Abstract
Ancrod is a purified fraction of venom from the Malayan pit viper, Calloselasma rhodostoma, currently under investigation for treatment of acute ischemic stroke. Treatment with ancrod leads to fibrinogen depletion. The present study investigated the mechanisms leading to the reduction of plasma fibrinogen concentration. Twelve healthy volunteers received an intravenous infusion of 0.17 U/kg body weight of ancrod for 6 hours. Blood samples were drawn and analyzed before and at various time points until 72 hours after start of infusion. Ancrod releases fibrinopeptide A from fibrinogen, leading to the formation of desAA-fibrin monomer. In addition, a considerable proportion of desA-profibrin is formed. Production of desA-profibrin is highest at low concentrations of ancrod, whereas desA-profibrin is rapidly converted to desAA-fibrin at higher concentrations of ancrod. Both desA-profibrin and desAA-fibrin monomers form fibrin complexes. A certain proportion of complexes carries exposed fibrin polymerization sites EA, indicating that the terminal component of the protofibril is a desAA-fibrin monomer unit. Soluble fibrin complexes potentiate tissue-type plasminogen activator-induced plasminogen activation. Significant amounts of plasmin are formed when soluble fibrin in plasma reaches a threshold concentration, leading to the proteolytic degradation of fibrinogen and fibrin. In the present setting, high concentrations of soluble fibrin are detected after 1 hour of ancrod infusion, whereas a rise in fibrinogen and fibrin degradation products, and plasmin-α2–plasmin inhibitor complex levels is first detected after 2 hours of ancrod infusion. Ancrod treatment also results in the appearance of cross-inked fibrin degradation productd-dimer in plasma.
Introduction
Ancrod is a purified fraction of venom from the Malayan pit viper, Calloselasma rhodostoma, containing a serine protease that cleaves fibrinopeptide A from fibrinogen.1 In contrast to thrombin, the enzyme does not cleave fibrinopeptide B2 and does not activate factor XIII.3 Therapeutic doses result in the formation of soluble fibrin complexes that are degraded by plasmin.4 5
Recent investigations have shown that release of fibrinopeptide B by thrombin occurs largely after the formation of particulate clots,6 which implies that the major proportion of soluble fibrin formed by the action of thrombin in vivo is desAA-fibrin, similar to the fibrin observed during the administration of ancrod. The major difference between ancrod- and thrombin-induced soluble fibrin complexes is that the latter contain factor XIIIa cross-linked γ-chains,7 because fibrinopeptide A release and factor XIII activation by thrombin are nearly simultaneous events in the course of fibrin formation.6 8 In contrast, ancrod-induced soluble fibrin complexes would be expected to consist of non–cross-linked fibrin monomer units.
In the present investigation we explored the structure of soluble fibrin complexes and fibrinogen and fibrin degradation products formed during ancrod therapy.
Patients, materials, and methods
Study population
Twelve healthy volunteers, 6 women and 6 men, aged 25 to 55 years, were included in the study, which was approved by the local ethical committee of the institution. After written informed consent and initial blood sampling, the volunteers received ancrod at a dosage of 0.17 U/kg body weight per hour by continuous intravenous infusion for a total of 6 hours, resulting in a cumulative dose of 1.0 U/kg body weight. Blood samples were obtained before ancrod infusion, and 1, 2, 3, 4, 5, 6, 8, 12, 15, 24, 48, and 72 hours after starting the infusion.
Samples for functional tests involving measurement of plasmin activity were drawn into syringes containing 1/10 volume of 0.11 mol/L trisodium citrate anticoagulant. For all other assays, we used syringes containing 1/10 volume of 0.11 mol/L trisodium citrate, 0.05 mol/L Tris, pH 7.4, with 1000 KIU/mL of aprotinin (Trasylol, Bayer, Leverkusen, Germany), and 7% of an antiserum against ancrod. The antiserum totally inhibits proteolytic activity of ancrod, without influencing thrombin or other human coagulant enzymes.4Samples were centrifuged at 2000g and 4°C for 10 minutes within 20 minutes after sampling. Aliquots of plasma were transferred to polypropylene sample tubes, snap-frozen in liquid nitrogen, and stored at −70°C.
Measurement of fibrinogen concentration
Total clottable protein (TCP-macro) was measured by the procedure described by Gaffney and Wong,9 which is a modification of the methods of Jacobsson10 and Blomback.11 Fibrinogen-related antigen was measured by immunoturbidimetric assay, using reagents from DAKO (Hamburg, Germany) and a Hitachi 904 automated photometric analyzer.
Soluble fibrin assays
Enzymun-Test FM (Enzymun FM) is based on antibodies against the neo–N-terminus of the α-chain of fibrin monomer exposed after cleavage of fibrinopeptide A from the parent fibrinogen molecule.12 13 Samples are pretreated with potassium thiocyanate (KSCN) solution for disaggregation of noncovalent fibrin complexes. The assay was performed with reagents from Roche Diagnostics (Mannheim, Germany) on an ES300 automated immunoassay analyzer.
DesA-profibrin14 was measured by enzyme-linked immunosorbent assay (ELISA), using streptavidin-coated microtiter plates from Roche Diagnostics, MAb 2B5 as biotinylated capture antibody, and a polyclonal immunoaffinity purified rabbit antihuman fibrinopeptide A antibody as tag. Bound rabbit IgG was detected by a monoclonal antibody against rabbit IgG conjugated with alkaline phosphatase (Sigma, Deisenhofen, Germany). For this assay, 20 μL of plasma was mixed either with 60 μL of 4.33 mol/L KSCN solution or 0.05 mol/L Tris, 0.1 mol/L NaCl, pH 7.4 buffer, incubated for 10 minutes, and an aliquot of 20 μL transferred to the streptavidin-coated microtiter plate. Serial dilutions of pooled plasma samples from 50 patients with disseminated intravascular coagulation, diluted with pooled plasma from healthy blood donors, were used as reference.
A similar assay was performed using biotinylated MAb 2B5 as capture antibody, and a polyclonal rabbit antiserum against human fibrinogen degradation product D as tag. This assay was performed without KSCN sample pretreatment.
Fibrinostika soluble fibrin (Fibrinostika SF) detects an epitope located in the stretch of Aα146-160, corresponding to a component of the tissue-type plasminogen activator (tPA) binding site,15 which is exposed on polymerization of fibrin monomers.16 A horseradish peroxidase conjugate of MAb G8, directed against an epitope on the C-termini of the α-chains is used as tag antibody. The assay kits were from Organon Teknika (Eppelheim, Germany).
The Chromogenix soluble fibrin assay Coatest SF (Chromogenix SF) (Chromogenix, Mölndal, Sweden) is based on the catalytic effect of soluble fibrin toward tPA-induced plasminogen activation.17 18 The assay was performed with microtiter plates containing co-lyophilized tPA, plasminogen, chromogenic substrate S-2403, and an antibody against plasmin inhibitor.
Thrombus precursor protein (TpP) was measured by ELISA from American Biogenetic Sciences (Boston, MA). The assay is based on a monoclonal antibody prepared by immunization with freeze-fractured cross-linked fibrin.19 Immunoreactivity of the antibody, MH-1, was shown to be limited to desAABB-fibrin.
Fibrinogen and fibrin degradation products
Fibrinogen degradation products were measured with the Fibrinostika FgDP assay (FgDP)20 from Organon Teknika. This assay is based on a monoclonal antibody (MAb FDP-14) against an epitope on the Bβ-chain (Bβ52-114), which reacts with fibrinogen and fibrin degradation products, but not with fibrinogen or non-degraded fibrin. A horseradish peroxidase-conjugated antibody against fibrinopeptide A is used for detection. Fibrin degradation products were measured with the Fibrinostika FbDP (FbDP) assay kit from the same manufacturer, which uses MAb FDP-14, combined with a monoclonal antibody (MAb DD13/HRP) elicited against d-dimer as peroxidase-conjugated tagging antibody.21
Fibrinogen/fibrin degradation products (FDP), and fibrinogen/fibrin degradation product E (FDP-E) were measured with latex-enhanced photometric immunoassays (LPIA) from Nippon Roche (Tokyo, Japan). Assays were performed on a Hitachi 904 photometric autoanalyzer. For these assays serum samples were prepared from citrated plasma samples, by addition of bovine thrombin (Test Thrombin, Behring, Marburg, Germany) at a final concentration of 1 NIH U/mL, 30 minutes of incubation, and centrifugation.
The D-dimer antigen was measured by Dimertest gold microtiter plate ELISA from AGEN (Brisbane, Australia),22 based on monoclonal antibody MAb DD3B6/22, specific for a conformational epitope related to the cross-linking of fibrin γ-chains by factor XIIIa, and by 3 latex-enhanced photometric immunoassays (LPIAs). Roche D-dimer LPIA was from Nippon Roche, STA-LIAtest D-di from Stago (Asnières, France), and TINAquant D-dimer from Roche Diagnostics.
TINAquant d-dimer uses monoclonal antibody MAb JIF-23 attached to latex microparticles.23,24 MAb JIF-23 detects an epitope formed when the D domain is cleaved from the parent molecule by plasmin.25 Agglutination of antibody-coated latex particles requires the presence of at least 2 such epitopes, resulting in reactivity of the assay with plasmin-degraded factor XIIIa–cross-linked fibrin complexes.7 The epitopes or structures recognized by STA-Liatest D-di, and Roche LPIA D-dimer have not been published.
Other laboratory assays
Plasminogen was measured by chromogenic assay from Roche Diagnostics. Plasmin inhibitor activity (antiplasmin) and plasminogen activator inhibitor 1 (PAI-1) activity were determined by functional chromogenic assays, tPA by microtiter plate ELISA from Chromogenix. Plasmin-α2-plasmin inhibitor complex was measured by ELISA from Dade Behring Diagnostics (Marburg, Germany). Prothrombin fragment F1.2 (F1.2), and thrombin-antithrombin complex (TAT) were measured by Enzygnost F1.2, and Enzygnost TAT ELISA kits from Dade Behring, respectively.
Immunoblotting experiments
Electrophoresis was performed with acrylamide gels prepared with ProSieve reagent from Biozym (Oldendorf, Germany). Proteins were transferred to Immobilon membranes (Millipore, Eschborn, Germany) by semidry immunoblotting. Membranes were blocked with gelatin blocking solution. Antibodies were applied in Tris-buffered saline (TBS) containing Tween-20 (TTBS). After final washing with TTBS and TBS, bands were stained using nitroblue tetrazolium/5-bromo-4-chloro-3-inodolyl phosphate (NBT/BCIP) staining solution (all reagents from Sigma).
Biotinylated MAb 2B5 from the Enzymun-Test FM (Roche Diagnostics) was used in conjunction with streptavidine-alkaline phosphatase conjugate from Sigma. MAb S4H9 against D-dimer was a generous gift from Nycomed (Oslo, Norway). Goat antimouse IgG alkaline phosphatase conjugate from Sigma was used for detection. The polyclonal rabbit antiserum against human fibrinogen was purchased from DAKO and was used with an alkaline phosphatase-conjugated monoclonal antibody against rabbit IgG from Sigma.
In vitro experiment on factor XIII activation
Pooled plasma from 20 healthy blood donors was desalted by gel filtration on a Sephadex G25M-column (Pharmacia, Freiburg, Germany) equilibrated with a buffer contatining 0.05 mol/L Tris, 0.1 mol/L NaCl, pH 7.4. Aliquots of 100 μL of desalted plasma were incubated with combinations of recombinant hirudin derivative lepirudin, calcium chloride, ancrod, and thrombin for 60 minutes at 37°C, resulting in a final volume of 150 μL. Final concentrations of reagents were: lepirudin (Refludan, Hoechst, Frankfurt, Germany) 0.65 mg/mL, calcium chloride 3 mmol/L, ancrod 1 U/ml, thrombin (Test Thrombin, Dade Behring) 0.4 NIH U/mL. After incubation, 400 μL of 0.5% sodium dodecyl sulfate (SDS), 3.0 mol/L urea buffer containing dithioerythritol (DTE) were added and samples incubated at 90°C for 30 minutes. Electrophoresis was performed using precast Tris/glycine gels from Invitrogen (Groningen, Netherlands). Proteins were transferred to Immobilon membranes by semidry immunoblotting. Membranes were blocked with gelatin blocking solution. Antibodies were applied in TBS containing Tween-20 (TTBS). After final washing with TTBS and TBS, bands were stained using NBT/BCIP staining solution. Antibodies used for detection were a polyclonal antibody against the activation peptide of human factor XIIIA prepared in rabbits and a rabbit polyclonal antiserum against the human fibrinogen γ-chain (Paesel, Frankfurt, Germany). Bound rabbit antibody was detected using alkaline phosphatase-conjugated monoclonal antibody against rabbit IgG from Sigma.
Statistical methods
Statistical evaluation included calculation of mean values, SD, minimum and maximum values, numeric coefficients of correlation, and Spearman rank correlation coefficients. Analyses were performed using StatView 4.5 software from Abacus Concepts (Berkeley, CA).
Results
Twelve healthy subjects received a 6-hour continuous intravenous infusion of ancrod, resulting in a final cumulative dose of 1 U/kg body weight. Blood samples were drawn before infusion (0 hour sample) and after 1, 2, 4, 6, 8, 12, 15, 24, 48, and 72 hours.
Immunoblotting experiments
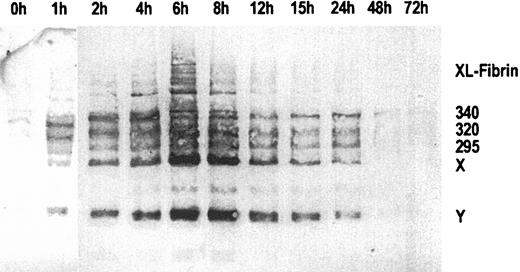
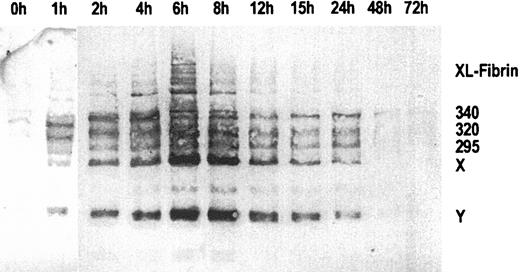
Figure 1 shows an immunoblot of sequential plasma samples obtained before, during, and after the 6-hour infusion of ancrod, using MAb 2B5 for immunodetection of the neo–N-terminus α-chain epitope. Before ancrod infusion, very little material is reactive with MAb 2B5. Ancrod infusion resulted in the formation of large amounts of material reactive with MAb 2B5, including fibrin monomer units, fibrin degradation products X and Y, and compounds with higher molecular weight than fibrinogen, corresponding to covalent fibrin complexes (XL-fibrin). According to densitometric analysis, the largest amount of MAb 2B5-reactive material is present in the fraction obtained at the end of the 6-hour ancrod infusion.
Immunoblots of sequential plasma samples.
Plasma samples were drawn before and 1 to 72 hours after start of ancrod infusion, using monoclonal antibody MAb 2B5 against the neo–N-terminus of the fibrin α-chain for detection. Nearly no fibrin antigen is detected in the pretreatment sample. After start of ancrod infusion, there is a gradual increase in the amount of antigen detected by Mab 2B5, consisting of fibrin monomer and fibrin degradation products X and Y. A small amount of reactive material with higher molecular weight than fibrinogen is found in plasma samples drawn between 2 and 12 hours after start of ancrod infusion.
Immunoblots of sequential plasma samples.
Plasma samples were drawn before and 1 to 72 hours after start of ancrod infusion, using monoclonal antibody MAb 2B5 against the neo–N-terminus of the fibrin α-chain for detection. Nearly no fibrin antigen is detected in the pretreatment sample. After start of ancrod infusion, there is a gradual increase in the amount of antigen detected by Mab 2B5, consisting of fibrin monomer and fibrin degradation products X and Y. A small amount of reactive material with higher molecular weight than fibrinogen is found in plasma samples drawn between 2 and 12 hours after start of ancrod infusion.
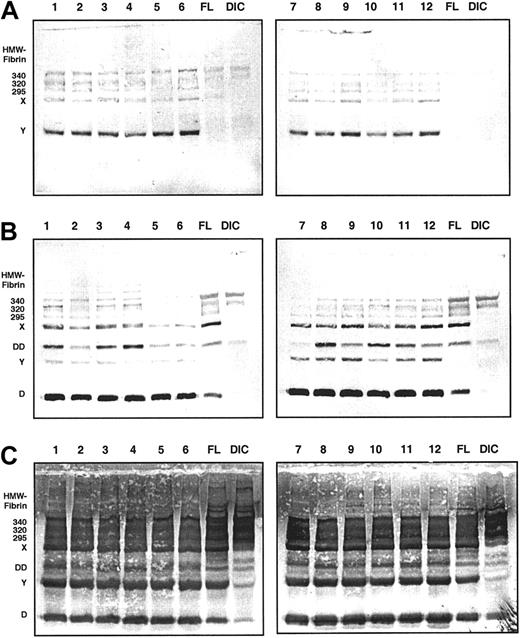
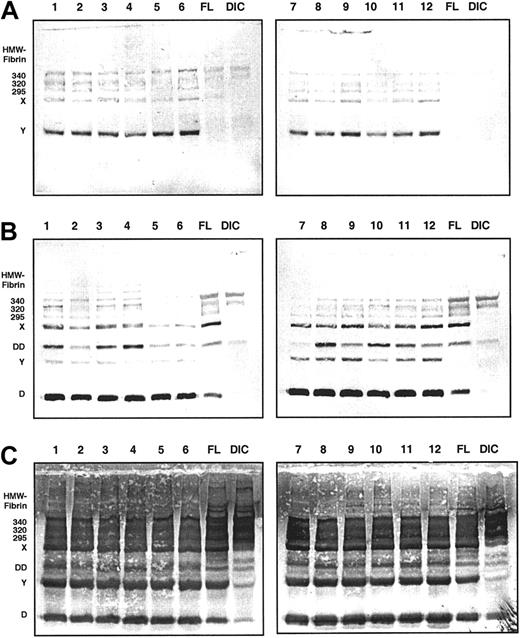
Immunoblots of the 6-hour samples from all 12 volunteers are displayed in Figure 2. MAb 2B5 was used for immunodetection in panel A, MAb S4H9, an antibody raised against fibrin fragment D-dimer, in panel B, and a polyclonal antiserum against fibrinogen in panel C. For reference, pooled plasma samples from patients with fibrinolytic therapy (FL) and disseminated intravascular coagulation (DIC) are included. Panel A shows that the major proportion of material reactive with MAb 2B5 during ancrod therapy is fibrin monomer units, along with fibrin degradation products X and Y. Fibrin fragment X also is present in the FL sample, whereas it appears to be nearly absent in the DIC pool sample. Bands of a molecular weight higher than fibrinogen are present in the ancrod samples, as well as in the FL and DIC samples. The D-dimer monoclonal antibody (panel B) detects a variety of derivatives, including monomeric fragment D, fragment Y, and fragment X. Despite the fact that ancrod does not activate factor XIII, fragment D-dimer, a specific degradation product of factor XIIIa–cross-linked fibrin, is present in the samples from ancrod-treated subjects. In addition to the lower molecular weight material, the monoclonal antibody detects a number of bands with a higher apparent molecular weight than fibrinogen, corresponding to cross-linked fibrin complexes.
Immunoblots of 6-hour samples from all 12 volunteers.
Monoclonal antibody MAb 2B5 against the neo–N-terminus of the fibrin α-chain was used for detection in panel A, MAb S4H9 against D-dimer antigen in panel B, and polyclonal antifibrinogen antiserum in panel C. For reference, pooled plasma samples from patients with fibrinolytic therapy (FL) and patients with disseminated intravascular coagulation (DIC) are included. MAb 2B5-reactive material consists primarily of fibrin monomer, fragment X, and fragment Y (panel A). MAb S4H9 detects several high-molecular-weight bands and fragment D-dimer of cross-inked fibrin, but also reacts with fragments X, Y, and D of non–cross-linked fibrin or fibrinogen (panel B). Immunostaining with the polyclonal antifibrinogen antiserum shows that fragments X, Y, and D are the prevailing proteolytic derivatives, whereas the D-dimer band is less prominent (panel C).
Immunoblots of 6-hour samples from all 12 volunteers.
Monoclonal antibody MAb 2B5 against the neo–N-terminus of the fibrin α-chain was used for detection in panel A, MAb S4H9 against D-dimer antigen in panel B, and polyclonal antifibrinogen antiserum in panel C. For reference, pooled plasma samples from patients with fibrinolytic therapy (FL) and patients with disseminated intravascular coagulation (DIC) are included. MAb 2B5-reactive material consists primarily of fibrin monomer, fragment X, and fragment Y (panel A). MAb S4H9 detects several high-molecular-weight bands and fragment D-dimer of cross-inked fibrin, but also reacts with fragments X, Y, and D of non–cross-linked fibrin or fibrinogen (panel B). Immunostaining with the polyclonal antifibrinogen antiserum shows that fragments X, Y, and D are the prevailing proteolytic derivatives, whereas the D-dimer band is less prominent (panel C).
Using the polyclonal antifibrinogen antiserum we see that fragments X, Y, and D are the prominent derivatives generated during ancrod therapy. The amount of fragments X, Y, and D appears to be quite similar to the FL pool sample, whereas in the DIC pool sample, these bands are less prominent. Higher molecular weight material corresponding to cross-linked fibrin complexes, as well as fibrin fragment D dimer bands are also detected by the polyclonal antiserum against fibrinogen.
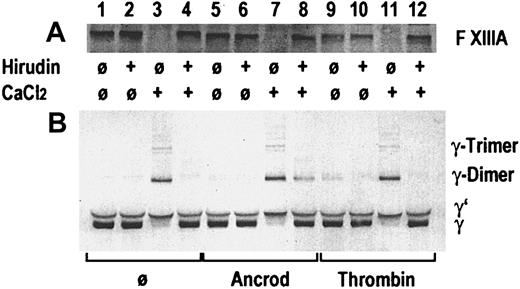
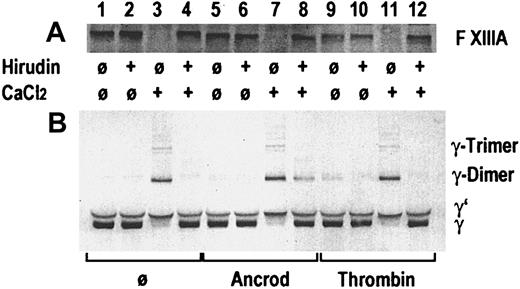
In an in vitro experiment, gel-filtered pooled plasma from healthy blood donors was incubated with ancrod or thrombin, in presence or absence of recombinant hirudin, and in presence or absence of 3 mmol/L calcium chloride (Figure 3). A buffer blank without enzyme was added as control. A buffer containing 0.05 mol/L Tris, 0.1 mol/L NaCl, pH 7.4, was used for gel filtration of plasma and for dilution. After 60 minutes of incubation at 37°C, SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer containing DTE as a reducing agent was added. Immunoblots were prepared, using a polyclonal rabbit antibody against the activation peptide of factor XIII subunit A in the first blot (Figure 3A) and a polyclonal rabbit antibody against the γ-chain of fibrinogen (Figure 3B). Addition of thrombin and calcium chloride (lane 11), addition of calcium chloride alone in the absence of hirudin (lane 3), and calcium chloride plus ancrod in absence of hirudin (lane 7) led to the disappearance of the factor XIII band, indicating proteolytic cleavage of the factor XIII activation peptide. The resulting activated factor XIII covalently links the γ-chains, resulting in the appearance of γ-dimers and γ-trimers, and disappearance of the γ-chain. In the absence of added thrombin, addition of calcium chloride at the given concentration leads to the autoactivation of the coagulation cascade, resulting in the formation of thrombin. Addition of hirudin prevents thrombin formation and proteolytic activation of factor XIII (lanes 4 and 12). Addition of ancrod in the absence of calcium chloride (lanes 5 and 6) causes no proteolysis of factor XIII and no γ-chain cross-linking. In contrast, addition of ancrod and calcium chloride leads to the generation of γ-chain dimers and trimers also in the presence of hirudin, whereas the hirudin prevents proteolysis of factor XIII (lane 8). This experiment shows that factor XIII γ-chain cross-linking activity may be generated also in the absence of proteolytic release of the factor XIII activation peptide, if polymeric desAA-fibrin and calcium chloride are present. This may be an explanation for the observation of cross-linked fibrin derivatives generated in the course of ancrod treatment.
Gel-filtered pooled plasma from healthy blood donors.
Immunoblots are shown from an in vitro experiment on the effect of ancrod and thrombin on proteolytic release of the factor XIII activation peptide and the catalytic activity of factor XIII leading to the formation of fibrin γ-chain dimers and trimers. Pooled plasma from healthy blood donors was incubated without enzyme, with ancrod, or with thrombin in absence or presence of recombinant hirudin and calcium chloride, as specified in the graph. Panel A shows an immunoblot using a polyclonal antiserum against the activation peptide of factor XIII. A polyclonal antiserum against the γ-chain of fibrinogen was used for the immunoblot in panel B. Addition of thrombin (lane 11) or intrinsic formation of thrombin after addition of calcium in the absence of hirudin (lane 3) led to disappearance of the factor XIII band and appearance of cross-linked γ-chains. Parallel addition of ancrod and calcium chloride led to the formation of γ-chain cross-links, also in presence of hirudin, even though there is no decrease in the factor XIII band. This indicates that the factor XIII zymogen is able to cross-link desAA-fibrin γ-chains formed by the action of ancrod in the presence of calcium.
Gel-filtered pooled plasma from healthy blood donors.
Immunoblots are shown from an in vitro experiment on the effect of ancrod and thrombin on proteolytic release of the factor XIII activation peptide and the catalytic activity of factor XIII leading to the formation of fibrin γ-chain dimers and trimers. Pooled plasma from healthy blood donors was incubated without enzyme, with ancrod, or with thrombin in absence or presence of recombinant hirudin and calcium chloride, as specified in the graph. Panel A shows an immunoblot using a polyclonal antiserum against the activation peptide of factor XIII. A polyclonal antiserum against the γ-chain of fibrinogen was used for the immunoblot in panel B. Addition of thrombin (lane 11) or intrinsic formation of thrombin after addition of calcium in the absence of hirudin (lane 3) led to disappearance of the factor XIII band and appearance of cross-linked γ-chains. Parallel addition of ancrod and calcium chloride led to the formation of γ-chain cross-links, also in presence of hirudin, even though there is no decrease in the factor XIII band. This indicates that the factor XIII zymogen is able to cross-link desAA-fibrin γ-chains formed by the action of ancrod in the presence of calcium.
DesA-profibrin and desAA-fibrin monomer
DesA-profibrin is the intermediate structure of fibrin formation, resulting from the cleavage of one fibrinopeptide A from fibrinogen. Combination of MAb 2B5 with an antibody against fibrinopeptide A yields an ELISA system for the measurement of desA-profibrin. We performed 2 variants of this assay, the first using native plasma samples for analysis, the second including a sample denaturation step by preincubating the plasma samples with 4.33 mol/L KSCN.
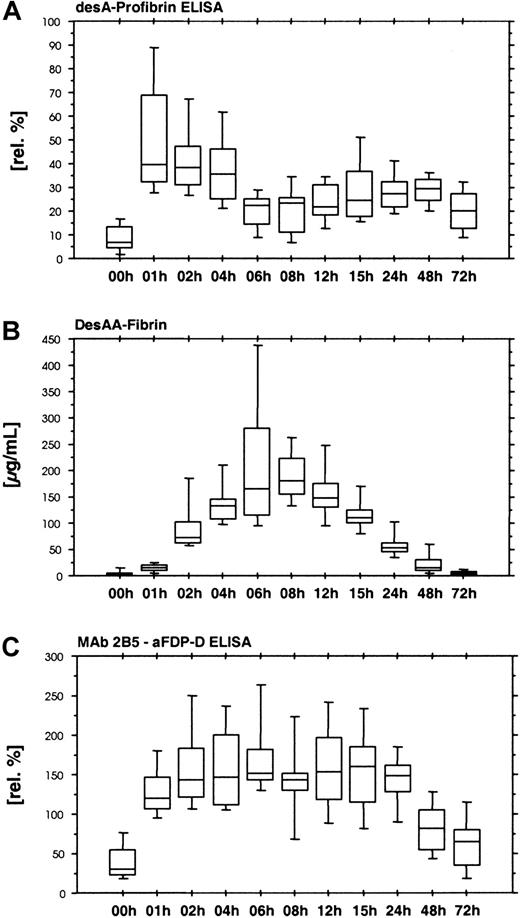
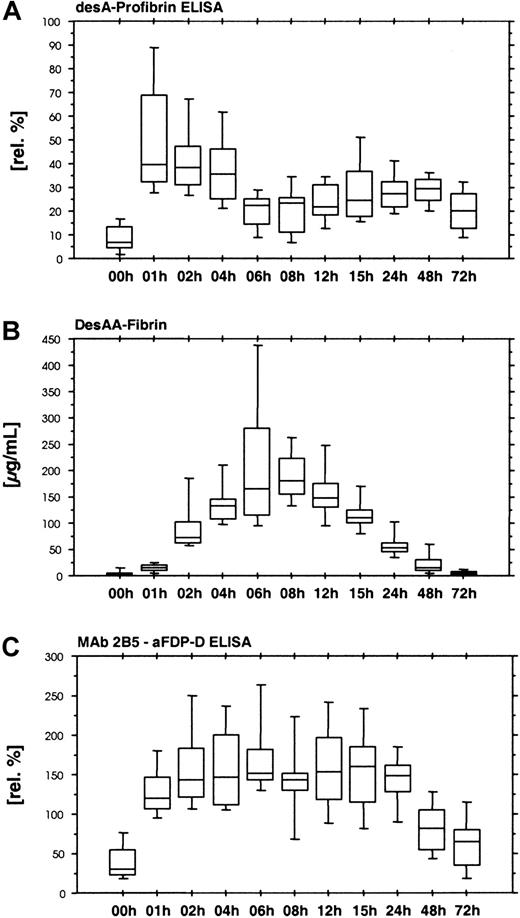
The desA-profibrin ELISA with KSCN sample pretreatment showed a rapid increase in desA-profibrin during the first hour of ancrod administration, followed by a plateau and subsequent decrease until the end of ancrod infusion (Figure 4A). A smaller increase was observed between 24 and 48 hours after the start of treatment. The assay without KSCN sample pretreatment showed only a slight increase in mean antigen concentration between 4 and 6 hours after the start of ancrod infusion, but levels were 30-fold lower than levels measured with the KSCN sample pretreatment variant of the assay (data not shown).
Time course of ancrod treatment of all 12 subjects (mean, SD, 95th percentile).
(A) DesA-profibrin; (B) desAA-fibrin; (C) Mab 2B5-aFDP-D ELISA. Release of one fibrinopeptide A from fibrinogen results in the formation of desA-profibrin. DesA-profibrin is detected by combining an antibody against the neo–N-terminus of the fibrin α-chain (MAb 2B5) with an antibody against fibrinopeptide A. Infusion of ancrod results in an increase in desA-profibrin in plasma, followed by a gradual decrease, and a more shallow second peak. At increasing plasma concentrations of ancrod, less desA-profibrin is generated in favor of desAA-fibrin. The Mab 2B5 epitope appears to be accessible without KSCN sample pretreatment, as exemplified by the result of the ELISA system combining MAb 2B5 with an antibody against the fibrin(ogen) D-domain, using native samples without KSCN sample pretreatment.
Time course of ancrod treatment of all 12 subjects (mean, SD, 95th percentile).
(A) DesA-profibrin; (B) desAA-fibrin; (C) Mab 2B5-aFDP-D ELISA. Release of one fibrinopeptide A from fibrinogen results in the formation of desA-profibrin. DesA-profibrin is detected by combining an antibody against the neo–N-terminus of the fibrin α-chain (MAb 2B5) with an antibody against fibrinopeptide A. Infusion of ancrod results in an increase in desA-profibrin in plasma, followed by a gradual decrease, and a more shallow second peak. At increasing plasma concentrations of ancrod, less desA-profibrin is generated in favor of desAA-fibrin. The Mab 2B5 epitope appears to be accessible without KSCN sample pretreatment, as exemplified by the result of the ELISA system combining MAb 2B5 with an antibody against the fibrin(ogen) D-domain, using native samples without KSCN sample pretreatment.
Enzymun FM uses biotinylated MAb 2B5 as capture antibody and enzyme-labeled MAb 2B5 as tag. The assay includes sample pretreatment with KSCN. Target antigens are fibrin compounds carrying 2 fibrin α-chain neo–N-termini exposed by fibrinopeptide A cleavage, such as desAA-fibrin monomer.
We observed increasing concentrations of desAA-fibrin antigen until 8 hours after starting ancrod infusion (Figure 4B). The increase was not linear in the initial phase, with relatively low values in the samples drawn after 1 hour of ancrod infusion and a sharp increase toward the samples drawn after 2 hours.
A similar assay without KSCN sample pretreatment yielded no detectable antigen in all plasma samples (data not shown). In contrast, an ELISA using the combination of MAb 2B5 with an antibody against fibrinogen degradation product D (MAb 2B5–aFDP-D ELISA) without KSCN sample pretreatment showed a rapid increase in antigen with no signs of delay during the first hour (Figure 4C). Highest levels were observed 6 hours after start of ancrod infusion, followed by a gradual decrease.
After starting ancrod infusion, desA-profibrin as well as desAA-fibrin monomers are formed. As the plasma concentration of ancrod increases, the amount of desA-profibrin decreases in favor of desAA-fibrin. Both desA-profibrin as well as desAA-fibrin monomer require KSCN sample pretreatment for detection, whereas combination of MAb 2B5 with the anti–D domain antibody detects antigenic compounds without KSCN sample pretreatment. This indicates that both desAA-fibrin monomer as well as desA-profibrin form complexes, which prevent the binding of a second antibody (directed against the remaining fibrinopeptide A in desA-profibrin or against the neo–N-terminus of the α-chain in desAA-fibrin) in the assay systems without KSCN sample pretreatment.
According to the results of the MAb 2B5–aFDP-D ELISA, a rather constant proportion of fibrin complexes carries at least one accessible α-chain N-terminus, which allows binding of MAb 2B5 without prior denaturation of the fibrin compounds. This exposed binding site is necessary for the longitudinal growth of the fibrin protofibril by binding of additional fibrin monomer units.
Detection of soluble fibrin
Whereas Enzymun FM detects the fibrin monomer units irrespective of size and structure of the fibrin polymer they form, due to the chemical disaggregation step included in the assay procedure, other soluble fibrin assays rely on specific structural properties of fibrin polymers for detection.
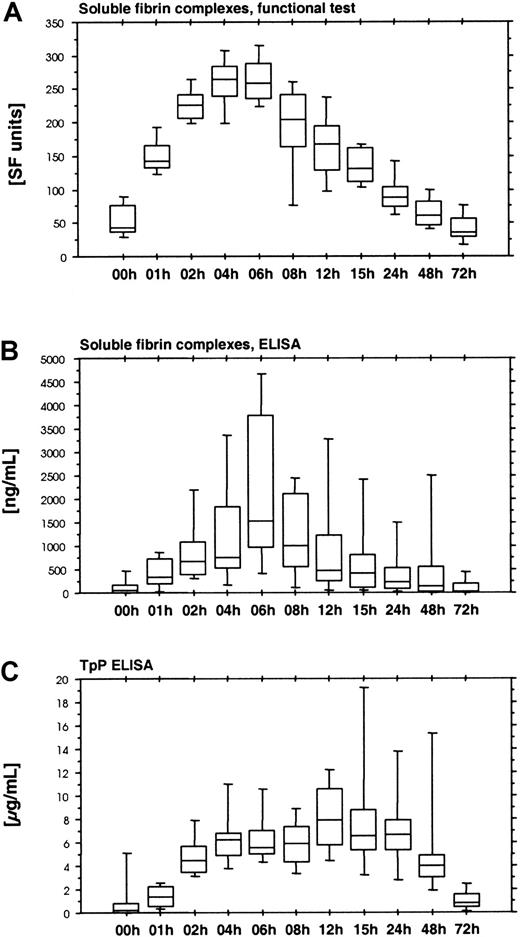
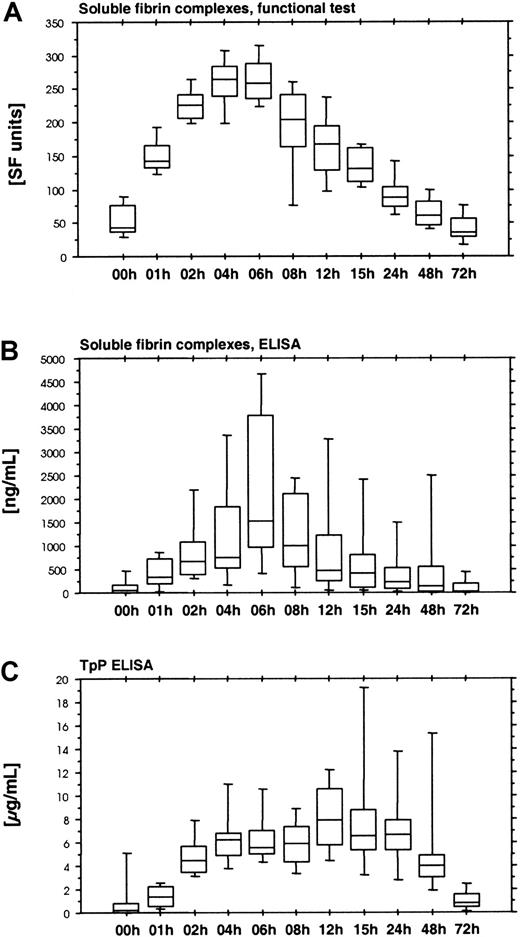
The functional soluble fiber assay (Chromogenix Coatest SF) is based on the catalytic effect of soluble fibrin complexes on the tPA-induced activation of plasminogen. This assay displayed a rapid increase during the first hours of ancrod infusion, reaching peak levels after 4 hours, followed by a gradual decrease (Figure5A). Fibrinostika SF detects an epitope within the fibrin α-chain remote from the N-terminal fibrin polymerization site. This epitope is exposed by fibrin polymerization and is identical with a major component of the tPA binding site. Fibrinostika SF assay showed a gradual increase in antigen during ancrod infusion, followed by a gradual decrease (Figure 5B). Highest values were observed at the end of the ancrod infusion. There was good correlation between Chromogenix SF and Fibrinostika SF (r = 0.82), reflecting the common relation of both assays to the process of tPA binding to soluble fibrin.
Time course of ancrod treatment of all 12 subjects (mean, SD, 95th percentile).
(A) Soluble fibrin complexes measured by Chromogenix SF. (B) Soluble fibrin complexes measured by immunologic test Fibrinostika SF. Both assays displayed a rather similar course, with a steady increase during the 6-hour ancrod infusion, followed by a gradual decrease. (C) TpP ELISA. This assay differed from the other soluble fibrin assays, showing a more extended course with a plateau between 6 and 24 hours after start of ancrod infusion, resembling the response of fibrin(ogen) degradation product assays.
Time course of ancrod treatment of all 12 subjects (mean, SD, 95th percentile).
(A) Soluble fibrin complexes measured by Chromogenix SF. (B) Soluble fibrin complexes measured by immunologic test Fibrinostika SF. Both assays displayed a rather similar course, with a steady increase during the 6-hour ancrod infusion, followed by a gradual decrease. (C) TpP ELISA. This assay differed from the other soluble fibrin assays, showing a more extended course with a plateau between 6 and 24 hours after start of ancrod infusion, resembling the response of fibrin(ogen) degradation product assays.
Another assay for polymeric fibrin is the TpP-ELISA. This assay showed a slight increase during the first hour of ancrod infusion, followed by a more rapid increase during the second hour, and a plateau between 4 and 24 hours after start of ancrod infusion (Figure 5C). This profile was different from all other soluble fibrin assays.
Fibrinogen and fibrin degradation products
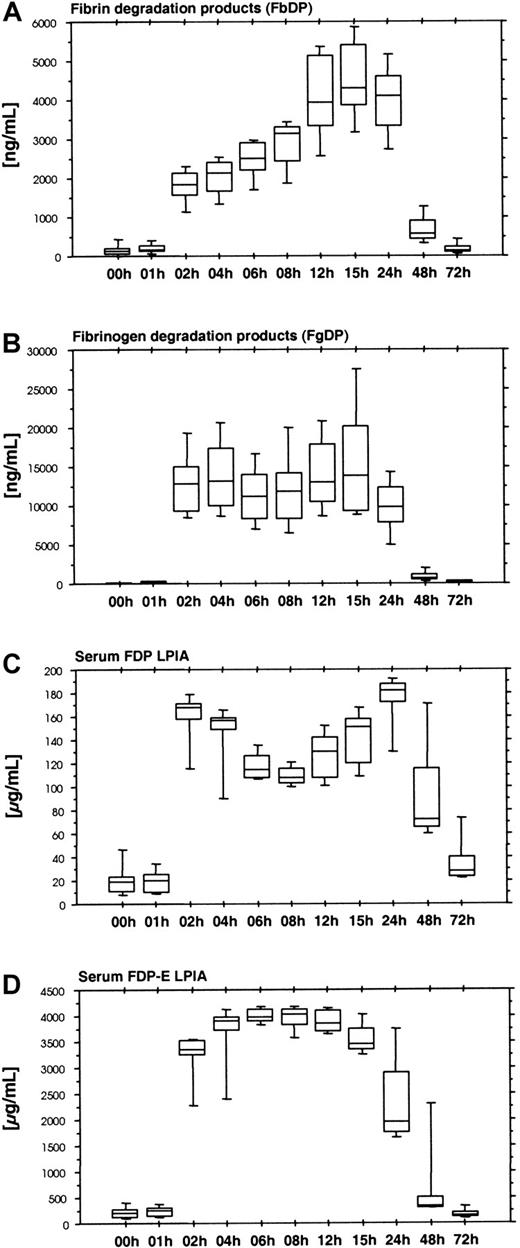
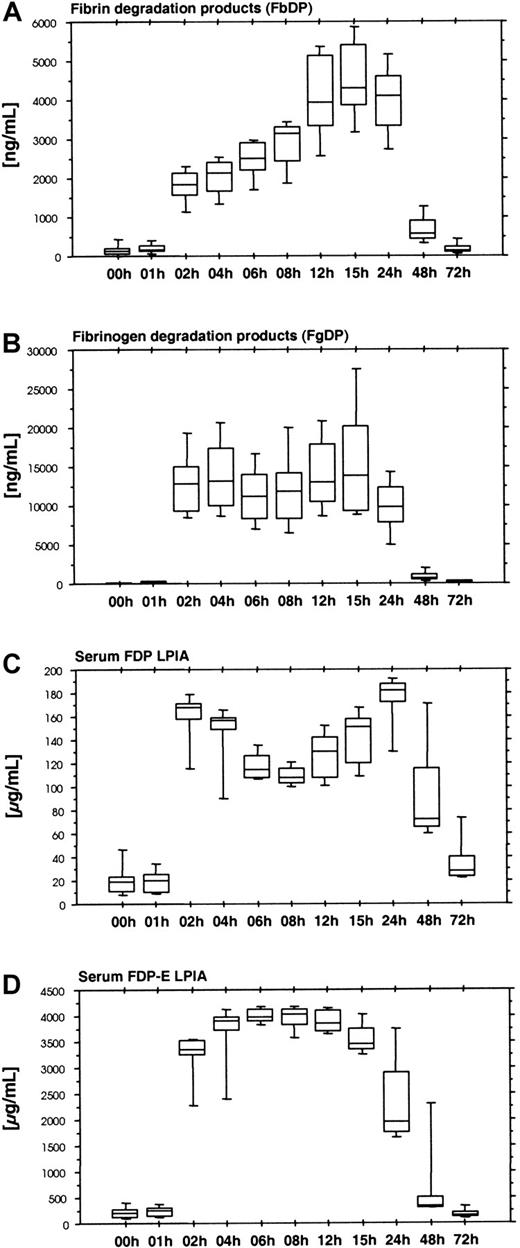
Ancrod therapy results in the formation of large amounts of soluble fibrin. Soluble fibrin displays a catalytic effect in the tPA-induced activation of plasminogen. The plasmin formed causes the degradation of fibrin and also fibrinogen. We used 2 ELISA systems: Fibrinostika FbDP for fibrin degradation products (Figure6A) and Fibrinostika FgDP for fibrinogen degradation products (Figure 6B). There was a lag phase in the formation of both FgDP and FbDP antigen, resulting in low levels of antigen in the samples obtained after 1 hour of ancrod infusion and high levels of degradation products between 2 and 24 hours after start of ancrod infusion. FbDP displayed a gradual increase between 2 and 15 hours after start of ancrod infusion, whereas FgDP showed a plateau during this period. The amount of FgDP detected by FgDP assay was about 4-fold the amount of FbDP antigen.
Time course of fibrin and fibrinogen degradation products after ancrod treatment in all 12 subjects (mean, SD, 95th percentile).
(A) Fibrin degradation products measured by Fibrinostika FbDP ELISA. (B) Fibrinogen degradation products measured by Fibrinostika FgDP ELISA. (C) Fibrinogen-fibrin degradation products measured by Roche FDP LPIA. (D) Fibrinogen-fibrin degradation product E measured by Roche FDP-E LPIA. All fibrin(ogen) degradation product assays display a lag of 1 hour after start of ancrod infusion, followed by a rapid increase at 2 hours. This indicates that fibrin(ogen) degradation is not a process directly related to the proteolytic action of ancrod and requires a threshold concentration of soluble fibrin complexes in plasma, which may be detected by the soluble fibrin assays shown above. After reaching this soluble fibrin threshold concentration, the generation of FgDP remains constant, whereas FbDP display a steady increase in plasma concentration until 15 hours after start (equals 9 hours after the end) of ancrod infusion. Low-molecular-weight fibrin(ogen) degradation products measured in serum samples (FDP LPIA and FDP-E LPIA) display high levels between 2 and 24 hours after start of ancrod infusion.
Time course of fibrin and fibrinogen degradation products after ancrod treatment in all 12 subjects (mean, SD, 95th percentile).
(A) Fibrin degradation products measured by Fibrinostika FbDP ELISA. (B) Fibrinogen degradation products measured by Fibrinostika FgDP ELISA. (C) Fibrinogen-fibrin degradation products measured by Roche FDP LPIA. (D) Fibrinogen-fibrin degradation product E measured by Roche FDP-E LPIA. All fibrin(ogen) degradation product assays display a lag of 1 hour after start of ancrod infusion, followed by a rapid increase at 2 hours. This indicates that fibrin(ogen) degradation is not a process directly related to the proteolytic action of ancrod and requires a threshold concentration of soluble fibrin complexes in plasma, which may be detected by the soluble fibrin assays shown above. After reaching this soluble fibrin threshold concentration, the generation of FgDP remains constant, whereas FbDP display a steady increase in plasma concentration until 15 hours after start (equals 9 hours after the end) of ancrod infusion. Low-molecular-weight fibrin(ogen) degradation products measured in serum samples (FDP LPIA and FDP-E LPIA) display high levels between 2 and 24 hours after start of ancrod infusion.
Two additional assays for fibrinogen/fibrin degradation products yielded rather similar results. The Roche FDP-LPIA (Figure 6C) and the FDP-E-LPIA (Figure 6D) are performed in serum samples, detecting proteolytic fibrinogen/fibrin derivatives not incorporated into fibrin clots during serum preparation. Large amounts of the respective antigens were present in the samples obtained after the initial 1-hour lag phase.
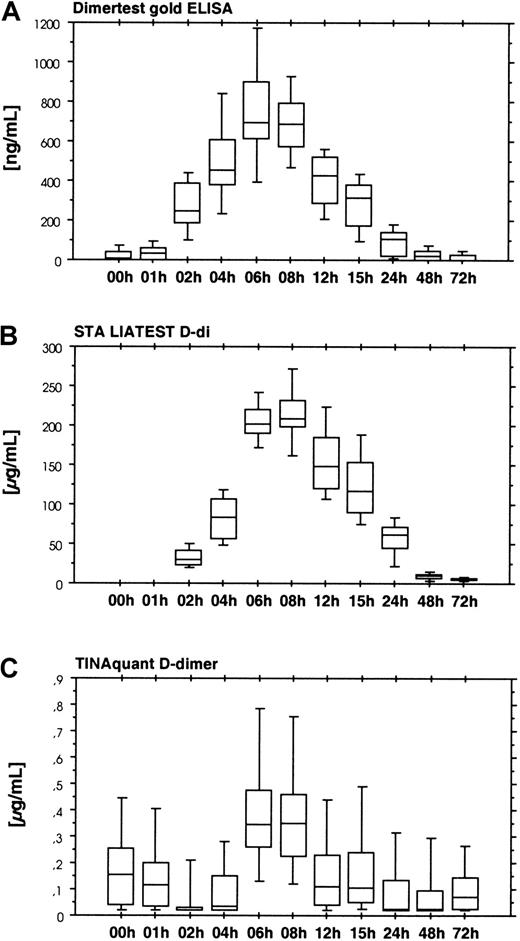
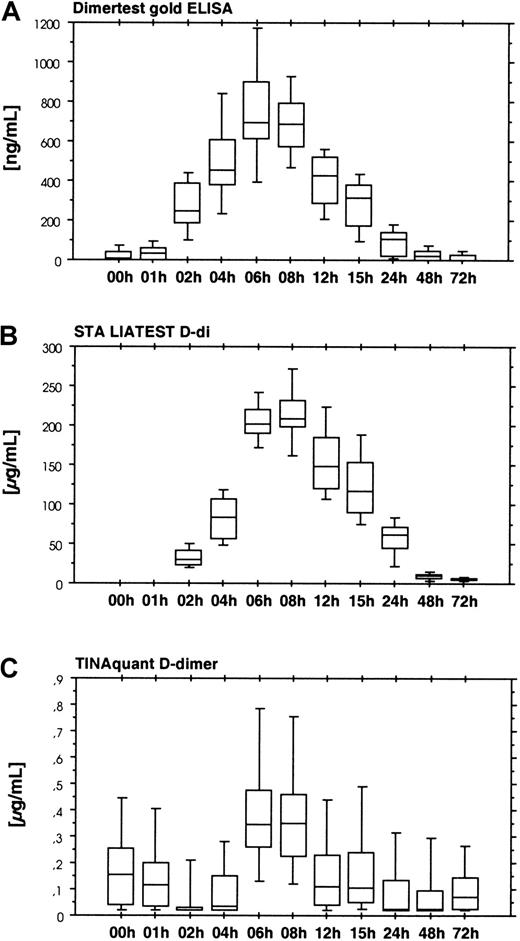
Four assays were used for the detection of cross-linked fibrin derivatives. There were 2 groups of assays. The first group consisting of the Dimertest gold ELISA (Figure 7A) and the STA LIAtest D-di (Figure 7, panel B) showed an increase in D-dimer antigen between 2 and 6 hours after start of ancrod infusion, with a lag phase similar to the fibrinogen/fibrin degradation product assays. Presence of D-dimer antigen in these assays, as well as the detection of D-dimer in the immunoblots indicates dissolution of cross-linked thrombin-induced fibrin or fibrin/fibrinogen oligomers in the course of the fibrinolytic response caused by ancrod therapy. Two additional assays for D-dimer antigen, TINAquant D-dimer (Figure 7C) and Roche D-dimer LPIA (data not shown) displayed a totally different response. Both assays failed to detect any significant increase in antigen during ancrod therapy.
Detection of cross-linked fibrin derivatives.
Results show the time course of ancrod treatment of all 12 subjects (mean, SD, 95th percentile). (A) D-dimer measured by Dimertest gold ELISA. (B) D-dimer measured by STA LIAtest D-di LPIA. (C) D-dimer measured by TINAquant D-dimer LPIA. Fibrin degradation product D-dimer is generated by plasmin degradation of factor XIIIa–cross-linked fibrin. Two D-dimer antigen assays, STA-Liatest D-di, and AGEN Dimertest gold, showed a gradual increase in D-dimer antigen in response to ancrod infusion, with highest levels 6 hours after start of infusion, followed by a gradual decrease. Similar to fibrin(ogen) degradation product assays, there was a 1-hour lag phase. Two other assays in clinical use for the detection of D-dimer antigen displayed no appreciable increase in D-dimer antigen concentration. Results of TINAquant D-dimer assay are shown in panel C. Values of both assays remained within the normal range throughout the experiment, indicating that the antigenic compounds detected by these assays are dissimilar to those of the former D-dimer antigen assays.
Detection of cross-linked fibrin derivatives.
Results show the time course of ancrod treatment of all 12 subjects (mean, SD, 95th percentile). (A) D-dimer measured by Dimertest gold ELISA. (B) D-dimer measured by STA LIAtest D-di LPIA. (C) D-dimer measured by TINAquant D-dimer LPIA. Fibrin degradation product D-dimer is generated by plasmin degradation of factor XIIIa–cross-linked fibrin. Two D-dimer antigen assays, STA-Liatest D-di, and AGEN Dimertest gold, showed a gradual increase in D-dimer antigen in response to ancrod infusion, with highest levels 6 hours after start of infusion, followed by a gradual decrease. Similar to fibrin(ogen) degradation product assays, there was a 1-hour lag phase. Two other assays in clinical use for the detection of D-dimer antigen displayed no appreciable increase in D-dimer antigen concentration. Results of TINAquant D-dimer assay are shown in panel C. Values of both assays remained within the normal range throughout the experiment, indicating that the antigenic compounds detected by these assays are dissimilar to those of the former D-dimer antigen assays.
Fibrinogen, plasminogen, and thrombin
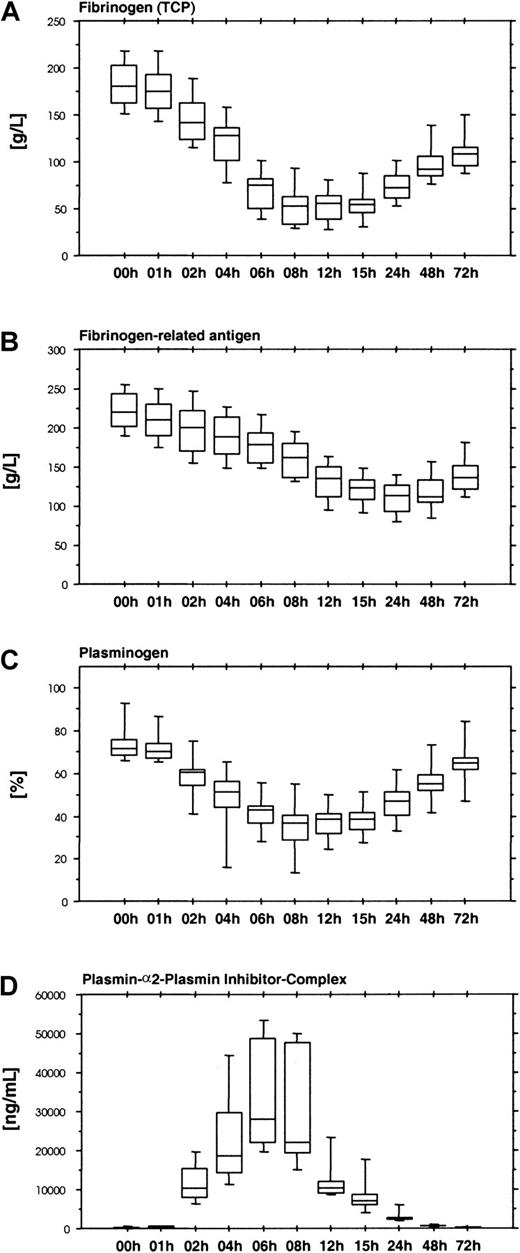
The fibrinolytic response induced by ancrod therapy results in a reduction of plasma fibrinogen levels. Large amounts of fibrinogen and fibrin degradation products cause a gap between the fibrinogen levels measured with functional fibrinogen assays (Figure8A) and immunologic assays based on polyclonal antifibrinogen antisera, which do not distinguish between intact fibrinogen and proteolytic fragments (Figure 8B).
Functional and immunologic fibrinogen assays, plasminogen, and plasmin-α2-plasmin inhibitor complex.
Results show the time course of ancrod treatment of all 12 subjects (mean, SD, 95th percentile). (A) Fibrinogen measured as total clottable protein. (B) Fibrinogen-related antigen measured with polyclonal antiserum. (C). Plasminogen measured by chromogenic assay. (D) Plasmin-α2-plasmin inhibitor complex measured by ELISA. Infusion of ancrod leads to a reduction in plasma fibrinogen concentration. Similar to the course of the fibrin(ogen) degradation product assays, the reduction in functional plasma fibrinogen concentration displayed a lag of 1 hour. Lowest values were observed between 8 and 15 hours after start of infusion. Fibrinogen-related antigen showed a more gradual decrease, with lowest levels after 24 hours. The discordance between functional fibrinogen levels and fibrinogen-related antigen can be explained by the presence of large amounts of fibrin(ogen) degradation products in plasma, which are not detected by the functional tests, but react with polyclonal antisera against fibrinogen. Plasminogen displays a decrease during ancrod infusion, mirrored by an increase in plasmin-α2-plasmin inhibitor complex concentration, indicating that reduction in plasminogen is caused by plasminogen activation.
Functional and immunologic fibrinogen assays, plasminogen, and plasmin-α2-plasmin inhibitor complex.
Results show the time course of ancrod treatment of all 12 subjects (mean, SD, 95th percentile). (A) Fibrinogen measured as total clottable protein. (B) Fibrinogen-related antigen measured with polyclonal antiserum. (C). Plasminogen measured by chromogenic assay. (D) Plasmin-α2-plasmin inhibitor complex measured by ELISA. Infusion of ancrod leads to a reduction in plasma fibrinogen concentration. Similar to the course of the fibrin(ogen) degradation product assays, the reduction in functional plasma fibrinogen concentration displayed a lag of 1 hour. Lowest values were observed between 8 and 15 hours after start of infusion. Fibrinogen-related antigen showed a more gradual decrease, with lowest levels after 24 hours. The discordance between functional fibrinogen levels and fibrinogen-related antigen can be explained by the presence of large amounts of fibrin(ogen) degradation products in plasma, which are not detected by the functional tests, but react with polyclonal antisera against fibrinogen. Plasminogen displays a decrease during ancrod infusion, mirrored by an increase in plasmin-α2-plasmin inhibitor complex concentration, indicating that reduction in plasminogen is caused by plasminogen activation.
By promoting plasminogen activation, ancrod therapy causes a decrease in plasminogen levels (Figure 8C) and the formation of plasmin-α2-plasmin inhibitor complexes (Figure 8D), with a parallel reduction in α2-plasmin inhibitor activity levels (Figure 9A). Reduction of plasminogen and α2-plasmin inhibitor and the increase in plasmin-α2-plasmin inhibitor complex display a lag similar to fibrinogen/fibrin degradation products.
Effect of ancrod therapy on α2-plasmin inhibitor, PAI-1, and prothrombin fragment F1.2
Results show the time course of ancrod treatment of all 12 subjects (mean, SD, 95th percentile). (A) α2-Plasmin inhibitor measured by chromogenic assay. (B) PAI-1 measured by ELISA. (C) Prothrombin fragment F1.2 measured by ELISA. The increase in plasmin-α2-plasmin inbibitor complex is mirrored by a reduction in plasma levels of α2-plasmin inhibitor. PAI-1 remains nearly unchanged during ancrod treatment. Ancrod infusion does not result in an increase in prothrombin fragment F1.2 levels.
Effect of ancrod therapy on α2-plasmin inhibitor, PAI-1, and prothrombin fragment F1.2
Results show the time course of ancrod treatment of all 12 subjects (mean, SD, 95th percentile). (A) α2-Plasmin inhibitor measured by chromogenic assay. (B) PAI-1 measured by ELISA. (C) Prothrombin fragment F1.2 measured by ELISA. The increase in plasmin-α2-plasmin inbibitor complex is mirrored by a reduction in plasma levels of α2-plasmin inhibitor. PAI-1 remains nearly unchanged during ancrod treatment. Ancrod infusion does not result in an increase in prothrombin fragment F1.2 levels.
The tPA levels remain constant during ancrod treatment (data not shown). PAI-1 levels displayed a minor and insignificant decrease during ancrod infusion (Figure 9B). Levels of prothrombin fragment F1.2 (Figure 9C), as well as thrombin-antithrombin complexes (TAT) (data not shown) did not increase in response to ancrod infusion, indicating the absence of prothrombin activation.
Discussion
The present study was undertaken to gain more information on the process leading to fibrinogen depletion during ancrod therapy. In the course of fibrin formation, desAA-fibrin monomers are formed from fibrinogen by the cleavage of the fibrinopeptide A from the N-termini of the Aα-chains of the fibrinogen molecule. The existence of desA-profibrin, the intermediate structure of fibrin formation carrying a single fibrinopeptide A, has been disputed.26 For detection of desA-profibrin, we developed an ELISA system, based on the soluble fibrin assay Enzymun-Test FM. In Enzymun FM, a biotinylated monoclonal antibody (MAb 2B5) against the neo–N-terminus of the fibrin α-chain is used for fixation of fibrin antigen to the walls of streptavidin-coated assay tubes. An enzyme conjugate of the same antibody is used for the detection of the antigen bound by binding to the unoccupied epitope on the other α-chain. Thus, Enzymun FM requires the presence of at least 2 accessible MAb 2B5 epitopes on a single analyte molecule, limiting the specificity of the assay to desAA-fibrin compounds. It was subsequently shown that reactivity in this assay system requires the disaggregation of the soluble fibrin compounds by incubation with strong denaturants such as KSCN,13 because the epitope is identical with a major component of fibrin polymerization site EA on the E domain of fibrin monomer. Cleavage of fibrinopeptide A exposes the epitope, but the interaction of polymerization site EA with its complementary polymerization site Da on the D domains of adjacent fibrin monomer units within the fibrin complex prevents the simultaneous binding of 2 monoclonal antibodies MAb 2B5 to each fibrin monomer. For detection of desA-profibrin, an antibody against fibrinopeptide A replaced the enzyme-labeled MAb 2B5 used in Enzymun FM.
From earlier experiments with desA-profibrin it was concluded that desA-profibrin may exist in monomeric form.14 We therefore performed the desA-profibrin ELISA with and without prior denaturation with KSCN solution. Using the assay with KSCN sample pretreatment, we found desA-profibrin to be formed during ancrod infusion. No significant amounts of desA-profibrin were detectable without KSCN sample pretreatment. This may either be the result of complex formation of desA-profibrin, making the remaining fibrinopeptide A inaccessible to antibody binding, or indicate that binding of MAb 2B5 requires denaturation of its epitope itself. Because MAb 2B5 binds to soluble fibrin compounds detected by the MAb 2B5-aFDP-D ELISA, we would favor the first theory, though improved binding of MAb 2B5 to denatured soluble fibrin compounds cannot be excluded.
DesA-profibrin displayed a rapid increase with highest values after 1 hour of ancrod infusion, whereas the formation of desAA-fibrin appeared to be more delayed, with highest values after 6 to 8 hours. DesA-profibrin decreased during further progression of ancrod infusion, with a secondary increase after termination of infusion. These results indicate that desA-profibrin is primarily formed at low plasma concentrations of ancrod, as observed in the early phase of ancrod infusion, and at the decline of plasma concentrations after the end of ancrod infusion. Due to the lack of an endogeneous inhibitor, ancrod displays a rather long half-life in vivo.27 28 At high plasma concentrations of enzyme, desA-profibrin is rapidly converted to desAA-fibrin, which is detected by Enzymun FM. The novel finding of the present study is that similar to the fibrin α-chain neo–N-termini, the remaining fibrinopeptide A of desA-profibrin is inaccessible to antibodies. Enzymatic cleavage of the second fibrinopeptide A of desA-profibrin therefore appears to occur after polymer formation, the delay between the release of the first and second fibrinopeptide A being dependent on enzyme activity.
The amount of fibrin α-chain N-terminus accessible to MAb 2B5 without prior denaturation with KSCN remains rather constant between 1 hour after start of infusion and 18 hours after end of ancrod infusion. This implies that the assay (MAb 2B5–aFDP-D ELISA) primarily detects fibrin protofibrils terminated with fibrin monomer units carrying one “open” polymerization site EA for potential longitudinal addition of further fibrin monomer units. Earlier experiments with monoclonal antibodies against the fibrin α-chain N-terminus in combination with polyspecific antifibrinogen antibodies29 30 similarly showed the presence of accessible α-chain N-terminal epitopes in soluble fibrin complexes.
The initial product of ancrod action in vivo is desA-profibrin, the product of the release of one fibrinopeptide A from fibrinogen. DesA-profibrin is converted to desAA-fibrin monomer by cleavage of the second fibrinopeptide A. Increasing the plasma concentration of ancrod results in a reduction in the ratio of desA-profibrin to desAA-fibrin, indicating more rapid conversion of desA-profibrin to desAA-fibrin monomer. Both desA-profibrin and desAA-fibrin monomer form polymeric structures. A certain proportion of these fibrin polymers carry an exposed fibrin polymerization site EA, which may react with complementary binding sites Da present on the D domains of fibrinogen as well as fibrin. Binding of fibrinogen, desA-profibrin, or desAA-fibrin monomer units results in longitudinal growth of the fibrin protofibril.
Soluble fibrin assays
Four commercially available soluble fibrin assays were compared in the present investigation. Enzymun FM detects fibrin monomer units within soluble fibrin complexes, including non–cross-linked as well as factor XIIIa–cross-linked fibrin complexes.7,13 Because ancrod does not activate factor XIII, we expected the major proportion of assay response in Enzymun FM to reflect non–cross-linked desAA-fibrin monomer. Chromogenix Coatest SF is a functional test probing the cofactor capacity of soluble fibrin compounds in the tPA-induced activation of plasminogen.17 Fibrinostika SF is an ELISA based on a monoclonal antibody against an epitope related to the binding site of fibrin for tPA, which is formed in the course of fibrin polymerization.16,31 Finally, the TpP ELISA is believed to detect a soluble fibrin structure indicating the imminent formation of insoluble clots, using a monoclonal antibody against a conformational epitope related to fibrin formation.19
The slight delay observed in Enzymun FM was not present in Chromogenix SF and Fibrinostika SF. Both assays displayed an early increase in the course of ancrod infusion, the former reaching half-maximal values 1 hour after start of infusion and maximum at 4 hours after start of infusion. In contrast to Enzymun FM, these assays appear not to distinguish between polymers of desA-profibrin and polymers of desAA-fibrin. Because Fibrinostika SF assay uses a monoclonal antibody against the fibrin α-chain C-terminus for detection of antigen bound to the anti-Aα-146-160 monoclonal antibody,15 it is to be expected that partially degraded fibrin monomer units devoid of their C-terminal α-chain appendages are not detected. Similarly, fibrin fragments X and Y (which are detected by Enzymun FM) would not be detected by this assay. Despite these limitations, there was a high degree of correlation between Fibrinostika SF and Chromogenix SF soluble fibrin, and a modest correlation of both assays with Enzymun FM, whereas correlation with TpP ELISA was low. TpP ELISA displayed a slight lag phase initially, but this was followed by a sustained elevation. Correlation of this assay was highest with Enzymun FM. From the initial description of the monoclonal antibody MH-1 used in the TpP ELISA, we did not expect to see any significant increase at all, because the authors claim that reactivity of the antibody is limited to desAABB-fibrin complexes.19 Consequently, the increase in TpP antigen either indicates the presence of soluble desAABB-fibrin complexes during ancrod treatment, cross-reactivity of the assay with desAA-fibrin complexes, or reaction of the assay with fibrin degradation products.
Fibrinogen/fibrin degradation products
An important finding of the present study is that proteolysis of fibrinogen and fibrin does not start immediately after ancrod infusion but is preceded by the formation of soluble fibrin complexes containing desA-profibrin and desAA-fibrin monomer. This delay in fibrinolytic activation also is visible in the plasma levels of plasminogen, α2-plasmin inhibitor, and especially, the plasmin-α2-plasmin inhibitor complex. In a previous investigation of Prentice and colleagues,4 this delay could not be seen because the first sample was drawn 3 hours after start of ancrod infusion.
Concentrations of fibrinogen degradation products measured by FgDP assay were about 4-fold the concentration of FbDP. Prentice and coworkers4 observed a reversal of the proportion of FgDP and FbDP in 6 patients receiving further subcutaneous doses of ancrod after completion of the 6-hour infusion, mirroring the reduction of plasma fibrinogen concentration.
As shown by the present study, treatment with ancrod also leads to the appearance of factor XIIIa–cross-linked fibrin degradation products, including fragment D-dimer, in plasma. Interestingly, the increase in D-dimer antigen levels was not detected by all D-dimer assays. Whereas Dimertest gold and STA Liatest D-di displayed high levels of D-dimer antigen, TINAquant D-dimer and Roche D-dimer LPIA failed to detect a significant increase in D-dimer during ancrod treatment. However, the generation of fibrin degradation product D-dimer during ancrod treatment was also shown by immunoblotting. The negative results of TINAquant D-dimer and Roche D-dimer LPIA thus may be the result of high concentrations of low-molecular-weight fibrinogen degradation products such as fragment D, which prevent the aggregation of antibody-coated latex particles. An alternative explanation is that these assays preferentially react with high-molecular-weight cross-linked fibrin derivatives, rather than with the low-molecular-weight fibrin fragment D-dimer.
Appearance of D-dimer and high-molecular-weight cross-linked fibrin compounds may be related to an intrinsic catalytic activity of the factor XIII zymogen,32 triggered by polymerization of desAA-fibrin. Alternatively, it could also be the result of plasmin proteolysis either of thrombin-induced particulate clots or thrombin-induced cross-linked fibrin/fibrinogen oligomers.
The delay in fibrinolytic activation after the start of ancrod infusion apparently is related to a threshold concentration of soluble fibrin complexes needed for efficient potentiation of tPA-induced plasminogen activation. This confirms earlier speculations that increased activity of tPA is of no consequence in the absence of a critical level of soluble fibrin.4
In conclusion, we have shown that the formation of soluble fibrin by ancrod involves the formation of desA-profibrin, which participates in polymer formation. Extension of the fibrin polymers occurs by binding of additional fibrinogen, desA-profibrin, or fibrin monomer units to exposed polymerization site EA of the polymer, indicating that the terminal structure of the growing polymer structures is desAA-fibrin. Early soluble fibrin complexes significantly enhance tPA-mediated plasminogen activation, leading to degradation of ancrod-induced desAA-fibrin, fibrinogen, and thrombin-induced desAABB-fibrin.
Supported by Knoll AG, Lugwigshafen, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carl-Erik Dempfle, Universitätsklinikum Mannheim, I. Med. Klinik, Theodor Kutzer Ufer 1-3, D-68167 Mannheim, Germany; e-mail: dempfle@verw.ma.uni-heidelberg.de.