Abstract
This study examined the expression of the platelet collagen receptor glycoprotein VI (GPVI) in megakaryocyte cell lines and primary megakaryocytes by reverse transcriptase-polymerase chain reaction and by flow cytometry and ligand blotting using the snake venom toxin convulxin. Expression of GPVI is increased in the megakaryoblastic cell lines HEL and CMK on differentiation with the phorbol ester phorbol 12-myristate 13-acetate (PMA), along with the Fc receptor γ-chain (FcR γ-chain). The increase in GPVI expression is associated with marked potentiation of tyrosine phosphorylation and Ca++ elevation in response to convulxin. Syk, linker for activated T cells, and phospholipase Cγ2 (PLCγ2) are among the proteins tyrosine phosphorylated on convulxin stimulation in PMA-differentiated HEL cells. Studies on primary murine megakaryocytes grown in vitro confirmed that GPVI is up-regulated in parallel with functional activation, assessed by measurement of [Ca++]i, during differentiation. The results demonstrate that expression of GPVI is up-regulated along with the FcR γ-chain during differentiation of megakaryocytes.
Introduction
Megakaryocytes originate in the bone marrow from pluripotent stem cells through a differentiation process that involves stem cell commitment, nuclear polyploidization, and cytoplasmic maturation leading to the production of platelets. They are unique when compared with other hematopoietic precursor cells because of their large size and high nuclear ploidy. Several cytokines and growth factors including interleukin (IL)-3, IL-6, IL-11, leukemia inhibitory factor, erythropoietin, and thrombopoietin (TPO), synergistically promote growth and maturation of megakaryocytes in the bone marrow.1-3 A number of megakaryoblastic cell lines have been characterized. Although each has unique features that distinguish them from bone marrow megakaryocytes, they can be induced to undergo further differentiation to varying degrees in the presence of cytokines and growth factors, or by phorbol esters such as phorbol 12-myristate 13-acetate (PMA).4-6 Thus, PMA stimulates further megakaryocytic development, resulting in an inhibition of cell proliferation, nuclear polyploidization, and an increase in the expression of platelet/megakaryocyte proteins such as the integrin GPIIb/IIIa (CD41/CD61).7-9
Glycoprotein VI (GPVI) is the collagen receptor underlying aggregation of platelets as shown by the selective impairment of response in GPVI-deficient patients.10,11 Cross-linking of GPVI is associated with tyrosine phosphorylation of a number of proteins such as Fc receptor γ-chain (FcR γ-chain), Syk, and phospholipase γ2 (PLCγ2), and subsequent increase in the levels of the messengers inositol 1,4,5-trisphosphate, 1,2-diacylglycerol, phosphatidylinositol 3,4,5-trisphosphate, and intracellular Ca++, leading to platelet shape change and aggregation.12-16 GPVI can be selectively activated by the snake venom toxin convulxin, a C-type lectin isolated from the venom of the South American rattlesnakeCrotalus durissus terrificus. Convulxin is comprised of 2 subunits, α and β, joined by disulfde bridges in an α3β3 structure.17
In this work we show that expression of GPVI and its associated FcR γ-chain are up-regulated during differentiation of the megakaryoblastic cell line HEL leading to potentiation of functional responses. Further, we use mouse megakaryocytes differentiated in vitro from bone marrow cells to confirm the increase in expression and up-regulation of activation with the stage of differentiation.
Material and methods
Materials
Convulxin was purified from the venom of the tropical rattlesnake Crotalus durissus terrificus and its concentration measured with a spectrophotometer at 280 nm. Its activity was assessed by bioassay on platelet aggregation.18RPMI-1640 medium, Iscove modified Dulbecco medium (IMDM), fetal bovine serum (FBS), and penicillin and streptomycin (P/S) antibiotics were from Gibco RBL (Life Technologies Ltd, Paisley, United Kingdom). Recombinant murine IL-6, IL-11, and TPO were from R & D Systems (Oxfordshire, United Kingdom). Antiphosphotyrosine monoclonal antibody (mAb) 4G10, anti-FcR γ-chain antibody and anti-linker for activated T cells (LAT) antibody were from Upstate Biotechnology Inc (TCS Biologicals Ltd, Bucks, United Kingdom). Anti-Syk N-19 and anti-PLCγ2 Q-20 antibodies were from Santa Cruz (Insight Biotechnology Limited, Middlesex, United Kingdom). For Syk and PLCγ2 immunoprecipitations, rabbit antiserum to either protein was generously provided by Drs Mike Tomlinson and Joe Bolen (DNAX Research Institute of Molecular and Cellular Biology, Palo Alto, CA), and used at a dilution of 1:500. Horseradish peroxidase (HRP)-conjugated anti-immunoglobulin antibodies, enhanced chemoluminescence (ECL) reagents, and Hyperfilm were from Amersham International (Buckinghamshire, United Kingdom). Fluorescein isothiocyanate (FITC)-conjugated antihuman GPIIIa and its isotype control were from DAKO Ltd (Denmark House, Cambridge, United Kingdom). Antimouse FcγRII-III and antimouse CD41 and its isotype control (rat IgG2a) antibodies were from Pharmingen (Becton Dickinson, Oxford, United Kingdom). FITC-conjugated antirabbit IgG (Fab′)2 fragments were from Sigma (Poole, Dorset, United Kingdom). RNAgents, M-MLV reverse transcriptase, and oligo(dT)15-primer were from Promega (Southampton, United Kingdom). Qiagen RNeasy kit was from Quiagen Ltd (Crawley, United Kingdom). AmpliTaq Gold was from Perkin Elmer (Warrington, United Kingdom). All other reagents were obtained from previously described sources.12 13
Cell culture
All cell lines were grown in RPMI-1640 medium supplemented with glutamine, P/S, and 10% heat-inactivated FBS under 5% CO2/95% air in a humidified incubator. Cells were kept at exponential phase of growth. To induce megakaryocytic differentiation, 2 × 105 cells/mL were exposed to 10 nmol/L PMA or an equal volume of DMSO as control medium for up to 3 days. To induce erythroid differentiation, 2 × 105 cells were stimulated with 25 μmol/L hemin for up to 3 days.19 Cell number and viability were determined by trypan blue dye exclusion method. Attached cells were harvested by adding phosphate-buffered saline (PBS) containing 2.5 mmol/L EDTA at 37°C for 5 minutes. Cells were washed and resuspended in Tyrodes-Hepes buffer (134 mmol/L NaCl, 0.34 mmol/L Na2HPO4, 2.9 mmol/L KCl, 12 mmol/L NaHCO3, 20 mmol/L Hepes, 5 mmol/L glucose, 1 mmol/L MgCl2 pH 7.3). HEL cells (107 cells/mL) were stimulated with 20 nmol/L convulxin at 37°C and terminated by the addition of an equal volume of Laemmli sample buffer, then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Platelets were obtained from drug-free volunteers on the day of the experiment, and suspended in Tyrodes-Hepes. They were isolated as previously described.20
RNA preparation and reverse transcriptase-polymerase chain reaction (RT-PCR)
For RNA isolation, platelets were prepared as above but steps were taken to avoid contamination of other cells by taking only the uppermost third of platelet-rich plasma (PRP) and filtering for leukocyte removal. Total RNA was extracted from platelets using RNAgents and from cultured cells using the Qiagen RNeasy kit. A 2-μg RNA sample was reverse-transcribed using M-MLV reverse transcriptase and oligo(dT)15-primer according to the manufacturer's instructions. One fifth of the reverse transcribed RNA mixture was subjected to PCR amplification using AmpliTaq Gold. The GPVI PCR in Figure 1 was performed for 40 cycles using primers 5′-AACCATGTCTCATCCCCGACC-3′ and 5′-CCGCTCGAGTGAACATAACCCGCG-3′ (1042-bp fragment).21β-Actin was amplified for 35 cycles using the primer pair: 5′-TACCACTGGCATCGTGATGGACT-3′ and 5′-TCCTTCTGCATCCTGTCGGCAAT-3′ (506-bp fragment).
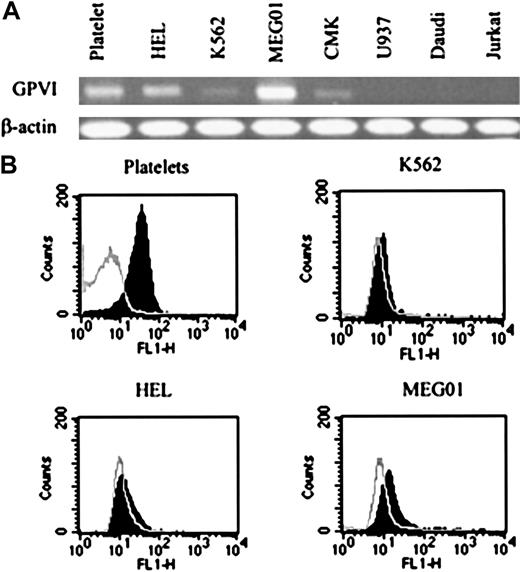
GPVI expression in megakaryoblastic cell lines and platelets.
(A) RNA (2 μg) was reverse transcribed and one fifth of the cDNA obtained used for PCR amplification. RT-PCR products were separated by agarose gels and stained with ethidium bromide to visualize GPVI and β-actin. (B) Fluorescent profile of representative cell lines. Cells were incubated with convulxin and a specific antibody to convulxin, then indirectly stained with FITC-conjugated antirabbit IgG (Fab′)2 fragment (shaded area). The nonshaded areas show background labeling observed in the absence of convulxin. Cytofluorographs were obtained on a FACsort. Results are representative of 5 experiments.
GPVI expression in megakaryoblastic cell lines and platelets.
(A) RNA (2 μg) was reverse transcribed and one fifth of the cDNA obtained used for PCR amplification. RT-PCR products were separated by agarose gels and stained with ethidium bromide to visualize GPVI and β-actin. (B) Fluorescent profile of representative cell lines. Cells were incubated with convulxin and a specific antibody to convulxin, then indirectly stained with FITC-conjugated antirabbit IgG (Fab′)2 fragment (shaded area). The nonshaded areas show background labeling observed in the absence of convulxin. Cytofluorographs were obtained on a FACsort. Results are representative of 5 experiments.
In Figure 2A the GPVI PCR was performed for 25 cycles and the primers used were 5′-AACCATGTCTCCATCCCC-3′ and 5′-TTCAGCGGTCATGAACATAA-3′ (1034-bp fragment). Glycophoryn A was amplified for 25 cycles using the primer pair 5′-AGCATCAAGTACCACTGGT-3′ and 5′-TTAAAGGCACGTCTCTGTC-3′ (359-bp fragment). GPIIIa was amplified for 25 cycles using the primer pair 5′-AGATGCGAAAGCTCACCA-3′ and 5′-TGAGCTCACTATAGTTCTG-3′ (553-bp fragment). Hypoxanthine phosphoribosyltransferase (HPRT) was amplified for 25 cycles using the primer pair 5′-AGTGATGATGAACCAGGT-3′ and 5′-GGCTTTGTATTTTGCTTTTC-3′ (620-bp fragment). Amplification products were electrophoresed on a 1.2% agarose gel and visualized by ethidium bromide staining. GPVI PCR products were directly sequenced on an ABI 377 sequencer.
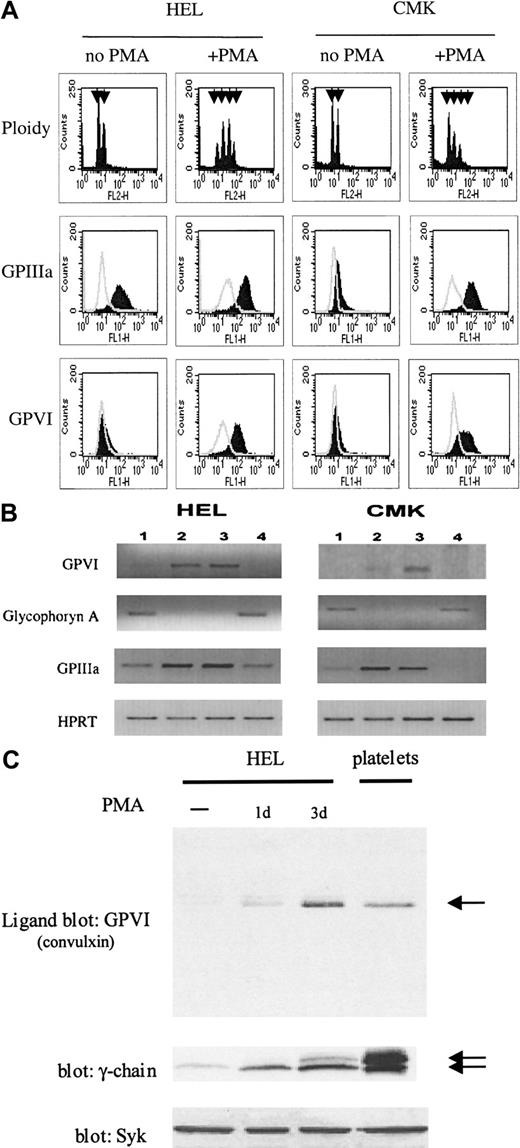
Flow cytometry, RT-PCR, and ligand blotting of GPVI in HEL and CMK cells.
(A) HEL and CMK cells were differentiated with PMA for 3 days and expression of GPVI detected by flow cytometry as in Figure 1. GPIIIa expression and ploidy values were measured with anti-GPIIIa and propidium iodide, respectively. Arrowheads in ploidy panels indicate DNA content, from 2n to 16n. (B) Semiquantitative RT-PCR of HEL and CMK cells shows expression of GPVI, glycophoryn A, GPIIIa (CD61), and HPRT before (lane 1) and after megakaryocytic differentiation with PMA for 1 (lane 2) and 3 days (lane 3) or erythroid differentiation using hemin for 3 days (lane 4). (C) Cells were differentiated with 10 nmol/L PMA for 1 and 3 days (1d, 3d). HEL extract (30 μg) was loaded per lane and subjected to SDS-PAGE under nonreducing conditions. GPVI was detected by ligand blotting using convulxin. FcRγ-chain expression was assessed by immunoblotting. Arrows indicate the relative position of GPVI (60 kd) and FcR γ-chain (22-24 kd). A platelet sample is included as a positive control; the platelet number used in either blot was different. The level of Syk measured by Western blotting is also shown for comparison. Results are representative of 3 experiments.
Flow cytometry, RT-PCR, and ligand blotting of GPVI in HEL and CMK cells.
(A) HEL and CMK cells were differentiated with PMA for 3 days and expression of GPVI detected by flow cytometry as in Figure 1. GPIIIa expression and ploidy values were measured with anti-GPIIIa and propidium iodide, respectively. Arrowheads in ploidy panels indicate DNA content, from 2n to 16n. (B) Semiquantitative RT-PCR of HEL and CMK cells shows expression of GPVI, glycophoryn A, GPIIIa (CD61), and HPRT before (lane 1) and after megakaryocytic differentiation with PMA for 1 (lane 2) and 3 days (lane 3) or erythroid differentiation using hemin for 3 days (lane 4). (C) Cells were differentiated with 10 nmol/L PMA for 1 and 3 days (1d, 3d). HEL extract (30 μg) was loaded per lane and subjected to SDS-PAGE under nonreducing conditions. GPVI was detected by ligand blotting using convulxin. FcRγ-chain expression was assessed by immunoblotting. Arrows indicate the relative position of GPVI (60 kd) and FcR γ-chain (22-24 kd). A platelet sample is included as a positive control; the platelet number used in either blot was different. The level of Syk measured by Western blotting is also shown for comparison. Results are representative of 3 experiments.
Immunoprecipitation
One to 2 × 106 PMA-differentiated HEL cells for 3 days were stimulated with 20 nmol/L convulxin for 90 seconds, lysed, and subjected to immunoprecipitation as previously described.12
Immunoblotting studies
Whole protein extracts (30 μg) in Laemmli sample buffer were loaded per lane and separated by SDS-PAGE using 10% gels, or 12.5% gels for FcR γ-chain detection. Primary and secondary antibodies were diluted in tris buffered saline–Tween 20 [0.1%] (TBS-T) containing 5% (w/v) BSA and incubated with Western blots. After washing, blots were developed using an ECL detection system.
Ligand blotting
For GPVI detection, membranes were blocked using PBS containing 5% skim milk then incubated with 10 nmol/L convulxin dissolved in PBS for 1 hour at room temperature, washed, and incubated with anticonvulxin antibody and secondary antibody, both dissolved in TBS-T. All HRP-conjugated secondary antibodies were used at a dilution 1:10 000.
Flow cytometry studies
Cells were resuspended in Tyrodes-Hepes buffer containing 1% human serum albumin (HSA) and 0.02% sodium azide. All incubation times were performed for 30 minutes unless otherwise indicated. For GPVI detection, HEL, CMK, and primary cultures cells were preincubated with either 10 μg/mL of antihuman FcγRIIA receptor monoclonal antibody IV.3 or antimouse FcγRII-III, to avoid nonspecific binding to the Fc receptor. Cells were incubated with 20 nmol/L convulxin, washed, and incubated with 0.4 μg/mL anticonvulxin antibody, washed again, and finally incubated with FITC-conjugated antirabbit IgG secondary antibody diluted 1:500. Incubation with convulxin was omitted to obtain background fluorescence. For GPIIIa detection, cells were incubated on ice with FITC-conjugated anti-GPIIIa antibody or its FITC-conjugated isotype control.
For GPIIb detection in primary cultures, cells were incubated for 30 minutes with antimouse GPIIb antibody or its isotype control (1:100), washed, and incubated with FITC-conjugated antirat IgG (1:500).
For ploidy analysis, cells were resuspended in Tyrodes-Hepes buffer or PBS and 50 μg/mL propidium iodide was added. Cells were permeabilized with NP-40 (0.1%) and analyzed immediately using a FACScalibur (Becton Dickinson). Data were recorded and analyzed using CellQuest software.
Bone marrow cell isolation and culture in vitro
Femurs and tibias from CD1 mice that were at least 8 weeks old were taken, and bone marrow cells flushed out with cold IMDM using a 25G5/8 needle, centrifuged, and resuspended for 5 minutes in lysis buffer (0.15 mol/L NH4Cl, 1 mmol/L KHCO3, 0.1 mmol/L Na2EDTA) to remove red cells. After centrifugation, cells were resuspended in IMDM supplemented with 5 mg/mL BSA, 0.2 mg/mL transferrin, 10 μg/mL insulin, 50 μmol/L β-mercaptoethanol, 40 μg/mL low-density lipoprotein, 20 μmol/L of each dNTP and NTP, P/S, 10 ng/mL IL-6, 10 ng/mL IL-11, and 50 ng/mL TPO. Cells were plated at a density of 1.5 × 106cells/mL and kept in culture for 4 days. Nonadherent cells were harvested for subsequent experiments. For acetylcholinesterase detection, cells were cytospined onto coverslips and incubated for 1 to 2 hours in a solution of 100 mmol/L sodium phosphate buffer, pH 6, containing 0.66 mg/mL acetylthiocholine iodide, 5 mmol/L sodium citrate, 3 mmol/L copper sulfate, and 0.5 mmol/L potassium ferricyanide. Cells were washed with sodium phosphate buffer and fixed with 95% ethanol for 5 minutes. Cells were air-dried and incubated for 20 seconds with Harris-hematoxylin, washed, and mounted on slides.
Measurement of intracellular Ca++ concentration
The HEL cells and megakaryocytes, identified on the basis of size and morphology,22 were viewed on an inverted microscope. [Ca++]i was measured by single cell digital imaging in FURA-2–labeled cells using Openlab software as described.20 Cells were stimulated with 20 nmol/L convulxin. Results are representative of at least 3 independent experiments.
Analysis of data
Results are shown as the mean ± SEM of at least 3 independent experiments. Statistical indications were made using Student t test.
Results
Expression of GPVI on different cell types
The presence of GPVI on several megakaryoblastic and nonmegakaryoblastic hematopoietic cell lines was investigated by RT-PCR and by flow cytometry. The results were compared to similar studies on platelets. Figure 1A shows that messenger RNA (mRNA) for GPVI was present in platelets and several megakaryoblastic cell lines, but was not detectable in the Daudi B-cell line, Jurkat T-cell line, and promonocytic U937 cell line. We confirmed the identity of the amplified band by sequencing. The absence of signal when M-MLV reverse transcriptase was omitted and the RNA mixture subjected to the same RT-PCR procedure confirmed that DNA synthesis resulted from complementary DNA (cDNA) (data not shown). All cell types expressed mRNA for β-actin detected by RT-PCR. Flow cytometry studies using the GPVI ligand convulxin and an antibody to convulxin revealed the surface expression of the receptor protein (Figure 1B). In all the cases the level of expression of GPVI in the megakaryoblastic cell lines in comparison to platelets was low (Figure 1B), a result that was confirmed by ligand blotting using convulxin (not shown).
GPVI and FcR γ-chain are up-regulated in HEL and CMK cells during differentiation
To further characterize the expression of GPVI, HEL and CMK cells were differentiated with PMA. The majority (> 99%) of nondifferentiated HEL and CMK cells had ploidy values of 2n (representative of cells in phase G1 of the cell cycle) and 4n (cells in G2/M). When exposed to PMA for 3 days, the ploidy values ranged from 2n to 16n confirming that differentiation had occurred (Figure 2A). Expression of GPVI mRNA increased on differentiation as measured by RT-PCR. This was accompanied by an increase in mRNA encoding GPIIIa, a marker of megakaryocytic differentiation, and a decrease in mRNA encoding glycophoryn A, a marker of erythroid differentiation (Figure 2B). When the cells were induced to undergo erythroid differentiation using hemin,19 expression of mRNA for GPIIIa was down-regulated and mRNA for GPVI was no longer detectable (Figure 2B). In contrast, the level of the “housekeeping” protein HPRT did not change significantly on differentiation with PMA or hemin as measured by RT-PCR (Figure 2B). Flow cytometric studies demonstrated that surface expression of GPVI and GPIIIa was also increased in both cell lines during megakaryocytic differentiation (Figure 2A). In addition, ligand and Western blotting studies in HEL cells revealed an up-regulation of the glycoprotein receptor and its associated FcR γ-chain, whereas expression of Syk did not change significantly (Figure 2C). Maximal increase in the expression of GPVI and FcR γ-chain was reached after 3 days of exposure to PMA. The correlation of expression of GPVI and FcR γ-chain corresponds to the observation made on platelets from patients deficient for GPVI.15
To investigate whether GPVI was functional on HEL cells, we measured tyrosine phosphorylation and [Ca++]ielevation in response to convulxin in differentiated and nondifferentiated cells. In nondifferentiated cells there was a slight increase in the overall level of tyrosine phosphorylation in response to convulxin, which was clearly seen after 90 seconds of stimulation and maintained up to 270 seconds (Figure3A). The increase in tyrosine phosphorylation in differentiated cells was stronger and more rapid, being evident after 30 seconds of convulxin stimulation and peaking at 90 seconds. Major protein bands of 36, 72, 76, 80, 100, 130, and 148 kd underwent increases in tyrosine phosphorylation on convulxin stimulation in cells differentiated with PMA, whereas in nondifferentiated cells only weak increases in the phosphorylation of these bands were seen. Increases in phosphorylation of several other, more minor bands were also seen. This increase in tyrosine phosphorylation corresponds to the increase in expression of GPVI with differentiation as shown in Figure 2. The identity of some of the proteins that underwent increases in tyrosine phosphorylation in response to convulxin stimulation was assessed after immunoprecipitation. The bands of 36, 72, and 148 kd contained LAT, Syk, and PLCγ2, respectively (Figure 3B). Additionally, FcR γ-chain was found to undergo tyrosine phosphorylation in response to convulxin stimulation (Figure 3B). This demonstrates that the major proteins undergoing phosphorylation in response to convulxin in HEL cells are the same as those in platelets.
Stimulation of HEL cells with convulxin.
(A) For 3 days, control and PMA-differentiated HEL cells were stimulated with convulxin (20 nmol/L) at different times, lysed, and subjected to SDS-PAGE, then blotted for tyrosine phosphorylation using mAb 4G10. Arrows indicate the major tyrosine phosphorylated bands. (B) Immunoprecipitation of the indicated proteins and tyrosine phosphorylation blot as in panel A. Membranes were reprobed for detection of the corresponding proteins to check equal loading. (C) [Ca++]i increase on convulxin and thrombin stimulation before and after differentiation with PMA. Values indicate increase in [Ca++]i ± SEM. Results are representative of 5 experiments, with 20 cells being measured in each experiment.
Stimulation of HEL cells with convulxin.
(A) For 3 days, control and PMA-differentiated HEL cells were stimulated with convulxin (20 nmol/L) at different times, lysed, and subjected to SDS-PAGE, then blotted for tyrosine phosphorylation using mAb 4G10. Arrows indicate the major tyrosine phosphorylated bands. (B) Immunoprecipitation of the indicated proteins and tyrosine phosphorylation blot as in panel A. Membranes were reprobed for detection of the corresponding proteins to check equal loading. (C) [Ca++]i increase on convulxin and thrombin stimulation before and after differentiation with PMA. Values indicate increase in [Ca++]i ± SEM. Results are representative of 5 experiments, with 20 cells being measured in each experiment.
We next determined if stimulation with convulxin leads to an increase in [Ca++]i. Cells were loaded with the reporter dye FURA-2, and [Ca++]i was measured by single cell digital imaging. Cells less than 15 μm in diameter did not undergo a significant increase in [Ca++]iin response to convulxin (Figure 3C). This population is thought to represent immature megakaryocytes. Fewer than 0.1% of the cells in the culture were more than 20 μm in diameter. Of these, the majority responded to convulxin with an elevation in [Ca++]i (not shown). This population is thought to represent cells that have undergone differentiation. In contrast, both populations of small and large cells responded to thrombin with an elevation in [Ca++]i (Figure3C). On differentiation with PMA, approximately 99% of cells responded to convulxin and thrombin with increases in [Ca++]i of 140 ± 23 and 212 ± 29 nmol/L above basal, respectively (Figure 3C).
In vitro differentiation of mouse bone marrow megakaryocytes
We examined the expression of GPVI in mouse megakaryocytes derived from bone marrow cells to determine if similar observations apply to primary cells. Cells were grown in vitro for up to 4 days in a medium designed to support megakaryocyte differentiation. Staining for acetylcholinesterase detection was used as a marker of megakaryocyte differentiation.23 Large, terminally differentiated bone marrow megakaryocytes make up less than 0.1% of total cell number at day 0, but undergo a significant expansion after 4 days in vitro, representing 2% to 5% of the cell population (Figure 4A). These in vitro grown cells were analyzed for expression of GPVI and GPIIb by flow cytometry. Three different cell populations were gated based on their different size and complexity as previously reported.24 Of these, the population of larger cells, consisting of more mature megakaryocytes, was detectable only after 4 days of culture. A second population, containing cells of intermediate size, and a third population of smaller cells represent less differentiated megakaryocytes and other cell types (Figure 4B). Almost 100% of the large cell population were GPIIb-positive, a megakaryocyte marker, whereas nearly 40% were positive for GPVI. In contrast, between 1% and 5% of the medium and small cells expressed GPVI (Table 1).
Mouse megakaryocytes differentiation in vitro and flow cytometry analysis of the primary cultures.
(A) Acetylcholinesterase staining of mouse bone marrow cells before (left panel) and after (right panel) in vitro differentiation for 4 days. Arrows indicate the position of mature megakaryocytes, which are easily distinguished from other cells by their large size. Bar indicates 25 μm. (B) Cells cultures characterized by flow cytometry showing dot plots for forward (FSC) and side (SSC) light scatter profiles of 10 000 cells. In each blot, 3 arbitrary analysis gates have been drawn. The lower gate includes small cells; the middle gate, intermediate-size cells; and the upper gate, large cells. Note a progressive increase in the proportion of intermediate-size and large cells with time. (C) In vitro differentiated mouse megakaryocytes were analyzed for calcium increase by single cell digital imaging. Top panel shows elevation of [Ca++]i on convulxin and thrombin stimulation, and table indicates the increase above basal level of calcium. Results are representative of 3 experiments, with 15 cells being measured in each experiment.
Mouse megakaryocytes differentiation in vitro and flow cytometry analysis of the primary cultures.
(A) Acetylcholinesterase staining of mouse bone marrow cells before (left panel) and after (right panel) in vitro differentiation for 4 days. Arrows indicate the position of mature megakaryocytes, which are easily distinguished from other cells by their large size. Bar indicates 25 μm. (B) Cells cultures characterized by flow cytometry showing dot plots for forward (FSC) and side (SSC) light scatter profiles of 10 000 cells. In each blot, 3 arbitrary analysis gates have been drawn. The lower gate includes small cells; the middle gate, intermediate-size cells; and the upper gate, large cells. Note a progressive increase in the proportion of intermediate-size and large cells with time. (C) In vitro differentiated mouse megakaryocytes were analyzed for calcium increase by single cell digital imaging. Top panel shows elevation of [Ca++]i on convulxin and thrombin stimulation, and table indicates the increase above basal level of calcium. Results are representative of 3 experiments, with 15 cells being measured in each experiment.
We examined the ability of convulxin to stimulate an increase in [Ca++]i in the primary megakaryocytes by single cell digital imaging. Only cells with a size greater than 20 μm were analyzed (Figure 4C). The cells responded to different degrees, with about 50% of the cells responding to convulxin stimulation with a robust increase in [Ca++]i, whereas all responded with a strong increase in response to thrombin. The percentage of cells responding to convulxin in this way correlates with the percentage of GPVI-positive megakaryocytes, as detected by flow cytometry.
Discussion
We have analyzed the expression of the collagen receptor GPVI in megakaryocytes. By RT-PCR we found the presence of GPVI mRNA in a number of megakaryoblastic cell lines but not in other hematopoietic cells, suggesting that it may be localized to megakaryocytes and platelets. We also observed increased expression of GPVI in HEL and CMK cells differentiated with PMA. Differentiated HEL cells showed an increase in the level of the FcR γ-chain, indicating that both proteins are up-regulated during the process of differentiation and consistent with their coexpression.
Nondifferentiated HEL cells, which express a low level of GPVI, respond with a weak increase in the overall level of tyrosine phosphorylation to convulxin, with only larger cells (>0.1% of the total cell population) exhibiting an increase in [Ca++]i. In contrast, PMA-differentiated HEL cells respond to convulxin with a powerful increase in tyrosine phosphorylation with nearly all undergoing increases in [Ca++]i. It is possible that the increase in tyrosine phosphorylation observed in the nondifferentiated megakaryocytes is primarily occurring at the subpopulation of larger cells, which exhibit an increase in [Ca++]iand which may represent cells that have undergone differentiation. A previous study on the megakaryocytic cell line DAMI also described an elevation of [Ca++]i in response to convulxin, which was increased when GPVI was introduced by transfection.21 Studies in mouse megakaryocytes grown in vitro also demonstrate that expression of GPVI increases on differentiation, accompanied by an increase in [Ca++]i elevation to convulxin.
We previously reported that nondifferentiated megakaryoblastic cell lines did not respond to collagen or collagen-related peptide with an increase in tyrosine phosphorylation or elevation of [Ca++]i.25 The major difference between that study and the present one is the use of convulxin. The trimeric α3β3 convulxin is a much more powerful ligand than collagen or collagen-related peptide, causing a much greater increase in response in platelets and megakaryoblastic cell lines (data not shown). A similar observation was made by Clemetson and colleagues,21 who reported that the megakaryocytic cell line DAMI responded to convulxin, but not collagen, through an increase in [Ca++]i.
This study presents evidence that GPVI is expressed in platelets and megakaryocytes and that expression increases toward the end of megakaryocyte differentiation. Although further work is required to confirm that GPVI is exclusively expressed on platelets and megakaryocytes, the results indicate that GPVI is likely to be a novel marker of end-stage megakaryocytopoiesis. The question arises as to why GPVI expression increases with late-stage megakaryocyte differentiation. One possible reason for this is to prevent activation of the immature, developing megakaryocyte through exposure to surrounding collagen. It is also possible that GPVI plays a role in the end-stage megakaryocyte differentiation/platelet formation. However, GPVI-deficient individuals have normal levels of platelets and show impairment in response only to collagen,10 11 indicating that the role of GPVI is primarily linked to the control of platelet function. Engineering of GPVI-deficient mice would enable a detailed investigation of this.
Supported by the British Heart Foundation, Wellcome Trust and Medical Research Council. S.P.W. is a British Heart Foundation Senior Research Fellow. J.F. is a Wellcome Trust Senior Research Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Oscar Berlanga, Department of Pharmacology, University of Oxford, Mansfield Rd, OX1 3QT, Oxford, United Kingdom; e-mail: oscar.berlanga@pharm.ox.ac.uk.

![Fig. 3. Stimulation of HEL cells with convulxin. / (A) For 3 days, control and PMA-differentiated HEL cells were stimulated with convulxin (20 nmol/L) at different times, lysed, and subjected to SDS-PAGE, then blotted for tyrosine phosphorylation using mAb 4G10. Arrows indicate the major tyrosine phosphorylated bands. (B) Immunoprecipitation of the indicated proteins and tyrosine phosphorylation blot as in panel A. Membranes were reprobed for detection of the corresponding proteins to check equal loading. (C) [Ca++]i increase on convulxin and thrombin stimulation before and after differentiation with PMA. Values indicate increase in [Ca++]i ± SEM. Results are representative of 5 experiments, with 20 cells being measured in each experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/8/10.1182_blood.v96.8.2740/5/m_h82000250003.jpeg?Expires=1769199963&Signature=k-LlJtl93wU5E7eSrN-c5mY4cLoGoS7~ZP9eHJIOFEG8-M4Cm5nXVf31U96Y7cKF0kGAm7WVJu8948yNQbGCh0cwxG3ssLFC9qBBkw16AbATnlH75cZ7wNX9u-YPAkmARgQTwO3b80TUanxgEmaL7zX4DHQ-Rp~tDNWtOTxXs4jRh-fsxee4fv-SKQfWCAZy67liaAcA72HNCGJ6j1kT3k2GrvZWWdVQqQJ8Up4ZopIauw9jZ7sq4LQDGj1~LVrBR4SLEJEmMvBhW-JY-yQKBbKhjJ1MBKFKEUx3KsqqETYsGF7-tsBSTnnlhdksGVUrH~RtIupW4IsRUAilhA7hDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Mouse megakaryocytes differentiation in vitro and flow cytometry analysis of the primary cultures. / (A) Acetylcholinesterase staining of mouse bone marrow cells before (left panel) and after (right panel) in vitro differentiation for 4 days. Arrows indicate the position of mature megakaryocytes, which are easily distinguished from other cells by their large size. Bar indicates 25 μm. (B) Cells cultures characterized by flow cytometry showing dot plots for forward (FSC) and side (SSC) light scatter profiles of 10 000 cells. In each blot, 3 arbitrary analysis gates have been drawn. The lower gate includes small cells; the middle gate, intermediate-size cells; and the upper gate, large cells. Note a progressive increase in the proportion of intermediate-size and large cells with time. (C) In vitro differentiated mouse megakaryocytes were analyzed for calcium increase by single cell digital imaging. Top panel shows elevation of [Ca++]i on convulxin and thrombin stimulation, and table indicates the increase above basal level of calcium. Results are representative of 3 experiments, with 15 cells being measured in each experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/8/10.1182_blood.v96.8.2740/5/m_h82000250004.jpeg?Expires=1769199963&Signature=z-AUvXPuNpWJSEvZ0Lrytmb-aXm6GklNR75B3pozmmxnr~RgXEewNN7bWJp~tPvGbJlgZjR-nuHks~aoQrrLOhzr63Y8VC9HXyyCc4zkcLle49GJOIvxIVK8w96kIgSMVgnwy2TfsHtnWbdmF6~0noGtnn6T69REJz0udezByGPqytA-fXihD8z9QRbWKGU2vzkDfEaWDF6d-m~8z3SmIDp~u1T2jpgPWEL5DfplBb8xl4RmDmu9sKu-02qfs50ATW5YfwvbY2X9IYScKPO5WgvH0V0ihVssMvy9tm6lfNeeLdBfz3SPuqcjel6UADZd8x30PlVwMhUfREoLP4bC3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
