Abstract
The common gamma-chain (γc) is a component of the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15 and is essential for their signal transduction. Western blotting and a newly established enzyme-linked immunosorbent assay detected substantial constitutive levels (50-250 ng/mL) of soluble γc (sγc) in sera of murine inbred strains. It was demonstrated that purified immune cells, such as T, B, and natural killer cells, and macrophages released this protein after activation. Transfection experiments with cDNA encoding the full-length γc showed that shedding of the transmembrane receptor led to the release of sγc. The shedding enzymes, however, appeared to be distinct from those cleaving other cytokine receptors because inhibitors of metalloproteases (eg, TAPI) did not influence sγc release. In vivo, superantigen-induced stimulation of T cells enhanced sγc serum concentrations up to 10-fold within 6 hours. Because these findings demonstrated regulated expression of a yet unknown molecule in the immune response, further experiments were performed to assess the possible function(s) of sγc. A physiological role of sγc was indicated by its capacity to specifically inhibit cell growth induced by γc-dependent cytokines. Mutational analysis revealed that the C-terminus and the WSKWS motif are essential for the cytokine inhibitory effect of the sγc and for binding of the molecule to cytokine receptor-expressing cells. Thus, competitive displacement of the transmembrane γc by excess sγc is the most likely mechanism of cell growth inhibition. It was implied that naturally produced sγc is a negative modulator of γc-dependent cytokines.
Introduction
The murine common gamma-chain (γc) is a 64-kd type 1 transmembrane protein of the cytokine receptor family.1 Although the γc alone is unable to bind to cytokines, it has been shown to be an essential component of the cell surface receptor complexes of IL-2, IL-4, IL-7, IL-9, and IL-15.1 Interaction of the respective cytokines with the γc within the receptor complexes results in the activation of the Janus kinase JAK3,2 which subsequently interacts with Pyk2, a member of focal adhesion tyrosine kinases, leading to the phosphorylation of this protein.3 Recent studies have shown that the γc is critical for lymphoid development because genetic defects of either this molecule4 or JAK35 in humans or targeted deletions of the γc6,7 or JAK38-10 in mice severely impair the development of T and B cells, leading to severe combined immune deficiency syndromes (SCID).11
Soluble cytokine receptors and growth factor receptors are present as immunomodulatory molecules in body fluids of humans and mice (reviewed in references 12 and 14). Two major nonexclusive mechanisms are responsible for the generation of soluble cytokine receptors: proteolytic cleavage of transmembrane receptors catalyzed mostly by metalloproteases15-19 or de novo synthesis of alternatively spliced mRNAs encoding soluble receptor molecules lacking a transmembrane domain.20-28
Soluble cytokine receptors appear to be potent regulators of cytokine activities. Interference with the binding of cytokines to their membrane receptors and, thus, inhibition of cytokine signaling has been demonstrated to occur in vitro and in vivo.13,14,29 On the other hand, several soluble cytokine receptors have been shown to intensify the activity of their respective cytokines in vivo—eg, by acting as carrier molecules—and to protect the cytokine against proteolytic cleavage30 or by forming agonistic complexes with their ligands able to interact with other signal-transducing molecules on cells.31 32
No reports concerning a murine soluble γc (sγc) have been published so far. In this study we investigated (1) whether sγc is present in sera of mice, (2) which cells are able to release this molecule, and (3) what mechanism(s) are responsible for the production of sγc. To assess the putative physiological function of the sγc, we further analyzed (4) its effects on cytokine-induced cell proliferation and (5) generated recombinant, mutated forms of the sγc to address the structure–function relationship of this molecule.
Our findings imply that the newly identified sγc of the mouse is released by several cell types of the immune system after activation and might be an important negative and highly selective regulator of cytokine responses.
Materials and methods
Mice and parasites
Female mice of the inbred strains BALB/c, C57BL/6, AKR/J, FVB/N, and BALB/c-SCID were obtained from Charles River breeding laboratories (Sulzfeld, Germany). Gld mice were kindly provided by Dr H. Körner from our institute. ICR mice were obtained from RCC Ltd (Füllinsdorf, Switzerland), and γc−/− mice6 and sex- and age-matched control animals were a generous gift from Dr W. Müller (Institute of Genetics, University of Cologne, Germany). RAG2−/−mice33 were a gift from Dr B. Arnold (DKFZ, Heidelberg, Germany). JAK3−/− mice10 were a gift from Dr M. Lutz (Department of Dermatology, University of Erlangen-Nuremberg, Germany). Leishmania major promastigotes of the strain MHOM/IL/81/FEBNI were grown in vitro in blood agar cultures as previously described.34 Stationary-phase promastigotes were washed in phosphate-buffered saline, and 2.5 × 106parasites were injected in a volume of 50 μL intradermally into the right hind footpad. A lysate of L major (LmAg) was prepared by freezing and thawing the pellet 5 times. Borrelia burgdorferi spirochetes (strain N40) were a gift from Dr D. Postic (Institute Pasteur, Paris, France).
Cytokines, antibodies, and reagents
Recombinant murine IL-4 (LPS content less than 100 pg/μg protein) was purchased from ICChemikalien (Ismaning, Germany), and recombinant murine IL-2, IL-9, and IL-15 (LPS content less than 100 pg/μg protein) were obtained from R&D Systems (Wiesbaden, Germany). IL-3 was produced in supernatants (IL-3-BPV-SN) of transfected X63Ag8-653 cells.35 An antimurine CD3 monoclonal antibody (mAb; clone 145-2C11) was purchased from Pharmingen (Hamburg, Germany). Rat anti-γc mAb TUGm2 was a generous gift from Dr Sugamura (Sendai, Japan). Purified, recombinant murine soluble IL-4 receptor was kindly provided by Dr F. Seiler Behringwerke AG (Marburg, Germany). A rat antimouse CD40 mAb (clone FGK) was a generous gift of Dr T. Winkler (Department of Immunology, University of Erlangen-Nuremberg). Streptavidin–fluorescein isothiocyanate (FITC) was purchased from Boehringer Mannheim (Mannheim, Germany). Concanavalin A (conA), PMA, and staphylococcal enterotoxin B were obtained from Sigma (Deisenhofen, Germany). Hygromycin, cyclosporin A, and rapamycin were purchased from Calbiochem (Bad Soden, Germany). The metalloprotease inhibitor TAPI was a kind gift of Dr R. Black (Immunex, Seattle, WA). Antipain, aprotinin, bestatin, pepstatin, phenylmethylsulfonyl fluoride (PMSF), leupeptin, EGTA-Na2, EDTA-Na2, and phosphoramidon were obtained from Boehringer Mannheim. N-Ac-Leu-Leu-norleucine (ALLN) was obtained from Calbiochem, and [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsuccinyl]-L-3-(5,6,7,8,-tetrahydro-1naphtyl)alanine-N-methylamide (KB8301) was purchased from Pharmingen.
RNA isolation, reverse transcription, and polymerase chain reaction
After RNA extraction from murine lymphocytes with acidic guanidinium thiocyanate,36 cDNA was synthesized with reverse transcriptase (Pharmacia Biotech, Freiburg, Germany) as previously described.37 The cDNA was synthesized in a 40 μL reaction volume containing 50 mmol/L Tris HCl, pH 8.3, 2.5 mmol/L MgCl2, 10 mmol/L dNTP, 1 U Taq polymerase (Pharmacia Biotech), and 100 nmol/L primers during 35 cycles (1 minute denaturation at 94°C, 1 minute annealing at 58°C to 63°C, and 1 minute extension at 72°C). For amplification of γc, primers used were γc-sense primer 5′-CCCAGAGAAAGAAGAGCAAGCACC-3′ and γc-antisense primer 5′-AAGGATTGATGTTCAGGCTTCCGG-3′. The resulting polymerase chain reaction (PCR) product was subcloned into the vector pSPT18 (Boehringer Mannheim). For expression of the 255N-Stop variant of the sγc, the coding region was amplified by using the γc-sense primer and the 255N-Stop–antisense primer 5′-GAAGCTTTCAATTCTCCTCTACAGTATGACTCCC-3′. All samples were analyzed on 1.5% agarose gels containing 0.2 μg/mL ethidium bromide.
Cloning, expression, and purification of murine sγc
The PCR fragment of the 255N-Stop variant was digested withPvuII and HindIII and was cloned into thePvuII/HindIII linearized eucaryotic expression plasmid pCEP4 (Invitrogen, Leek, Netherlands). The episomal pCEP-255N-Stop-sγc expression plasmid was transfected into the human embryonic kidney cell line 293/EBNA (Invitrogen) by electroporation in 0.8 mL medium at 900 μF and 260 V using an Easyject electroporation unit (Eurogentec, Seraing, Belgium). Transfected cells, which were selected by the addition of 300 μg/mL hygromycin B and 250μg/mL neomycin, constitutively produced the recombinant pCEP4-255N-Stop-sγc. For the expression of sγc in SF9 insect cells, the plasmids pCEP4-255N-Stop-sγc and pCEP4-GSKGS-255N-Stop-sγc were linearized with KpnI, treated with T4 polymerase to generate blunt ends, and subsequently digested with BamHI. The resultant fragments were inserted into the plasmid pVL1392 (Pharmingen), which had been linearized with XbaI, treated with T4 polymerase, and digested with BamHI. The generation of recombinant baculovirus and the infection of SF9 cells were performed as recommended by the manufacturer (BaculoGold system; Pharmingen). For expression in Escherichia coli, the cDNA encoding murine γc was amplified using the sense primer 5′-GAAGCTTTCAATTCTCCTCTACAGTATGACTCCC-3′ and the antisense primer 5′-GAAGCTTTCAATTCTCCTCTACAGTATGACTCCC-3′. The resulting PCR fragment was purified by gel electrophoresis, digested with BamHI andHindIII, and cloned into theBamHI/HindIII linearized His-tag expression plasmid pQE30 (QIAGEN, Hilden, Germany). The resultant plasmid pQE30-255N-Stop-sγc was transformed into the E coli strain C600 (Stratagene, Heidelberg, Germany). To analyze the expression of the 255N-Stop protein, transformed bacterial cells were induced with 2 mmol/L isopropyl-β-D-thiogalactoside for 3 hours, and total bacterial proteins were analyzed on 15% sodium dodecyl sulfide–polyacrylamide gel electrophoresis (SDS-PAGE). The bacterial cell pellets were mixed thoroughly in 2 vol homogenization buffer (50 mmol/L Tris HCl, pH 7.4–10 mmol/L MgCl2–0.2 mol/L KCl–5% glycerol) and passed through a French press (SLM Aminco, Rochester). Cell homogenates were centrifuged at 20 000g for 30 minutes at 4°C. Pellets were washed in 50 mmol/L Tris HCl, pH 7.4–10 mmol/L MgCl2 and treated with 6 mol/L guanidine HCl–0.1 mol/L NaH2PO4–0.01 mol/L Tris, pH 8.0, for 4 hours at room temperature. The extracted protein was purified by affinity chromatography on Ni-NTA-resin (QIAGEN) and eluted by acidification, as recommended by the manufacturer. Purified protein was dialyzed against PBS, pH 7.4. All cloned cDNAs were sequenced using the Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Warrington, United Kingdom) as recommended by the manufacturer.
Site-directed mutagenesis
Mutations of the 255N-Stop-sγc variant were introduced by use of the QuikChange Site-Directed Mutagenesis Kit (Stratagene) following the instructions of the manufacturer. For creating a 244P-Stop-sγc, the sense primer 5′-GTAAATGGAGCCAGCCTTAGCACTGGGGGAGTCATACTG-3′ and the antisense primer 5′-CAGTATGACTCCCCCAGTGCTAAGGCTGGCTCCATTTAC-3′ were used. The 235S-Stop-sγc variant was generated with the sense primer 5′-CCCAATCTGTGGAAGTTAGCAACAGTGGAGTAAATGG-3′ and the antisense primer 5′-CCATTTACTCCACTGTTGCTAACTTCCACAGATTGGG-3′. For exchange of the WSKWS motif we used the sense primer 5′-GTTCTCAACAGGGGAGTAAAGGGAGCCAGCCTG-3′ and the antisense primer 5′-CAGGCTG GCTCCCTTTACTCCCCTGTTGAGAAC-3′.
Transient transfection of COS-7.1 cells
The cDNA encoding the complete transmembrane murine γc was amplified, and the PCR fragment was subcloned into the pSPT18 vector as described above. After digesting the vector with HindIII andSmaI and after treatment of the 5′-end using Klenow enzyme (Pharmacia Biotech), the γc-encoding fragment was ligated into theHindIII/NotI–linearized eucaryotic expression plasmid pCDM7.38 After confirmation of the nucleotide sequence, 15 μg purified receptor plasmid DNA was electroporated into 1 × 107 COS-7.1 cells kindly provided by Dr S. Rose-John (Mainz, Germany) in 0.8 mL medium at 900 μF and 260 V in an Easyject electroporation unit (Eurogentec). Transiently transfected cells were used for analysis of γc expression 48 to 72 hours after transfection. Control cells were transfected with vector only.
Cell culture and proliferation assays
EL-4 cells39 were used for FACS analysis of membrane-bound γc and cultured as described below. L1/1,40 MC-9,41 and CTLL-242cells were used for cell proliferation studies. The cells were grown in complete medium (Clicks/RPMI medium; Life Technologies, Eppenstein, Germany), supplemented with 10% fetal calf serum (Biochrom, Berlin, Germany), 2 mmol/L L-glutamine, 10 mmol/L HEPES, 100 μg/mL penicillin, 60 ng/mL streptomycin, 13 mmol/L NaHCO3, and 5 × 10−5 mol/L 2-ME, and stimulated in the presence or absence of various concentrations of IL-2, IL-3, IL-4, IL-9, IL-15, and the purified and concentrated supernatants containing the sγc or an equally treated control supernatant, respectively, in 96-well flat-bottom microtiter plates (Nunc, Wiesbaden, Germany). The cells were pulsed after 48 hours of culture with [3H] thymidine (18.5 kBq/well; Amersham, Braunschweig, Germany) for 16 hours and processed for beta-counting.
B-cell and natural killer-cell enrichment by magnetic cell sorting and analysis by flow cytometry
B cells were purified from naive murine spleen cells by magnetic separation with a MACS column (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of B cells negatively selected with anti-CD4, anti-CD8, and anti-CD11b mAb-coupled microbeads (Miltenyi Biotech) was 95% to 97% as analyzed by flow cytometry. Purified splenic B cells (1 × 106) were cultured in the presence or absence of stimuli as indicated. Natural killer (NK) cells were purified from naive C57BL/6 spleen cells by magnetic separation using biotinylated DX-5 mAb (Pharmingen) and streptavidin-conjugated Dynabeads (Deutsche Dynal, Hamburg, Germany) according to the manufacturer's instructions. More than 90% of the isolated cells were NK cells, and no T cells were detectable by flow cytometry. Purified splenic NK cells (2.5 × 105) were cultured in the presence or absence of stimuli as indicated. The concentrations of sγc were measured by enzyme-linked immunosorbent assay (ELISA) as described below.
Preparation and stimulation of macrophages
Macrophage monolayers were prepared and tested for purity as described elsewhere.43 Briefly, thioglycolate-elicited peritoneal exudate cells were seeded at a concentration of 1 × 106/mL, and nonadherent cells were removed after 4 hours of incubation by 3 washings. Macrophages were then stimulated with total lysate of B burgdorferi (bacteria–cell ratio, 10:1), conA, or PMA in the respective medium from 6 to 72 hours. Measurements of the sγc in the cell culture supernatants were made using ELISA.
In vitro stimulation of spleen cells
Spleen cells from BALB/c mice were prepared as single-cell suspensions and cultured in vitro for 48 to 72 hours at densities indicated in complete medium in the presence or absence of various concentrations of conA and PMA, respectively. After 12 to 96 hours of culture, the release of the sγc into the supernatant was determined by ELISA.
SDS-PAGE and Western blot analysis
Column elutions containing sγc from sera or concentrated culture supernatants containing the recombinant sγc were separated under reducing conditions using 15% SDS-PAGE. Immunodetection of sγc on nitrocellulose blots was performed with the rabbit antiserum K20 (Santa Cruz Biotechnology, Heidelberg, Germany) followed by a goat antirabbit immunoglobulin conjugated to horseradish peroxidase (Dianova, Hamburg, Germany) and the enhanced chemiluminescence Western blotting system (Amersham).
sγc ELISA
The concentrations of murine sγc in sera or supernatants of cells were measured by a 2-site ELISA. Monoclonal antibody 4G3 (Pharmingen) served as capture antibody. Bound sγc was detected by rabbit–antimouse γc antiserum K20 followed by biotinylated donkey–antirabbit IgG (Dianova), using StreptAB (DAKO, Hamburg, Germany) and PNPP (Sigma) for visualization in an ELISA reader (Dynatech, Denkendorf, Germany). This ELISA detects murine sγc in the range of 0.1 to 20 ng/mL. We used recombinant sγc expressed in 293/EBNA cells and purified by affinity chromatography at known concentrations for the standardization of this ELISA.
sIL-4R ELISA
Monoclonal antibodies were raised against BHK-derived recombinant murine sIL-4R by standard techniques and were used for a sandwich ELISA for quantitative determination of murine sIL-4R. This ELISA has a working range of 50 to 2000 pg/mL, as described previously.23 Recombinant murine sIL-4R at known concentrations was used as a standard.
Flow cytometry analysis
The surface expression of murine γc was determined by staining with rat–antimouse γc mAb TUGm2 and FITC-labeled goat–antirat antibody (Dianova), analyzed by flow cytometry (FACScan; Becton Dickinson, Heidelberg, Germany). A rat IgG2b antibody of unknown specificity (R35-38; Pharmingen) was used as isotype control.
Affinity chromatography
Mice were bled from the retro-orbital veinplexus. After allowing the whole blood to coagulate at 4°C for 4 hours, it was centrifuged for 15 minutes at 3000g. Serum was collected and stored at −20°C. Five milliliters serum was diluted in 95 mL PBS. The sγc from serum or from supernatants of transfected 293/EBNA cells or infected SF9 insect cells was bound to a Hi-Trap affinity column (Pharmacia Biotech) coupled with anti-γc mAb 4G3 (Pharmingen) and eluted with 100 mmol/L glycine, pH 2.7.
Concentration of serum-free cell supernatants
Serum-free supernatants of transfected 293/EBNA cells were collected and concentrated 10- to 20-fold by ultrafiltration using YM10 membranes (Amicon, Witten, Germany). The concentration of sγc was measured using ELISA.
Sodium iodide I 125-sγc–binding assays
Twenty-five micrograms recombinant murine sγc was iodinated using Iodogen-coated glass tubes (Pierce Chemical, Rockford, IL) according to the manufacturer's instructions. Iodinated sγc was repurified by the use of an anti- γc affinity column. Equilibrium binding of 125I-sγc to murine IL-4R expressed on human TF-1 cells44 was measured after the incubation of 2 × 106 cells in the presence of 125I-sγc and 100 ng/mL IL-4, in a final volume of 200 μL in microfuge tubes at 4°C for 120 minutes. To separate nonbound 125I-sγc from cell-bound 125I-sγc, the reaction mixture was centrifuged through an oil gradient.44 Specificity of binding was determined by the addition of a 200-fold excess of unlabeled sγc.
Results
Sγc can be detected in sera of inbred mouse strains
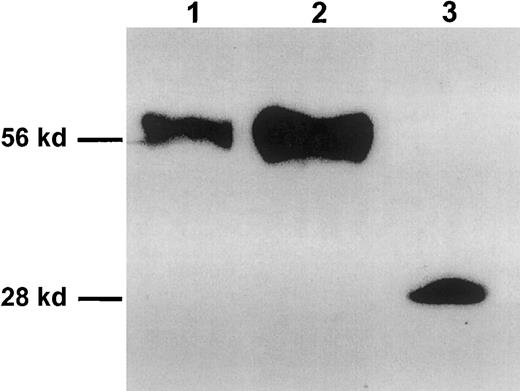
To identify soluble γc in the body fluids of mice, BALB/c serum was subjected to affinity chromatography using an anti-γc mAb, and eluted proteins were analyzed by SDS-PAGE followed by Western blot analysis. A signal representing a protein with a molecular weight of approximately 56 kd was detected with an anti-γc antiserum K20 (Figure 1, lane 1) and with the anti-γc mAb 4G3 (data not shown). Bands of comparable length were detected with these antibodies when culture supernatants of conA-stimulated murine spleen cells or supernatants of EL-4 cell stimulated with phorbol esters were tested (data not shown). The molecular weight of the natural sγc is approximately twice as high as the calculated mass of the extracellular domain of the γc (27.6 kd), which might result from the presence of 6 potential N-glycosylation sites within the extracellular part of the γc.45 Therefore, we analyzed the migration behavior of 2 recombinant variants of the sγc in parallel. The coding region of the extracellular domain of the γc, with a stop codon introduced after Asn255 (2-amino acidN-terminal of the transmembrane region), was inserted into a eucaryotic and a procaryotic expression vector. Recombinant 255N-Stop-sγc expressed in eucaryotic cells had a molecular weight similar to that of natural sγc (Figure 1, lane 2), whereas the protein expressed in E coli lackingN-glycosylation migrated as a single-band 28 kd (Figure 1, lane 3).
Visualization of natural and recombinant soluble forms of the membrane-bound γc by Western blot.
Lane 1: naturally occurring sγc concentrated and purified from serum of BALB/c mice by affinity chromatography using the 4G3 mAb. Lane 2: Rsγc (255N-Stop-sγc) expressed in 293/EBNA cells. Lane 3: Rsγc expressed in E coli. Molecular mass markers are indicated on the left.
Visualization of natural and recombinant soluble forms of the membrane-bound γc by Western blot.
Lane 1: naturally occurring sγc concentrated and purified from serum of BALB/c mice by affinity chromatography using the 4G3 mAb. Lane 2: Rsγc (255N-Stop-sγc) expressed in 293/EBNA cells. Lane 3: Rsγc expressed in E coli. Molecular mass markers are indicated on the left.
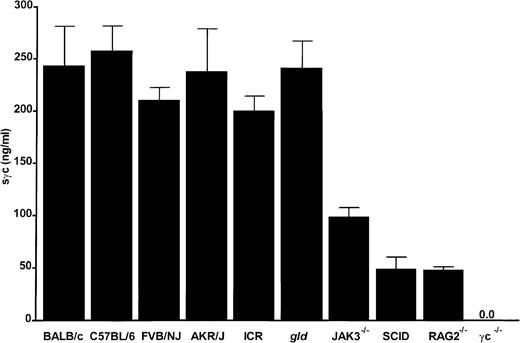
Reduced concentrations of sγc in sera of immunocompromised mice as compared with immunocompetent mice
Sera taken from at least 5 mice per strain were tested in an sγc-ELISA using a recombinant protein of known concentration as a standard (Figure 2). BALB/c, C57BL/6, FVB/NJ, AKR/J, ICR, and gld mice had similar levels of circulating sγc (200-250 ng/mL) that were independent of age and sex (data not shown). Immune-deficient animals with severe T- and B-cell defects, such as BALB/c-SCID, JAK3−/−, or RAG2−/− mice, had 2- to 5-fold reduced concentrations of sγc in their sera. The specificity of the newly developed sγc-ELISA was convincingly demonstrated by the complete absence of detectable protein in the sera of γc−/− mice (Figure 2).
Serum levels of the sγc in mice.
Mice were bled from retro-orbital veins, and sera were analyzed by sγc ELISA. Bars represent the mean of at least 5 mice per strain.
Serum levels of the sγc in mice.
Mice were bled from retro-orbital veins, and sera were analyzed by sγc ELISA. Bars represent the mean of at least 5 mice per strain.
Sγc is released after activation by different subsets of immune cells
To define the potential cellular source(s) of the sγc, different cell types were stimulated in vitro, and the kinetics of sγc release were determined by ELISA. The Th2 cell clone L1/1 released sγc with nearly identical kinetics after stimulation with the respective specific antigen (LmAg, mitogens conA, PMA) (Figure3A), whereas only a slight increase of sγc was observed in control cultures. Purified primary splenic B cells released sγc with a somewhat later maximum at 72 hours after stimulation with lipopolysaccharide (LPS) (Figure 3B) and after stimulation using an anti-CD40 mAb (clone FGK) (data not shown). Because sera of mice devoid of T and B cells (Figure 2) had reduced but still clearly detectable levels of sγc, NK cells were analyzed for their capacity to produce sγc. NK cells purified from spleens of mice showed an enhanced production of sγc only in the presence of PMA but not with conA, arguing against the presence of significant numbers of contaminating T cells (Figure 3C). Thioglycolate-elicited peritoneal exudate macrophages showed an enhanced release of sγc in the presence of LPS and B burgdorferi spirochetes (Figure 3D). Interestingly, the presence of the cytokines IL-2, IL-4, IL-7, IL-9, and IL-15, using the γc in their receptor complexes for signal transduction, did not alter the release of sγc with any of the cell types tested (data not shown).
Kinetics of release of the sγc into culture supernatants after stimulation of immune cells analyzed by ELISA.
(A) Th2 cells of the L major- specific clone L1/1 (4 × 106 cells/mL) were stimulated with PMA (500 ng/mL), conA (5 μg/mL), or soluble L major antigen (LmAg) (3 × 106/well). (B) B cells purified by MACS from spleens of BALB/c mice (1 × 106 cells/mL, 95%-97% purity) were stimulated with PMA (500 ng/mL) or LPS (10 μg/mL). (C) NK cells from C57BL/6 mice were purified using Dynabeads yielding purity greater than 90%. 2.5 × 105 cells/mL were stimulated with PMA or conA, as above. (D) Peritoneal exudate cells (1.5 × 106 cells/mL) of BALB/c mice were stimulated with PMA, conA, LPS as above, or B burgdorferi (10 spirochetes per macrophage).
Kinetics of release of the sγc into culture supernatants after stimulation of immune cells analyzed by ELISA.
(A) Th2 cells of the L major- specific clone L1/1 (4 × 106 cells/mL) were stimulated with PMA (500 ng/mL), conA (5 μg/mL), or soluble L major antigen (LmAg) (3 × 106/well). (B) B cells purified by MACS from spleens of BALB/c mice (1 × 106 cells/mL, 95%-97% purity) were stimulated with PMA (500 ng/mL) or LPS (10 μg/mL). (C) NK cells from C57BL/6 mice were purified using Dynabeads yielding purity greater than 90%. 2.5 × 105 cells/mL were stimulated with PMA or conA, as above. (D) Peritoneal exudate cells (1.5 × 106 cells/mL) of BALB/c mice were stimulated with PMA, conA, LPS as above, or B burgdorferi (10 spirochetes per macrophage).
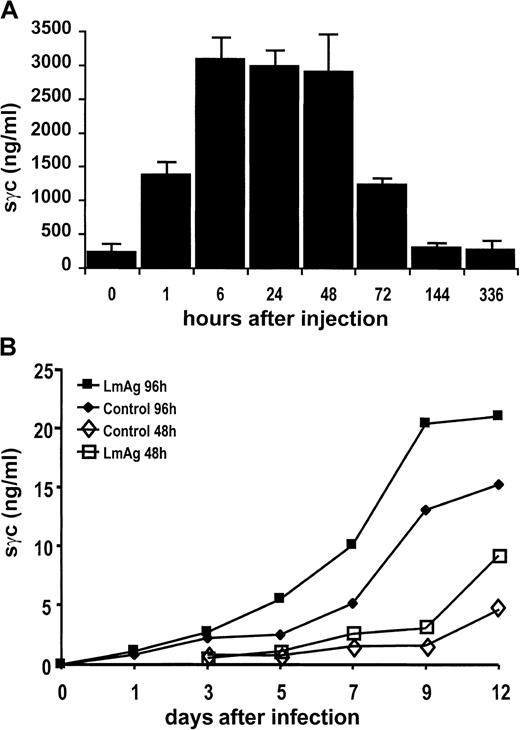
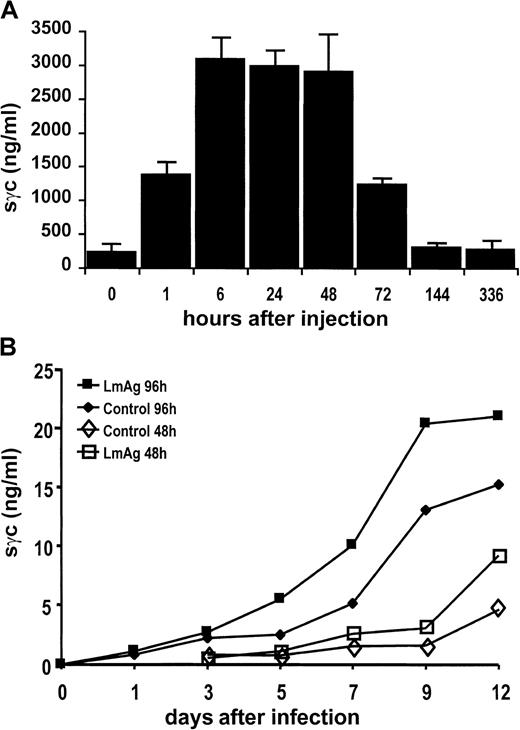
In vivo activation of T cells leads to the release of sγc
BALB/c mice were treated with staphylococcal enterotoxin B to induce polyclonal T-cell activation in vivo. As shown in Figure4A, there was a rapid increase of sγc in the sera of mice 1 hour after injection that reached a maximum of approximately 3 μg/mL after 6 to 24 hours. These 10-fold elevated sγc concentrations returned to basal levels within 6 days. To address the question whether T cells activated during a primary immune response are capable of releasing sγc in an antigen-specific manner, BALB/c mice were infected subcutaneously with L majorpromastigotes. Cells of the lymph nodes draining the site of infection released sγc, cumulating in their supernatants during 96 hours of in vitro culture. There was a steep increase from day 3 to day 9 after infection (Figure 4B), a period in which T-cell activation has been shown to take place in this experimental infection. Furthermore, the addition of parasite lysates (LmAg) to cell suspensions already containing parasites enhanced sγc production. Together with our finding that L major-specific CD4 T cells respond to their antigen with sγc production (Figure 3A), these data strongly argue for sγc release by T cells after priming in vivo.
Release of sγc after activation of immune cells in vivo.
(A) Increase of sγc in sera of BALB/c mice after intraperitoneal injection of 10 μg staphylococcal enterotoxin B per mouse. Sera of 3 mice per time-point were analyzed for their sγc content by ELISA. Bars indicate the means of 3 mice. (B) Sγc concentrations in supernatants of lymph node cell cultures obtained from BALB/c mice during the early course of an infection with L major. Mice were infected with 2 × 106L majorpromastigotes subcutaneously into the right hind footpad. At the time-points indicated, the popliteal lymph nodes were obtained, and their cells (2 × 106 cells/mL) were cultured for 48 and 96 hours in the presence or absence of LmAg. Concentrations of sγc were determined by ELISA.
Release of sγc after activation of immune cells in vivo.
(A) Increase of sγc in sera of BALB/c mice after intraperitoneal injection of 10 μg staphylococcal enterotoxin B per mouse. Sera of 3 mice per time-point were analyzed for their sγc content by ELISA. Bars indicate the means of 3 mice. (B) Sγc concentrations in supernatants of lymph node cell cultures obtained from BALB/c mice during the early course of an infection with L major. Mice were infected with 2 × 106L majorpromastigotes subcutaneously into the right hind footpad. At the time-points indicated, the popliteal lymph nodes were obtained, and their cells (2 × 106 cells/mL) were cultured for 48 and 96 hours in the presence or absence of LmAg. Concentrations of sγc were determined by ELISA.
Proteolytic shedding of membrane-anchored γc molecules is responsible for the release of the sγc
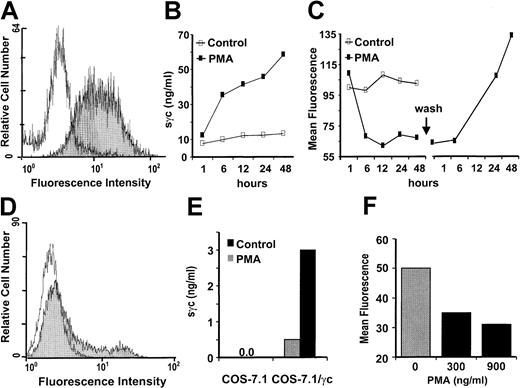
We next analyzed the mechanisms of sγc release. When EL-4 thymoma cells, constitutively expressing high numbers of γc on their surfaces (Figure 5A), were stimulated with PMA, up to 40 ng/mL sγc were released into the culture medium during the first 6 hours (Figure 5B). A corresponding decrease in the number of cell surface γc molecules was demonstrated by the 2-fold reduction of the mean fluorescence in flow cytometry analysis (Figure5C). A complete re-expression of the transmembrane γc was detected within 24 hours of washing the cells to remove the PMA (Figure 5C). As expected, because of the relatively rapid reappearance of γc, there was no influence on cytokine responsiveness of T cells after PMA-induced shedding in proliferation tests, which lasted 48 to 72 hours (data not shown).
Sγc is generated by proteolytic shedding of membrane-bound γc.
(A) On the surfaces of EL-4 cells, γc expression was detected by flow cytometry with an isotype-matched control mAb (white graph) or TUGm2 mAb (gray graph). (B) PMA induces the release of sγc by EL-4 cells. 2 × 106 cells/mL were cultured in the presence or absence of PMA (250 ng/mL), and at the time-points indicated the sγc concentrations in the culture supernatants were measured by ELISA. (C) Decreased expression of membrane-bound γc of EL-4 cells in the presence of PMA and re-expression after PMA withdrawal. In parallel to the sγc measurements in the supernatants (see B), cell surface expression of γc was analyzed by flow cytometry. Forty-eight hours after stimulation, the cells were washed and the re-occurrence of the γc was measured. Ratios of the mean fluorescence (MF) of stimulated cells divided by the MF of nonstimulated cells are depicted. (D) Expression of full-length transmembrane γc by transfected COS-7.1 cells. Graphs appear as described for panel A. (E) Enhanced release of sγc by COS-7.1/γc, but not control transfected COS-7.1, cells in the presence of PMA (300 ng/mL). (F) PMA dose-dependent decrease of the MF after staining the surface-bound γc on COS-7.1/γc cells.
Sγc is generated by proteolytic shedding of membrane-bound γc.
(A) On the surfaces of EL-4 cells, γc expression was detected by flow cytometry with an isotype-matched control mAb (white graph) or TUGm2 mAb (gray graph). (B) PMA induces the release of sγc by EL-4 cells. 2 × 106 cells/mL were cultured in the presence or absence of PMA (250 ng/mL), and at the time-points indicated the sγc concentrations in the culture supernatants were measured by ELISA. (C) Decreased expression of membrane-bound γc of EL-4 cells in the presence of PMA and re-expression after PMA withdrawal. In parallel to the sγc measurements in the supernatants (see B), cell surface expression of γc was analyzed by flow cytometry. Forty-eight hours after stimulation, the cells were washed and the re-occurrence of the γc was measured. Ratios of the mean fluorescence (MF) of stimulated cells divided by the MF of nonstimulated cells are depicted. (D) Expression of full-length transmembrane γc by transfected COS-7.1 cells. Graphs appear as described for panel A. (E) Enhanced release of sγc by COS-7.1/γc, but not control transfected COS-7.1, cells in the presence of PMA (300 ng/mL). (F) PMA dose-dependent decrease of the MF after staining the surface-bound γc on COS-7.1/γc cells.
Because these findings were compatible with shedding as the main mechanism of sγc production, we tested this possibility by transfection experiments. COS-7.1 cells were transiently transfected with an expression vector containing the non-spliceable cDNA for the transmembrane γc. The transfectants expressed cell surface γc as detected after staining with specific mAb (Figure 5D). Stimulation of the transfected, but not the control COS-7.1, cells resulted in sγc production (Figure 5E) accompanied by a reduction of cell surface γc expression, as shown by flow cytometry in a PMA dose-dependent manner (Figure 5F).
Proteolytic shedding appears to be independent of metalloproteases responsible for the cleavage of other cytokine receptors
Pharmacologic inhibitors for several proteases and for immunosuppressive agents and kinase inhibitors were used to define the molecular mechanisms and enzyme(s) responsible for the shedding of membrane-bound γc. Spleen cells obtained from BALB/c mice were stimulated with conA, PMA, or anti-CD3 mAb in the presence or absence of inhibitors in nontoxic concentrations, as determined by trypan blue exclusion. As shown in Table 1, none of the compounds tested significantly reduced the concentrations of sγc in the respective supernatants. Other inhibitors, such as aprotinin (1 μg/mL), leupeptin (2 μg/mL), pepstatin (1.3 μg/mL), and PMSF (5 μmol/L), also were unable to inhibit the shedding process of γc. Of special interest, neither staurosporin, a kinase inhibitor, nor TAPI, a hydroxamic acid-based inhibitor of zinc-metalloproteases,15 influenced the sγc release, whereas they clearly suppressed the release of sIL-4R as measured in the same supernatants.
Inhibition of γc-dependent cell growth by a recombinant form of sγc
To test sγc for its biologic functions, a recombinant form of this molecule comprising the extracellular part lacking only the 2 last C-terminal aa (255N-Stop-sγc) was analyzed in cell proliferation tests. When sγc was added 30 minutes before the γc-dependent cytokines, such as IL-2 (Figure 6A), IL-4 (Figure 6B), IL-9 (Figure 6C), and IL-15 (data not shown), a dose-dependent inhibition of cytokine-induced proliferation of T (Figure 6A) and MC-9 mast cells (Figure 6B-C) was observed, irrespective of whether the sγc was expressed in 293 EBNA or SF9 insect cells. Proliferation of MC-9 cells after stimulation with IL-3, a γc-independent cytokine, remained unaffected, excluding unspecific toxicity of the 255N-Stop-sγc preparations. The inhibitory effects of sγc were absent when the recombinant protein was added later than 60 minutes after the cytokines (data not shown).
Recombinant sγc inhibits cytokine-induced proliferation in a dose-dependent and specific manner.
Cells were preincubated with serial dilutions of concentrated supernatants of 293/EBNA cells, expressing 255N-Stop-sγc (black bars) or the equally treated supernatants of vector-transfected cells (control, open bars) 30 minutes before the addition of cytokines. (A) L1/1 T cells were stimulated using IL-2 (2 ng/mL). (B) MC-9 mast cells were stimulated using IL-4 (1 ng/mL), (C) IL-9(1 ng/mL), or (D) IL-3 (1.25% IL-3-BPV-SN). After 48 hours, [3H]thymidine was added, and 16 hours later, cells were harvested and radioactivity was measured in a β-counter. The data presented are representative of at least 5 comparable assays per cytokine tested. Bars marked with w/o represent cytokine proliferation in the absence of inhibitor (100% control value).
Recombinant sγc inhibits cytokine-induced proliferation in a dose-dependent and specific manner.
Cells were preincubated with serial dilutions of concentrated supernatants of 293/EBNA cells, expressing 255N-Stop-sγc (black bars) or the equally treated supernatants of vector-transfected cells (control, open bars) 30 minutes before the addition of cytokines. (A) L1/1 T cells were stimulated using IL-2 (2 ng/mL). (B) MC-9 mast cells were stimulated using IL-4 (1 ng/mL), (C) IL-9(1 ng/mL), or (D) IL-3 (1.25% IL-3-BPV-SN). After 48 hours, [3H]thymidine was added, and 16 hours later, cells were harvested and radioactivity was measured in a β-counter. The data presented are representative of at least 5 comparable assays per cytokine tested. Bars marked with w/o represent cytokine proliferation in the absence of inhibitor (100% control value).
Cytokine-inhibitory effects of sγc require the C-terminus and the WSKWS motif of the protein
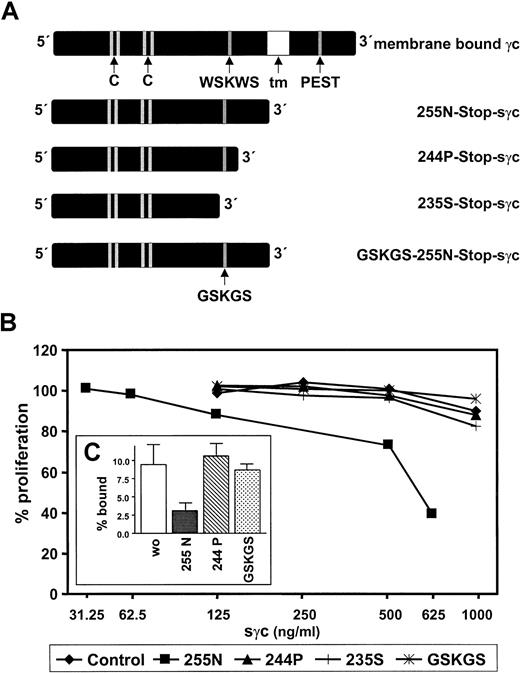
To analyze the structural requirements for cytokine inhibition, we cloned and expressed mutants of the biologically active 255N-Stop-sγc, as shown schematically in Figure7A. Because we considered the interaction of sγc with other membrane-bound receptors and not the direct binding of cytokines as the most likely mechanism, the C-terminus of the protein was subjected to site-directed mutagenesis. Three mutated forms of the sγc expressed in 293 EBNA cells were secreted, displayed the expected molecular sizes in Western blots, and were bound by the conformation-dependent mAbs 4G3 and 3E12 (not shown). These facts argue against the possible misfolding of these mutated receptor proteins. As shown in Figure 7B, deletion of the last 11 aa (244P-Stop-sγc) completely abrogated the growth-inhibitory effect of sγc. Of special interest, the GSKGS mutant of the 255N-Stop-sγc also did not suppress cytokine-induced cell growth, illustrating the functional importance of the WSKWS motif conserved among the cytokine receptor family.45 46
Requirement of the C-terminus and the WSKWS motif of the sγc for cytokine-inhibitory activity and binding to cells.
(A) Two C-terminal deletions (244P, 235S), and a double-point mutant (GSKGS instead of WSKWS) were generated by site-directed mutagenesis and expressed in 293 EBNA cells. tm, transmembrane domain. (B) CTLL-2 cells were preincubated with different sγc variants at concentrations indicated or equally treated control supernatant 30 minutes before adding IL-2 (2 ng/mL). Cell proliferation was determined as described in the legend to Figure 6. (C) Affinity-purified 125I-sγc (255N) and IL-4 were added to human TF-1 cells expressing murine IL-4R in the presence or absence of 200-fold excess amounts of unlabeled 255N-Stop-sγc, 244P-Stop-sγc, or GSKGS-255N-Stop-sγc, respectively. The percentages of cell-bound 125I-sγc were determined as described in “Materials and methods.” Data given are the mean (± SEM) of triplicate determinations.
Requirement of the C-terminus and the WSKWS motif of the sγc for cytokine-inhibitory activity and binding to cells.
(A) Two C-terminal deletions (244P, 235S), and a double-point mutant (GSKGS instead of WSKWS) were generated by site-directed mutagenesis and expressed in 293 EBNA cells. tm, transmembrane domain. (B) CTLL-2 cells were preincubated with different sγc variants at concentrations indicated or equally treated control supernatant 30 minutes before adding IL-2 (2 ng/mL). Cell proliferation was determined as described in the legend to Figure 6. (C) Affinity-purified 125I-sγc (255N) and IL-4 were added to human TF-1 cells expressing murine IL-4R in the presence or absence of 200-fold excess amounts of unlabeled 255N-Stop-sγc, 244P-Stop-sγc, or GSKGS-255N-Stop-sγc, respectively. The percentages of cell-bound 125I-sγc were determined as described in “Materials and methods.” Data given are the mean (± SEM) of triplicate determinations.
To evaluate the mechanism of cytokine inhibition by the sγc cells, binding studies were performed. It has been shown47 by surface plasmon resonance that the extracellular domain of the γc binds to a preformed binary IL-4R/IL-4 complex, but not to immobilized IL-4R or IL-4 alone. Thus, affinity-purified sγc was labeled with125I and added together with saturating concentrations of IL-4 to transfected TF-1 cells,44 expressing approximately 20 000 molecules of the mouse IL-4Rα per cell. As depicted in Figure7C, 125I-sγc bound to the cells in a specific manner because binding competition was seen with excess of the wild-type sγc (255 N-Stop-sγc). In contrast, an excess of biologically inactive mutants of the sγc (244P-Stop-sγc, GSKGS mutant) did not interfere with 125I-sγc binding to the cells. Thus, our data, together with the findings of Letzelter et al,47 support the hypothesis that interaction of the sγc in trimolecular complexes with IL-4 and the IL-4R results in competitive displacement of the transmembrane γc, which, in turn, results in an inhibition of cytokine response.
Discussion
In this study we have shown that a soluble form of the murine γc is present in blood at high concentrations. This molecule is released after activation by all immune cells analyzed and is able to inhibit specifically cell proliferation induced by γc-dependent cytokines.
The size of the newly identified, naturally occurring sγc, as determined after purification from sera by Western blotting, was indistinguishable from that of a recombinant form of the nearly complete extracellular domain of the γc expressed in eucaryotic cells. Thus, as previously shown for other soluble cytokine receptors, the sγc represents a molecule with a C-terminus located in proximity to the transmembrane domain of the cell surface anchored receptor.13 14
To investigate the regulation of sγc expression, we developed an ELISA-based assay. The specificity of this assay was convincingly demonstrated by the fact that sγc was undetectable in sera and in cell culture supernatants of γc−/− mice. The constitutive concentration of sγc in the sera of immunocompetent mice (more than 200 ng/ mL) was 10- to more than 100-fold higher than those reported previously for the soluble forms of the IL-4R (1-2 ng/mL),44 IL-6R (less than 10 ng/mL),48 or the tumor necrosis factor receptors (TNF-Rs; p55 60 pg/mL, p75 5 ng/mL),49 respectively.
Dummer et al50 reported that preparations of soluble IL-2Rα and IL-2Rβ molecules, enriched from human sera by affinity chromatography, contained proteins showing reactivity with antibodies specific for the γc. Thus, it can be assumed that a soluble form of the human γc in sera is at least partially complexed with other cytokine receptor molecules.
The γc is expressed on hematopoietic cells such as granulocytes, B, T, and NK cells,51 and dendritic and Langerhans cells,52 which, therefore represent potential sources for the sγc. Indeed, all immune cell types tested in vitro were capable of releasing high amounts of sγc after appropriate stimulation. TCR-mediated activation of primary or cloned T cells and stimulation of B cells or macrophages by bacteria or their products was followed by an enhanced release of sγc. A very potent stimulus, previously shown to enhance the proteolytic shedding of several other cytokine receptors,15,18,23 is the phorbol ester PMA, demonstrating the critical involvement of PKC-dependent pathways in the process of sγc release. Importantly and in contrast to other soluble cytokine receptors, the presence of γc-dependent cytokines such as IL-2, IL-4, IL-7, IL-9, and IL-15 did not influence sγc production over wide range of concentrations and in all cell types tested. All γc-dependent cytokines have been shown to mediate their cell activation by the induction of JAK3 tyrosine phosphorylation.53 Thus our finding that the sγc levels in sera of JAK3−/− mice were similar compared to those of other mice with severe B- and T-cell defects, such as SCID and RAG2−/− mice (Figure 2) support the notion that signaling by the γc is not essential for the release of its soluble receptor fragment. The observation that despite the absence of B and T cells sγc was detectable in the sera of immunodeficient mice at concentrations up to 100 ng/mL is most likely a consequence of production by NK cells and macrophages/monocytes capable of releasing this molecule (Figure 3). Nevertheless, cells of the specific immune system appear to be the main producers of sγc in vivo. The importance of activated T cells as a source for sγc was shown by the rapid 10-fold rise in sγc levels after polyclonal activation in vivo using the superantigen staphylococcal enterotoxin B. Of special interest, the kinetics of increase was significantly slower than that of cytokines, such as IL-2 and TNF, which show their concentration maximus within the first 4 hours after staphylococcal enterotoxin B injection returning to baseline levels after 8 hours.54These findings suggest that the processes involved in the up-regulation of sγc appear to be distinct from those of cytokine up-regulation and that sγc might possess a longer t½ in vivo than typical cytokines. However, the elucidation of the exact pharmacokinetics of the sγc awaits further studies.
Using the well-established infection model of mice with the protozoon parasite L major, we studied the kinetics of sγc up-regulation during an anti-infectious immune response. Although only a slight increase of 30% to 50% of sγc in sera of infected mice was detected (data not shown), we observed a dramatic increase of sγc production when cells of lymph nodes draining the site of infection were analyzed in vitro. The kinetics of expression and the enhancement of sγc production after the addition of soluble Leishmaniaantigens to the cultures strongly argue for parasite-specific T cells being the main producers of the sγc. In agreement with this idea, cloned L major-specific Th2 cells were stimulated to release sγc after recognition of their specific antigen (Figure 3A).
Two main nonmutually exclusive mechanisms for the production of solubilized cell surface molecules have been described. The receptors for TNF, IL-1, IL-2, macrophage–colony-stimulating factor (M-CSF), platelet-derived growth factor (PDGF), and nerve growth factor (NGF) appear to be subject to proteolytic cleavage, resulting in the shedding of the extracellular part of the respective molecules.13,14 On the other hand, alternative splicing of the receptor mRNAs resulting in proteins lacking a transmembrane domain have been described for several cell surface molecules, including the receptors for GM-CSF, G-CSF, IL-4, IL-5, IL-7, IL-9, EGF, LIF, erythropoietin, and thrombopoietin.13,14 However, as analyzed in detail for the sIL-4R of the mouse,23 for one soluble receptor both mechanisms can occur in the same cell but appear to be activated by distinct pathways. Several considerations and observations make shedding the most likely mechanism responsible for sγc production. No alternatively spliced message has been described so far, and we found no up-regulation of the γc-encoding mRNA by RNAase protection assays after stimulation of T cells, B cells, or mixed spleen cell populations in vitro (Blum and Meiβner, unpublished data). Furthermore, there was a reproducible inverse correlation between activation-induced decrease of cell surface γc and increasing amounts of sγc in the cell culture supernatants. To test directly whether sγc is generated by shedding, COS-7.1 cells were transfected with complete cDNA for the transmembrane γc. Because these cells produced sγc with a molecular weight indistinguishable from that of the recombinantly expressed extracellular domain of the γc (255N-Stop-sγc) (data not shown), alternative splicing could be excluded as being involved in sγc generation at least in the transfectants. Numerous sheddases responsible for the secretion of other cytokine receptors are activated after protein kinase C activation by phorbol esters.55 However, it is still unclear whether the sheddase(s) are directly activated by phosphorylation events56 or whether the phosphorylation of the receptor molecule itself renders it accessible for proteolytic enzymes, as has been suggested for the TNFR.57
We used a number of protease inhibitors to define the sheddase(s) responsible for the cleavage of γc. Although TAPI and KB8301, well-characterized inhibitors of zinc-metalloproteases, markedly suppressed sIL-4R production, no inhibition of sγc production was observed in the same cultures. Because of a PEST-like sequence in the intracellular domain of the γc and a recent publication showing that the protease calpain mediates the intracellular cleavage of γc at the PEST-like site,58 we used the calpain inhibitors ALLN and antipain to analyze whether this protease is also involved in the release of the sγc. None of the agents tested led to a significant reduction of sγc release. Thus, based on these experiments with inhibitors, the shedding protease(s) responsible for γc cleavage appears to be distinct from other enzymes identified as essential for the proteolysis of other receptors and cytokines (eg, TNF alpha-converting enzyme).16 Our future experiments aiming to define the C-terminus of the naturally occurring sγc and using transfected mutants of the γc will help to elucidate the shedding mechanism.
An important function of the newly identified sγc was found when we analyzed the influence of this molecule on cytokine-induced cell proliferation. Specific inhibition of γc-dependent cytokines was observed with sγc concentrations similar to those occurring in vivo. In line with a potent immune-regulatory role of the sγc, this growth suppression was accompanied by the inhibition of cytokine-induced JAK-STAT activation (Gessner et al, manuscript in preparation).
Because no direct interaction of the γc with cytokines takes place in the absence of additional cytokine receptor molecules,59cytokine neutralization in solution by this molecule appears to be unlikely. Soluble gp 130 is the only other cytokine receptor described so far that shows cytokine-suppressive capacity despite its inability to bind to the respective ligands directly. Narazaki et al60 reported that the sgp130 in human sera inhibited IL-6 effects, and they proposed the competition of sgp130 with membrane-bound gp130 in IL-6R complexes as the most likely mechanism of cytokine suppression. To address this possibility, we cloned and expressed variants of the inhibitory sγc (255N-Stop-sγc) carrying mutations not in the N-terminal, presumably ligand-interacting, but in the C-terminal part. These recombinant proteins were unable to reduce cytokine-induced proliferation, supporting our hypothesis that competitive replacement of the membrane-bound γc in excess of the sγc leads to a loss of cytokine responsiveness of the cell. The WSXWS motif, previously shown to be critical for the function of the human membrane-bound γc,61 also appears to be essential for the interaction of soluble and membrane-bound cytokine receptors. As shown recently by Letzelter et al47 using biosensor techniques, the extracellular domain of the γc interacts with binary complexes consisting of IL-4 and IL-4Rα chain, whereas no binding of the γc to the IL-4Rα chain was detected in the absence of IL-4. Along these lines, we detected specific binding of purified and iodinated sγc to cells expressing large numbers of the mouse IL-4 R when IL-4 was present. Thus, sγc-mediated cytokine inhibition is most likely due to an association of sγc with cytokine–cytokine receptor complexes already preformed on the cell surface. Additional experiments are necessary to precisely define the mechanisms and kinetics of the inhibitory interaction of the sγc with cytokine-responsive cells.
In conclusion, the shedding of γc is a regulated process with 2 important functional consequences, the transient depletion of a cell surface molecule required for the signaling of at least 5 cytokines and the generation of a soluble inhibitor capable of modulating an ongoing immune response. Because the initiation of the γc shedding process appears to be independent of cytokines, this mechanism represents paracrine and retrocrine modes of controlling cytokine functions in vivo.
Acknowledgments
We thank Dr H. Körner and Dr P. Curley for critical reading of the manuscript.
Supported by the Deutsche Forschungsgemeinschaft (SFB263, A6) and the Graduiertenkolleg, Immunologische Mechanismen bei Infektion, Entzündung und Autoimmunität (U.M.).
U.M. and H.B. have contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
André Gessner, Institute for Clinical Microbiology, Immunology and Hygiene, University of Erlangen-Nuremberg, Wasserturmstrasse 3, 91054 Erlangen, Germany; e-mail:gessner@mikrobio.med.uni-erlangen.de.


![Fig. 6. Recombinant sγc inhibits cytokine-induced proliferation in a dose-dependent and specific manner. / Cells were preincubated with serial dilutions of concentrated supernatants of 293/EBNA cells, expressing 255N-Stop-sγc (black bars) or the equally treated supernatants of vector-transfected cells (control, open bars) 30 minutes before the addition of cytokines. (A) L1/1 T cells were stimulated using IL-2 (2 ng/mL). (B) MC-9 mast cells were stimulated using IL-4 (1 ng/mL), (C) IL-9(1 ng/mL), or (D) IL-3 (1.25% IL-3-BPV-SN). After 48 hours, [3H]thymidine was added, and 16 hours later, cells were harvested and radioactivity was measured in a β-counter. The data presented are representative of at least 5 comparable assays per cytokine tested. Bars marked with w/o represent cytokine proliferation in the absence of inhibitor (100% control value).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/1/10.1182_blood.v97.1.183/6/m_h80110531006.jpeg?Expires=1764475168&Signature=Y7bKWZyNarrAi~Zi3jCqqHROdjRo3SLx3lZhYlzY980GOOYcc20qHrYJ9aPdCtrudhiIDv7xw6lcEYDaojUqoBF88cWEcxGDymrHvF0i-cjbD6UTPue8dN~LlwvOI6TUcIMckOccurBRSIPRNusvaODZg4GqXDlxjO-yDo3ZnfoLmsvcW937sS5RXgAALYCJS~3uhUqkah62HLy-OJX6LxpiZVchH5XTQ9GNjE05BOgqOtfA~f7PYEdhq7DHFu3rHFrp8-jBuZ2UFm-TU9H~XK6beRXoQOvaqyzzzDi0CZ5oJEwmWwJoEP3Ejm0r4Dx~nfQvfOqQD9jJGq4dSl6QlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 6. Recombinant sγc inhibits cytokine-induced proliferation in a dose-dependent and specific manner. / Cells were preincubated with serial dilutions of concentrated supernatants of 293/EBNA cells, expressing 255N-Stop-sγc (black bars) or the equally treated supernatants of vector-transfected cells (control, open bars) 30 minutes before the addition of cytokines. (A) L1/1 T cells were stimulated using IL-2 (2 ng/mL). (B) MC-9 mast cells were stimulated using IL-4 (1 ng/mL), (C) IL-9(1 ng/mL), or (D) IL-3 (1.25% IL-3-BPV-SN). After 48 hours, [3H]thymidine was added, and 16 hours later, cells were harvested and radioactivity was measured in a β-counter. The data presented are representative of at least 5 comparable assays per cytokine tested. Bars marked with w/o represent cytokine proliferation in the absence of inhibitor (100% control value).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/1/10.1182_blood.v97.1.183/6/m_h80110531006.jpeg?Expires=1764475169&Signature=yVh6ZvCZIioGEbeLMNbe3FybzMVqVN3fzSWpzz5OF1vTSFdsLHAQcZSywEin~bwX7LkKpQzYSbMB9o92kD04raNJCwA7sA-4~X83yemkgcDMKsccXVZbWJppswgL3H6XOiSChG76ScJZTGJatpCZ7iRP60W5Ml5d2exWzJKfVtrwrBAundxftIOJ6hoZzWPB7Mo2etkoOKqRBV5M0w0R1y7wToQ5Di8zkx3ywSgDiKkEeIodIDQe-Lm-UhJnG9Ztf6Su4Qy-BSDNcu4LPAiFHleIOMSx~mFfj53-0ttOtdaYysk7EFMt6gPsGzZPlgzq0K6gT4pq9l46Z8C7e9J~QQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)