushima Medical University (A. Kikuta); Chiba Children's Hospital (Y. Okimoto); Kiyose Children's Hospital (T. Kaneko); Tokyo Medical and Dental University (Y. Ohkawa); Osaka City General Hospital (M. Sako); Nagoya University (S. Kojima, K. Horibe); Jichi Medical School (T. Yamauchi); Mie University (E. Azuma); Sapporo Medical University (T. Kudo); Kagoshima University (K. Kawakami); Shiga University of Medical Science (S. Ohta); The Jikei University School of Medicine (K. Fujisawa); Toyohashi City Hospital (Y. Nishimura); Okayama University (M. Oda); Hokkaido University (R. Kobayashi); Hiroshima University (K. Ueda); Fukuoka University (K. Nibu); Sapporo National Hospital (Y. Hatae); Nara Medical University (Y.-D. Park); Nagoya National Hospital (J. Yoshida); Akita University School of Medicine (A. Watanabe); Kyushu University (S. Ohga); Yamagata University (Y. Shimizu); Kinki University School of Medicine (H. Miyata); Osaka University Faculty of Medicine (J. Hara); Showa University School of Medicine (K. Isoyama); Tohoku University (M. Imaizumi); Kyoto First Red Cross Hospital (H. Ikuta); Maebashi Red Cross Hospital (M. Shimoda); Kyoto University (Y. Akiyama); Osaka Medical College (M. Miyake); Okazaki City Hospital (N. Nagai); Ohtsu Red Cross Hospital (T. Takimoto); Fukui Red Cross Hospital (S. Hayashi); Kyoto Second Red Cross Hospital (S. Matsuo); Kurume University (H. Eguchi); Yokohama City University (K. Ikuta); and Dokkyo University School of Medicine (K. Sugita).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Seiji Kojima, Department of Developmental Pediatrics, Nagoya University School of Medicine, 65 Tsurumai-chou, Shouwa-ku, Nagoya 466-8550, Japan; e-mail: kojimas@med.nagoya-u.ac.jp.