Abstract
Two randomized, placebo-controlled trials previously showed that fluconazole (400 mg/d) administered prophylactically decreases the incidence of candidiasis in blood and marrow transplant (BMT) recipients. However, there exists conflicting data regarding the optimal duration of fluconazole administration, specifically whether prophylaxis through acute graft-versus-host disease (GVHD) results in improved survival in allograft recipients. Reported here are the results of long-term follow-up and a detailed analysis of invasive candidiasis and candidiasis-related death in 300 patients who received fluconazole (400 mg/d) or placebo for 75 days after BMT at the Fred Hutchinson Cancer Research Center. Patients in both treatment arms were compared for survival, causes of death, and the incidence of invasive fungal infections early (less than 110 days) and late (more than 110 days) after BMT. After 8 years of follow-up, survival is significantly better in fluconazole recipients compared with placebo recipients (68 of 152 vs 41 of 148,P = .0001). The overall incidence of invasive candidiasis was increased in patients who received placebo compared with fluconazole (30 of 148 vs 4 of 152, P < .001). More patients who received placebo died with candidiasis early (13 of 148 vs 1 of 152, P = .001) and late (8 of 96 vs 1 of 121,P = .0068) after BMT. The incidence of severe GVHD involving the gut was higher in patients who did not receive fluconazole (20 of 143 vs 8 of 145, P = .02), and fewer patients who received fluconazole died with this complication. Thus, administration of fluconazole (400 mg/d) for 75 days after BMT appears to be associated with decreased gut GVHD, a persistent protection against disseminated candidal infections and candidiasis-related death, resulting in an overall survival benefit in allogeneic BMT recipients.
Introduction
Two large randomized, placebo-controlled trials performed in the early 1990s documented that fluconazole (400 mg/d), administered for prophylaxis after allogeneic and autologous blood and marrow transplantation (BMT), confers a decreased risk for superficial and invasive fungal infections.6,18 These studies led to the routine use of fluconazole for prophylaxis after BMT. However, the 2 trials differed in several ways. One used fluconazole only during the period of neutropenia and enrolled primarily autologous graft recipients6 and the other used fluconazole during neutropenia and acute graft-versus-host disease (GVHD) (75 days) and enrolled primarily allogeneic graft recipients.18 Both trials found that fluconazole prophylaxis is associated with a decrease in invasive fungal infections, but only the latter trial showed a decrease in overall mortality in fluconazole recipients. Curiously, this overall mortality benefit did not seem to be explained by a decrease in fungal infections in the initial study.18However, this study did not analyze candidiasis- and mold-related deaths separately, which may have obscured the effect of fluconazole on candidiasis-related death.
Since the publication of these 2 studies, much debate has centered around the optimal duration of fluconazole prophylaxis in BMT recipients.4,8,9 12 Many authorities, including a recent Centers for Disease Control and Prevention study group, recommend the use of fluconazole only until engraftment in allogeneic graft recipients (C. Dykewitz, written communication, July 1999). This is largely because the mortality benefit associated with 75-day fluconazole administration has not been adequately explained. It has been suggested that the mortality benefit observed in the original 75-day prophylaxis trial might actually represent a statistical aberration. Also, because fluconazole prophylaxis might only delay inevitable mortality in BMT recipients, the observed mortality benefit might wane late after BMT. To resolve this issue, we examined the long-term outcome of patients enrolled in our initial randomized trial, with emphasis placed on the anti-Candida selectivity of fluconazole and specific host risks.
Patients, materials, and methods
Patients and randomized study design
Patients who were enrolled in the previous randomized, placebo-controlled, double-blinded study underwent BMT at Fred Hutchinson Cancer Research Center (FHCRC), Seattle, WA, between June 1990 and February 1992.18 Both autologous and allogeneic BMT recipients were included in the trial and randomized with stratification for type of BMT (88% allogeneic vs 12% autologous), HLA-matched status, and type of isolation (laminar airflow vs conventional). A total of 152 patients were randomized to receive fluconazole (400 mg/d) and 148 patients were randomized to receive placebo. Demographic and baseline clinical characteristics (age, gender, underlying disease, cytomegalovirus [CMV] serostatus, and transplant characteristics [type, HLA match, receipt of BMT in a laminar airflow room, conditioning regimen, GVHD prophylaxis, and marrow cell dose]) were evenly distributed between the treatment arms (Table 1).
Patient demographics and transplant characteristics
| Factor . | Fluconazole (n = 152) . | Placebo (n = 148) . | P . |
|---|---|---|---|
| Mean age, years (range) | 36.4 (13, 60) | 36.1 (11, 65) | .80 |
| Gender | |||
| Male | 85 | 89 | .46 |
| Female | 67 | 59 | |
| Underlying disease | .06 | ||
| Acute lymphocytic leukemia | 9 | 22 | |
| Acute nonlymphocytic leukemia | 35 | 24 | |
| Chronic lymphocytic leukemia | 62 | 50 | |
| Hodgkin lymphoma | 7 | 13 | |
| Non-Hodgkin lymphoma | 18 | 16 | |
| Other | 21 | 23 | |
| Transplant type | .99 | ||
| Autologous | 18 | 17 | |
| HLA matched allogeneic | 78 | 76 | |
| HLA mismatched or unrelated allogeneic | 56 | 55 | |
| Transplant in LAF | .51 | ||
| Yes | 52 | 56 | |
| No | 100 | 92 | |
| Conditioning regimen | |||
| BU + CY | 24 | 32 | |
| BCNU, CY, VP-16 | 13 | 16 | |
| CY + TBI (≤1200 cGy) | 52 | 38 | |
| CY + TBI (>1200 cGy) | 61 | 59 | .43 |
| Other | 2 | 3 | |
| GVHD prophylaxis* | .518 | ||
| Cyclosporin A | 21 | 26 | |
| Cyclosporin A and methotrexate | 101 | 95 | |
| Methotrexate | 6 | 8 | |
| Other | 5 | 2 | |
| Marrow cell dose: mean (range) | 192 (23, 641) | 199 (58, 656) | .51 |
| Recipient CMV serology | |||
| Positive | 62 | 56 | .60 |
| Negative | 90 | 92 |
| Factor . | Fluconazole (n = 152) . | Placebo (n = 148) . | P . |
|---|---|---|---|
| Mean age, years (range) | 36.4 (13, 60) | 36.1 (11, 65) | .80 |
| Gender | |||
| Male | 85 | 89 | .46 |
| Female | 67 | 59 | |
| Underlying disease | .06 | ||
| Acute lymphocytic leukemia | 9 | 22 | |
| Acute nonlymphocytic leukemia | 35 | 24 | |
| Chronic lymphocytic leukemia | 62 | 50 | |
| Hodgkin lymphoma | 7 | 13 | |
| Non-Hodgkin lymphoma | 18 | 16 | |
| Other | 21 | 23 | |
| Transplant type | .99 | ||
| Autologous | 18 | 17 | |
| HLA matched allogeneic | 78 | 76 | |
| HLA mismatched or unrelated allogeneic | 56 | 55 | |
| Transplant in LAF | .51 | ||
| Yes | 52 | 56 | |
| No | 100 | 92 | |
| Conditioning regimen | |||
| BU + CY | 24 | 32 | |
| BCNU, CY, VP-16 | 13 | 16 | |
| CY + TBI (≤1200 cGy) | 52 | 38 | |
| CY + TBI (>1200 cGy) | 61 | 59 | .43 |
| Other | 2 | 3 | |
| GVHD prophylaxis* | .518 | ||
| Cyclosporin A | 21 | 26 | |
| Cyclosporin A and methotrexate | 101 | 95 | |
| Methotrexate | 6 | 8 | |
| Other | 5 | 2 | |
| Marrow cell dose: mean (range) | 192 (23, 641) | 199 (58, 656) | .51 |
| Recipient CMV serology | |||
| Positive | 62 | 56 | .60 |
| Negative | 90 | 92 |
Data from original study reproduced with permission of editor.18 Note small differences in numbers occurred as a result of updating of computerized database and correction of typographical error.
BU indicates busulfan; CY, cyclophosphamide; BCNU, bischloroethylnitrosourea; TBI, total body irradiation; GVHD, graft-versus-host disease; CMV, cytomegalovirus.
GVHD prophylaxis for allogeneic patients. No data was available for one fluconazole recipient.
Patients received the study drug in a blinded fashion from the day of conditioning chemotherapy until 75 days after BMT or until the patient developed a documented fungal infection or severe adverse effects. Patients thus received the drug for a mean duration of 64 days.18 In the original analysis, patients were evaluated for invasive fungal infection and survival at the end of study drug administration, 2 weeks after the drug was discontinued (89 days), and at the time of discharge (110 days).18 This analysis concentrates on the long-term follow-up of this cohort.
Long-term follow-up
Day 110 after BMT corresponds with the approximate day on which most patients are discharged from the FHCRC system. Clinical records from patients beyond discharge were maintained in a computerized database at FHCRC by contact with the patient, family, and primary care provider, and follow-up outpatient appointments. Also, copies of discharge summaries, death summaries, and autopsy reports were retrieved for all patients admitted to local hospitals.
For this study, the computerized database was examined to determine outcome (including date of death) of patients in both treatment arms. Additional information extracted from these records included the development of relapse and chronic GVHD (cGVHD). To determine conditions present at death, chart reviews were performed by 2 physicians who were blinded to patient treatment arm. This information was extracted from death certificates, discharge summaries, and autopsy reports, when available.
Definitions and statistical analysis
Acute and chronic GVHD were diagnosed and graded according to standard criteria by clinicians who were not involved in this study and thus were blinded to treatment arm.16,20 Only clinically extensive disease was considered of interest for the purposes of this analysis.20 Relapse was defined as clinical evidence of recurrent underlying disease.
Candidemia was defined as any positive culture from blood. The diagnosis of tissue infection required histologic evidence and microbiologic confirmation of candidal infection from biopsy (premortem or postmortem) of a normally sterile site. Death associated with candidiasis was defined as bloodstream infection within 2 weeks of death or documentation of invasive infection from biopsy premortem, within 4 weeks of death, or postmortem. Death associated with mold infection required a diagnosis of definite (microbiologic and histologic documentation) or probable (either microbiologic or histologic documentation) infection within 4 weeks before death or postmortem. Death associated with bacterial infection required a diagnosis of bacteremia within 2 weeks before death or tissue invasion at autopsy. Deaths associated with protozoan (toxoplasmosis) and viral infections required histologic evidence of infection within 6 weeks of death or at autopsy.
Because infection-related death was an important end point in this study, patients who experienced infection in the presence of GVHD were considered to have infection-related death. However, the presence of GVHD was documented. To avoid bias from additional chemotherapy regimens, patients who died with an infectious complication of relapsed disease were considered to have died of the underlying disease, not of the complication.19
Kaplan-Meier estimates of probability of survival were computed. Survival was compared between patients who received fluconazole or placebo over the entire follow-up period (approximately 8 years), using the log-rank test. To determine whether factors other than fluconazole administration could account for the observed difference in survival, multivariable Cox regression analysis was performed with the inclusion of factors previously found to affect outcome in patients undergoing BMT. These factors included those used in the original randomization stratification (LAF isolation, transplant type, and HLA matching), plus patient age, gender, CMV serostatus, underlying diagnosis, and remission status of disease. A multivariable Cox regression analysis was also performed to determine relapse-free survival in the 2 groups, with inclusion of the variables used in the survival analysis. Also, Cox regression analysis was performed to determine relative risks (RRs) of development of relapse and acute and chronic GVHD for patients in the fluconazole arm versus placebo. Cumulative incidences were calculated to summarize the cause of death during and after the initial study period (day 110 after BMT). As there were no censored observations before day 110, testing for differences in the rate of death due to a specific cause was carried out using χ2 or Fisher exact tests. In the latter time window, however, censoring for incomplete follow-up does occur, so a log rank testing procedure was used instead. All analyses were performed at FHCRC.
Results
Outcome
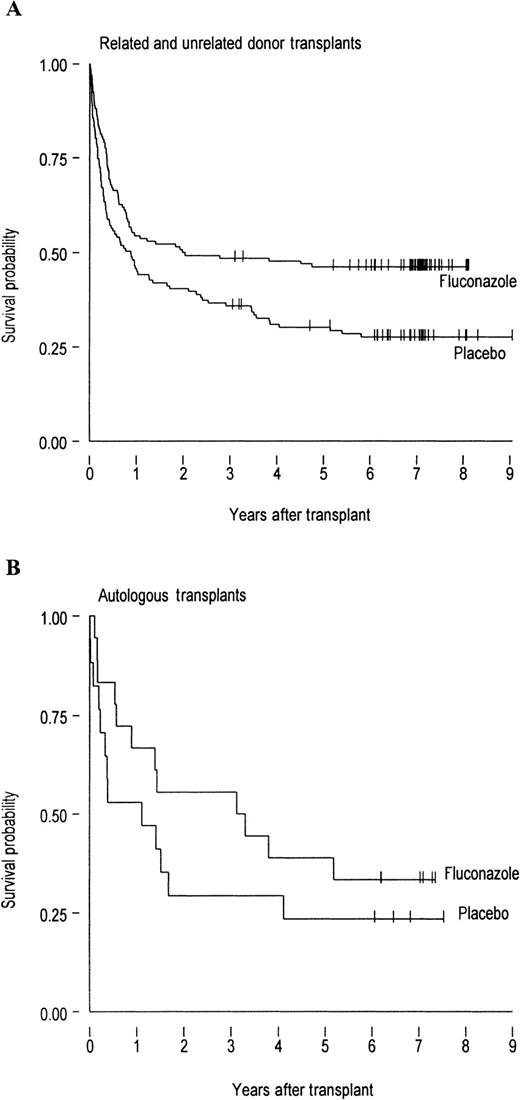
A total of 107 of the 148 patients who received placebo and 84 of the 152 patients who received fluconazole died during the 8-year period after BMT. The Kaplan-Meier survival curves (Figure1) demonstrate a continued survival benefit in patients who received fluconazole compared with placebo. This survival benefit was highly significant among patients who received allogeneic grafts (P = .0018, Figure 1A), but was not significant among the smaller subset of autologous graft recipients (P = .60, Figure 1B). Among allograft recipients, a survival benefit was evident in both related HLA-matched graft recipients and related HLA-mismatched or unrelated graft recipients (data not shown).
The survival probability in allogenic and autologous BMT patients.
Probability of survival in (A) 134 allogeneic BMT recipients who received fluconazole and 131 who received placebo (P = .0018) and (B) 18 autologous BMT recipients who received fluconazole and 17 who received placebo (P = .6). The allogeneic recipients include 213 patients who received HLA-matched grafts and 52 patients who received mismatched grafts. Tick marks represent patients alive at the last follow-up.
The survival probability in allogenic and autologous BMT patients.
Probability of survival in (A) 134 allogeneic BMT recipients who received fluconazole and 131 who received placebo (P = .0018) and (B) 18 autologous BMT recipients who received fluconazole and 17 who received placebo (P = .6). The allogeneic recipients include 213 patients who received HLA-matched grafts and 52 patients who received mismatched grafts. Tick marks represent patients alive at the last follow-up.
The probabilities of death, development of relapsed disease, and acute and chronic GVHD in the 2 treatment arms were summarized separately for patients before and after the end of the initial study (Table2). The protection from death associated with fluconazole administration was apparent during both periods examined. The number of patients who developed acute GVHD grades 2 to 4 and chronic clinically extensive GVHD involving any organ system was not different between study arms.
Outcome variables before and after day 110 in patients who received fluconazole compared with placebo
| Outcome . | On or before day 110 (%)* . | P† . | After day 110 (%)‡ . | P† . |
|---|---|---|---|---|
| Deaths | ||||
| Placebo | 52 (35) | .004 | 55 (57) | .043 |
| Fluconazole | 31 (20) | 53 (44) | ||
| Relapse | ||||
| Placebo | 8 (5) | .37 | 31 (32) | .09 |
| Fluconazole | 5 (3) | 27 (22) | ||
| Acute GVHD grade ≥ 2 | ||||
| Placebo | 82 (55) | .17 | na | na |
| Fluconazole | 96 (63) | na | ||
| Chronic GVHD2-153 | ||||
| Placebo | 21 (14) | .49 | 27 (28) | .75 |
| Fluconazole | 26 (17) | 32 (21) |
| Outcome . | On or before day 110 (%)* . | P† . | After day 110 (%)‡ . | P† . |
|---|---|---|---|---|
| Deaths | ||||
| Placebo | 52 (35) | .004 | 55 (57) | .043 |
| Fluconazole | 31 (20) | 53 (44) | ||
| Relapse | ||||
| Placebo | 8 (5) | .37 | 31 (32) | .09 |
| Fluconazole | 5 (3) | 27 (22) | ||
| Acute GVHD grade ≥ 2 | ||||
| Placebo | 82 (55) | .17 | na | na |
| Fluconazole | 96 (63) | na | ||
| Chronic GVHD2-153 | ||||
| Placebo | 21 (14) | .49 | 27 (28) | .75 |
| Fluconazole | 26 (17) | 32 (21) |
GVHD indicates graft-versus-host disease; cGVHD, chronic graft-versus-host disease.
Among 148 patients in placebo group and 152 patients in fluconazole group.
Calculated from χ2 tests.
Among 96 placebo and 122 fluconazole arm patients alive at day 110 after transplant.
Only clinically extensive cGVHD is included.
To determine whether the survival benefit was secondary to a factor that was not included in the protocol's stratification, a multivariable analysis was performed at the last follow-up. This analysis confirmed that receipt of placebo was independently associated with poor survival (RR for death 1.5, 95% CI 1.1, 2.0). Other factors that were significantly associated with a poor outcome in this data set included age greater than 50 years (RR for death 2.2, 95% CI 1.2, 4.0), absence of LAF isolation (RR for death 1.5, 95% CI 1.0, 2.2), and receipt of BMT from a related mismatched (RR for death 3.4, 95% CI 2.1, 5.4) or unrelated (RR for death 3.0, 95% CI 1.9, 4.8) donor. The type and stage of the patients' underlying disease (7 categories,P < .001) also significantly impacted survival, with chronic myelogenous leukemia (CML) in chronic phase having the best prognosis. Factors that were not associated with differential survival included CMV seropositivity and patient gender.
Because there was a trend to increased relapse disease in recipients of placebo compared with fluconazole (Table 2), we verified that the receipt of fluconazole is associated with a survival benefit, independent of relapsed underlying disease, by determining transplant-related mortality in the 2 groups. The multivariable model for nonrelapse mortality confirmed that more recipients of placebo died (RR 1.67, 95% CI 1.2, 2.4), independent of relapsed disease, age greater than 50 years (RR 3.7, 95% CI 1.7, 8.3), transplant in LAF isolation (RR 0.5, 95% CI 0.3, 0.8), receipt of BMT from a related mismatched (RR 3.4, 95% CI 2.0, 5.9) or unrelated donor (RR 3.7, 95% CI 2.2, 6.4), or type and stage of underlying disease (7 categories,P < .001). As in the Cox regression analysis for survival, neither gender nor CMV serostatus were significant. These analyses indicate that the long-term survival advantage conferred on fluconazole recipients is independent of other important pretransplant characteristics and relapsed underlying disease.
As noted previously,18 there was a lower risk of death in patients who received fluconazole relative to placebo during the 110-day study period, with 121 patients (80%) alive in the fluconazole arm and 96 patients (65%) alive in the placebo arm (Table 2). Because the initial data analysis combined infections caused by fluconazole-susceptible yeasts and fluconazole-resistant molds, we performed a repeat cause of death analysis of patients who died during the initial study period, with emphasis on specific causes of fungal infection-related deaths (Table 3). As opposed to the original data analysis, which examined causes of death relative to the total number of deaths in each study arm, we analyzed these data in relation to the entire cohort randomized to fluconazole or placebo. This repeat analysis revealed a lower incidence of candidiasis-related death in fluconazole recipients (less than 1%) compared with placebo (8%, P = .001). All mold infections were caused by Aspergillus spp, except for one patient in the fluconazole arm who developed disseminated infection withRhizopus spp. The only breakthrough yeast infection in the fluconazole arm was caused by Candida albicans. By day 110, the placebo arm contained 7 patients with disseminated C albicans, 2 with C tropicalis, 2 with C glabrata, and 2 patients with infections by Candida(species not identified).
Conditions present in patients who died during the study period (on or before day 110) and late after BMT (after day 110)
| Condition present at death . | On or before day 1103-150 . | After day 1103-151 . | ||||
|---|---|---|---|---|---|---|
| Fluconazole (%) . | Placebo (%) . | P . | Fluconazole (%) . | Placebo (%) . | P . | |
| Fungal infection | ||||||
| Mold | 10 (7) | 6 (4) | .33 | 10 (8) | 6 (6) | .60 |
| Candida spp. | 1 (< 1) | 13 (9) | .001 | 1 (< 1) | 8 (8) | .007 |
| Other infection | ||||||
| Bacteremia | 5 (3) | 2 (1) | .45 | 6 (5) | 10 (11) | .11 |
| CMV pneumonia | 2 (1) | 0 (0) | .50 | 3 (2) | 1 (1) | .44 |
| Bacterial pneumonia | 1 (< 1) | 2 (1) | .62 | 1 (< 1) | 0 (0) | .38 |
| Toxoplasmosis | 0 (0) | 2 (1) | .24 | 0 (0) | 0 (0) | — |
| Respiratory virus | 0 (0) | 1 (< 1) | .49 | 1 (< 1) | 0 (0) | .37 |
| Pneumocystis carinii | 0 (0) | 0 | — | 0 (0) | 1 (1) | .26 |
| Relapse3-152 | 4 (3) | 7 (5) | .33 | 20 (16) | 18 (19) | .57 |
| Idiopathic pneumonia | 3 (2) | 7 (5) | .18 | 6 (5) | 3 (3) | .54 |
| GVHD (no infection) | 2 (1) | 8 (5) | .049 | 1 (< 1) | 3 (3) | .15 |
| Multiorgan failure3-153 | 3 (2) | 4 (3) | .72 | 1 (< 1) | 4 (3) | .20 |
| Unrelated3-155 | 0 (0) | 0 (0) | — | 3 (2) | 3 (3) | .67 |
| Condition present at death . | On or before day 1103-150 . | After day 1103-151 . | ||||
|---|---|---|---|---|---|---|
| Fluconazole (%) . | Placebo (%) . | P . | Fluconazole (%) . | Placebo (%) . | P . | |
| Fungal infection | ||||||
| Mold | 10 (7) | 6 (4) | .33 | 10 (8) | 6 (6) | .60 |
| Candida spp. | 1 (< 1) | 13 (9) | .001 | 1 (< 1) | 8 (8) | .007 |
| Other infection | ||||||
| Bacteremia | 5 (3) | 2 (1) | .45 | 6 (5) | 10 (11) | .11 |
| CMV pneumonia | 2 (1) | 0 (0) | .50 | 3 (2) | 1 (1) | .44 |
| Bacterial pneumonia | 1 (< 1) | 2 (1) | .62 | 1 (< 1) | 0 (0) | .38 |
| Toxoplasmosis | 0 (0) | 2 (1) | .24 | 0 (0) | 0 (0) | — |
| Respiratory virus | 0 (0) | 1 (< 1) | .49 | 1 (< 1) | 0 (0) | .37 |
| Pneumocystis carinii | 0 (0) | 0 | — | 0 (0) | 1 (1) | .26 |
| Relapse3-152 | 4 (3) | 7 (5) | .33 | 20 (16) | 18 (19) | .57 |
| Idiopathic pneumonia | 3 (2) | 7 (5) | .18 | 6 (5) | 3 (3) | .54 |
| GVHD (no infection) | 2 (1) | 8 (5) | .049 | 1 (< 1) | 3 (3) | .15 |
| Multiorgan failure3-153 | 3 (2) | 4 (3) | .72 | 1 (< 1) | 4 (3) | .20 |
| Unrelated3-155 | 0 (0) | 0 (0) | — | 3 (2) | 3 (3) | .67 |
BMT indicates bone marrow transplantation; CMV, cytomegalovirus; GVHD, graft-versus-host disease.
All conditions present at death, except for specific infections, were extracted from previous publication.18 Specific infections present at death were determined with an additional data review. Percentage deaths are calculated relative to total cohort (see text for details).
Percentage deaths are calculated relative to number of patients alive at day 110 (96 placebo, 122 fluconazole). Multiple causes of death are listed for 4 patients (see text).
Included in this group are patients who died with all relapse-related complications.
Included in this group are patients who died with veno-occlusive disease before day 110 (n = 4).
Causes of death thought unrelated to BMT or unrelated disease during the follow-up period included traumatic cerebral hemorrhage (n = 2), gastrointestinal ulcer with bleed (n = 1), pulmonary embolism (n = 1), and ethanol-induced hepatic failure (n = 1).
During the first 110 days after BMT, the frequency of acute GVHD (grade more than or equal to 2) and cGVHD was equally distributed between patients who received placebo compared with fluconazole (Table 2). The presence of acute GVHD grades 3 to 4 was also equivalent in both arms (data not shown). Although there was an equivalent risk for acute GVHD, fewer patients in the fluconazole arm died with this as a diagnosis in the absence of infection (2 vs 8, P = .049, Table 3). Because gut mucosal breakdown is a risk factor for candidiasis,7 and antimicrobial administration might impact the development of gut GVHD by decreasing microbial colonization,1 we compared the development of gut GVHD in patients who received placebo compared with fluconazole. Receipt of fluconazole was associated with a decreased incidence of severe gut GVHD grades 3 to 4 (Figure 2), although the numbers of patients who developed skin and liver GVHD grades 3 to 4 were equivalent between groups (P = .42 and .93, respectively). Finally, more allogeneic graft recipients who received placebo died with severe gut GVHD (grades 3 to 4) compared with those who received fluconazole (10 of 125 vs 2 of 125,P = .018).
Development of gastrointestinal GVHD.
Of the 143 placebo recipients and 145 fluconazole recipients who had gut GVHD data available, 95 (66%) placebo recipients and 88 (61%) fluconazole recipients had grade 0 gut GVHD, and 18 (13%) placebo recipients and 35 (24%) fluconazole recipients had grade 1 gut GVHD. Compared with placebo patients (open bars), patients who received fluconazole (solid bars) had significantly less severe gut GVHD develop (P = .019). Gut GVHD (x-axis) was graded according to published definitions.16
Development of gastrointestinal GVHD.
Of the 143 placebo recipients and 145 fluconazole recipients who had gut GVHD data available, 95 (66%) placebo recipients and 88 (61%) fluconazole recipients had grade 0 gut GVHD, and 18 (13%) placebo recipients and 35 (24%) fluconazole recipients had grade 1 gut GVHD. Compared with placebo patients (open bars), patients who received fluconazole (solid bars) had significantly less severe gut GVHD develop (P = .019). Gut GVHD (x-axis) was graded according to published definitions.16
The conditions present at death in patients after day 110 are also listed in Table 3. During this period, the only difference between the 2 groups was the number of patients who died with candidiasis. Table 3includes 4 patients who had multiple infections at the time of death. One patient had both bacterial sepsis and candidemia, 1 had CMV pneumonia and aspergillosis, and 2 had both aspergillosis and candidal invasion in the gastrointestinal tract at autopsy. The one patient in the fluconazole arm who died with candidiasis also had a diagnosis of disseminated aspergillosis at the time of death. All mold infections were caused by Aspergillus species. Two infections were caused by C glabrata (both in the placebo arm) and there were 6 disseminated candidal infections in which the species ofCandida was not determined. During this late period after BMT, the presence of cGVHD was equally distributed between patients who received fluconazole compared with placebo (Table 2).
Since the completion of the randomized trial, no patients have been lost to follow-up. However, because of incomplete records, we were unable to determine the cause of death for 3 patients in the placebo arm.
Incidence of candidiasis and Candida-related death
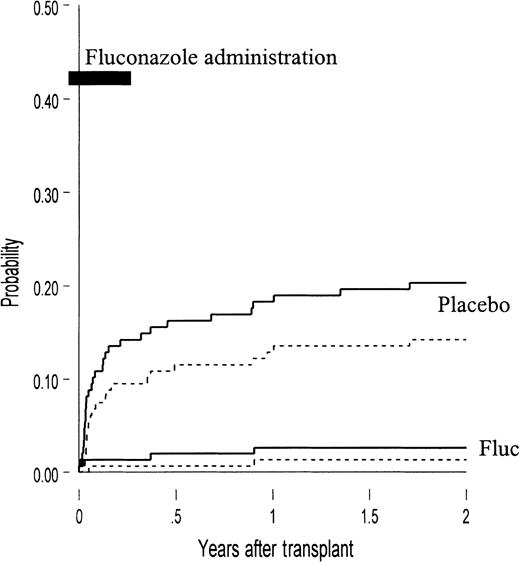
The probabilities of developing invasive candidiasis and candidiasis-related death during the first 2 years after BMT are compared graphically in Figure 3. There were no additional cases after 2 years post-BMT (with 8 years of follow-up). As demonstrated in this figure, receipt of fluconazole compared with placebo was associated with a protection against candidal infection that persisted beyond the 75-day drug administration period.
The development of invasive candidiasis and candidiasis-related death.
Probability of the development of invasive candidiasis (bloodstream and tissue infection, solid lines) and candidiasis-related death (dashed lines) in placebo recipients and fluconazole (fluc) recipients. Compared with fluconazole recipients, more patients in the placebo arm had candidal infection (20% vs 3%,P < .001) and candidiasis-related death (14% vs 1%,P < .001). Also presented is the duration of fluconazole administered (conditioning through day 75, bold line).
The development of invasive candidiasis and candidiasis-related death.
Probability of the development of invasive candidiasis (bloodstream and tissue infection, solid lines) and candidiasis-related death (dashed lines) in placebo recipients and fluconazole (fluc) recipients. Compared with fluconazole recipients, more patients in the placebo arm had candidal infection (20% vs 3%,P < .001) and candidiasis-related death (14% vs 1%,P < .001). Also presented is the duration of fluconazole administered (conditioning through day 75, bold line).
The days on which patients developed candidiasis after day 110 are presented in Figure 4. Included in this figure are the 9 patients who died with candidiasis and 4 additional patients who developed candidemia (3 placebo recipients and 1 fluconazole recipient) but did not die with the infection. Of the 9 patients who died with candidiasis, 5 had candidemia, 2 had hepatosplenic candidiasis, 1 had pneumonia, and 1 had gastrointestinal (GI) invasion (colitis).
Frequency of candidal infection after day 110 of BMT.
The number of placebo recipients (■) and fluconazole recipients (▪) in whom invasive candidiasis developed after day 110 is shown.
Frequency of candidal infection after day 110 of BMT.
The number of placebo recipients (■) and fluconazole recipients (▪) in whom invasive candidiasis developed after day 110 is shown.
All patients who developed candidal infection late after BMT had received allogeneic BMTs. Nine (64%) patients had received marrow from a mismatched or unrelated donor, and 9 of 12 (75%) patients who developed candidiasis late after BMT had documented clinically extensive cGVHD. One patient who developed late candidiasis did not have cGVHD data available.
Discussion
This study demonstrates that the use of fluconazole (400 mg/d) for 75 days after allogeneic donor BMT not only results in a decrease in mortality early after BMT, but it is also associated with an independent, long-term mortality benefit. Fluconazole prophylaxis during the early transplant period appears to provide a persistent protection against candidiasis and candidiasis-related death, even after discontinuation of fluconazole at day 75 post-BMT. In addition, fluconazole administration appears to be associated with a decreased incidence of severe gut GVHD. The previously unexplained survival benefit can now be clearly attributed to a reduction in candidiasis-related mortality.
The results of this follow-up study demonstrate the persistence of the overall mortality benefit observed in our original randomized study, as 17.5% more patients in the fluconazole arm survived until 8 years after BMT (Figure 1). This represents one of the most impressive reductions in BMT-related mortality associated with one prophylactic strategy.2,3,5,10,15 These findings are also in agreement with the results of another study at our institution that indicated that fluconazole prophylaxis increased the probability of survival in patients who received allogeneic BMT for CML, a cohort largely different than that described in this study.10
This analysis also revealed that fewer patients who received fluconazole developed severe acute GVHD involving the GI tract. The protection associated with fluconazole was only apparent for GVHD involving the gut, as the overall incidence of acute GVHD did not differ between groups (Table 2). More patients in the fluconazole arm developed grade 1 GVHD, which is primarily defined by nausea.16 This might be explained by drug-related nausea.18 More importantly, gut GVHD that involves colonic mucosa (grades 3 to 4) was decreased in fluconazole recipients compared with placebo recipients. Protection against GI mucosal breakdown, combined with decreased Candida colonization, as documented in the early study,18 might explain the overall decrease in candidiasis and candidiasis-related mortality. Previous studies have shown that GVHD and gut mucosal breakdown serve as risk factors for disseminated candidiasis.7 14 In this study, the low incidence of both candidiasis and severe gut GVHD makes a statistically sound risk factor analysis difficult. However, the hypothesis is supported by a decreased mortality due to overall acute GVHD and gut GVHD in the fluconazole arm.
One possible explanation for the fluconazole-associated decrease in gut GVHD is decreased local antigenic stimulation as a result of intestinal microbial “decontamination.”11,21 A recent randomized study noted a decreased risk for the development of acute GVHD in patients who received prophylactic antimicrobials having anaerobic activity.1 In that study, antimicrobial-associated protection against GVHD was only apparent in recipients of matched-related grafts. In our study, fluconazole-associated protection against gut GVHD did not appear to be stronger among matched-related grafts (data not shown). Further studies are warranted to enhance our understanding of the interactions between microbes, antimicrobials, and GVHD.
The overall mortality benefit at 8 years after BMT, which is independent of pretransplant factors previously known to affect outcome (patient age, transplantation conditions, CMV seropositivity, type of BMT, underlying disease, and stage), is associated with a decreased number of infections caused primarily by the yeasts C albicans and C tropicalis (Table 3). This finding is not surprising given the fact that these 2 species, which cause the majority of candidal infections in patients not receiving fluconazole, are also considered very virulent and usually fluconazole-susceptible.23,24 A recent study examining the microbial epidemiology of candidal infections at FHCRC subsequent to the adoption of this 75-day prophylaxis regimen has confirmed an absence of fluconazole-susceptible C albicans and C tropicalis and an overall decreased mortality associated with infections caused by resistant Candida species (C glabrata, C krusei).13
A novel finding of this study is that patients who were treated with fluconazole for 75 days had significantly fewer systemic candidal infections “late” (more than 110 days) after BMT. Furthermore, more patients who received placebo died with candidiasis during this late period. These findings are in agreement with a recent autopsy study that documented that fluconazole decreases the incidence of chronic hepatosplenic candidiasis after BMT.22 This is again likely to be secondary to a low amount of gastrointestinal colonization after 75 days of fluconazole in patients who have cGVHD involving GI mucosal barriers. Although we do not have data describing the use of antifungals in patients after discharge to community physicians, it is unlikely that fluconazole was continued beyond this time because the drug was not approved by the FDA for use in BMT recipients until January 1994, which is more than 2 years after enrollment of the last patient in this trial.18 Our data suggests that a significant decrease in candidal burden during the first 75 days after BMT results in continued protection, even if immunosuppression continues.
The original determination of cause of death did not separate out death with candidal infection from death associated with fluconazole-resistant fungi such as the Aspergillusspecies.18 When this original analysis was performed, data on the resistance of molds to high doses of fluconazole was not available. Also, the previous publication presented the relative frequencies of cause-specific death among those who had died in each arm. That approach does not take into account the differing overall mortality rates in the 2 treatment arms. In this analysis, we chose to summarize the data with a focus on the incidence of death associated with specific causes among all patients at risk. This method, which is perhaps more meaningful, highlights small to moderate reductions in almost every category of mortality in patients who received fluconazole.
The determination of cause of death in BMT recipients has been difficult, given the overlapping conditions present (GVHD, relapse) that may ultimately lead to infectious death. Therefore, in recent publications, an operational definition of “infection-related death” has been used to analyze the mortality associated with infections after BMT.3 5 Although some patients in this study also had concomitant GVHD, this definition did not appear to create bias, as fluconazole prophylaxis was associated with protection after controlling for underlying disease and BMT type in the multivariate model. Furthermore, we used a very conservative definition of candidiasis-related death (death within 2 weeks of diagnosis or requiring autopsy confirmation of candidal involvement). Because we considered patients with relapse to have died with this underlying condition instead of with the resultant infectious complication, the protection against candidiasis may actually be an underestimate. Overall, this study demonstrates that the excess deaths in the placebo group are predominantly associated with candidiasis (Table 3).
The results of this study emphasize the differences between infectious risks between types of BMT, as the mortality benefit was not apparent in the subset of patients consisting only of autologous donor BMT. This observation is consistent with another recent randomized trial17 and intuitive given the association between gut GVHD and candidiasis in allograft recipients. Unfortunately, differences in host risks have been previously ignored both in the interpretation and design of several studies. This common omission is perhaps most apparent in a recent meta-analysis comparing prophylactic fluconazole versus placebo studies that included results from trials consisting of all “neutropenic” patients (acute leukemia patients, autologous BMT recipients, and allogeneic BMT recipients).8 The results of this study indicate no apparent survival benefit of antifungal drugs administered prophylactically or empirically. A complete understanding of specific host risks is necessary both in the development of prophylactic antifungal studies and clinical policies. In this context, the impact of fluconazole administered until engraftment on late candidiasis, candidiasis-related death and GVHD specific to organ system should be reevaluated in high-risk (ie, allogeneic) patients only.
The collection of patient microbiology data late after discharge is frequently incomplete, as it relies on data collected at different hospitals. Although this may contribute to an underestimation of invasive candidiasis in patients from both arms, it is unlikely to bias one group or affect cause of death data because of the availability of clinical summaries and autopsy reports. Another limitation of a study that assesses patient outcome late after discharge is potentially incomplete data regarding antimicrobial utilization. In this study, the lack of data describing the use of antifungals and other antimicrobials after discharge to community physicians might affect the overall incidence of Candida colonization and resultant disease. However, the randomized design of this study and the equivalent frequency of noncandidal infections both early18 and late after discharge (this study) suggests that the likelihood of a bias is small. And, as discussed previously, community use of fluconazole is believed to be extremely low because of the lack of FDA approval of the drug. Finally, whether 75-day administration of lower doses of fluconazole or other antifungal agents have a profound effect on infection-related mortality cannot be determined from these data.
In conclusion, we have shown that fluconazole, administered prophylactically for 75 days after BMT, is associated with a persistent mortality benefit in allogeneic BMT recipients. This survival benefit is unequivocally explained by a reduction in candidiasis-related deaths, which are especially prominent in the setting of GVHD. These data support the continuation of antifungal prophylaxis until day 75 in allogeneic BMT recipients. Further studies are necessary to determine effective strategies for the prevention of non-Candidafungal infections in BMT recipients.
Supported by National Institutes of Health grants CA-18029 to the Fred Hutchinson Cancer Research Center and K08-AI1571 (K.A.M). The original clinical trial was supported by Pfizer Inc. (New York, New York).
Informed consent was obtained from study patients according to Institutional Review Board guidelines.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kieren A. Marr, Fred Hutchinson Cancer Research Center, Program in Infectious Diseases, 1100 Fairview Ave N, D3-100, Seattle, WA 98109-1024; email: kmarr@fhcrc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal