Abstract
Anaplastic large cell lymphoma (ALCL) is a distinct entity of non-Hodgkin lymphoma, characterized by a proliferation of pleomorphic large lymphoid cells that express CD30. Recent studies have found that a subset of ALCL aberrantly expresses a chimeric anaplastic lymphoma kinase (ALK) protein as a result of t(2;5)(p23;q35) or variant translocations. ALK-positive ALCLs feature good prognosis, but some of them lead to poor outcomes. Since CD56 is expressed in some ALCLs, its clinical significance was examined in a series of T/null cell type ALCLs. Of 143 patients, 83 (58%) showed ALK-positive staining, and of 140 patients, 25 (18%) expressed CD56. The ALK-positive subgroup was characterized by a younger age of onset (P < .0001), lower serum lactate dehydrogenase level (P = .01), better performance status (P = .03), less frequent extranodal involvement (P = .01), lower international prognostic index (IPI) categories (P = .002), and superior survival (P = .0009) in comparison with the ALK-negative group, suggesting that ALK is a specific marker defining a distinct subtype. CD56+ cases showed a significantly poor prognosis overall (P = .002) as well as in both ALK-positive and ALK-negative subgroups (P = .02 andP = .04, respectively). Multivariate analysis confirmed that CD56 is independent of other prognostic factors, including IPI. Although CD56+ cases showed a higher incidence of bone involvement, no other differences in clinicopathologic parameters were found between the CD56+ and CD56− groups. These findings suggest that CD56 is not a marker to identify a distinct subtype of ALCL, but a strong clinical prognostic factor. Effective therapeutic approaches should be explored for high-risk ALCL patients, who can be identified by means of a prognostic model, including CD56.
Introduction
Anaplastic large cell lymphoma (ALCL) was first described by Stein et al1 as a large-cell non-Hodgkin lymphoma (NHL) characterized by a bizarre morphology that often shows intrasinusoidal and paracortical infiltration of lymph nodes. The tumor cells of ALCL express CD30 antigen, which is also expressed on Reed-Sternberg cells in Hodgkin disease (HD) and on a subset of various T-cell neoplasms.1-3 Both the B- and T/null cell type ALCLs were initially recognized in the updated Kiel Classification,4,5 but only T/null cell type ALCL has been included in the Revised European American Lymphoma (REAL) classification6 and the World Health Organization (WHO) classification.7 Although some morphological variants were proposed afterward, ALCL has been recognized as a distinct disease entity.
A nonrandom chromosomal translocation t(2;5)(p23;q35) has been reported in ALCL.8-10 This translocation has been cloned and shown to result in the fusion of the NPM gene on chromosome 5 and the ALK gene on chromosome 2, resulting in the expression of an aberrant fusion protein, p80NPM/ALK.11,12The polyclonal antibody against p80NPM/ALK, which recognizes anaplastic lymphoma kinase (ALK),13 and the subsequently established monoclonal antibodies ALK114 and ALKc15 have made it possible to further categorize ALCL as an entity separate from HD, lymphomatoid papulosis, and primary cutaneous ALCL.16-23 Accumulated evidence, such as immunohistochemical, cytogenetic, and reverse genetic detection, also supports the recognition of ALK-positive ALCL as a distinct subtype with a much younger age distribution, nodal predilection, and good prognosis.14,15,18-21 24-32 However, these issues are as yet only marginally dealt with within the REAL/WHO classifications because they have not been sufficiently confirmed by data from large series of ALCL cases.
A further issue is the expression of CD56, a neural cell-adhesion molecule, which is expressed on natural killer (NK) cells and a subset of T cells and monocytes.33,34 Its expression is well recognized in hematolymphoid malignancies of NK-cell lineage,35,36 but also in some cases of acute myeloid leukemia (AML). CD56 expression has been found to be a risk factor for AMLs with t(8;21) and t(15;17),37 38 but its significance in malignant lymphomas other than those of NK-cell lineage awaits further clarification. For this study, we investigated 143 cases of T/null cell type ALCL to determine the biologic and prognostic significance of p80/ALK and CD56 for the category of ALCL.
Patients, materials, and methods
Patient selection
From the patient records of Aichi Cancer Center and collaborating institutions, 143 patients with ALCL of T/null cell phenotype were identified. These included 42 patients of cytotoxic-molecule–positive ALCL previously reported by us.39 All specimens were obtained at the initial presentation of the patients and were reviewed by 2 independent pathologists (T.S. and S.N.). Patients meeting the original criteria of Stein et al,1 supplemented by the description of Suchi et al,5 were enrolled in this study. Excluded were those occasional patients whose primary diagnostic material was not optimal for the identification of features relevant to this series, including minute biopsy specimens, tissues with extensive necrosis, and tissue materials used for rapid frozen-section diagnosis. Patients with ALCL of B-cell phenotype, primary cutaneous ALCL, and secondary ALCL were also excluded from this study, as were patients with retrovirus (human T-cell leukemia/lymphoma virus type 1 and human immunodeficiency virus) infection. The patients' records and clinical data were investigated retrospectively.
Histopathology
Tissue was fixed in 10% formalin and embedded in paraffin. Sections (5 μm thick) were stained with hematoxylin and eosin, periodic acid–Schiff, Giemsa, and Gomori silver impregnation.
Immunophenotypic study
Immunoperoxidase studies for the following antigens were performed on the formalin-fixed, paraffin-embedded sections by means of the avidin-biotin peroxidase complex method.40 The antibodies comprised Ber-H2/CD30 (Dako, Santa Fe, CA), LeuM1/CD15 (Becton Dickinson, Sunnyvale, CA), L26/CD20 (Dako), CD79a (Dako), UCHL1/CD45RO (Dako), MT1/CD43 (Bio-Science Products, Emmenbrucke, Switzerland), CD3 (Dako), CD4 (Novocastra Laboratories, Newcastle, UK), CD8 (Dako), E29/EMA (Coulter, Hialeah, FL), CD56 (Novocastra), Leu7/CD57 (Becton Dickinson), LMP-1 (Dako), DO-7/p53 (Dako), BCL-2 (Dako), TIA-1 (Coulter), granzyme B (Monosan, Uden, The Netherlands), βF1 (T Cell Science, Cambridge, MA), ALK1 (Dako), and p80 (courtesy of S. Mori, University of Tokyo, Japan). Detection of Epstein-Barr virus (EBV) small RNAs by means of in situ hybridization using EBV-encoded small RNA (EBER) oligonucleotides was also performed on formalin-fixed paraffin-embedded sections by means of the Dako hybridization kit with a cocktail of fluorescein-isothiocyanate–labeled EBER oligonucleotides (one oligonucleotide corresponding to EBER-1 and one to EBER-2, both 30 bases long).41 The cell lineage of each case was identified as previously described.28 Briefly, cases were classified as T lineage if they reacted with one or more antibodies against the T-cell antigens CD45RO, CD43, and CD3 and lacked reactivity for the B-cell–associated antigens CD20 and CD79a. They were classified as B-lineage if the opposite pattern of reactivity was observed. A null phenotype was assigned to cases that did not express either T- or B-cell–associated markers.
Statistical analysis
Correlation between the 2 groups was examined with the χ2 test, the Fisher exact test, the Student ttest, and the Mann-Whitney U test. Patient survival data were analyzed with the Kaplan-Meier method and were compared by means of the log-rank test. Univariate and multivariate analyses were performed with the Cox proportional hazard regression model, and variables were selected with the stepwise method. Data were analyzed with the SAS system (SAS Institute Inc, Cary, NC).
Results
ALK immunohistochemistry and histopathological features
With the use of p80 and/or ALK1 antibodies, 83 of the 143 patients (58%) were shown to have ALK-positive ALCL cases, 1 of which showed nuclear-restricted ALK staining and 49 of which showed nuclear-positive cytoplasmic staining (ALK-N/NC), suggesting that the tumor cells of these cases harbored the NPM-ALK chimeric protein.42Another 25 cases displayed cytoplasmic-restricted staining (ALK-C), which indicated that the ALK gene may remain intact or may fuse with genes other than NPM. The staining pattern (ALK-N/NC vs ALK-C) could not be determined for the remaining 8 cases, mainly owing to the unsuitable condition of the paraffin blocks.
Histologically, 128 patients were categorized as classical type ALCL, 11 as HD-like ALCL, and 4 as lymphohistiocytic (LH)/small-cell (SC) variants. All of the 4 LH/SC variants showed ALK expression, but only 1 of the 11 HD-like ALCL types did. The diagnosis of HD-like ALCL was based on the histological appearance. All of these 11 cases showed sinusoidal involvements and a cohesive growth pattern of neoplastic cells, which led to a diagnosis of NHL rather than HD. These cases also had occasional Hodgkin/Reed-Sternberg–like cells, the absence of which would result in a diagnosis of classical or common type ALCL.
Clinical features
There were 97 males and 44 females with an age range from 1 to 85 years (median age, 32 years). Patients' clinical characteristics and subgroups according to ALK expression are summarized in Table1. The ALK-positive group showed a dramatically younger age distribution (mean: 25.0 ± 17.6 vs 50.6 ± 20.6 years). ALK-negative cases were male predominant, although the difference was not statistically significant. No differences in stage or B symptoms between ALK-positive and ALK-negative subgroups were observed. In ALK-positive cases, the performance status (PS) showed significantly better distribution (P = .03), and the serum lactate dehydrogenase (LDH) level was lower (P = .01). Most of the patients in both groups showed nodal presentation of the lymphoma, but the incidence of extranodal involvement was significantly higher in the ALK-negative group (P = .01). The incidence of BM or skin involvement tended to be higher in the ALK-negative group, and that of bone disease higher in the ALK-positive group, although the difference was not significant. The incidence of extranodal involvement at 2 or more sites did not show any difference. The international prognostic index (IPI) categories of the ALK-positive group showed lower distribution than those of the ALK-negative group (P = .002).
Expression of phenotypic markers and cytotoxic molecules
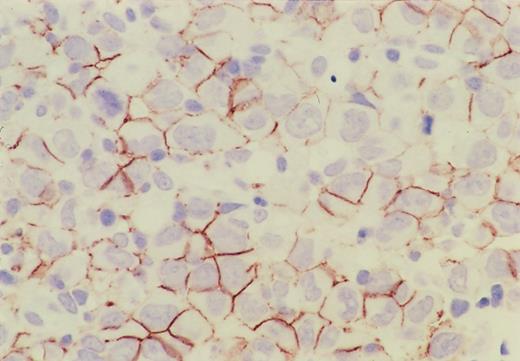
The results are summarized in Table2 and categorized according to ALK-positive and ALK-negative subgroups. Immunohistochemical profile of a CD56-positive case is shown in Figure1. All cases but 1 were positive for CD30, and CD56 was positive in 13 of 81 cases (18%) of the ALK-positive group and in 12 of 59 cases (20%) of the ALK-negative group, so that the incidence of expression was almost the same. None of the ALK-positive group showed any expression of CD15, BCL-2, or EBV, but most of them were positive for epithelial membrane antigen (EMA). On the other hand, the expression of these markers was somewhat heterogeneous for the ALK-negative group, resulting in a statistically significant difference between these 2 groups. In HD-like ALCL, the expression of CD15 was found in 4 out of 10, the expression of EMA in 7 out of 9, and the presence of EBV in 2 out of 10 cases. Of the 4 CD15+ cases, 3 were also positive for EMA; the remaining case was not examined for EMA. Only one case showed simultaneous expression of CD15 and EBV, but the neoplastic cells were also positive for EMA and CD45RO. The expression of cytotoxic molecules (TIA-1 and granzyme B) was significantly higher in the ALK-positive group (P < .0001 and P = .007, respectively).
Immunohistochemistry of CD56 in ALCL.
CD56 is expressed on the cell surface membrane of the lymphoma cells, and its expression is more intense on the adjacent membrane of neighboring cells.
Immunohistochemistry of CD56 in ALCL.
CD56 is expressed on the cell surface membrane of the lymphoma cells, and its expression is more intense on the adjacent membrane of neighboring cells.
Therapeutic response and prognosis
The treatment consisted of chemotherapeutic regimens containing doxorubicin for 125 patients and without doxorubicin for 9. Five patients with stage I disease did not receive chemotherapy and were treated with radiation or operative resection alone; the 3 patients who had not received any therapy because of their poor PS died of the disease; and 1 patient was lost to follow-up before receiving any therapy. In total, 100 of the 139 patients (71.9%) attained complete remission, and 18 (12.9%) partial remission. Therapeutic response was significantly better for the ALK-positive group (P = .009, Mann-Whitney U test).
The overall survival curves of ALK-positive and ALK-negative ALCLs, shown in Figure 2A, demonstrate a significantly better survival for ALK-positive ALCLs (P = .0009). The ALK-negative group showed no differences in survival between HD-like and common ALCL. The ALK-N/NC and ALK-C groups showed almost identical survival (Figure 2B).
Overall survival of ALK-positive and ALK-negative ALCL.
(A) ALK-positive ALCL shows significantly better prognosis (P = .0009). (B) No difference is seen in the pattern of ALK positivity (nuclear/nuclear + cytoplasmic vs cytoplasmic) (P = .61).
Overall survival of ALK-positive and ALK-negative ALCL.
(A) ALK-positive ALCL shows significantly better prognosis (P = .0009). (B) No difference is seen in the pattern of ALK positivity (nuclear/nuclear + cytoplasmic vs cytoplasmic) (P = .61).
Comparison of CD56+ and CD56− cases
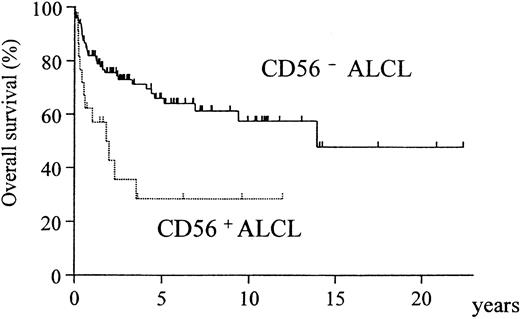
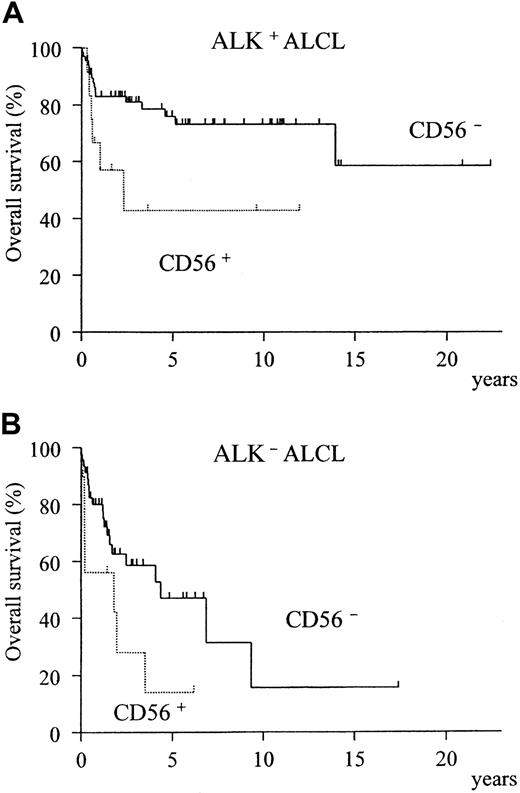
A comparison of the clinical characteristics of CD56+and CD56− cases is summarized in Table3. Although CD56+ cases showed a significant preponderance of bone disease (P = .005), none of the other clinical factors or the expression of phenotypic markers and cytotoxic molecules registered any significant difference between the CD56+ and CD56− groups (Table 4). However, the overall survival was significantly different, with the CD56+ cases showing a much poorer prognosis (Figure3, P = .002). In both the ALK-positive and ALK-negative subgroups, CD56+ cases showed a poorer prognosis than CD56− cases (Figure4A-B).
Overall survival of CD56+ and CD56− ALCL cases.
The CD56+ group has a significantly worse prognosis (P = .002).
Overall survival of CD56+ and CD56− ALCL cases.
The CD56+ group has a significantly worse prognosis (P = .002).
Prognostic difference between CD56+ and CD56− ALCL according to ALK expression.
The CD56+ group shows a significantly lower survival for both ALK-positive (A, P = .02) and ALK-negative (B,P = .04) subtypes.
Prognostic difference between CD56+ and CD56− ALCL according to ALK expression.
The CD56+ group shows a significantly lower survival for both ALK-positive (A, P = .02) and ALK-negative (B,P = .04) subtypes.
Prognostic factors for ALCL
Univariate Cox analysis identified the following prognostic factors: age, clinical stage, PS, ALK expression, CD56 expression, EBV positivity, serum LDH level, presence of B symptoms, extranodal involvement of more than one site, and IPI (Table5). Multivariate analysis excluding IPI categories showed age older than 60, advanced stage (III or IV), CD56 positivity, and PS greater than one to be significant and independent prognostic factors (Table 5).
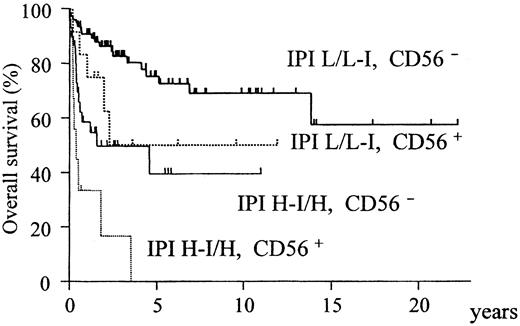
When the IPI was included instead of its constitutive factors, only IPI (relative risk [RR] = 4.0; confidence interval [CI], 2.2-7.2;P = .00001) and CD56 (RR = 2.6 CI; 1.3-5.0;P = .004) were identified as independent and significant prognostic factors. According to these findings, all patients were divided into 4 groups with different prognoses on the basis of IPI and CD56 (Figure 5).
Overall survival of all ALCL patients stratified according to CD56 expression and IPI category.
The CD56+ and IPI high-intermediate/high subgroups have an extremely poor prognosis (P < .0001).
Overall survival of all ALCL patients stratified according to CD56 expression and IPI category.
The CD56+ and IPI high-intermediate/high subgroups have an extremely poor prognosis (P < .0001).
Discussion
In our series of ALCL patients, clear clinicopathologic differences were found between ALK-positive and ALK-negative subtypes, which is consistent with most of the studies in the literature.24 30-32 ALK-positive ALCLs are characterized by a younger age distribution, lower serum LDH level, better PS, less frequent extranodal involvement, lower IPI categories, and better prognosis. Although we could demonstrate that the expression of CD56 on the lymphoma cells is an independent prognostic factor for T/null cell type ALCL, the clinical manifestations of CD56+ and CD56− ALCLs were quite similar. This suggests that CD56 expression is not a relevant factor for the identification of a novel subtype of ALCL but a purely clinical risk factor.
Initial investigation did not identify the IPI risk category as prognostic for ALCL,43 but recent studies with large populations have shown that the IPI is highly prognostic for ALCL.30-32 Our study also confirmed the prognostic significance of IPI for T/null cell type ALCL. In our study, however, multivariate analyses did not identify ALK expression as an independent prognostic factor, but this does not contradict the fact that ALK-positive ALCL is a distinct subtype, because the expression of ALK is closely correlated with age and IPI. It is therefore not fruitful to discuss whether age or ALK expression is more prognostically significant for ALCL, since these 2 factors are interrelated and have the same impact on the prognosis for ALCL. When age or the IPI was not included in the multivariate analysis models, ALK instead of age or the IPI was identified as prognostic in both models. These results confirm that ALK-positive ALCL constitutes a distinct entity.
CD56 has been well documented as being expressed in a variety of NK-cell neoplasms,44-47 but it is also expressed in hematolymphoid malignancies other than those of NK-cell lineage, ie, AML,37,38,48,49 acute lymphoblastic leukemia of both T- and B-cell lineage,49 and some types of T- and B-cell lymphoma.50-53 Its expression is rare in diffuse large B-cell lymphoma,54,55 but common and well investigated in multiple myeloma.56,57 CD56 is expressed mostly on myeloma cells in bone marrow but less frequently on those in peripheral blood (plasma cell leukemia) or extramedullary sites (plasmacytoma), suggesting its adhesive function to bone marrow stroma cells.58-60 In ALCL, Felgar et al61 detected CD56 expression in 8 of 17 cases (47%) of T/null cell type ALCL, but Krenacs et al62 reported much lower frequency of CD56+ cases (1 of 32), and Foss et al63 no CD56 expression in 13 cases. In our larger series, CD56 was expressed in 25 of 140 cases (17%) and was shown to be a prognostic factor. The expression of CD56 has also been shown to be a risk factor in AMLs with t(8;21) and t(15;17),37,38 but is controversial in multiple myeloma. In an early report by Van Camp et al,56CD56− patients were shown to have aggressive clinical courses, but this might simply represent the tumor cell localization of myeloma cells. Mathew et al64 reported similar survival curves for CD56+ and CD56− myeloma, whereas Garcia-Sanz et al65 showed poorer prognosis of myeloma cases with high CD56+ CD3-plasma cells in peripheral blood. For none of the specific entities of malignant lymphoma have any prognostic implications of CD56 expression been argued. The expression of CD56 might also be a prognostic factor for other types of hematolymphoid malignancy. The clinical and prognostic significance of CD56 expression in various types of leukemias and lymphomas therefore deserves to be examined.
The reason CD56 functions as a prognostic factor in ALCL remains unclear. Although CD56 is a neural cell adhesion molecule, the frequency of extranodal involvements for the CD56+ and CD56− groups was not different in our ALCL cases. The expression of CD56 is associated with disease localization in multiple myeloma,58-60 but did not correlate with extramedullary involvement in AML studies with large numbers.48,49 The role of CD56 might be different in different subtypes of hematolymphoid malignancy. Integrin β1, an adhesion molecule that interacts with the extracellular matrix, was recently shown to mediate the anti-apoptotic signal resulting in drug resistance of small-cell lung cancer cells.66 CD56 is a homophilic-binding adhesion molecule, and its expression often appears to be more intense on the adjacent membrane of neighboring cells. It is therefore possible that CD56 also mediates certain intercellular signals and functions as an adverse prognostic factor in various hematolymphoid malignancies, as well as in those with NK-cell lineage.
From the viewpoint of lymphoma classification, HD-like ALCL is not a well-defined entity, although it was included in the REAL classification as a provisional entity.6 A workshop report on HD and related diseases67 and the recently published WHO classification7 emphasized that HD-like ALCL should be separated into T-lineage ALCL and B-lineage HD. Expression of T-cell–related antigens (CD3, CD43, CD45RO) or EMA is strongly in favor of a diagnosis of ALCL; the presence of B-cell markers (CD20) EBV or CD15 favors a diagnosis of HD. For our 11 cases of HD-like ALCL, 4 were positive for CD15. However, 3 of the 4 CD15+ cases were also positive for EMA, and 2 coexpressed T-lineage antigens (CD45RO and CD4, respectively). Phenotypical results do not conflict with the inclusion of these HD-like cases in a category of ALCL. CD15 and EBV were also found, respectively, in 7 and 8 cases without HD-like appearance, indicating that the presence of CD15+ or EBV-positive cases in the ALK-negative group were not because of the inclusion of HD-like ALCLs. The proportions of CD15+ or EBV-positive cases in common and HD-like ALCLs are consistent with those reported in a previous study by Zinzani et al,68although B-cell type ALCLs were also included in their study. In addition, no prognostic differences were found for the HD-like cases in the ALK-negative ALCL, so that we have included the HD-like cases in this study. However, the possibility that these HD-like ALCLs may represent tumor-cell–rich cases of HD deserves further investigation before a strict border is drawn between ALCL and HD.
In our study, 65 of 81 (85.0%) ALK-positive ALCL cases and 30 of 57 (52.6%) ALK-negative ALCL cases expressed cytotoxic molecules, granzyme B, and/or TIA-1. This is consistent with the observations by others of high-frequency cytotoxic molecule expression in ALCL and suggests a possible derivation of ALCL from cytotoxic T cells.30 61-63 In our cases, no differences between cytotoxic-molecule–positive and cytotoxic-molecule–negative cases, including differences in clinicopathologic features and prognosis, could be identified (data not shown). The negative cases, however, especially those in the ALK-positive group, may express cytotoxic molecules other than TIA-1 or granzyme B. Further investigations are needed to determine the origin of these cytotoxic-molecule–negative ALCLs.
Recently, Falini et al42 established that the staining pattern of ALK is defined by the chimeric partner of ALKgene as a result of its oncogenic translocations. They showed that 44 of 59 ALK-positive ALCL cases (75%) possess NPM-ALK and 15 to have variant ALK chimera. ALK-N/NC staining means that lymphoma cells have an NPM-ALK fusion protein as a result of t(2;5)(p23;q35), whereas ALK-C staining is derived from other variant ALK fusion and 2p23 abnormalities.14,69-73 Some of the fusion partners of ALK in these variant translocations have recently been cloned and identified as TPM3, TFG, AITC, andCLTCL genes.74-79 Although these 4 genes have no homologous region or function, they were fused to the ALKgene at a similar break point, at just 3′ of the transmembrane region. As a result, this transmembrane portion was lost in the x-ALK chimeric products, whereas the tyrosine kinase domain was preserved. These findings suggest that the oncogenic event accounting for these 2p23 translocations is the deregulation of the aberrant ALK gene expression by the promoter/enhancer of the fusion partners. We determined that 50 of 75 cases (67%) showed ALK-N/NC staining, but could not identify any clinicopathologic, immunophenotypic, or prognostic differences between ALK-N/NC and ALK-C groups. Our result suggests that the staining pattern of ALK does not define a distinct subtype. This is consistent with the speculation that the oncogenicity of the aberrant ALK expression is not affected by the fusion partners.
Several recent studies have been performed on the basis of age categorization as pediatric80,81 or adult.32,67 82 In our ALK-positive ALCL cases, however, both pediatric and adult patients showed identical clinicopathologic characteristics. The entity of ALK-positive ALCL therefore transcends the arbitrary boundaries of 15 or 20 years of age, so that there seems to be no good reason to divide this disease into 2 age categories, pediatric and adult, for a more accurate understanding of the disease. Pediatric and adult cases tended to be treated with different therapeutic protocols, however, mainly owing to the physician's specialization, either pediatrics or internal medicine. The appropriate therapeutic approach for ALCL should be investigated from the viewpoint of a continuous spectrum of ALCL, at least for children and adolescents/young adults. A prospective clinical trial is needed to explore an effective therapeutic approach for ALCL. For this, we recommend that both pediatric and adult patients be treated with a consistent strategy.
In conclusion, we propose that for clinical studies of ALCL, CD56 expression as well as the IPI should be included in the prognostic factors used for patient stratification.
Acknowledgments
We thank H. Ishida and Y. Tokoro for technical assistance, and the collaborators from the following institutions for providing the patients' data and specimens: National Sapporo Hospital; Sapporo Municipal Hospital; Akita University School of Medicine; Japanese Red Cross Ashikaga Hospital; Gunma University School of Medicine; Kitazato University School of Medicine; Tsukuba University School of Medicine; Saitama Cancer Center; Chiba University School of Medicine; Hamamatsu Medical School; Seirei Hamamatsu Hospital; Iida Municipal Hospital; Takaoka Hospital; Toyama Central Hospital; Toyohashi Municipal Hospital; Japanese Red Cross Nagoya First Hospital; Aichi Prefectural Hospital; Okazaki Municipal Hospital; Kariya General Hospital; Kousei Hospital; Tokoname Municipal Hospital; Ichinomiya Municipal Hospital; Nagoya University School of Medicine; Nagoya City University School of Medicine; Higashi Municipal Hospital; Nagoya Ekisaikai Hospital; Nagoya Memorial Hospital; National Nagoya Hospital; National Higashi Nagoya Hospital; Aichi Medical School; Showa Hospital; Gifu Municipal Hospital; Yokkaichi Municipal Hospital; Mie University School of Medicine; Suzuka Central General Hospital; Fukui Saiseikai Hospital; Youka Hospital; National Kyoto Hospital; National Osaka Hospital; Chugoku-chuou Hospital; Okayama Saiseikai Hospital; Okayama Rousai Hospital; Japanese Red Cross Okayama Hospital; Mitoyo General Hospital; Fukuyama National Hospital; Kawasaki Medical School; Japanese Red Cross Takamatsu Hospital; Fukuoka University School of Medicine.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ritsuro Suzuki, Division of Molecular Medicine, Aichi Cancer Center, 1-1 Kanokoden, Chikusa-ku, Nagoya 464-8681, Japan; e-mail: rsuzuki@aichi-cc.pref.aichi.jp.