Abstract
Cross-linking of IgE or a bacterial product (f-Met-Leu-Phe; FMLP) induces the release of leukotriene C4 (LTC4) and histamine in human basophils. However, the signaling mechanisms in human basophils are only partially understood. It has been demonstrated that extracellular signal-regulated kinases (ERK1/2) specifically regulate the pathway for LTC4 generation, but not for histamine release and interleukin-4 production. More recent studies have suggested that tyrosine kinase (syk)-mediated phosphorylation of shc is responsible for the ras-ERK cascade via the formation of shc-Grb2-Sos2 following stimulation with anti-IgE antibody, but not FMLP, in human basophils. However, while characterizing the role of phosphatidylinositol (PI)-3 kinase in signaling pathways leading to basophil mediator release, it was noted that this pathway might also regulate p21ras activation. Anti-IgE antibody, but not FMLP, resulted in phosphorylation of p85 (regulatory subunit of PI3 kinase), suggesting activation of PI3 kinase. Inhibition of PI3 kinase by selective inhibitor (LY294002) abolished anti-IgE antibody- but not FMLP-induced phosphorylation of MEK1 (MAPK kinase/ERK kinase) and ERKs while inhibiting LTC4 generation as well as histamine release. IgE-mediated activation of ras (upstream of MEK-ERK) was also inhibited. But, further upstream, phosphorylation of syk and of shc and inducible association between shc and Grb2 were not affected. Furthermore, the IgE-mediated cytosolic calcium response ([Ca++]i) was also diminished. These results suggest that functional responses may be dependent on the activity of PI3 kinase, which regulates at least 2 important signaling pathways: by regulating activation of ras for the MEK-ERK pathway and the increase in [Ca++]i.
Introduction
Human basophils are characterized as important effector cells in allergic inflammation, such as bronchial asthma, allergic rhinitis, and atopic dermatitis, by releasing chemical mediators (eg, histamine and leukotriene C4 [LTC4]) and cytokines (eg, interleukin [IL]-4).1,2 Human basophils, as well as human mast cells, express high-affinity IgE receptors (FcεRI) on their surface and release mediators in response to aggregation of these receptors by antigens. Although the signaling mechanisms underlying IgE-mediated activation have been elucidated in rodent cell models (eg, rat basophilic leukemia cells [RBL-2H3], mouse mast cell lines, and bone marrow–derived mast cells),3 4 the counterparts in human basophils are only partially understood.
We have previously reported that extracellular signal-regulated kinases (ERK1/2) selectively regulate the pathway for free arachidonic acid (AA)/LTC4 generation by phosphorylating cytosolic phospholipase A2 (cPLA2) after stimulation with cross-linking of FcεRI (by anti-IgE antibody [Ab]) or f-Met-Leu-Phe (FMLP), whereas little role for ERKs was found for histamine release and IL-4 production in human basophils.5,6 The pathway from activation of FcεRI to ERK/cPLA2 phosphorylation has been examined closely in RBL-2H3 cells,7-9 and this information seems likely to have some application to understanding the pathway in human basophils. In RBL-2H3 cells, the events that link the activation of syk to ERK (ERK2) include syk-dependent tyrosine phosphorylation of adapter protein, shc; and the association of phosphorylated shc with another adapter protein, Grb2, which is constitutively associated with the ras guanine nucleotide exchange factor, son of sevenless (Sos1). These associations lead to the conversion of ras to its active GTP-bound state as well as the activation of raf-1, MEK (MAPK kinase/ERK kinase), and ERK2. We have recently examined the events upstream of ERK1/2 phosphorylation in human basophils and found tyrosine kinase (presumably syk)-dependent phosphorylation of shc, association of shc with Grb2-Sos2 complex, and activation of ras-MEK1-ERK1/2. PP1 (src-family kinase inhibitor) inhibited the activation of this cascade by inhibiting the initial activation of lyn and syk (manuscript submitted). In the same study, we found that FMLP-mediated activation of ERKs is independent of these tyrosine kinases or the formation of shc-Grb2-Sos2, suggesting that an alternative pathway is involved in FMLP-mediated activation of ras-ERKs.
Phosphatidylinositol (PI)-3 kinase phosphorylates the D-3 position of the inositol ring of PI to generate phosphatidylinositol triphosphate (PIP3). The enzyme is activated by receptor tyrosine kinases, nonreceptor kinases, and G protein–coupled receptors and is involved in signaling pathways regulating rearrangement of the cytoskeleton, cell proliferation, and protein transcription.10-12 Several lines of evidence from studies using rodent mast cells (RBL-2H3 cells, mouse mast cell lines, and mouse bone marrow–derived mast cells) stimulated through FcεRI have suggested that PI3 kinase activity is necessary for degranulation, whereas this kinase is not likely to be involved in the signaling leading to ERK activation and the generation of AA metabolites (eg, LTC4). These conclusions have been reached in part by testing the effects of specific PI3 kinase inhibitors (wortmannin and LY294002) on secretion.13-16 Similarly, these inhibitors abolished IgE-mediated release of histamine and IL-4 in human basophils, indicating that PI3 kinase may regulate signaling leading to degranulation and cytokine (IL-4) production in these cells.17,18 In contrast to the results in the rodent models, these inhibitors also inhibit activation of ERKs as well as LTC4 generation in human basophils.17 These results suggest that human basophils use PI3 kinase in ways distinct from rodent mast cells.
In the current studies, we have explored some of the signaling elements regulated by PI3 kinase during stimulation with anti-IgE Ab and compared these results with stimulation with FMLP. Causal testing of the role of PI3 kinase was examined by using selective inhibitors of its function.
Materials and methods
Materials
The following were purchased: piperazine-N,N-bis-2-ethanesulfonic acid (PIPES), bovine serum albumin, ethyleneglycol-bis-(β-amino ethyl ether) N,N′-tetraacetic acid (EGTA), EDTA, d-glucose, NaF, Na4P2O7, Na3VO4, 2-mercaptoethanol (ME), and NP-40 (Sigma, St. Louis, MO); crystallized human serum albumin (HSA) (Miles Laboratories, Elkhart, IN); fetal calf serum (FCS) and RPMI 1640 containing 25 mmol/L N-2-hydroxyethyl-piperizine N-2-ethanesulfonic acid (HEPES) and l-glutamine (Biowhittaker, Walkersville, MD); Percoll (Pharmacia, Piscataway, NJ); Tris (hydroxymethyl)-aminomethane and Tween-20 (Bio-Rad, Hercules, CA); leupeptin, dithiothreitol (DTT), and phenylmethylsulfonyl fluoride (PMSF) (Boehringer Mannheim, Indianapolis, IN); antiphosphotyrosine monoclonal antibody (mAb) (4G10) and rabbit anti-p85 Ab (for Western blotting) (Upstate Biotechnology, Lake Placid, NY); rabbit anti–phospho-ERK Ab, rabbit anti–phospho-MEK Ab, and biotinylated molecular-weight markers (New England Biolabs, Beverly, MA); anti-shc Ab (for immunoprecipitation and Western blotting), anti-shc mAb (for Western blotting), anti-Grb2 mAb (for Western blotting), and anti-ras mAb (Transduction Laboratories, San Diego, CA); rabbit anti-Grb2 Ab, rabbit anti-p85 Ab (for immunoprecipitation), and anti-syk mAb (Santa Cruz Biotechnology, Santa Cruz, CA); anti-MEK1 Ab (MBL, Watertown, MA); peroxidase-linked donkey anti-rabbit Ig Ab, peroxidase-linked sheep anti-mouse Ig Ab, and protein G sepharose beads (Amersham Life Science, Arlington Heights, IL); LY294002 and Ro-31-8220 (Calbiochem, La Jolla, CA); PP1 (Biomol, Plymouth Meeting, PA); and fura-2/am(Molecular Probes, Eugene, OR). Goat antihuman IgE was prepared as described previously.19 Stock solutions of LY294002, PP1, and Ro-31-8220 were prepared in dimethyl sulfoxide (DMSO) at 100 mmol/L, 100 mmol/L, and 10 mmol/L, respectively. Controls were incubated with an equal concentration of DMSO.
Buffers
PIPES-albumin-glucose (PAG) buffer consisted of 25 mmol/L PIPES, 110 mmol/L NaCl, 5 mmol/L KCl, 0.1% glucose, and 0.003% HSA. PAGCM consisted of PAG supplemented with 1 mmol/L CaCl2 and 1 mmol/L MgCl2. TBST contained 12 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, and 0.05% Tween 20.
Basophil purification
Basophils were purified from residual cells of normal donors undergoing leukapheresis with Percoll density gradient and countercurrent-flow elutriation.20,21 The cells were further purified by negative selection using Miltenyi reagents (basophil isolation kit containing anti-CD3, -CD7, -CD14, -CD15, -CD16, -CD36, -CD45RA, and -HLA-DR) and columns (Miltenyi Biotec, Auburn, CA).22 In all experiments involving the use of Western blot analysis of phosphorylated proteins, basophil purities were greater than 90% as determined by Alcian blue staining.23
Phosphorylation of ERKs and MEK
The phosphorylation of ERKs and MEK was assessed using phospho-ERKs Ab5,24,25 and phospho-MEK Ab,26respectively. After the basophils (0.5-1 × 106 cells per sample) were stimulated in PAGCM buffer, the reactions were stopped by adding ice-cold PAG and the cells were microfuged for 5 to 10 seconds. After collecting the supernatant (for measurement of histamine and LTC4), we immediately lysed the cell pellets in 1 × Laemmli sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 10% glycerol, 1.25% sodium dodecyl sulfate [SDS], 0.0025% bromophenol blue, 2.5% 2-ME) (NOVEX, San Diego, CA) and analyzed them on 10% or 4% to 20% Tris glycine gels (NOVEX). Electrophoresis and transfer were performed as described previously.5,6 The membranes were immersed in TBST containing 5% nonfat dried skim milk (Carnation, Los Angeles, CA) overnight to block nonspecific binding. Immunoreactive proteins were detected with anti–phospho-MEK Ab, which was diluted in TBST containing 1% skim milk for 4 hours. After washing, the membranes were incubated with horseradish peroxidase–conjugated antirabbit Ab for 1 hour. After washing, enhanced chemiluminescence (ECL) detection was performed as described previously.5,6 In some experiments, the same membranes were reblotted with phospho-ERK and anti-MEK1 Ab. Between each blot, the membranes were stripped with stripping buffer (62.5 mmol/L Tris-HCl, pH 6.7, 100 mmol/L 2-ME, 2% SDS) for 1 hour at 50°C.5
Immunoprecipitation
After the basophils (3-5 × 106 cells per sample) were stimulated in PAGCM buffer at 37°C, the reactions were stopped by adding ice-cold PAG and the cells were microfuged for 5 to 10 seconds. The cell pellets were immediately lysed in immunoprecipitation (IP) buffer (20 mmol/L Tris-HCl, pH 7.5, 2 mmol/L EDTA, 2 mmol/L EGTA, 100 μg/mL aprotinin, 10 mmol/L benzamidine, 5 mmol/L DTT, 1 mmol/L PMSF, 100 μg/mL leupeptin, 50 mmol/L NaF, 5 mmol/L Na4P2O7, 1 mmol/L Na3VO4, and 1% NP-40). Lysates were precleared with protein G sepharose beads for 1 hour at 4°C to remove any nonspecific binding to the beads. The lysates were then incubated with 1 μg/mL of specific Ab prebound to protein G sepharose beads at 4°C. After 1 hour of incubation, the beads were washed 3 times with IP buffer. The immunoprecipitated proteins were eluted by boiling in Laemmli sample buffer. Electrophoresis, transfer, and immunoblotting were performed as described previously.5
Activated ras affinity precipitation assay
The activated ras affinity precipitation assay was performed as described previously, with slight modifications.27 28 A glutathione S-transferase (GST) fusion protein containing the Ras-binding domain (RBD) of raf (amino acids 1-149 of raf-1), which binds only GTP-bound (activated) ras, was immobilized on glutathione-agarose beads (Upstate Biotechnology). After the basophils (approximately 5 × 106 cells) were stimulated, the reactions were stopped by adding ice-cold PAG and the cells were microfuged for 5 to 10 seconds. The cell pellets were immediately lysed in ras affinity precipitation (AP) buffer (25 mmol/L HEPES, pH 7.5, 2 mmol/L EGTA, 150 mmol/L NaCl, 10 mmol/L MgCl2, 10% glycerol, 50 μg/mL aprotinin, 5 mmol/L benzamidine, 50 μg/mL leupeptin, 25 mmol/L NaF, 1 mmol/L Na3VO4, 1% NP-40, and 1 mmol/L PMSF). Clarified lysates were incubated with GST-RBD (5 μL per sample) for 1 hour at 4°C with rocking. The GST-RBD beads were washed 3 times with ras AP buffer. Bound proteins were eluted by boiling in Laemmli sample buffer. Affinity-precipitated ras was detected by immunoblotting with antiras mAb.
LTC4 and histamine measurements
A total of 50 000 basophils were challenged in a final volume of 100 μL of PAGCM at 37°C. The reactions were terminated with 900 μL of ice-cold PAG-EDTA, and the cells were then centrifuged in a microfuge at 14 000 rpm for 10 seconds. A radioimmunoassay was performed using 100 μL of supernatant to determine LTC4 levels, as described previously.19,29 An additional 500 μL of supernatants was mixed with an equal volume of PAG to measure histamine by automated fluorimetry.30 The percentage of total histamine release was calculated for the diluted supernatants after subtraction of spontaneous histamine release.31 Each condition tested was performed in duplicate.
[Ca++]i measurements
Basophils were labeled with 1 μmol/L fura-2/am for 20 minutes at 37°C in RPMI 1640 containing 2% FCS (300 000-500 000 cells in 200 μL). After washing, the cells were resuspended in PAG for loading in the microscope observation chamber.6,32[Ca++]i changes were determined by digital videomicroscopy using techniques previously described in detail.32 33 Briefly, 15 μL of cells (20 000-30 000) were loaded onto the siliconized coverslip of the microscope chamber and, after settling, overlaid with 1 mL of PAGCM buffer. After warming to 37°C, monitoring of the cells was begun. After several frames (each frame is a single ratio measurement of a field of 30-100 cells) of prechallenge [Ca++]i levels were acquired, the cells were challenged with 1 mL of stimulus in buffer. Data were then acquired for 50 to 150 frames at intervals of 1 to 10 seconds to determine the subsequent [Ca++]iresponse.
Results
Effect of PI3 kinase inhibitor (LY294002) on release of LTC4 and histamine after stimulation with anti-IgE Ab or FMLP
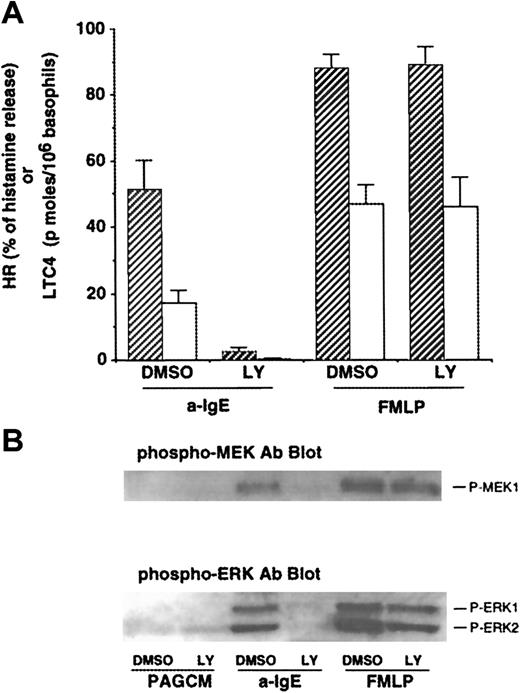
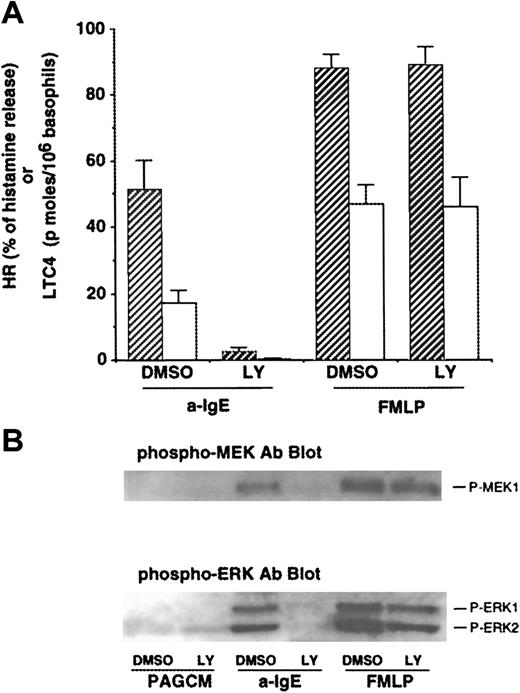
As noted earlier, some of the effects of PI3 kinase inhibitors, wortmannin and LY294002, have been studied in human basophils.17,18 However, the effects of PI3 kinase inhibitors on LTC4 release or phosphorylation of ERKs induced by FMLP have not been reported. We first examined the concentration dependence of inhibition by both PI3 kinase inhibitors on IgE- or FMLP-mediated release of histamine and LTC4. Both wortmannin and LY294002 inhibited IgE-mediated histamine release with a 50% inhibitory concentration (IC50) of ≈4 nmol/L and ≈1 μmol/L, respectively (with complete inhibition at 30 nmol/L and 10 μmol/L, respectively), results that were consistent with previous studies.17 In contrast, at concentrations up to 10 nmol/L or 10 μmol/L, respectively, neither wortmannin nor LY294002 was able to affect FMLP-induced histamine release (some of these data are shown in Figure 1A). The IC50 for inhibition of IgE-mediated LTC4 release was similar to that for histamine release, and these drugs had little effect on FMLP-mediated LTC4 release except at high concentrations. Wortmannin has been reported to affect other enzymes at concentrations of greater than 30 nmol/L (eg, myosin light chain kinase and phospholipase D10 34), and these effects have not been described for LY294002. Across a range of FMLP concentrations (30-300 nmol/L) that induced LTC4 release that was similar to anti-IgE–induced release (ie, titrating for a similar “strength” of signaling), 10 μmol/L LY294002 caused no inhibition of LTC4 release following FMLP but completely inhibited LTC4 release induced by anti-IgE Ab (data not shown). Therefore, we used 10 μmol/L LY294002 in subsequent experiments to determine the roles of PI3 kinase for signaling events and functions in human basophils.
Effect of LY294002 on release of histamine and LTC4 and phosphorylation of MEK1 and ERKs after stimulation with anti-IgE Ab or FMLP.
(A) Effect of LY294002 on release of histamine and LTC4. Basophils were preincubated with DMSO (1:10 000 dilution) or LY294002 (10 μmol/L) for 10 minutes and were stimulated with or without FMLP (1 μmol/L) for 5 minutes or anti-IgE Ab (0.5 μg/mL) for 10 minutes. Reactions were stopped with the addition of ice-cold PAG, and the cells were microfuged. Supernatants were collected for histamine (closed column) and LTC4 (open column) measurements (n = 3). (B) Effect of LY294002 on phosphorylation of MEK1 and ERKs. Cell pellets were lysed and subjected to Western blot analysis as described in “Materials and methods.” The anti-MEK1 blot indicated essentially equal protein loading (data not shown). The Western blot shown is representative of 3 separate experiments.
Effect of LY294002 on release of histamine and LTC4 and phosphorylation of MEK1 and ERKs after stimulation with anti-IgE Ab or FMLP.
(A) Effect of LY294002 on release of histamine and LTC4. Basophils were preincubated with DMSO (1:10 000 dilution) or LY294002 (10 μmol/L) for 10 minutes and were stimulated with or without FMLP (1 μmol/L) for 5 minutes or anti-IgE Ab (0.5 μg/mL) for 10 minutes. Reactions were stopped with the addition of ice-cold PAG, and the cells were microfuged. Supernatants were collected for histamine (closed column) and LTC4 (open column) measurements (n = 3). (B) Effect of LY294002 on phosphorylation of MEK1 and ERKs. Cell pellets were lysed and subjected to Western blot analysis as described in “Materials and methods.” The anti-MEK1 blot indicated essentially equal protein loading (data not shown). The Western blot shown is representative of 3 separate experiments.
Effect of LY294002 on phosphorylation of MEK1 and ERKs after stimulation with anti-IgE Ab or FMLP
Gibbs et al17 have reported that PI3 kinase inhibitors abolish IgE-mediated phosphorylation of ERKs in human basophils. It has been reported that PI3 kinase may regulate the activation of ERKs after stimulation with FMLP in other cell types.35 The effect of LY294002 on FMLP-mediated ERK activation in human basophils has not been reported. As shown in Figure1B, anti-IgE–induced phosphorylation of ERKs (ERK1/2) was significantly inhibited, consistent with previous observations.17 We have recently found that human basophils express MEK1 (ERK kinase1) and that MEK1 is phosphorylated after stimulation with anti-IgE Ab or FMLP. Thus, the effect of this compound on phosphorylation of MEK1 was examined. Anti-IgE–induced phosphorylation of MEK-1 was also inhibited (Figure 1B). By contrast, FMLP-induced phosphorylation of MEK-1 and of ERKs was not affected by LY294002. In these experiments, supernatants were tested for histamine and LTC4 release. LY294002 (10 μmol/L) almost completely inhibited IgE-mediated release of histamine and LTC4 (94% ± 2% and 94% ± 4% inhibition, respectively), although no effect on FMLP-induced release was observed (Figure 1A), as described previously. Activation of protein kinase C (PKC) by phorbol 12-myristate 13-acetate (PMA) or calcium ionophore (ionomycin) also resulted in phosphorylation of ERKs in human basophils. LY294002 also failed to inhibit phosphorylation of ERKs induced by these stimuli (data not shown). These results suggest that PI3 kinase is specifically involved in IgE-mediated ERK activation.
Effect of LY294002 on activation of ras or phosphorylation of syk and shc after stimulation with anti-IgE Ab
We attempted to determine the effect of LY294002 on IgE-mediated signaling events upstream of MEK-ERK. Recently, a novel technique to detect activation of ras was established using the RBD of raf-1 (amino acids 1-149 of raf-1) conjugated to agarose beads (see “Materials and methods”). The effect of LY294002 on activation of p21ras after stimulation with anti-IgE Ab was examined. An A431 cell lysate was used as a p21ras reference standard. RBD-agarose alone (without incubation with cell lysates) was also used as a negative control. As shown in Figure 2, the mass of ras (GTP-bound form) induced by anti-IgE Ab was significantly decreased by LY294002, suggesting that PI3 kinase might regulate the activation of ras (previous studies demonstrated that peak ras activation occurred at about 5 minutes after stimulation).
Effect of LY294002 on activation of ras after stimulation with anti-IgE Ab.
Basophils were resuspended in PAGCM (containing 1 mmol/L Ca++) or PAG (without Ca++) and preincubated with or without LY294002 (10 μmol/L) for 10 minutes. The cells were stimulated with or without anti-IgE (0.5 μg/mL) for 5 minutes. Reactions were stopped with the addition of ice-cold PAG, and the cells were microfuged. Clarified lysates were subjected to affinity precipitation with RBD-GST. Affinity-precipitated GTP-ras was detected by immunoblotting with anti-ras Ab. The immunoblot shown is representative of 2 separate experiments.
Effect of LY294002 on activation of ras after stimulation with anti-IgE Ab.
Basophils were resuspended in PAGCM (containing 1 mmol/L Ca++) or PAG (without Ca++) and preincubated with or without LY294002 (10 μmol/L) for 10 minutes. The cells were stimulated with or without anti-IgE (0.5 μg/mL) for 5 minutes. Reactions were stopped with the addition of ice-cold PAG, and the cells were microfuged. Clarified lysates were subjected to affinity precipitation with RBD-GST. Affinity-precipitated GTP-ras was detected by immunoblotting with anti-ras Ab. The immunoblot shown is representative of 2 separate experiments.
Cross-linking of IgE results in cytosolic free calcium ([Ca++]i) elevations; LY294002 at 10 μmol/L also inhibited IgE-mediated elevations of [Ca++]i (Figure3). Therefore, it was possible that inhibition of ras by LY294002 was due to its ability to inhibit [Ca++]i elevations. However, as shown in Figure 2, IgE-mediated activation of p21ras was similar in the presence or absence of extracellular calcium.
Effect of LY294002 on the [Ca++]i response after stimulation with anti-IgE Ab.
Basophils were labeled with fura-2/am. After washing, the cells were treated with or without LY294002 for 10 minutes and stimulated with anti-IgE Ab (0.5 μg/mL). The [Ca++]i response was analyzed as described in “Materials and methods.” The result shown is the average of 2 separate experiments.
Effect of LY294002 on the [Ca++]i response after stimulation with anti-IgE Ab.
Basophils were labeled with fura-2/am. After washing, the cells were treated with or without LY294002 for 10 minutes and stimulated with anti-IgE Ab (0.5 μg/mL). The [Ca++]i response was analyzed as described in “Materials and methods.” The result shown is the average of 2 separate experiments.
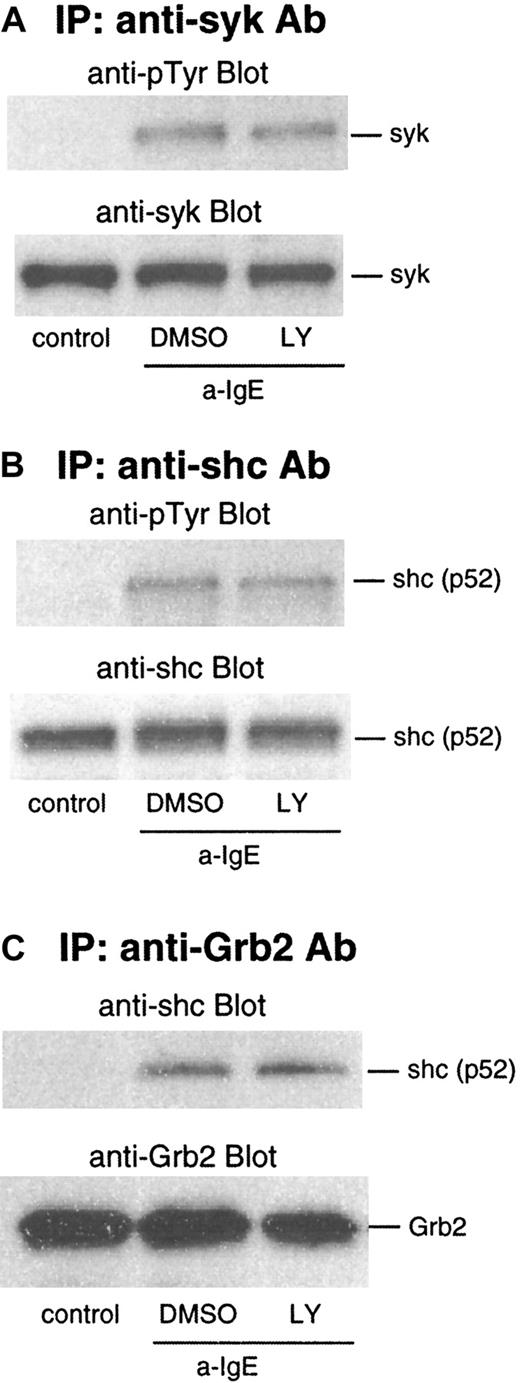
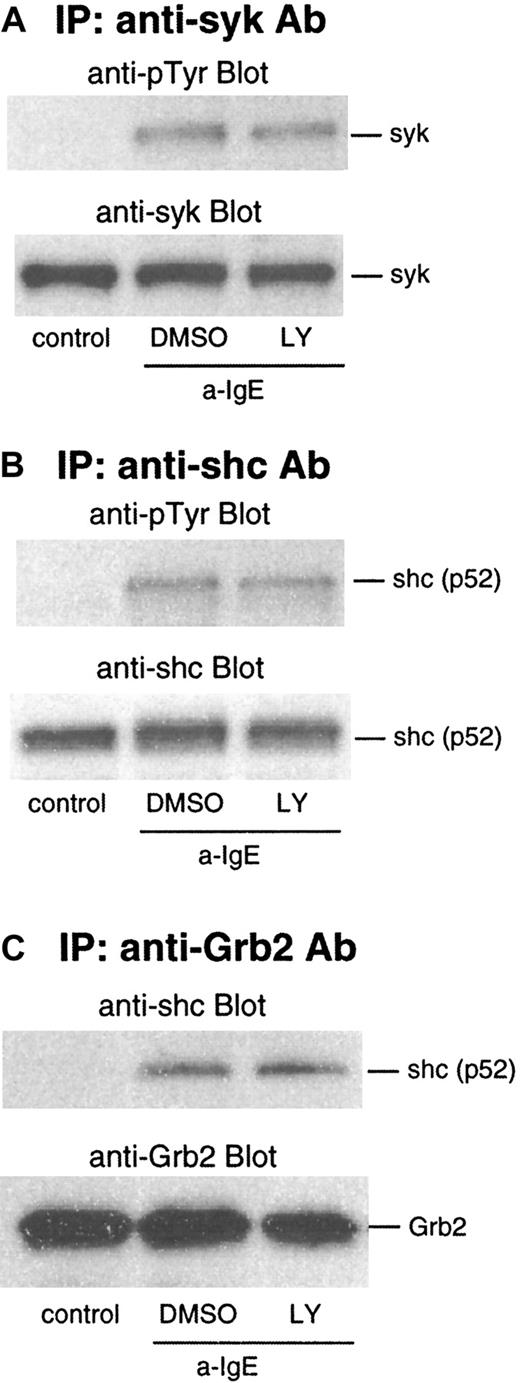
We next examined the effect of LY294002 on events upstream of ras: phosphorylation of syk and shc. As shown in Figure4A and B, phosphorylation of syk and shc was not affected by this inhibitor. IgE-mediated activation also results in an association of shc to the Grb2/Sos2 complex, which is dependent on phosphorylation of shc. In lysates from the cells stimulated by anti-IgE Ab, shc was coimmunoprecipitated with Grb2 captured by anti-Grb2/protein G beads. This association was not affected by LY294002 (Figure 4C).
Effect of LY294002 on phosphorylation of syk and shc and association of Grb2 and shc after stimulation with anti-IgE Ab.
Basophils were preincubated with DMSO (1:10 000 dilution) or LY294002 (10 μmol/L) for 10 minutes and were stimulated with anti-IgE (0.5 μg/mL) for 5 minutes. Reactions were stopped with the addition of ice-cold PAG, and the cells were microfuged. Clarified lysates were immunoprecipitated with anti-syk (A), anti-shc (B), or anti-Grb2 (C). The immunoprecipitated proteins were subjected to Western blot analysis with the indicated Abs, as described in “Materials and methods.” The same membranes were stripped and reblotted with the indicated Ab. The anti-syk (A), anti-shc (B), and anti-Grb2 (C) blots indicate essentially equal protein loading. Each Western blot shown is representative of 2 separate experiments.
Effect of LY294002 on phosphorylation of syk and shc and association of Grb2 and shc after stimulation with anti-IgE Ab.
Basophils were preincubated with DMSO (1:10 000 dilution) or LY294002 (10 μmol/L) for 10 minutes and were stimulated with anti-IgE (0.5 μg/mL) for 5 minutes. Reactions were stopped with the addition of ice-cold PAG, and the cells were microfuged. Clarified lysates were immunoprecipitated with anti-syk (A), anti-shc (B), or anti-Grb2 (C). The immunoprecipitated proteins were subjected to Western blot analysis with the indicated Abs, as described in “Materials and methods.” The same membranes were stripped and reblotted with the indicated Ab. The anti-syk (A), anti-shc (B), and anti-Grb2 (C) blots indicate essentially equal protein loading. Each Western blot shown is representative of 2 separate experiments.
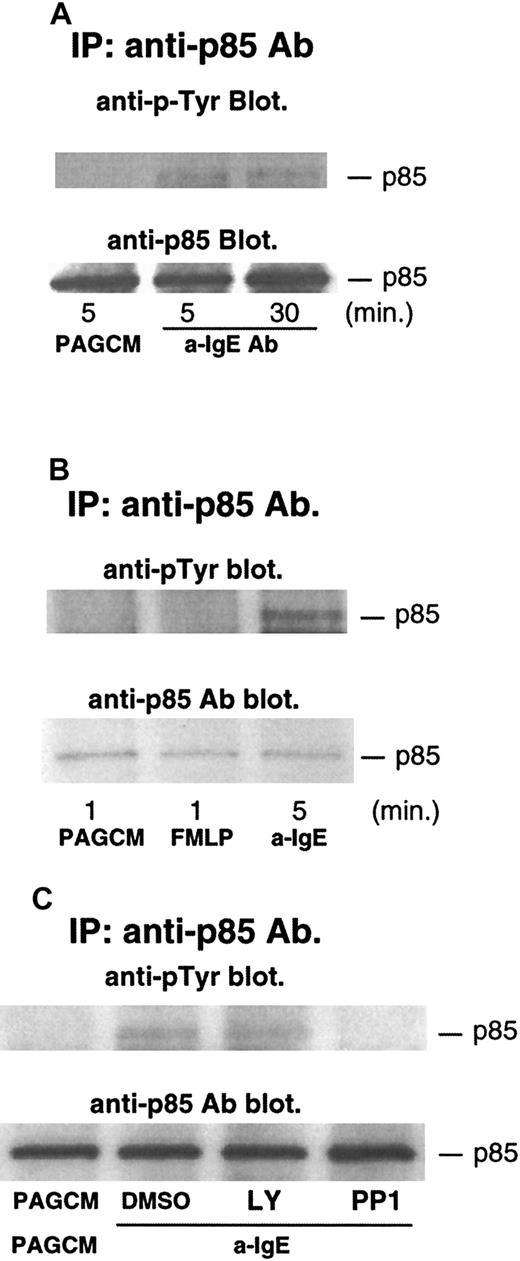
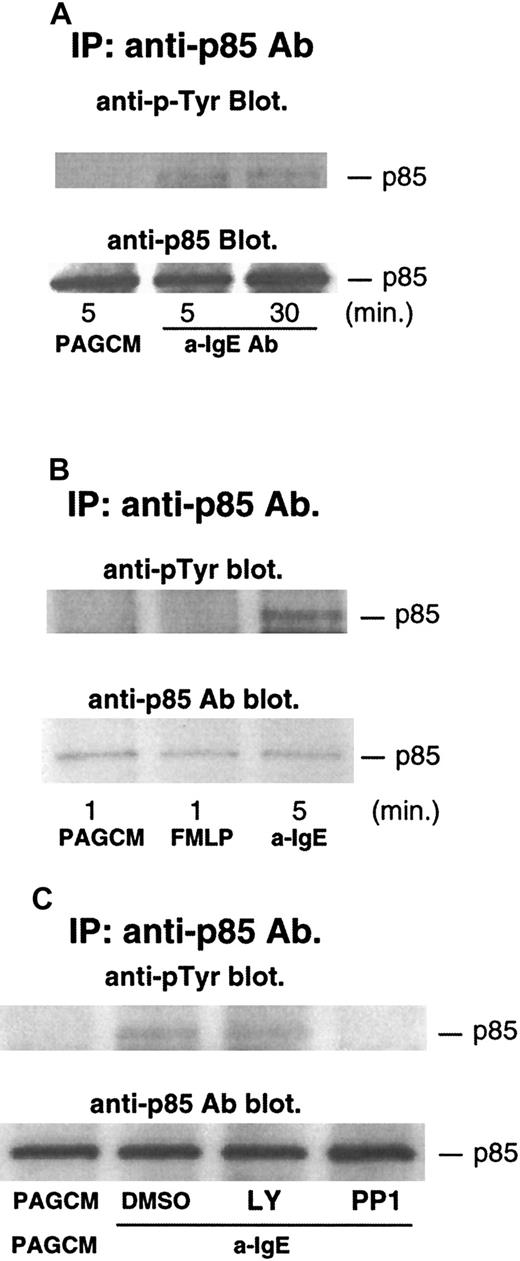
Phosphorylation of p85 (regulatory subunit of PI3 kinase) after stimulation with anti-IgE Ab or FMLP
Several studies have shown that phosphorylation of p85 (regulatory subunit of PI3 kinase) accompanies increases in the enzymatic activity of PI3 kinase.36 37 Thus, we examined the phosphorylation of p85 after stimulation with anti-IgE Ab. As shown in Figure5A, anti-IgE Ab induced significant tyrosine phosphorylation of p85. The level of phosphorylation was sustained, with phosphorylation being similar at 5 and 30 minutes after stimulation. In contrast to stimulation with anti-IgE Ab, FMLP-induced phosphorylation of p85 was not detected (anti-IgE Ab induced significant phosphorylation of p85 in the same experiments) (Figure5B). It should be noted that release of histamine and LTC4 induced by FMLP was much higher than that induced by anti-IgE Ab in this experiment: histamine and LTC4 induced by FMLP were 93% and 75 pmol/106 basophils, respectively; histamine and LTC4 induced by anti-IgE Ab were 75% and 29 pmol/106 basophils, respectively. A similar result was obtained in a separate experiment. We have recently found that PP1 (src-family specific inhibitor) inhibited phosphorylation of syk, presumably by inhibiting the activity of lyn kinase. PP1 inhibited IgE-induced phosphorylation of p85 (Figure5C), a result consistent with the model in which lyn kinase starts the entire signaling cascade, including activation of PI3 kinase. In contrast, LY294002 did not affect the phosphorylation of p85 (Figure 5C).
Tyrosine phosphorylation of p85 after stimulation with anti-IgE Ab and FMLP, and the effect of LY294002 or PP1.
Basophils were stimulated with or without anti-IgE (0.5 μg/mL) (A) or FMLP (1 μmol/L) (B) for the times indicated. (C) Basophils were pretreated with or without DMSO, LY294002 (10 μmol/L), or PP1 (10 μmol/L) for 10 minutes and stimulated by anti-IgE Ab for 5 minutes. Reactions were stopped with the addition of ice-cold PAG, and the cells were microfuged. Clarified lysates were immunoprecipitated with anti-p85 Ab. The immunoprecipitated proteins were subjected to Western blot analysis with antiphosphotyrosine Ab as described in “Materials and methods.” The same membranes were stripped and reblotted with anti-p85 Ab. The anti-p85 blot indicates essentially equal protein loading. Each Western blot shown is representative of 2 separate experiments.
Tyrosine phosphorylation of p85 after stimulation with anti-IgE Ab and FMLP, and the effect of LY294002 or PP1.
Basophils were stimulated with or without anti-IgE (0.5 μg/mL) (A) or FMLP (1 μmol/L) (B) for the times indicated. (C) Basophils were pretreated with or without DMSO, LY294002 (10 μmol/L), or PP1 (10 μmol/L) for 10 minutes and stimulated by anti-IgE Ab for 5 minutes. Reactions were stopped with the addition of ice-cold PAG, and the cells were microfuged. Clarified lysates were immunoprecipitated with anti-p85 Ab. The immunoprecipitated proteins were subjected to Western blot analysis with antiphosphotyrosine Ab as described in “Materials and methods.” The same membranes were stripped and reblotted with anti-p85 Ab. The anti-p85 blot indicates essentially equal protein loading. Each Western blot shown is representative of 2 separate experiments.
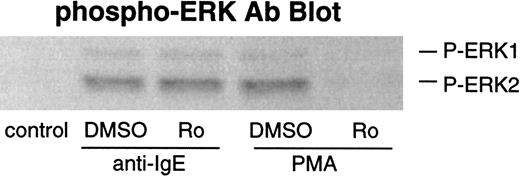
Effect of PKC inhibitor on phosphorylation of ERKs and LTC4 release after stimulation with anti-IgE Ab
It has been reported that elevated PIP3 levels generated by PI3 kinase may activate PDK1 (which contains a PH domain), which in turn phosphorylates and activates PKC family members.38,39 Thus, it is possible that PKCs may be involved in PI3 kinase–mediated ERK activation. We have previously demonstrated that PKCs do not play a prodegranulatory role in IgE-mediated secretion in human basophils with the use of specific PKC inhibitors (bisindolylmaleimide II and Ro-31-8220).21 In the present study, we also examined the effects of PKC inhibitor Ro-31-8220 on phosphorylation of ERKs and LTC4 release after stimulation with anti-IgE Ab. This compound did not inhibit IgE-mediated ERK phosphorylation, although PMA-induced phosphorylation was completely inhibited (Figure 6). Consistent with its lack of effect on ERK phosphorylation or the absence of inhibition of histamine release,21 Ro-31-8220 did not inhibit LTC4 release (at concentrations up to 1 μmol/L) induced by anti-IgE Ab (data not shown).
Effect of Ro-31-8220 on phosphorylation of ERKs after stimulation with anti-IgE Ab or FMLP.
Basophils were preincubated with DMSO (1:10 000 dilution) or Ro-31-8220 (1 μmol/L) for 10 minutes and were stimulated with or without anti-IgE Ab (0.5 μg/mL) for 10 minutes or PMA (50 ng/mL) for 20 minutes. Reactions were stopped with the addition of ice-cold PAG, and the cells were microfuged. Cell pellets were lysed and subjected to Western blot analysis as described in “Materials and methods.” The anti-ERK1 and anti-ERK2 blots indicated essentially equal protein loading (data not shown). The Western blot shown is representative of 2 separate experiments.
Effect of Ro-31-8220 on phosphorylation of ERKs after stimulation with anti-IgE Ab or FMLP.
Basophils were preincubated with DMSO (1:10 000 dilution) or Ro-31-8220 (1 μmol/L) for 10 minutes and were stimulated with or without anti-IgE Ab (0.5 μg/mL) for 10 minutes or PMA (50 ng/mL) for 20 minutes. Reactions were stopped with the addition of ice-cold PAG, and the cells were microfuged. Cell pellets were lysed and subjected to Western blot analysis as described in “Materials and methods.” The anti-ERK1 and anti-ERK2 blots indicated essentially equal protein loading (data not shown). The Western blot shown is representative of 2 separate experiments.
Discussion
IgE-mediated activation of ERK1/2 has previously been shown to be transient.5 We have recently determined that ras and MEK1, signaling elements upstream of ERK1/2, are also transiently activated after stimulation with anti-IgE Ab or FMLP. These results suggest that it is the activation state of ras that regulates the activation state of the MEK-ERK pathway (presumably through activation of raf-1 kinase). We have further found that anti-IgE Ab induced phosphorylation of shc and an association of shc to the Grb2-Sos2 complex in human basophils. In addition, the src-family kinase inhibitor, PP1, prevented IgE-mediated phosphorylation of shc and ERKs by inhibiting phosphorylation of syk. In contrast to the transient activation of ras, MEK1, and ERK1/2, the phosphorylation of syk and shc and the association of shc with Grb2/Sos2 were sustained for more than 30 minutes (manuscript submitted). Collectively, these results would be difficult to reconcile if it were assumed that the association of shc/Grb2/Sos2 were responsible for the activation of p21ras. These results suggest that other pathways might contribute to the activation state of p21ras, and it is the down-regulation of these other pathways that might explain the transient activation of p21ras.
In the current study, we explored the involvement of PI3 kinase in both IgE- and FMLP-mediated activation of p21ras. We found that LY294002 effectively inhibits IgE-mediated activation of p21ras. Provided that this inhibitor is acting selectively on PI3 kinase, these results provide evidence for another pathway leading to the activation of p21ras. It appears to be a pathway distinct from the shc/Grb2/Sos2 interaction because LY294002 does not inhibit phosphorylation of syk or shc or the association of shc/Grb2/Sos2. A direct interaction between p21ras and PI3 kinase has been suggested previously. Several groups have reported that PI3 kinase may regulate the activation of ras,34,37,40 whereas others have reported that ras may be upstream of PI3 kinase and regulate this kinase.41,42 On the basis of the inhibition by LY294002, we suggest that in human basophils, PI3 kinase lies upstream of p21ras. Future studies will determine whether down-regulation of PI3 kinase activity, or any intervening elements between PI3 kinase and p21ras activation, is responsible for down-regulation of p21ras even while the association of shc/Grb2/Sos2 is sustained. However, in this context, the current studies indicate that phosphorylation of the p85 regulatory subunit of PI3 kinase is sustained during IgE-mediated activation. If p85 phosphorylation state is reflective of PI3 kinase activity, then this result suggests that PI3 kinase activity is also sustained. This conclusion would be compatible with the observation that PI3 kinase activity is required for IL-4 secretion, a process that requires hours, but it is less compatible with the transient nature of p21ras activation. The precise molecular mechanism of regulation of ras by PI3 kinase in IgE-mediated activation remains to be explored. However, it is known that some guanine nucleotide exchange factors for ras (eg, Sos1 and Sos2) contain PH domains and might be influenced by the presence of membrane PIP3. Human basophils predominantly express Sos2 (manuscript submitted), and Sos2 may require an increase of PIP3 for its localization to the plasma membrane to activate ras effectively. In contrast to the results in human basophils, it has been reported that LY294002 or wortmannin does not inhibit IgE-mediated activation of ERKs or the release of AA/LTC4 in rodent cell models for IgE-mediated activation (RBL-2H3 cells or mouse bone marrow–derived mast cells).13-16
In direct comparisons with IgE-mediated events, FMLP-mediated events (release of histamine and LTC4 and ERK phosphorylation) were not affected by PI3 kinase inhibitors. In addition, FMLP does not induce tyrosine phosphorylation of p85. It was considered possible that FMLP induced high levels of PIP3 generation and that only low levels of PIP3 were necessary for maximal activation of the ERK pathway. Under these conditions, higher concentrations of LY294002 might be necessary to observe inhibition of FMLP-induced signaling through this pathway. However, we found that when FMLP concentrations were titrated to result in levels of LTC4 release that were the same or less than those induced by anti-IgE Ab, LY294002 continued to cause no inhibition of FMLP-induced release. Unless there are different requirements for PIP3 generation in the context of FMLP versus anti-IgE Ab, these results suggest that PI3 kinase does not play a critical role in the FMLP-mediated response in human basophils.
We have also shown that LY294002 inhibited anti-IgE–induced [Ca++]i elevations in human basophils (Figure3). Inhibition of IgE-mediated [Ca++]i elevations by this inhibitor may partially account for inhibition of histamine release and IL-4 production17 because previous studies have demonstrated that these events are dependent on sustained [Ca++]i elevations.43,44 Similar results have been reported from studies of IgE-mediated [Ca++]i in RBL-2H3 cells and mouse bone marrow–derived mast cells using wortmannin and LY294002.45,46 The precise mechanisms regulating [Ca++]i elevations are still unclear. However, based on studies in B cells, it has been proposed that PI3 kinase indirectly regulates the sustained elevation of [Ca++]i. It does this by regulating levels of PIP3, which in turn regulate recruitment of Btk (Btk/Tec-family tyrosine kinase) to the plasma membrane. Once recruited, Btk is activated by phosphorylation, and its activity leads to phosphorylation of phospholipase Cγ, causing further generation of IP3 and a sustained emptying of intracellular stores of calcium.47 48 Alternatively, PI3 kinase may regulate a variety of pathways distinct from the p21ras pathways or [Ca++]i elevations but nevertheless relevant to degranulation and secretion of cytokines.
In summary, we have shown that PI3 kinase may regulate ras activation, which in turn regulates the MEK-ERK pathway leading to LTC4 release. This enzyme also regulates the [Ca++]iresponse in human basophils.
Acknowledgments
We thank Dr S. E. Lavens-Phillips and Dr J. Gong for helpful discussion and Ms Val Alexander, Ms Kris Chichester, and Ms Ellen Roche for technical assistance.
Supported by National Institutes of Health Grants AI20253 and AI07290.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donald W. MacGlashan Jr, Johns Hopkins Asthma and Allergy Center, 5501 Hopkins Bayview Circle, Baltimore, MD 21224; e-mail: dmacglas@welch.jhu.edu.


![Fig. 3. Effect of LY294002 on the [Ca++]i response after stimulation with anti-IgE Ab. / Basophils were labeled with fura-2/am. After washing, the cells were treated with or without LY294002 for 10 minutes and stimulated with anti-IgE Ab (0.5 μg/mL). The [Ca++]i response was analyzed as described in “Materials and methods.” The result shown is the average of 2 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2199/5/m_h81800175003.jpeg?Expires=1763705804&Signature=YNkrre-CBcIp4p-KZHh~Axhf8qD~WvG-lzo7US~YdNlTgCvVS730y9PLBROeDC6BneCNvfXHHZhDLhMNQ1cpyG2Jtz7zreBXeECR1NurRWH8cplYUUMb7-t26orlLvSeTUf6LgY1t3-ht8QJo-YSfj65tecz5QnzgC12iEQtCWiLNpK0tWFGXF2kWPhrYgLJTam1ndIUIUqQMAwtnHfDRsL5CXyGVPVEKpYp9LEQv6wCxlMzMcgbkeVIQnHDY-aSJIQJBnBoTfp5mbu7t0Xtam1UZf-BmUwA4HZh1U0Iwr8ZMVjyeNZONV25zS0PYT~4buIsjuwcs-Ytwp7w868-JA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 3. Effect of LY294002 on the [Ca++]i response after stimulation with anti-IgE Ab. / Basophils were labeled with fura-2/am. After washing, the cells were treated with or without LY294002 for 10 minutes and stimulated with anti-IgE Ab (0.5 μg/mL). The [Ca++]i response was analyzed as described in “Materials and methods.” The result shown is the average of 2 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/6/10.1182_blood.v96.6.2199/5/m_h81800175003.jpeg?Expires=1763705805&Signature=bP7P-xQxTHrQgM5V7FleAOcOkYMKHl3PyZMuw5KYdghVwNitUXgCHN1uONUpdZccfA9z8MI~mR5vC0s5a3hBr2n9WOR0~Q9CezQ9kt6LTQTq82CqHDidPSaaWA-lP13Brmed40gnIL0Y8hVMQBmhXqPopNLZZYyAxj7lx77zPW8Kqiz~Rs0RWXWAfLAedVYLk46MFJRem-aSCrEOdomuGi-P5ju3exTpDt1AP7J4cbaKd71-lBuzIIk6phzd9ze4k1VevAlntZp9AfuxHvK2ej2hZHpbM5vHhHqR-PNqe2f2Z0LttODhFb9iyd9aXz8dJWywOST9poh~5-laBjh49A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)