Abstract
Sickle cell anemia is characterized by painful vaso-occlusive crises. It is hypothesized that monocytes are activated in sickle cell disease and can enhance vaso-occlusion by activating endothelium. To test this hypothesis, human umbilical vein endothelial cells (HUVEC) and human microvascular endothelial cells (MVEC) with sickle and normal mononuclear leukocytes were incubated, and endothelial activation was measured. Endothelial cells incubated with sickle mononuclear leukocytes were more activated than those incubated with normal mononuclear leukocytes, as judged by the increased endothelial expression of adhesion molecules and tissue factor and the adhesion of polymorphonuclear leukocytes (PMNL). Monocytes, not lymphocytes or platelets, were the mononuclear cells responsible for activating endothelial cells. Sickle monocytes triggered endothelial nuclear factor-kappa B (NF-κB) nuclear translocation. Cell-to-cell contact of monocytes and endothelium enhanced, but was not required for, activation. Antibodies to tumor necrosis factor-alpha (TNF-α) and interleukin-1-beta (IL-1β) blocked activation of the endothelium by monocytes. Peripheral blood monocytes from patients with sickle cell disease had 34% more IL-1β (P = .002) and 139% more TNF-α (P = .002) per cell than normal monocytes. Sixty percent of sickle monocytes expressed the adhesion molecule ligand CD11b on their surfaces compared with only 20% of normal monocytes (P = .002). Serum C-reactive protein, a marker of systemic inflammation, was increased 12-fold in sickle serum than in normal serum (P = .003). These results demonstrate that sickle monocytes are activated and can, in turn, activate endothelial cells. It is speculated that vascular inflammation, marked by activated monocytes and endothelium, plays a significant role in the pathophysiology of vaso-occlusion in sickle cell anemia.
Introduction
Mutation of the β-globin gene of hemoglobin in sickle cell anemia has pleiotropic effects in patients with sickle cell disease, including vaso-occlusion, strokes, hemolytic anemia, increased infection, and ischemic organ damage.1 Acute painful episodes, often called vaso-occlusive crises, are the most frequent complication of sickle disease and result in frequent hospitalizations.
The vascular endothelium plays a critical role in vaso-occlusion and ischemic organ damage by several mechanisms.1,2 These include the regulation of hemostasis and vascular tone, adhesion of red blood cells (RBCs) and leukocytes, and possibly injury caused by ischemia–reperfusion. Evidence indicates the vascular endothelium is damaged in sickle disease.2 This damage includes histopathologic changes in many vascular beds, such as the spleen, brain, and retina of patients with sickle disease3-6; formation of thromboses at sites of underlying intimal hyperplasia4-6; abnormal presence of circulating endothelial cell adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin in the plasma7; and increased numbers of circulating endothelial cells in the blood of patients in acute painful crisis.8-10
Circulating endothelial cells found in patients in crisis are activated as judged by the expression of adhesion molecules and tissue factors on their surfaces.10,11 The adhesion molecules ICAM-1, VCAM-1, P-selectin, and E-selectin and the procoagulant molecule tissue factor are expressed on activated endothelium and play key roles in the recruitment of leukocytes12,13 and the promotion of thrombosis14 at sites of vascular inflammation.
Activation of endothelial cells is regulated in part by the translocation of a transcription factor, NF-κB, from the cytoplasm to the nucleus.15 Translocation to the nucleus occurs after an inhibitory protein subunit, inhibitor kappa B-alpha, is phosphorylated and degraded exposing a nuclear localization site on the p65 subunit of NF-κB.16,17 NF-κB turns on the transcription of a large number of genes associated with inflammation, including adhesion molecules,18 tissue factor,19 cytokines,15 and acute-phase proteins.15 NF-κB translocation is initiated by a wide variety of stimuli15 18 including reactive oxygen species, cytokines such as TNF-α and IL-1β, mitogens such as phorbol esters and calcium ionophores, viruses, and bacterial products such as lipopolysaccharide. We hypothesize that the monocytes of patients with sickle cell anemia are activated and can enhance vaso-occlusion through an inflammatory response promoted by the NF-κB–mediated up-regulation of adhesion molecules and tissue factor on the surfaces of endothelial cells.
Patients, materials, and methods
Reagents were obtained from Sigma Aldrich (St. Louis, MO) or Gibco BRL (Grand Island, NY) unless otherwise indicated. Buffer A contained 0.8% (wt/vol) NaCl, 1 mmol/L EDTA, 10 mmol/L HEPES, and 0.5% bovine serum albumin (BSA), pH 7.4. Buffer B contained Hank's balanced salt solution, 25 mmol/L HEPES, and 0.5% BSA, pH 7.4. Buffer C contained phosphate-buffered saline (PBS), 0.5% BSA, and 0.1% Tween-20, pH 7.4.
Subjects
Male and female patients with sickle cell anemia were recruited from the University of Minnesota-Fairview Hospital and Clinics, Minneapolis Children's Hospital, and St. Paul Children's Hospital. Informed consent was obtained from the patient or the patient's parent and assent from the minor if the patient was younger than 18 years of age. Patients were in the hospital during crisis or in the clinic for a routine visit. Normal healthy male and female control subjects without hemoglobinopathy were recruited from the University of Minnesota and included African-American, white, and Asian volunteers. Venous blood (8-100 mL) was collected from the antecubital vein into Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) containing EDTA anticoagulant, unless otherwise indicated. Blood samples were processed within 30 minutes of collection.
Endothelial cell culture
Mononuclear leukocyte isolation
Mononuclear leukocytes used for incubation with endothelial cells were isolated on Ficoll-Hypaque density gradients.23Mononuclear leukocytes were washed in buffer A and resuspended in buffer B.
Monocyte and lymphocyte separation
Monocytes and lymphocytes were isolated by rate zonal density gradient centrifugation using OptiPrep medium.24Monocytes and lymphocytes were washed in buffer A and resuspended in buffer B. Lymphocyte preparations contained less than 1% monocytes and no polymorphonuclear leukocytes (PMNL), and the monocyte preparations contained less than 10% lymphocytes and no PMNL, as judged by fluorescence-activated cell sorting (FACS) forward and side scatter and CD14 immunostaining.
Monocytes also were purified by FACS. Mononuclear leukocytes were isolated on Ficoll-Hypaque density gradients. Five percent of the mononuclear leukocytes were labeled with monoclonal anti-CD14–fluorescein isothiocyanate conjugate and analyzed on a FACStar (Becton Dickinson, San Jose, CA). A gate was placed around monocytes based on forward and side scatter and CD14 immunofluorescence. Monocytes were collected within the gate from the remaining unlabeled mononuclear leukocytes. The monocytes were washed in buffer A and resuspended in buffer B. These preparations contained less than 10% lymphocytes and platelets.
Platelet isolation
Platelet-rich plasma (PRP) was prepared from citric acid–citrate–dextrose (CACD) blood at 130g for 20 minutes. PRP was diluted 1:1 with 0.1 mol/L CACD, pH 6, and centrifuged at 830g for 20 minutes. The platelets were washed in CACD and resuspended in buffer B.
Polymorphonuclear leukocyte isolation
Normal PMNL were isolated from blood containing heparin anticoagulant using hydroxyethylstarch and Percoll gradients as described.25
Endothelial cell incubations with leukocytes and platelets
Given the differences in endothelial cell preparations, all experiments were performed on the same day, with the same preparation of endothelial cells, for any given pair of leukocyte or platelet preparations from patients with sickle cell disease and normal subjects. Leukocytes (1-18 mononuclear leukocytes, monocytes, or lymphocytes per endothelial cell), platelets (100 platelets per endothelial cell), or TNF-α (10 ng/mL) were added to the endothelial cell media and incubated for 1 to 6 hours. After incubation, the endothelial monolayer was washed 3 times in buffer B.
For experiments in which endothelial cell expression of E-selectin, ICAM-1, VCAM-1, and tissue factor were measured, incubations of 4 or 5 hours with mononuclear leukocytes were sufficient for all proteins to be expressed. For E-selectin experiments, 3 hours were sufficient.
In time-course experiments, HUVEC were incubated with monocytes or lymphocytes for 5 minutes to 5 hours. After the indicated incubation time, HUVEC were washed and replenished with fresh media. All HUVEC incubations lasted a total of 5 hours, including the initial incubations with monocytes, lymphocytes, or TNF-α.
In experiments designed to block leukocyte–HUVEC contact, mononuclear leukocytes were incubated above HUVEC monolayers inside cell culture inserts containing polyethylene terephthalate membranes with 0.4-μm pores and pore densities of 1 × 108pores/cm2 (Becton Dickinson, Franklin Lakes, NJ). These membranes allow high rates of basolateral diffusion.
The role of cytokines in leukocyte activation of HUVEC was evaluated using monoclonal antibodies (10 μg/mL) to block TNF-α and IL-1β activity (R&D Systems, Minneapolis, MN). Mononuclear leukocytes, IL-1β, and TNF-α were preincubated with the blocking antibodies for 15 minutes before the leukocyte/antibody or cytokine/antibody mixtures were added to HUVEC.
PMNL adhesion to HUVEC
Normal PMNL were isolated as described above and labeled with 50 to 100 μCi 51Cr for 60 minutes. HUVEC were incubated with sickle or normal mononuclear leukocytes, TNF-α, or media only for 4 hours. After these initial preincubations, HUVEC were washed and incubated with 51Cr-labeled PMNL at a concentration of 1 to 5 PMNL per HUVEC for 15 minutes. After the second incubation, HUVEC were washed 3 times, and the supernatant and washes from each well were combined. Bound PMNL and HUVEC were removed from wells after overnight incubation in 1 N NaOH. Bound and unbound51Cr-labeled–PMNL were counted in a gamma counter. The percentage of PMNL bound to HUVEC was calculated as CPM bound/(CPM bound + unbound). Adhesion was measured in either triplicate or quadruplicate wells for each of the 5 experiments.
Enzyme immunoassays for HUVEC E-selectin, ICAM-1, VCAM-1, and tissue factor
Washes were in buffer B for adhesion molecules and in buffer C for tissue factor. After incubation of HUVEC with mononuclear leukocytes, monocytes, lymphocytes, platelets or TNF-α in 24-well tissue culture plates, HUVEC were washed 3 times, fixed for 15 minutes in 4% paraformaldehyde, washed, and permeabilized with cold methanol (−20°C) for 15 minutes. Tissue factor assays were not permeabilized. Thus, tissue factor represents cell surface protein, and E-selectin, ICAM-1, and VCAM-1 represent intracellular and cell surface protein. Next, HUVEC were washed and incubated 2 hours with primary mouse monoclonal antibodies (2 μg/mL) against E-selectin, VCAM-1 (Pharmingen, San Diego, CA), ICAM-1 (R& D Systems, Minneapolis, MN), or tissue factor (a gift from Dr Ron Bach, Veterans Administration Medical Center, Minneapolis, MN). HUVEC were washed 3 times and incubated for 1 hour with the secondary antibody, donkey antimouse IgG alkaline phosphatase conjugate (Jackson ImmunoResearch Laboratories, West Grove, PA). HUVEC were washed 3 times and incubated for 60 minutes with alkaline phosphatase substrate P-nitrophenyl phosphate in 1 mol/L diethanolamine buffer, 0.5 mmol/L MgCl2, pH 9.8. The reaction was stopped with 3 mol/L NaOH, and 100-μL aliquots were transferred in triplicate to 96-well plates and read at 405 nm in a microtiter plate reader (Molecular Devices, Palo Alto, CA). Background optical density (OD) values were measured in wells containing substrate only and subtracted from all other values. Results are expressed as a percentage of control (media only) HUVEC. Background OD values from nonspecific binding of secondary antibody in the absence of primary antibody were small, so these data were not used in the calculations.
RNA extraction and reverse transcription–polymerase chain reaction
RNA was extracted from washed endothelial cells using a chloroform phenol extraction kit, RNAzol B (Tel-Test, Friendswood, TX). RNA quantity and quality were measured spectrophotometrically at 260/280 nm (DU-70; Beckman, Fullerton, CA). An extinction coefficient of 12 mg × μL−1 × OD−1 × cm−1at 260 nm was used to calculate RNA concentrations.
All reagents for cDNA synthesis and amplification were obtained from Life Technologies, (Gaithersburg, MD). One microgram total RNA was used for cDNA synthesis using SuperScript II RT and a poly dTTP primer. Polymerase chain reactions (PCR) were conducted in an Attotech (Minneapolis, MN) thermocycler. A 1000-bp region of E-selectin cDNA was amplified using the following primer sequences: 5′-GCGGTACCCCTGTACATTTGACTGTGAAG-3′ and 5′-GCTCTAGAAAGGCTTTGGCAGCTGCTGGCA-3′. A 462-bp region of tissue factor cDNA was amplified using the following sequences: 5′-ACTCCCCAGAGTTCACACCTTACC-3′ and 5′-GGAGCTGTGGCATTTGTGGTCA-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as a control. A 600-bp region of GAPDH cDNA was amplified using primers 5′-CCACCCATGGCAAATTCCATGGCA-3′ and 5′-GGTGGACCTGACCTGCCGTCAAGA-3′. cDNA products were separated on 2% agarose gels in TAE buffer containing 40 mmol/L Tris-acetate, 2 mmol/L Na2 EDTA, pH 8.5, in the presence of 1μg/mL ethidium bromide.
Extraction of endothelial cell nuclear proteins
Nuclear proteins were extracted from washed (3×) endothelial cells (5.5 × 106) using a modification of previously described methods.26 Cells were harvested with trypsin-EDTA and washed once in ice-cold media. Cells were washed twice more in PBS and resuspended 5 minutes in 100 μL cell lysis buffer containing 10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.3 mol/L sucrose, 1.5 mmol/L MgCl2, 0.5 mmol/L dithiothreitol, 0.5% NP-40 (Calbiochem, La Jolla, CA), and protease inhibitors. Nuclei were pelleted at 16 000g and washed in 100 μL cell lysis buffer. Nuclear proteins were extracted into 100 μL nuclear extraction buffer containing 20 mmol/L HEPES, pH 7.9, 0.1 mol/L KCl, 0.1 mol/L NaCl, 0.5 mmol/L dithiothreitol, 20% glycerol, and protease inhibitors. Protein concentrations were measured by bicinchoninic acid protein assay after trichloroacetic acid precipitation.27
Electrophoretic mobility shift assay
One hundred nanograms of ssDNA 5′-TCTCAACAGAGGGGACTTTCCGAGAGGCCATCTGG-3′ containing the consensus sequence of the NF-κB DNA binding site (underlined) was made into radioactive dsDNA by fill-in labeling with α-[32P]dGTP, α-[32P]dCTP, dATP, and dTTP according to the manufacturer's protocol (Boehringer Mannheim, Indianapolis, IN). DNA-protein binding reactions contained 10 μg nuclear protein extract and 2 ng labeled dsDNA. Reactions were carried out in 20 mmol/L HEPES, pH 7.9, 5 mmol/L KCl, 0.5 mmol/L EDTA, 5% glycerol, 1 mmol/L dithiothreitol, 0.5 mmol/L PMSF, 1 mg/mL BSA, 0.1% NP-40, and 250 ng poly dI/dC. Some reactions contained a 25-fold excess of unlabeled NF-κB–specific dsDNA to serve as a cold competitor with32P-labeled dsDNA or 2 μg of goat anti-NF–κB p65 or p50 IgG (Santa Cruz Biotechnology, Santa Cruz, CA) to supershift the NF-κB band. Binding reactions were incubated for 30 minutes at room temperature and separated on a 6% nondenaturing polyacrylamide gel using 0.5× TBE running buffer containing 44.5 mmol/L Tris, 44.5 mmol/L boric acid, and 1 mmol/L EDTA, pH 8.
Measurement of monocyte activation by FACS
Leukocytes were obtained from blood after lysis of RBCs in isotonic ammonium chloride buffer.28 They were labeled with monoclonal anti-CD14–FITC (Becton Dickinson, San Jose, CA) and either anti-CD11b–phycoerythrin (PE; Becton Dickinson), anti-TNF-α-PE (Pharmingen, San Diego, CA), or anti-IL-1β-PE (R&D Systems) conjugate antibodies. Leukocytes were fixed and permeabilized (Pharmingen) before immunostaining for CD14/TNF-α or CD14/IL-1β. Labeled cells were washed and resuspended in 0.5% formaldehyde in PBS–EDTA.
FACS was performed on a FACSCalibur (Becton Dickinson). Mononuclear leukocytes were identified and gated by forward and side scatter. Monocytes were identified within the gate using CD14-specific immunofluorescence. Monocyte activation was measured as the mean fluorescent intensity of monocyte TNF-α and IL-1β and as the percentage of monocytes with CD11b on their surfaces.
C-reactive protein measurement
C-reactive protein (CRP) was measured by rate nephelometry on a Beckman Coulter Array 360 system (Beckman Coulter, Brea, CA) in the clinical laboratory of Fairview University Medical Center.
Statistics
Normal and sickle groups were compared using a Studentt test or a Mann-Whitney rank sum test run on SigmaStat 2.0 for Windows (SPSS, Chicago, IL). Data presented without statistics are from individual experiments, which are representative of 2 or more experiments.
Results
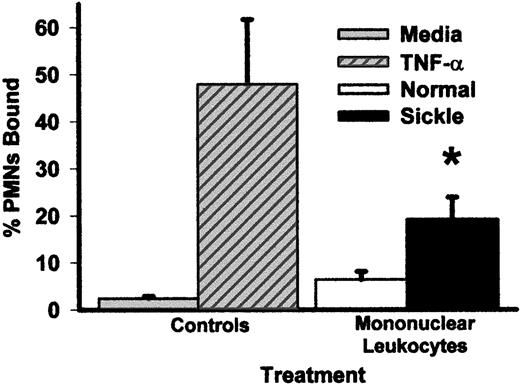
Pretreatment of HUVEC for 4 hours with mononuclear leukocytes from patients with sickle disease increased the adhesion of normal PMNL to HUVEC 3-fold compared with HUVEC pretreated with mononuclear leukocytes from normal subjects (P < .05) and 8-fold compared with control HUVEC (Figure 1). TNF-α (10 ng/mL), a positive control for HUVEC activation, increased PMNL adhesion 20-fold compared with the control. Similar PMNL adhesion results were seen on MVEC (data not shown).
Pretreatment of HUVEC with mononuclear leukocytes from patients with sickle cell disease increases the adhesion of normal PMNL to HUVEC relative to media alone or to mononuclear leukocytes from normal subjects.
HUVEC were pretreated with media alone, TNF-α (10 ng/mL), or sickle or normal mononuclear leukocytes (2 leukocytes/endothelial cell) for 4 hours at 37°C. After the 4-hour pretreatment period, the endothelial monolayer was washed 3 times, and 51Cr-labeled normal PMNL were added to HUVEC and incubated for 15 minutes. HUVEC were washed 3 times, and PMNL adhesion to endothelium was calculated by counting radioactivity in the cell bound and unbound wash fractions. Results are expressed as percentage of PMNL bound to HUVEC. *P < .05 for sickle versus normal mononuclear leukocytes.
Pretreatment of HUVEC with mononuclear leukocytes from patients with sickle cell disease increases the adhesion of normal PMNL to HUVEC relative to media alone or to mononuclear leukocytes from normal subjects.
HUVEC were pretreated with media alone, TNF-α (10 ng/mL), or sickle or normal mononuclear leukocytes (2 leukocytes/endothelial cell) for 4 hours at 37°C. After the 4-hour pretreatment period, the endothelial monolayer was washed 3 times, and 51Cr-labeled normal PMNL were added to HUVEC and incubated for 15 minutes. HUVEC were washed 3 times, and PMNL adhesion to endothelium was calculated by counting radioactivity in the cell bound and unbound wash fractions. Results are expressed as percentage of PMNL bound to HUVEC. *P < .05 for sickle versus normal mononuclear leukocytes.
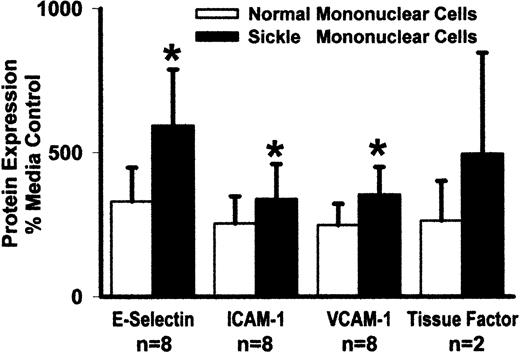
Sickle and normal mononuclear leukocytes were compared for their ability to activate adhesion molecule and tissue factor expression on endothelial cells. HUVEC were incubated 5 hours with sickle or normal mononuclear leukocytes. Adhesion molecule and tissue factor protein expression on HUVEC were measured by enzyme immunoassay (Figure2). Sickle mononuclear leukocytes increased E-selectin expression 1.8-fold compared with normal mononuclear leukocytes (594% vs 329%, P < .05; control HUVEC = 100%). ICAM-1 expression was increased 1.3-fold (338% vs 254%, P < .05) VCAM-1 expression was increased 1.4-fold (354% vs 248%, P < .05), and tissue factor expression was increased 1.9-fold (495% vs 263%, n = 2). TNF-α (10 ng/mL), a positive control for endothelial cell activation, increased E-selectin 13-fold, ICAM 6-fold, VCAM 7-fold, and tissue factor 2-fold (data not shown). Similar results were seen in MVEC; however, adhesion molecule and tissue factor expression in MVEC were generally less than that seen in HUVEC (data not shown).
Sickle mononuclear leukocytes activate HUVEC expression of E-selectin, ICAM-1, VCAM-1, and tissue factor relative to normal mononuclear leukocytes.
HUVEC were incubated with media alone or sickle or normal mononuclear leukocytes (2 leukocytes/endothelial cell) for 5 hours at 37°C. HUVEC adhesion molecule and tissue factor proteins were measured by enzyme immunoassay, and the results are expressed as a percentage of control (media only) HUVEC (100%). *P < .05 for sickle versus normal mononuclear leukocytes.
Sickle mononuclear leukocytes activate HUVEC expression of E-selectin, ICAM-1, VCAM-1, and tissue factor relative to normal mononuclear leukocytes.
HUVEC were incubated with media alone or sickle or normal mononuclear leukocytes (2 leukocytes/endothelial cell) for 5 hours at 37°C. HUVEC adhesion molecule and tissue factor proteins were measured by enzyme immunoassay, and the results are expressed as a percentage of control (media only) HUVEC (100%). *P < .05 for sickle versus normal mononuclear leukocytes.
The data presented for E-selectin, ICAM-1, and VCAM-1 represent total protein expression (intracellular + cell surface expression) by HUVEC because the cells were permeabilized with methanol during the immunoassay procedure. However, the tissue factor data represent cell surface expression by HUVEC because the cells were not permeabilized for the tissue factor immunoassays. In one study, HUVEC were stimulated with sickle mononuclear leukocytes, and subsequent immunoassays for E-selectin and VCAM-1 were run with and without cold methanol permeabilization to differentiate between intracellular and cell surface protein expression. Intracellular and cell surface E-selectin expression peaked at 4 and 5 hours, respectively (data not shown). Virtually all the E-selectin was found on the cell surface after 5 hours. Intracellular and cell surface VCAM-1 expression peaked at 5 and 6 hours, respectively (data not shown); however, VCAM-1 expression was not measured beyond 6 hours. Virtually all the VCAM-1 protein was found on the cell surface at 6 hours.
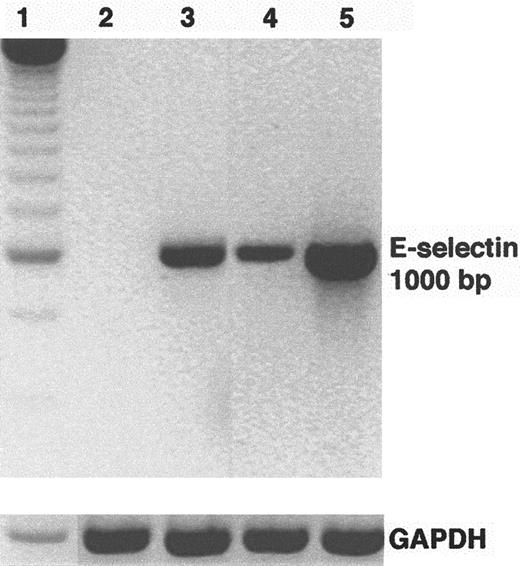
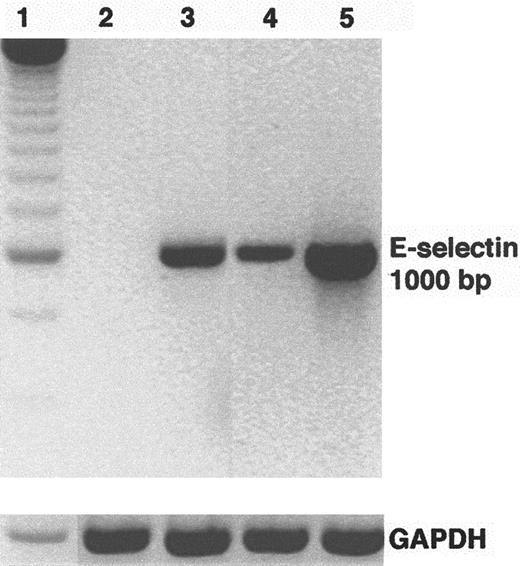
HUVEC E-selectin mRNA levels were examined by reverse transcription (RT)-PCR after incubation for 5 hours with sickle and normal mononuclear leukocytes. E-selectin mRNA levels were up-regulated in HUVEC incubated with sickle and normal mononuclear leukocytes compared with control HUVEC (Figure 3). E-selectin up-regulation was greater in HUVEC incubated with sickle mononuclear leukocytes than in normal mononuclear leukocytes. HUVEC pretreated with TNF-α for 2 hours served as a positive control for endothelial activation. GAPDH mRNA, an internal control, was similar across all lanes.
Sickle mononuclear leukocytes up-regulate E-selectin mRNA in HUVEC.
HUVEC were incubated with sickle or normal mononuclear leukocytes (2 leukocytes/endothelial cell) for 5 hours or TNF-α (10 ng/mL) for 2 hours. E-selectin mRNA was isolated from the endothelial cells, reverse transcribed into cDNA, and amplified by PCR as described in “Materials and methods.” RT-PCR products were separated by electrophoresis on a 2% agarose gel and stained with ethidium bromide. The figure is a reverse image of the stain. Lane 1, 250-bp DNA ladder; lane 2, control (media only) HUVEC; lane 3, TNF-α–treated HUVEC; lane 4, normal mononuclear leukocyte-treated HUVEC; lane 5, sickle mononuclear leukocyte-treated HUVEC. GAPDH mRNA, a control, was also amplified by RT-PCR from the same RNA extracts and run on a separate agarose gel shown at the bottom. The E-selectin and the GAPDH products measured 1000 bp and 600 bp, respectively, as predicted from the gene sequences. Results are from a single representative experiment of 2.
Sickle mononuclear leukocytes up-regulate E-selectin mRNA in HUVEC.
HUVEC were incubated with sickle or normal mononuclear leukocytes (2 leukocytes/endothelial cell) for 5 hours or TNF-α (10 ng/mL) for 2 hours. E-selectin mRNA was isolated from the endothelial cells, reverse transcribed into cDNA, and amplified by PCR as described in “Materials and methods.” RT-PCR products were separated by electrophoresis on a 2% agarose gel and stained with ethidium bromide. The figure is a reverse image of the stain. Lane 1, 250-bp DNA ladder; lane 2, control (media only) HUVEC; lane 3, TNF-α–treated HUVEC; lane 4, normal mononuclear leukocyte-treated HUVEC; lane 5, sickle mononuclear leukocyte-treated HUVEC. GAPDH mRNA, a control, was also amplified by RT-PCR from the same RNA extracts and run on a separate agarose gel shown at the bottom. The E-selectin and the GAPDH products measured 1000 bp and 600 bp, respectively, as predicted from the gene sequences. Results are from a single representative experiment of 2.
Because both monocytes and endothelial cells can express tissue factor in response to an inflammatory stimulus,29-31 it was possible that the observed tissue factor expression was associated with monocytes bound to the endothelial monolayer. However, this possibility was unlikely because virtually all the mononuclear cells were removed from endothelial cells by washing 3 times after incubation. We used immunofluorescence staining and laser confocal microscopy to confirm that tissue factor was expressed on endothelial cells. Tissue factor immunofluorescence was strongly up-regulated on endothelial cells after incubation with TNF-α or sickle, but not normal, mononuclear leukocytes (data not shown).
Genes for E-selectin, ICAM-1, VCAM-1, and tissue factor all have NF-κB binding sites in their promoters. We examined nuclear NF-κB levels in endothelial cells (MVEC) incubated with sickle and normal mononuclear leukocytes for 1 hour (Figure4). MVEC may be a more relevant target than HUVEC because vaso-occlusion occurs primarily in the microvasculature. Sickle mononuclear leukocytes up-regulated nuclear NF-κB levels in MVEC relative to control MVEC and MVEC incubated with normal mononuclear leukocytes. Nuclear NF-κB also was strongly up-regulated by TNF-α. The NF-κB bands in MVEC disappeared in the presence of a 25-fold excess of unlabeled NF-κB dsDNA and was composed primarily of the p65 and p50 subunits (data not shown). Similar results were seen when sickle and normal mononuclear leukocytes were incubated with HUVEC (data not shown). The sickle mononuclear leukocytes themselves did not contribute to the observed NF-κB. Washing the endothelial monolayer removed virtually all the leukocytes, and the concentration of NP-40 detergent (0.5%) used during the endothelial cell lysis step resulted in the loss of all nuclear proteins associated with leukocytes.
Sickle mononuclear leukocytes activate NF-κB in MVEC.
Mononuclear leukocytes (15 leukocytes/endothelial cell) or TNF-α (10 ng/mL) were incubated with MVEC for 1 hour. After incubation, MVEC nuclear proteins were extracted for NF-κB analysis. Nuclear extracts (10 μg protein) were incubated with 2 ng [32P]-labeled dsDNA containing a consensus NF-κB sequence and analyzed by electrophoretic mobility shift assay. Lane 1, [32P]-labeled dsDNA probe alone; lane 2, control (media only)-treated MVEC; lane 3, TNF-α–treated MVEC; lane 4, normal mononuclear leukocyte-treated MVEC; lane 5, sickle mononuclear leukocyte-treated MVEC. Results are from a single representative experiment of 2.
Sickle mononuclear leukocytes activate NF-κB in MVEC.
Mononuclear leukocytes (15 leukocytes/endothelial cell) or TNF-α (10 ng/mL) were incubated with MVEC for 1 hour. After incubation, MVEC nuclear proteins were extracted for NF-κB analysis. Nuclear extracts (10 μg protein) were incubated with 2 ng [32P]-labeled dsDNA containing a consensus NF-κB sequence and analyzed by electrophoretic mobility shift assay. Lane 1, [32P]-labeled dsDNA probe alone; lane 2, control (media only)-treated MVEC; lane 3, TNF-α–treated MVEC; lane 4, normal mononuclear leukocyte-treated MVEC; lane 5, sickle mononuclear leukocyte-treated MVEC. Results are from a single representative experiment of 2.
The data presented in Figures 1 to 4 were generated using mononuclear leukocytes prepared by Ficoll-Hypaque density gradient centrifugation. These preparations contained monocytes, lymphocytes, and platelets. To determine which cell type(s) was responsible for endothelial cell activation and to exclude the possibility that the observed differences were caused by an artifact or contamination of the mononuclear leukocyte preparations, 2 additional isolation techniques were used—monocytes isolated by rate zonal density gradient centrifugation using OptiPrep medium and monocytes isolated by FACS. These monocyte preparations were highly enriched with CD14 monocytes and had less contamination with CD3 lymphocytes and CD61 platelets. These monocyte preparations from patients with sickle cell disease continued to activate HUVEC adhesion molecule expression more than preparations from normal subjects (data not shown).
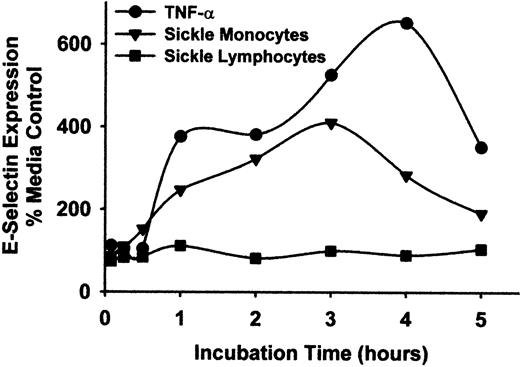
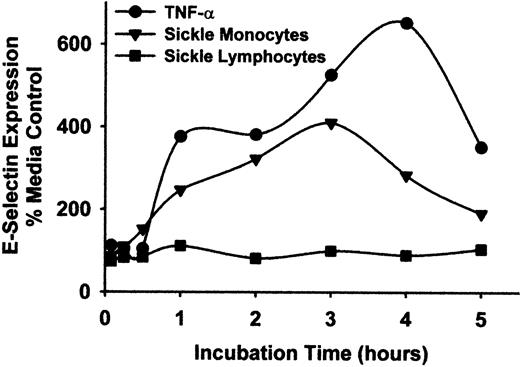
To exclude the possibility that lymphocytes contributed to endothelial cell activation, lymphocytes and monocytes from patients with sickle disease were separated by rate zonal density gradient centrifugation using OptiPrep medium. The lymphocyte preparations contained less than 1% monocytes and no PMNL, and the monocyte preparations contained less than 10% lymphocytes and no PMNL, as judged by flow cytometry analysis of forward and side scatter and immunostaining of CD14. However, some CD61+ platelets remained within the monocyte preparation. HUVEC were incubated with these purified sickle monocytes, lymphocytes, or TNF-α for varying lengths of time from 5 minutes to 5 hours, including the initial incubations with monocytes, lymphocytes, or TNF-α. After the indicated incubation times, monocytes, lymphocytes, or TNF-α were removed from the HUVEC by washing and were replaced with fresh media. All incubations lasted a total time of 5 hours. Purified sickle monocytes increased HUVEC E-selectin expression steadily throughout the first 3 hours of the time-course, peaking at 410% over control HUVEC after 3 hours and then declining to 190% after 5 hours (Figure 5). In contrast, purified sickle lymphocytes had little or no effect on HUVEC E-selectin expression at any time point. TNF-α increased HUVEC E-selectin expression throughout the time-course, peaking at 653% of control HUVEC after 4 hours.
Sickle monocytes, but not lymphocytes, activate HUVEC E-selectin expression.
Sickle monocytes and lymphocytes were separated by rate zonal density gradient centrifugation using OptiPrep medium as described in “Materials and methods.” HUVEC were incubated with TNF-α (10 ng/mL) or purified sickle monocytes or lymphocytes (2 leukocytes/endothelial cell) for increasing lengths of time, from 5 minutes to 5 hours. After the indicated incubation time, the TNF-α, monocytes, or lymphocytes were removed from the HUVEC by washing and were replaced with fresh media. All HUVEC were incubated for a total of 5 hours at 37°C, including the incubation time with TNF-α or leukocytes. After the 5-hour incubation, the endothelial cells were washed and E-selectin protein expression was measured by enzyme immunoassay. Results are expressed as a percentage of control (media only) HUVEC (100%) and are from a single representative experiment of 2.
Sickle monocytes, but not lymphocytes, activate HUVEC E-selectin expression.
Sickle monocytes and lymphocytes were separated by rate zonal density gradient centrifugation using OptiPrep medium as described in “Materials and methods.” HUVEC were incubated with TNF-α (10 ng/mL) or purified sickle monocytes or lymphocytes (2 leukocytes/endothelial cell) for increasing lengths of time, from 5 minutes to 5 hours. After the indicated incubation time, the TNF-α, monocytes, or lymphocytes were removed from the HUVEC by washing and were replaced with fresh media. All HUVEC were incubated for a total of 5 hours at 37°C, including the incubation time with TNF-α or leukocytes. After the 5-hour incubation, the endothelial cells were washed and E-selectin protein expression was measured by enzyme immunoassay. Results are expressed as a percentage of control (media only) HUVEC (100%) and are from a single representative experiment of 2.
Mononuclear leukocyte preparations obtained by Ficoll-Hypaque density gradient centrifugation and the monocyte preparations obtained by OptiPrep rate zonal density gradient centrifugation contained platelets. However, preparations from patients with sickle disease and normal subjects contained similar numbers of platelets. Mononuclear leukocytes isolated by Ficoll-Hypaque from normal subjects contained on average 57 ± 41 (mean ± SD, n = 3) platelets per mononuclear leukocyte, and preparations from patients with sickle disease contained 40 ± 28 (n = 3). To exclude the possibility that platelets were contributing to endothelial cell activation, purified platelets from patients with sickle disease and normal subjects were incubated with HUVEC for 4 hours in the presence and absence of mononuclear leukocytes. Incubation of HUVEC with sickle or normal platelets (100 platelets per HUVEC) for 4 hours did not stimulate HUVEC expression of E-selectin and ICAM-1 above control HUVEC incubated with media alone (Table 1). Moreover, in a separate representative experiment, the addition of platelets to mononuclear leukocytes did not augment, and in most cases slightly decreased, HUVEC expression of E-selectin and ICAM-1 (Table 1). These data suggest that platelets did not contribute to endothelial cell activation.
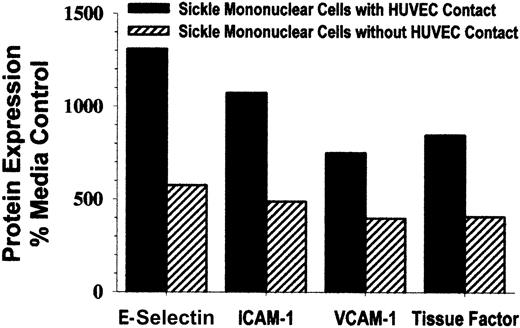
Because sickle monocytes were extremely potent in their ability to activate endothelium, we wondered whether cell-to-cell contact of monocytes and endothelial cells was required to achieve activation. To prevent cell-to-cell contact, cell culture inserts separated monocytes and endothelial cells with 0.4-μm, high-pore density membranes during a 4-hour incubation. HUVEC E-selectin, ICAM-1, VCAM-1, and tissue factor expression were increased by 400% to 600% above control HUVEC (Figure 6). These results demonstrate that HUVEC can be activated by sickle monocytes without cell-to-cell contact. However, when HUVEC were incubated with sickle monocytes in the absence of a cell culture insert membrane, E-selectin, ICAM-1, VCAM-1, and tissue factor expression was increased 800% to 1300% above control HUVEC (Figure 6), indicating that HUVEC activation is further enhanced by cell-to-cell contact of sickle monocytes and endothelial cells.
Cell-to-cell contact between sickle mononuclear leukocytes and HUVEC enhances, but is not required for, up-regulation of adhesion molecules and tissue factor.
To prevent cell-to-cell contact, mononuclear leukocytes and endothelial cells (1.5 mononuclear leukocyte/endothelial cell) were separated by cell culture inserts with 0.4-μm, high-pore density membranes during the 4-hour incubation. HUVEC E-selectin, ICAM-1, VCAM-1, and tissue factor were measured by enzyme immunoassay, and the results are expressed as a percentage of control (media only)-treated HUVEC (100%). Results are from a single representative experiment of 2.
Cell-to-cell contact between sickle mononuclear leukocytes and HUVEC enhances, but is not required for, up-regulation of adhesion molecules and tissue factor.
To prevent cell-to-cell contact, mononuclear leukocytes and endothelial cells (1.5 mononuclear leukocyte/endothelial cell) were separated by cell culture inserts with 0.4-μm, high-pore density membranes during the 4-hour incubation. HUVEC E-selectin, ICAM-1, VCAM-1, and tissue factor were measured by enzyme immunoassay, and the results are expressed as a percentage of control (media only)-treated HUVEC (100%). Results are from a single representative experiment of 2.
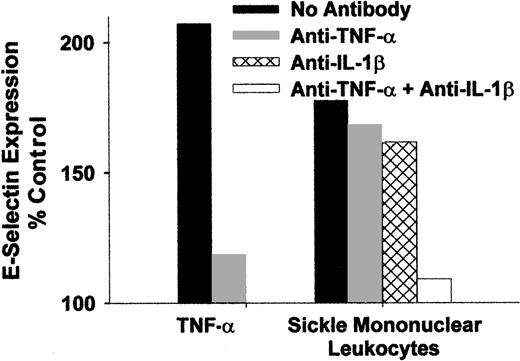
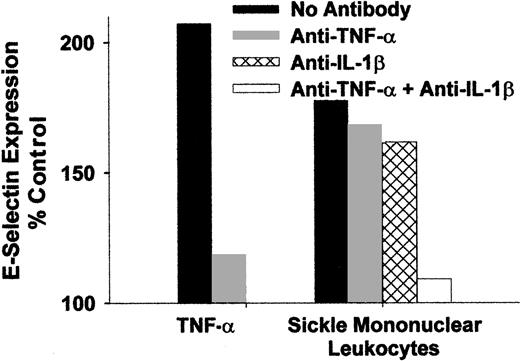
Because monocytes are known to secrete cytokines TNF-α and IL-1β during states of activation, it was of interest to determine whether antibodies that block the activity of these cytokines can prevent the activation of endothelial cells by sickle monocytes. Mononuclear leukocytes from a patient with sickle cell disease were isolated and preincubated for 15 minutes at room temperature with blocking antibodies to TNF-α or IL-1β individually and in combination. The leukocyte/antibody mixtures were then incubated with HUVEC for 4 hours at 37°C. Antibodies to TNF-α or IL-1β individually only slightly decreased HUVEC E-selectin expression (Figure7), but when both antibodies were combined, HUVEC E-selectin expression was almost completely reduced to the level found in control untreated HUVEC. Similar results were seen for VCAM-1 expression (data not shown). These data indicate that sickle monocytes were activated and produced TNF-α and IL-1β that in turn activated the endothelium. Moreover, monocyte activation of endothelial cells can be primarily attributed to TNF-α and IL-1β.
Endothelial activation caused by sickle monocytes is inhibited by anti-TNF-α and IL-1β monoclonal antibodies.
Sickle mononuclear leukocytes were isolated and preincubated for 15 minutes at room temperature with blocking antibodies to TNF-α or IL-1β, individually and in combination (10 μg/mL of each IgG). The leukocyte/antibody mixtures were incubated with HUVEC for 4 hours at 37°C (1.5 mononuclear leukocyte/endothelial cell). HUVEC E-selectin was measured by enzyme immunoassay, and the results are expressed as a percentage of control (media only)-treated HUVEC (100%). Results are from a single representative experiment of 2.
Endothelial activation caused by sickle monocytes is inhibited by anti-TNF-α and IL-1β monoclonal antibodies.
Sickle mononuclear leukocytes were isolated and preincubated for 15 minutes at room temperature with blocking antibodies to TNF-α or IL-1β, individually and in combination (10 μg/mL of each IgG). The leukocyte/antibody mixtures were incubated with HUVEC for 4 hours at 37°C (1.5 mononuclear leukocyte/endothelial cell). HUVEC E-selectin was measured by enzyme immunoassay, and the results are expressed as a percentage of control (media only)-treated HUVEC (100%). Results are from a single representative experiment of 2.
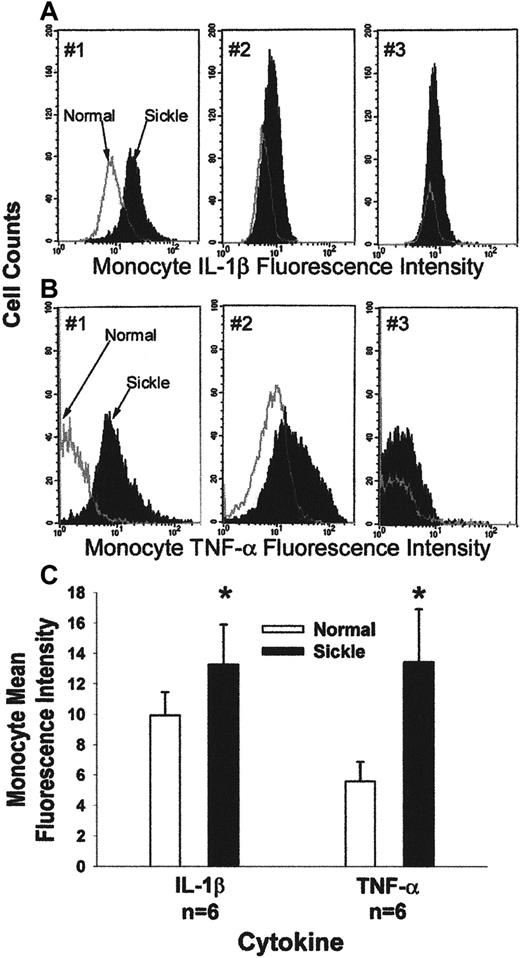
We measured TNF-α and IL-1β in sickle and normal monocytes. Leukocytes were obtained from blood after the lysis of RBCs. Leukocytes were fixed, permeabilized, and immunostained with fluorescent antibodies against CD14 and TNF-α or CD14 and IL-1β. CD14 is a 55-kd glycoprotein expressed on the surfaces of monocytes, macrophages, and PMNL, and it is a receptor for lipopolysaccharides.31 32 Immunostained leukocytes were analyzed by FACS. Mononuclear leukocytes were identified and gated by their forward and side scatter. Monocytes were identified within the gate based on CD14-specific immunofluorescence. Monocyte activation was measured as the mean fluorescence intensity of monocyte TNF-α and IL-1β. Figure 8, panels A and B, show typical monocyte IL-1β (Figure 8A) and TNF-α (Figure 8B) histograms for 3 pairs of normal (white under the curve) and sickle (black under the curve) subjects. The x-axis measures mean fluorescence intensity of monocyte IL-1β and TNF-α, and the y-axis measures cell counts. The mean fluorescence intensity of monocyte IL-1β and TNF-α was higher each time (n = 6) for the patient with sickle disease than for the paired normal subject. Figure 8C summarizes the data from 6 pairs of patients and normal subjects. Five of the 6 patients were in the hospital for pain crisis. Sickle monocytes had 34% more IL-1β (P = .002) and 139% more TNF-α (P = .002) per cell than normal monocytes, based on the measurement of mean fluorescence intensity for each cytokine. These data suggest that sickle monocytes are more activated than normal monocytes.
Typical flow cytometry analysis of IL-1β (A) and TNF-α (B) in peripheral blood monocytes from 3 pairs of patients with sickle disease and normal subjects.
Leukocytes were obtained from blood after the lysis of RBCs. Leukocytes were fixed and permeabilized; then they were immunostained for the expression of CD14, IL-1β, and TNF-α. Immunostained monocytes were analyzed by flow cytometry. A gate was placed around the mononuclear leukocytes based on their forward and side scatter. Monocytes within that gate were identified based on their expression of CD14-specific immunofluorescence. The x-axis measures mean fluorescence intensity of monocyte TNF-α or IL-1β, and the y-axis measures the cell counts. Each of the 3 graphs shows data from a pair of normal (white under the curve) and sickle (black under the curve) subjects that were collected and processed at the same time. (C) Sickle monocytes are activated: summary of IL-1β and TNF-α flow cytometry results. Expression of monocyte CD14, IL-1β, and TNF-α was measured by flow cytometry in 6 pairs of patients with sickle cell disease and normal subjects, as described in Figure 8A,B. Results are presented as the mean fluorescence intensity of monocyte IL-1β and TNF-α in patients and normal subjects. Mean fluorescence intensity of monocyte IL-1β was 13.3 in patients and 9.9 in normal subjects (P = .002). Mean fluorescence intensity of monocyte TNF-α was 13.4 in patients and 5.6 in normal subjects (P = .002). Data were normalized before statistical analysis. Five of 6 patients were in the hospital for sickle pain crisis.
Typical flow cytometry analysis of IL-1β (A) and TNF-α (B) in peripheral blood monocytes from 3 pairs of patients with sickle disease and normal subjects.
Leukocytes were obtained from blood after the lysis of RBCs. Leukocytes were fixed and permeabilized; then they were immunostained for the expression of CD14, IL-1β, and TNF-α. Immunostained monocytes were analyzed by flow cytometry. A gate was placed around the mononuclear leukocytes based on their forward and side scatter. Monocytes within that gate were identified based on their expression of CD14-specific immunofluorescence. The x-axis measures mean fluorescence intensity of monocyte TNF-α or IL-1β, and the y-axis measures the cell counts. Each of the 3 graphs shows data from a pair of normal (white under the curve) and sickle (black under the curve) subjects that were collected and processed at the same time. (C) Sickle monocytes are activated: summary of IL-1β and TNF-α flow cytometry results. Expression of monocyte CD14, IL-1β, and TNF-α was measured by flow cytometry in 6 pairs of patients with sickle cell disease and normal subjects, as described in Figure 8A,B. Results are presented as the mean fluorescence intensity of monocyte IL-1β and TNF-α in patients and normal subjects. Mean fluorescence intensity of monocyte IL-1β was 13.3 in patients and 9.9 in normal subjects (P = .002). Mean fluorescence intensity of monocyte TNF-α was 13.4 in patients and 5.6 in normal subjects (P = .002). Data were normalized before statistical analysis. Five of 6 patients were in the hospital for sickle pain crisis.
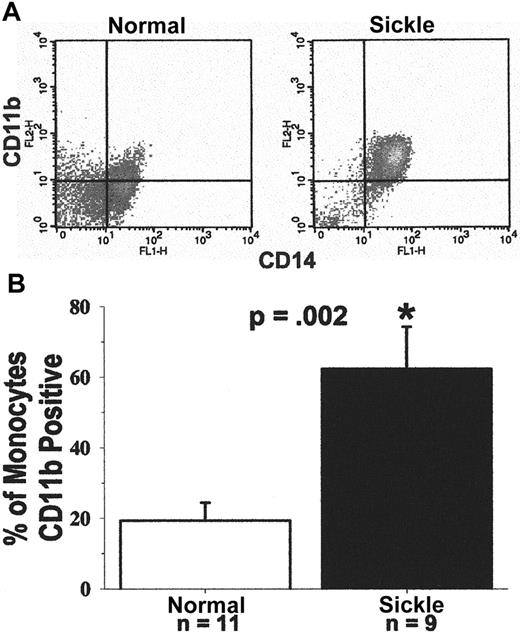
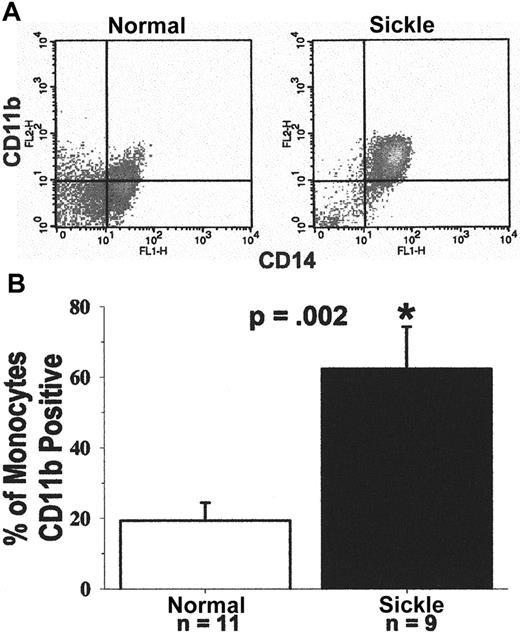
CD11b expression on the monocyte cell surface was measured as an additional marker of activation. Leukocytes were obtained from blood after the lysis of RBCs. Leukocytes were immunostained for surface expression of CD11b and CD14. CD11b, an α-chain integrin, is expressed on the surface of activated leukocytes in a heterodimer complex with CD18, a β-chain integrin. The complex, also known as MAC-1, is a ligand for ICAM-1.33 Figure9, panel A, shows a typical FACS analysis of CD11b and CD14 expression on the surface of monocytes from a patient with sickle disease and a normal subject. The crosshatch separates specific and nonspecific immunofluorescence. Cells to the right of the crosshatch are CD14+ monocytes, and cells above the crosshatch are CD11b+ monocytes. Therefore, cells in the upper right quadrant are activated monocytes, and cells in the lower right quadrant are resting monocytes. It is apparent in Figure 9, panel A, that most of the patient's monocytes are activated (97% activated), and most of the normal subject's monocytes are resting (63% resting). The FACS results are summarized in Figure 9, panel B. Results are presented as the percentage of CD14+ monocytes positive for CD11b. On average, 62% of sickle monocytes (n = 9) were activated, as judged by CD11b surface expression, compared to only 19% of normal monocytes (n = 11, P = .002). Six of the 9 patients were in crisis. There was no statistical difference in monocyte CD11b expression between patients in crisis and those not in crisis. Both crisis (P = .029) and noncrisis (P = .007) subgroups had higher monocyte CD11b expression than normal subjects. These results confirm that sickle monocytes were activated compared with normal monocytes.
(A) Typical flow cytometry analysis of CD11b and CD14 expression on the surfaces of monocytes from a patient with sickle cell disease and a normal subject.
Leukocytes were obtained from blood after the lysis of RBCs. They were immunostained for the surface expression of CD11b and CD14. Immunostained monocytes were analyzed by flow cytometry. A gate was placed around the mononuclear leukocytes based on their forward and side scatter. Monocytes within that gate were identified based on their expression of CD14-specific immunofluorescence. The crosshatch separates specific and nonspecific immunofluorescence. Cells to the right of the crosshatch are CD14+ monocytes, and cells above the crosshatch are CD11b+ monocytes. Monocytes in the upper right quadrant are activated, and monocytes in the lower right quadrant are resting. In this example, based on CD11b surface expression, 97% of the patient's monocytes are activated and 3% are resting; 37% of the normal subject's monocytes are activated and 63% are resting. (B) Sickle monocytes are activated: summary of CD11b flow cytometry results. Expression of CD11b and CD14 on monocytes was measured by flow cytometry in 9 patients and 11 normal subjects, as described in A. Results are presented as the percentage of CD14+ monocytes that are CD11b+. On average, 62% of sickle monocytes were activated as judged by CD11b surface expression compared to only 19% of normal monocytes (P = .002). Six of the 9 patients were admitted to the hospital for pain crisis. Surface expression of monocyte CD11b in the crisis (P = .029) and noncrisis patient groups (P = .007) was statistically greater than normal.
(A) Typical flow cytometry analysis of CD11b and CD14 expression on the surfaces of monocytes from a patient with sickle cell disease and a normal subject.
Leukocytes were obtained from blood after the lysis of RBCs. They were immunostained for the surface expression of CD11b and CD14. Immunostained monocytes were analyzed by flow cytometry. A gate was placed around the mononuclear leukocytes based on their forward and side scatter. Monocytes within that gate were identified based on their expression of CD14-specific immunofluorescence. The crosshatch separates specific and nonspecific immunofluorescence. Cells to the right of the crosshatch are CD14+ monocytes, and cells above the crosshatch are CD11b+ monocytes. Monocytes in the upper right quadrant are activated, and monocytes in the lower right quadrant are resting. In this example, based on CD11b surface expression, 97% of the patient's monocytes are activated and 3% are resting; 37% of the normal subject's monocytes are activated and 63% are resting. (B) Sickle monocytes are activated: summary of CD11b flow cytometry results. Expression of CD11b and CD14 on monocytes was measured by flow cytometry in 9 patients and 11 normal subjects, as described in A. Results are presented as the percentage of CD14+ monocytes that are CD11b+. On average, 62% of sickle monocytes were activated as judged by CD11b surface expression compared to only 19% of normal monocytes (P = .002). Six of the 9 patients were admitted to the hospital for pain crisis. Surface expression of monocyte CD11b in the crisis (P = .029) and noncrisis patient groups (P = .007) was statistically greater than normal.
Another marker of systemic inflammation is serum CRP, an acute-phase reactant. CRP was measured in 13 patients and normal subjects. Patients had mean ± SD CRP of 3.0 ± 5.1 mg/dL, and normal subjects had mean CRP of 0.3 ± 0.1 mg/dL (P = .003) (data not shown). Taken together, these data indicate that the vasculature of patients with sickle cell disease is in a state of inflammation.
Discussion
These studies support our hypothesis that monocytes from patients with sickle cell anemia are activated and can enhance vaso-occlusion through an endothelial inflammatory response promoted by the NF-κB–mediated up-regulation of adhesion molecules and tissue factor. A surprising observation was the potency of sickle monocytes; sickle monocytes were able to activate endothelial cells at concentrations as low as one monocyte per endothelial cell. Additionally, most patients with sickle cell disease have monocytosis,34 further predisposing the endothelium toward an activated state. The Multicenter Study of Hydroxyurea35reported that decreases in neutrophil, monocyte, reticulocyte, and platelet counts were directly associated with decreases in 3-month crisis rates.
We observed that blocking antibodies to TNF-α or IL-1β individually only slightly decreased HUVEC E-selectin and VCAM-1 expression in response to monocytes, but when both antibodies were combined, HUVEC E-selectin and VCAM-1 expression were almost completely inhibited. These data are consistent with our observations that cell-to-cell contact enhanced the monocyte activation of endothelial cells because some of the TNF-α and IL-1β produced by monocytes can be membrane associated.36,37 Our data suggest that the activation of endothelial cells by sickle monocytes in vitro can be attributed primarily to TNF-α and IL-1β. These cytokines are elevated in the plasma of some patients with sickle cell disease.38-41TNF-α and IL-1β are markers of monocyte activation. This was confirmed by FACS analysis of monocyte TNF-α and IL-1β expression and by CD11b expression on the monocyte surface.
One may ask, how are sickle monocytes activated? How does the mutation in hemoglobin translate into monocyte activation? In any given patient, multiple pathways for monocyte activation may be operational. Perhaps unique features of the sickle erythrocyte promote activation of the monocyte. One possibility is that increased erythrophagocytosis of senescent sickle RBCs by monocytes stimulates monocyte activation. Another pathway could be RBC microparticles; human RBCs shed plasma membrane–derived exocytic microvesicles or microparticles in vivo. RBC microparticles have been found in increased numbers in several vascular diseases, including sickle cell disease and β-thalassemia.42 These microparticles activate inflammatory responses in cultured monocyte/macrophages (J.D.B. and G.M.V., unpublished data). In addition, sickle RBC-derived hemoglobin and heme-derived oxidants could activate inflammatory responses in monocytes. Or, perhaps in vivo it is activated endothelium that primes the monocytes. Sultana et al43 have recently reported that adherence/contact of sickle RBCs to HUVEC in the presence of von Willebrand–containing media induces oxidant stress, leading to NF-κB nuclear translocation and transendothelial migration of HL60 and normal peripheral blood monocytes.
An important mechanism whereby vascular inflammation could contribute to vaso-occlusive crisis is through the up-regulation of tissue factor expression. Tissue factor is the coagulation system's triggering mechanism.14 Previous studies have shown that whole blood tissue factor procoagulant activity associated with mononuclear leukocytes is elevated in sickle disease.44 Tissue factor expression is significantly elevated on circulating endothelial cells isolated from patients with sickle cell disease11 compared with normal subjects. Expression is greater when patients have acute vaso-occlusive episodes. Elevated tissue factor expression on activated monocytes and endothelium in sickle cell disease may be a powerful determinant of vaso-occlusive crisis. Moreover, platelets from patients with sickle cell disease are activated45-47 providing ideal conditions for vaso-occlusive crisis.
Platelets can adhere to monocytes through thrombospondin cross-linking of glycoprotein IV on the surface of both kinds of cells.48 Mononuclear leukocyte preparations obtained by Ficoll-Hypaque density gradient centrifugation contained substantial numbers of platelets and lymphocytes. However, there were no significant differences in platelet or lymphocyte contamination between sickle and normal mononuclear leukocyte preparations. Moreover, the addition of purified sickle or normal platelets or lymphocytes to endothelial cells in culture in the presence and absence of sickle or normal monocytes did not augment the expression of endothelial cell adhesion proteins.
In addition to activated monocytes, platelets, endothelial cells, and circulating cytokines, there are other signs of inflammation in sickle disease. These include elevated serum CRP (this paper and49-51) and the abnormal presence of adhesion molecules in plasma, such as E-selectin, ICAM-1, and VCAM-1.7 Moreover, many clinical features of sickle cell disease are similar to other inflammatory processes, such as fever, local edema, erythema, leukocytosis, and elevated erythrocyte sedimentation rate.
These observations provide a molecular framework for treating sickle cell disease as an inflammatory disease of the vasculature. An important question pertains to the old chicken-and-egg dilemma: which comes first, inflammation or vaso-occlusion? Does vascular inflammation—with its attendant increase in endothelial stickiness to RBCs, leukocytes, and platelets and its procoagulant balance—precede vaso-occlusion, or does vaso-occlusion—its resultant ischemia–reperfusion injury—precede inflammation? Perhaps both pathways are operational in sickle disease, resulting in a vicious circle. In that case, the question of which comes first is moot. More important, is inflammation required for vaso-occlusion? This question is answerable. Anti-inflammatory trials should be given serious consideration for the treatment and prevention of vaso-occlusive crisis and stroke. Griffin et al52 found that a short course of high-dose methylprednisolone decreased the duration of severe pain in children and adolescents with sickle disease, but patients who received methylprednisolone had more rebound attacks after therapy was discontinued. Our results suggest that therapeutics, which inhibit NF-κB, might be especially beneficial in preventing vaso-occlusive crisis. Preliminary studies in our laboratory suggest that sulfasalazine, an NF-κB inhibitor used in treating inflammatory bowel disease, has potent anti-inflammatory activity in patients with sickle cell disease. TNF-α and IL-1β are other potential therapeutic targets. We speculate that anti-inflammatory drugs will become an important source of therapy in the prevention and treatment of vaso-occlusive crisis and stroke in sickle disease.
Acknowledgments
The authors are deeply indebted to the many patients with sickle cell disease and the control subjects who donated blood for these studies. We thank Ms Jean Harkness for her help in coordinating and procuring blood samples from our patients. We also thank Dr Steve Nelson at the Minneapolis Children's Hospital and Dr Chris Moertel at St. Paul Children's Hospital for coordinating and procuring blood samples from their patients. We thank Mr Chris Bryant for his excellent technical skills and perseverance in the laboratory and Ms Julia Nguyen for her extraordinary help and skills in the isolation and culture of endothelial cells.
Supported by National Institutes of Health grant HL55552.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John D. Belcher, Department of Medicine, Division of Hematology, Oncology and Transplantation, University of Minnesota, Box 480 UMHC, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: belcher@tc.umn.edu.

![Fig. 4. Sickle mononuclear leukocytes activate NF-κB in MVEC. / Mononuclear leukocytes (15 leukocytes/endothelial cell) or TNF-α (10 ng/mL) were incubated with MVEC for 1 hour. After incubation, MVEC nuclear proteins were extracted for NF-κB analysis. Nuclear extracts (10 μg protein) were incubated with 2 ng [32P]-labeled dsDNA containing a consensus NF-κB sequence and analyzed by electrophoretic mobility shift assay. Lane 1, [32P]-labeled dsDNA probe alone; lane 2, control (media only)-treated MVEC; lane 3, TNF-α–treated MVEC; lane 4, normal mononuclear leukocyte-treated MVEC; lane 5, sickle mononuclear leukocyte-treated MVEC. Results are from a single representative experiment of 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/7/10.1182_blood.v96.7.2451/5/m_h81900196004.jpeg?Expires=1767854535&Signature=IdZtN2x~N7nSSb2enLc0O5JeVJr34ou2-CRFpYQwaCoux8cSRqoUpEhuiFjFww1YXy2UBm6FCSzc-uJqTdYheVjpBmodV3M0uMCo4qTnflWtQZWK64R4j03T7lFKeIj32NdjUi5SLo6U7EA-njvlKSKyaWnjl5jU4E8Uqr5v~B~fCKDebI-9h9Wgm8dsjn7lPVdH9gH7UBBNCESoHcfUpSVyQGA-7UkHTbKP2C6chu7aF~MG1~DYYsQWfEaXzHOyP5c~KvSnSNiRLJ589vmDR-oVoUKRzUehbBinwLv5axy0Tmr0BPtki0WdK1u-wyJPaFG~A-RgKot3hucoJdBh3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Fig. 4. Sickle mononuclear leukocytes activate NF-κB in MVEC. / Mononuclear leukocytes (15 leukocytes/endothelial cell) or TNF-α (10 ng/mL) were incubated with MVEC for 1 hour. After incubation, MVEC nuclear proteins were extracted for NF-κB analysis. Nuclear extracts (10 μg protein) were incubated with 2 ng [32P]-labeled dsDNA containing a consensus NF-κB sequence and analyzed by electrophoretic mobility shift assay. Lane 1, [32P]-labeled dsDNA probe alone; lane 2, control (media only)-treated MVEC; lane 3, TNF-α–treated MVEC; lane 4, normal mononuclear leukocyte-treated MVEC; lane 5, sickle mononuclear leukocyte-treated MVEC. Results are from a single representative experiment of 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/7/10.1182_blood.v96.7.2451/5/m_h81900196004.jpeg?Expires=1767997218&Signature=CqZqNuPn~aDya-IO89Vv8JeqXI7lyVDv4d8v1sWDlfs7jvcqZFI9rlBVHn47Ob6h3iQDbrRJ6mx2kU3bnS5uThFQu9nvtf9wkLcaECwptRAT3mrSc2m3uMXNJOjrJ87PjKjMAdHD3DaSt69tVspaH-q~3Tz9FNW7CcVplxbwgrj1k-3M8168pJ7DoJEmNTaID7p6o9U2M~ZQ3EI~DQoihvmmp2AoIV58Bw0aaCVPd-iVfN4SVC63OmMOY4hLi0BThSbCdaJaH81WyU1v8jBZJbWRVaA-hO3JCbe~UWuJHnu7A-8WqQ~z2JCztd~vqTImQSRIp0iS~Y6cUkmsAnakGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)