Abstract
Administration of donor T cells expressing the herpes simplex–thymidine kinase (HS-tk) with a hematopoietic stem cell (HSC) transplantation could allow, if graft-versus-host disease (GVHD) was to occur, a selective in vivo depletion of these T cells by the use of ganciclovir (GCV). The study evaluates the feasibility of such an approach. Escalating numbers of donor HS-tk–expressing CD3+ gene-modified cells (GMCs) are infused with a T-cell–depleted bone marrow transplantation (BMT). Twelve patients with hematological malignancies received 2 × 105(n = 5), 6 × 105 (n = 5), or 20 × 105(n = 2) donor CD3+ GMCs/kg with a BMT from a human leukocyte antigen (HLA)–identical sibling. No acute toxicity was associated with GMC administration. An early increase of circulating GMCs followed by a progressive decrease and long-lasting circulation of GMCs was documented. GCV treatment resulted in significant rapid decrease in circulating GMCs. Three patients developed acute GVHD, with a grade of at least II, while one patient developed chronic GVHD. Treatment with GCV alone was associated with a complete remission (CR) in 2 patients with acute GVHD, while the addition of glucocorticoids was necessary to achieve a CR in the last case. Long-lasting CR occurred with GCV treatment in the patient with chronic GVHD. Unfortunately, Epstein-Barr virus–lymphoproliferative disease occurred in 3 patients. Overall, the administration of low numbers of HS-tk–expressing T cells early following an HLA-identical BMT is associated with no acute toxicity, persistent circulation of the GMCs, and GCV-sensitive GVHD. Such findings open the way to the infusion of higher numbers of gene-modified donor T cells to enhance post-BMT immune competence while preserving GCV-sensitive alloreactivity.
Introduction
A hematopoietic graft comprises stem cells capable of durably reconstituting multilineage hematopoiesis and mature immunocompetent cells such as T cells. These T cells are capable of recognizing the host as “foreign” and are thus responsible for one of the most severe and limiting complications of allogeneic hematopoietic stem cell (HSC) transplantation: graft-versus-host disease (GVHD).1 On the other hand, these same T lymphocytes have an important role in facilitating engraftment, and they mediate long-lasting antitumor effects: the graft-versus-leukemia (GVL) effect.2 This GVL effect is the most potent cancer immunotherapy available at the present time3 and justifies, by itself, the use of allogeneic HSC transplantation in the treatment of a wide spectrum of hematological malignancies. Although some evidence suggests that GVHD and GVL are separable, most data support the notion that donor T cells are the dominant effector cells for both the harmful GVHD and the beneficial GVL.
Ex vivo T-cell depletion (TCD) of the graft4 and post-transplantation immunosuppression5 are the 2 methods presently used routinely to prevent the deleterious effects of allogeneic reactivity after transplantation. Although very efficient in preventing GVHD, ex vivo TCD is associated with increased graft rejection and leukemia relapse as well as overall immune incompetence.4 In contrast, posttransplantation immunosuppression does not interfere with marrow engraftment and allows for a significant GVL effect, even in patients not experiencing significant GVHD.5 For these reasons, post-transplantation immunosuppression is often preferred to TCD for GVHD prophylaxis. Unfortunately, this approach is only partly successful in preventing GVHD, and results in significant acute and chronic GVHD-induced mortality and morbidity, especially after matched-unrelated or partly matched-related transplantation.1 Furthermore, severe GVHD remains difficult to treat, and the broad immunosuppressive agents used in such a setting often increase the incidence of lethal infectious complications.6 The difficulties encountered in dealing with post-HSC transplantation alloreactivity have been highlighted by the demonstration that the use of an anti–interleukin-2 (anti–IL-2) receptor monoclonal antibody (mAb) during the first 28 days after non-TCD bone marrow transplantation (BMT) could result in an unchanged GVHD incidence, reduced GVL effect, and significantly reduced disease-free survival.7 Lastly, donor lymphocyte infusion to treat relapse after transplantation has proven to be a very efficient treatment in patients with chronic myelogenous leukemia (CML) and, to a lesser extent, in other hematological malignancies.8 Unfortunately, such an approach, even late after transplantation, can result in severe GVHD including marrow aplasia.8 Overall, adequate modulation of alloreactivity remains an elusive goal, and new therapeutic strategies are clearly needed.
The introduction of a gene encoding a susceptibility factor (“suicide gene”) can make target cells sensitive to a chemotherapeutic agent that is ordinarily not toxic.9 The most established among the suicide genes is the thymidine kinase (tk) enzyme from the herpes simplex 1 virus (HS-tk).10 11 In contrast to mammalian tk, HS-tk is capable of phosphorylating specific nucleoside analogues, such as ganciclovir (GCV), to nucleoside monophosphate. The nucleoside monophosphate is then phosphorylated by a cellular kinase to nucleoside triphosphate and incorporated into DNA, thereby leading to inhibition of DNA synthesis and resulting in the death of dividing cells.
The ex vivo transfer of the HS-tk gene into T cells before their infusion with a TCD graft could allow for selective in vivo depletion of these T cells with GCV if subsequent GVHD developed. Thus, with the early infusion of such cells, one could preserve the beneficial effects of the T cells on engraftment and tumor control early after transplantation and throughout the post-transplantation period for patients not experiencing severe GVHD. In addition, the GCV-induced selective immunosuppression restricted to proliferating donor mature T cells infused with HSCs could result in less toxicity than the broad immunosuppressive agents presently used for GVHD treatment. Similarly, as reported by Bonini et al,12 the use of such HS-tk–expressing donor T cells with or without GCV could also contribute to preventing or treating GVHD associated with the use of donor lymphocytes for the treatment and/or prevention of tumor relapse3 or Epstein Barr virus–lymphoproliferative disease (EBV-LPD).13
We have established that anti-CD3 + IL-2–stimulated peripheral blood mononuclear cells (PBMCs) can be successfully transduced with a retroviral vector containing the HS-tk and neomycin phosphotransferase (NeoR) genes (G1Tk1SvNa; Genetic Therapy Inc. [GTI]/Novartis, Palo Alto, CA).14 A 5-day GCV exposure of the gene-modified cells (GMCs) resulted in a greater than 80% specific growth inhibition. GCV is effective at killing GMCs in vitro at concentrations found in vivo during GCV treatment for cytomegalovirus (CMV) infection.15 Gene-modified T cells are alloreactive with persisting GCV sensitivity. Culture of the GMCs for 2-4 weeks, as well as transduced HUT-78 (CD4+ T-cell lymphoma cell line) for several months, suggests satisfactory stability of HS-tk expression over time. GCV-induced growth inhibition of primary T cells is not associated with a bystander effect. Only T cells expressing HS-tk are killed. Lastly, transduced T lymphocytes maintain their functional activity and GCV sensitivity after cryopreservation.
We are conducting a phase I/II study involving the use of HS-tk–expressing donor T cells in conjunction with a TCD BMT.16 The primary objectives of our study are to evaluate the safety and toxicity of the GMCs administered in conjunction with a T-cell–depleted allogeneic marrow graft, the survival and functional capabilities of the GMCs in peripheral blood, and the effects of GCV treatment on GMC survival. Secondary objectives include defining the effects of the GMCs on GVHD occurrence, severity, and response to GCV treatment. We report the results concerning the first 12 patients given donor CD3+ GMCs with a TCD marrow graft from a human leukocyte antigen (HLA)–identical sibling.
Materials and methods
Clinical protocol and patient characteristics
Details of the protocol have been reported earlier.16 Twelve adult patients with an HLA-identical sibling donor received escalating doses of CD3+ GMCs with a TCD BMT. In all cases, the recipient age was greater than 40 years, and/or a female donor–male recipient sex-mismatch was present, as required by the protocol. Both of these factors have been associated with an increased risk of acute GVHD in the absence of T-cell depletion of the graft. Patient characteristics are detailed in Table1. After obtaining written informed consent from the prospective donor and the recipient, peripheral blood was harvested from the donor, and GMCs were prepared as described below and cryopreserved. The recipient then received an intensified conditioning regimen including 60 mg/kg cyclophosphamide 2 times, 10 mg/kg thiothepa, and 2 Gy fractionated total body irradiation 6 times followed by a TCD marrow graft. T-cell depletion involved complement-dependent cytotoxicity (anti-CD2, CD7 mAb; [Diaclone, Besançon, France]); rabbit complement [ETS Franche-Comté, Besançon, France] (n = 11) or CD34+ selection (Isolex 300I; Baxter Healthcare, Deerfield, IL) of the graft (n = 1, patient no. 8). Because of the occurrence of EBV-LPD, the protocol was amended to include a B-cell depletion (patients, nos. 6-12), which was obtained by the addition of an anti-CD19 mAb (Diaclone) to the anti–T-cell mAb or a positive selection of the graft CD34+ cells. Marrow graft characteristics, as well as the number of GMCs infused, are detailed in Table 2.
The median number of CD34+ cells in the graft was 1.63 × 106 cells per kg (range, 0.55-3.95 × 106 cells per kg), and the median number of unmodified CD3+ cells in the graft was 0.81 × 105 cells per kg (range, 0.05-2.36 × 105 cells per kg). Thawed GMCs were infused 15 minutes before the infusion of the marrow graft. Escalating amounts (semi-log increase) of HS-tk–expressing T cells were administered in the absence of refractory GVHD in prior patients included in the protocol. The starting dose level was 2 × 105 donor CD3+ GMCs/kg of recipient. This number of T cells was chosen based on previous studies suggesting that the threshold of donor T lymphocytes for GVHD in HLA-matched recipients is approximately 1 × 105 clonable T cells per kg.17 Because of theoretical concerns that there might be an added GVHD risk with the GMCs as a result of the period of ex vivo culture, all patients received cyclosporine from day −1 (prior to transplantation) to days 60-90 after rapid tapering for GVHD prophylaxis. For CMV and herpes simplex virus prophylaxis, we administered 60 mg/kg per day intravenous (IV) Foscarnet, starting at day −1, followed by 200 mg oral acyclovir for 4 times a day starting between day 20 and 30.18 We initiated 5 mg/kg GCV treatment twice a day in the case of suspected or proven systemic CMV infection, biopsy-proven acute GVHD classified greater than grade I (Glücksberg classification), or extensive chronic GVHD. Grade I acute GVHD was not treated. Glucocorticoid treatment (2 mg/kg per day methylprednisolone) was added to the GCV in the absence of response or in the presence of grade III to IV acute GVHD. Study end points included toxicity of GMC infusion as well as in vivo survival and GCV sensitivity of the GMCs. Presently, patient accrual is interrupted because of clinical-grade retroviral vector unavailability.
Retroviral vector
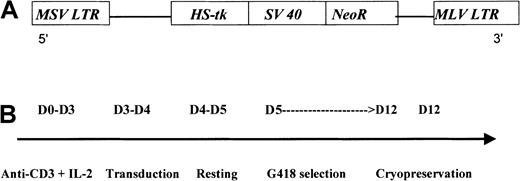
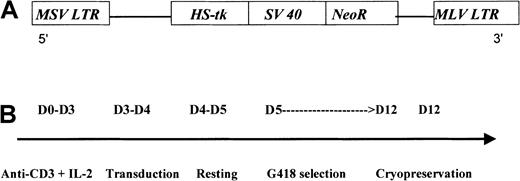
G1Tk1SvNa is a retroviral vector derived from the Moloney murine leukemia virus. This vector contains an HS-tk gene cDNA transcribed from the viral LTR and a bacterial neomycin resistance (NeoR) gene transcribed from an internal simian virus 40 (SV40) early promoter (LTR/HS-tk/SV40-LTR) in a G1 vector backbone (GTI/Novartis, Gaithersburg, MD) (Figure1A). This G1-based vector has been modified for increased safety by alteration of the gag start codon and by elimination of viral sequences needed for the formation of the virus. The vector construct was transfected into the PA317 packaging cell line. The G1TKSVNa cells were selected in G418 and cloned. Both vector and producer cell line have been previously described in detail.19
G1Tk1SvNa vector and preparation of the HS-tk–expressing T cells.
G1TK1SvNA vector (A) and steps for GMC preparation (B). MSV indicates Moloney sarcoma virus; LTR, long terminal repeat; MLV, Moloney leukemia virus.
G1Tk1SvNa vector and preparation of the HS-tk–expressing T cells.
G1TK1SvNA vector (A) and steps for GMC preparation (B). MSV indicates Moloney sarcoma virus; LTR, long terminal repeat; MLV, Moloney leukemia virus.
Preparation of the GMCs
PBMC isolation and activation (step 1).
We collected 150-300 mL peripheral blood from the HSC donor. PBMCs were then isolated by centrifugation over Ficoll (Pharmacia-Biotech, Uppsala, Sweden) and washed before being transferred into a LifeCell bag (Baxter) containing culture media (CM) comprising Roswell Park Memorial Institute medium (RPMI 1640) with 25 mmol/L 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), penicillin, and streptomycin (Biowhittaker, Verviers, Belgium) in addition to 5% to 10% autologous or allogeneic serum (ETS-Franche-Comté, Besançon, France). The cells were then cultured at 37°C and 5% carbon dioxide (CO2) for 3 days in the presence of 10 ng/mL CD3 mAb/OKT-3 (Janssen-Cilag, Levallois, France) and 500 U/mL IL-2 (Chiron, Emeryville, CA).
Retroviral-mediated PBMC transduction (step 2).
Activated cells were then transferred to the retroviral vector–containing medium (multiplicity of infection of 3) supplemented with 1000 U/mL IL-2 plus 5 μg/mL protamine sulfate (Choay, Gentilly, France) and cultured at 37°C and 5% CO2 for 24 hours. A cell aliquot was cultured in parallel in CM with IL-2 without the retroviral vector (nontransduced control cells).
Cell washing and resting period (step 3).
At day 4, the cells were washed and further cultured in fresh CM with 500 U/mL IL-2 for an additional 24 hours.
Selection of transduced cells (step 4).
The cells were subsequently cultured in CM containing 500 U/mL IL-2 and 800 μg/mL G418 (Sigma, Saint Quentin Fallavier, France) from days 5-12. An aliquot of transduced cells was cultured in parallel without G418 (transduced-unselected cells). Other controls included non-transduced–selected cells (positive control for G418 toxicity) and non-transduced–unselected cells cultured in parallel in CM plus IL-2 with or without G418, respectively.
Dead cell removal and cryopreservation of the GMCs (step 5).
At day 12, dead cells were removed by centrifugation over Ficoll. Viable cells were washed twice and cryopreserved in 4% human albumin (LFB, Les Ullis, France) and 8% to 10% DMSO (Braun Medical, Boulogne, France) before being stored in liquid nitrogen until clinical use. The cost estimation for GMC preparation (excluding costs related to viral supernatant production and testing) is $4000 per donor.20
Quality-control of the GMCs
GCV sensitivity of GMCs was evaluated as previously described14 by hydrogen 3 (3H)-thymidine incorporation (3HdT, 0.037 MBq [1 μCi] per well; specific activity, 7.4 × 1010 Bq/mmol [2 Ci/mmol]) (Amersham, Les Ulis, France) after a 10-day culture in the presence of 500 U/mL IL-2 with or without GCV. The assay was considered positive if the inhibition of the GMCs by 1 μg/mL GCV was greater than 80%.
Quantification of gene-modified cells
IL-2 dependence.
After dead cell removal on day 12, a small fraction of the GMCs were not cryopreserved and further cultured in CM in the presence or absence of 500 U/mL IL-2. Cell growth was assessed until complete death of cultured cells without IL-2.
Cell phenotype.
Cells were stained with CD3-FITC (fluorescein isothiocyanate), CD4-FITC, CD8-PE (phycoerythrin), and CD56-PE mAb (Becton Dickinson, Le Pont de Claix, France) and analyzed by flow cytometry using a fluorescence-activated cell sorter, FACScan, and CellQuest program (both from Becton Dickinson).
Cell viability.
Cell viability was assessed on cultured cells or on frozen cells after thawing by the trypan blue dye exclusion assay.
Monitoring for mycoplasma contamination.
Mycoplasma contamination was assessed by polymerase chain reaction (PCR) with the use of mycoplasma group-specific primers and by enzyme-linked immunosorbent assay (ELISA) (Boehringer Mannheim, Meylan, France).
Sterility controls and endotoxin levels.
Standard sterility controls were performed on the culture supernatant. Endotoxin levels were determined using a Limulus Amoebocyte Lysate assay (Euromedex, Souffelweyersheim, France).
Screening for replication competent recombinants.
GMCs and culture supernatant were screened for replication competent recombinants (RCRs) both by biological assays and by PCR. Mus dunni amplification assays21 were performed by Q-one Biotech (Glasgow, Scotland) or by GTI (Gaithersburg, MD).22 RCR detection by PCR involved both the detection of a sequence of the amphotropic envelope gene (adapted from Dunbar et al23 and previously described20) and the detection of a putative recombinant sequence, as predicted from homologous recombination of the 5′-portions of the vector and helper sequences present in the packaging cell.22 All samples were negative for RCRs.
RCR detection in vivo
The presence of circulating RCRs was assessed on peripheral blood leukocytes by either PCR or a vector rescue culture assay22 at baseline and every 3 months for the first year, then yearly. In the case of PCR positivity, confirmation of RCR presence by a culture assay was required. All samples were negative either by PCR or culture assay. In addition, a cocultivation of peripheral blood leukocytes with Mus dunni cells and retrovirus detection by feline S+L− (PG4) assay was performed in the 3 patients who developed EBV-LPD (Q-one Biotech). RCR was not detected.
In vitro and in vivo detection and quantification of GMCs
For in vitro and in vivo quantification of GMCs, we developed a competitive PCR assay for the NeoR gene based on coamplification of an internal homologous competitor with the wild-type DNA, which was extracted from cultured cells or PBMCs.24We engineered, by mutagenesis PCR, a plasmid construct with a 5′-end 30 base pair (bp)–deleted NeoR DNA sequence. The primers used to amplify a part of the wild-type NeoR gene as well as the NeoR competitor were: Fam-Rml3 [44]: 5′-Fam-GGTGGAGAGGCTATTCGGCTATGA-3′, and Rml4 [467]: 5′-TCCTGA- TCGACAAGACCGGCTTCG-3′. The numbers in brackets represent the position at which each primer sequence begins with respect to the start codon. The sense primer was labeled with the 6-Fam fluorescent dye in order to label the PCR products. Each 50-μL PCR reaction contained, in a final concentration, 1.5 mmol/L magnesium dichloride (MgCl2), 1 μmol/L of each PCR primer, and 0.5 UTaq DNA polymerase (Eurogentec, Seraing, Belgium) and was performed in a PTC 200 thermocycler (MJ Research, Watertown, MA). Amplification profile was 3 minutes at 94°C followed by cycles of 1 minute at 94°C, 1.5 minutes at 60°C, 1 minute at 72°C, and a final extension of 5 minutes at 72°C. The PCR was stopped during the predetermined exponential phase of the reaction.
For each sample quantification, a large scale of known serial dilution of the competitor DNA was added to a constant amount of target DNA (1 μg genomic DNA). The second quantification was performed with a more restricted interval of standard concentrations. Fluorescent dye–labeled PCR products (423 bp and 393 bp in length for the wild-type and standard DNA, respectively) were separated on 6% acrylamide-urea gel electrophoresis on a 373A DNA sequencer, and fluorescence intensity was analyzed with 672 Genescan Software (Applied Biosystems, Foster City, CA). A regression line was designed for each competition assay with 3 or 4 determinations by plotting the log of the ratio (R) of amplified products against the log of the initial number of standard molecules added into the PCR reaction. The original number of target molecules in the sample before amplification corresponds to the intersection point of the regression curve and the abscissa axis where log R = 0, so R = 1 (target equals standard sequences). The equation of the regression line and its regression coefficient were determined. A regression coefficient of greater than 0.9 was required for each quantification. The assay was validated by quantification of a blinded panel of 7 dilutions of transduced cells in untransduced cells. All the transduced cells (provided by GTI) derived from a cell line containing only one proviral copy of the gene, as determined by insertion site Southern blot analysis.19 In all cases, calculation of the percentage of GMCs was made assuming one transgene copy per GMC.25 26
A semiquantitative PCR assay was used in some instances to assess for the presence of GMCs among PBL or in skin biopsies. For negative first-run NeoR-PCR samples, we designed a nested pair of primers: Rml5 [103]: 5′-GCCGTGTTCCGGCTGTCAGC-3′, and Rml6 [432]: 5′- GCTTGGTGGTCGAATGGGC-3′. The second run of NeoR PCR was performed, using 2 μL of the first-run PCR products, with the same conditions as described above. The sensitivity of this nested PCR, determined by amplification of diluted positive DNA, was 10−5. Briefly, DNA from the packaging cell line was diluted and amplified by PCR using the same conditions as described above. PCR products were analyzed on agarose gels, Southern blotted, and hybridized with a radio-labeled oligomeric-specific NeoR probe: [101]: 5′-TGACAGCCGGAACACGGC-3′. Hybridization levels from samples were quantified by comparing the sample signal with the diluted cell line DNA PCR signals.
Pathology
Skin biopsies were obtained from all patients, and a salivary gland biopsy was obtained from patient No. 9, who had suspected GVHD. Biopsy specimens were fixed in 10% formaldehyde or Bouin fixative, embedded in paraffin, and stained with hematoxylin-eosin-soffron for microscopic examination. Histological changes were graded according to Lerner et al.27
Results
Preparation and characteristics of TK+ cells
All GMC preparations met the microbiological quality control criteria (eg, sterility and absence of RCRs). Functional and phenotypical quality control criteria are reported in Table3. A median transduction efficiency (before G418 selection) of 8.3% (range, 1.5% to 19.7%), combined with the anti-CD3 +IL-2–induced in vitro cell proliferation, resulted in 6.1 more GMCs (range, 1.5-15.4 GMCs) at day 12 than PBMCs at input (day 0). A majority of the GMCs were T cells (median, 90.5 cells; range, 71.4-97.2 cells) with a significant fraction of CD56+ natural killer (NK) cells (median, 13.0 cells; range, 4-45 cells) (Table 2). Within the T cells the fraction of CD4+ and CD8+ varied significantly, with a median 39.8% CD4+ (range, 28.3% to 76.4%) and 52.5% CD8+ (range, 23% to 67.4%). Median inhibition of GMCs by 1 μg/mL GCV was 87.0% (range, 80% to 93%). Over the same time period, GMC preparation failure was observed twice in 2 additional donors due to inadequate G418 selection of GMCs in one case and low transduction efficiency in the second case.
GMC administration
Administration of GMCs was associated with no acute toxicity. The number of CD3+ T cells within the GMCs and infused with the marrow graft was 2 × 105 cells per recipient kg for the first 5 patients, 6 × 105 cells per kg for the next 5 patients, and 20 × 105 cells per kg for the last 2 patients (Table 1). Three patients received a second GMC infusion; 2 patients (Nos. 1 and 5) received GMCs to treat EBV-LPD (2 × 106 CD3+ cells per kg at day 49 and 1.7 × 106 cells per kg at day 62, respectively). Patient No. 7, allografted for Ph+ acute lymphocytic leukemia (ALL), received 5.7 × 106 CD3+ cells per kg at day 133 because of mixed chimerism associated with the recurrence of a bcr/abl transcript (PCR) in PBMCs.
Engraftment
Initial engraftment was demonstrated in all patients. However, 2 patients (Nos. 7 and 10) experienced late graft failure (10 and 4 months after BMT, respectively) with severe pancytopenia, which required the infusion of a second unmodified HSC graft from the same donor. Both of these second grafts resulted in long-lasting donor engraftment.
Detection and quantification of circulating GMCs
After administration, circulating GMCs could be detected in vivo as early as one hour after infusion and for periods of more than one year in evaluable patients. Circulating GMCs were found in all 12 patients early after transplantation. At time of analysis, 11 of 12 patients were evaluable for GMC detection 2 months after transplantation; 7 patients were evaluable 4 months after transplantation; 5 patients at 6 months; and 4 patients at more than 12 months. Two months after transplantation, circulating GMCs could be found in 10 of 11 patients. The patient (No. 6) without detectable GMCs at day 60 had very low levels of circulating GMCs early after BMT and before receiving GCV at day 20 for acute GVHD. Unfortunately, quantification data were not available for 3 patients (Nos. 2, 3, and 4) because of an insufficient amount of stored biological material.
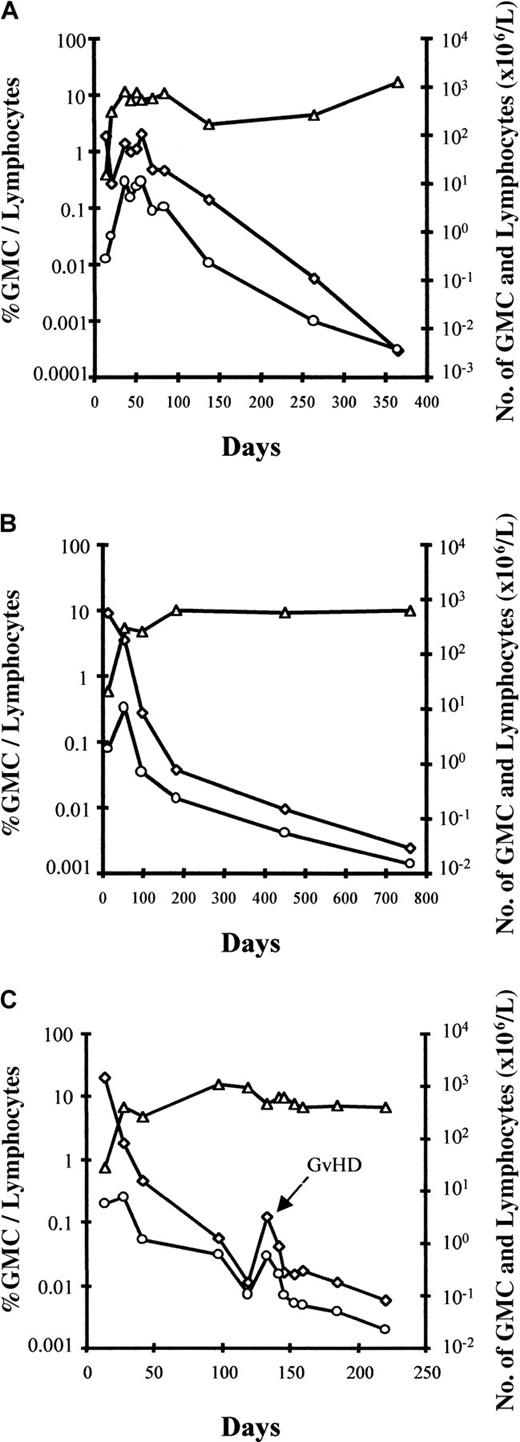
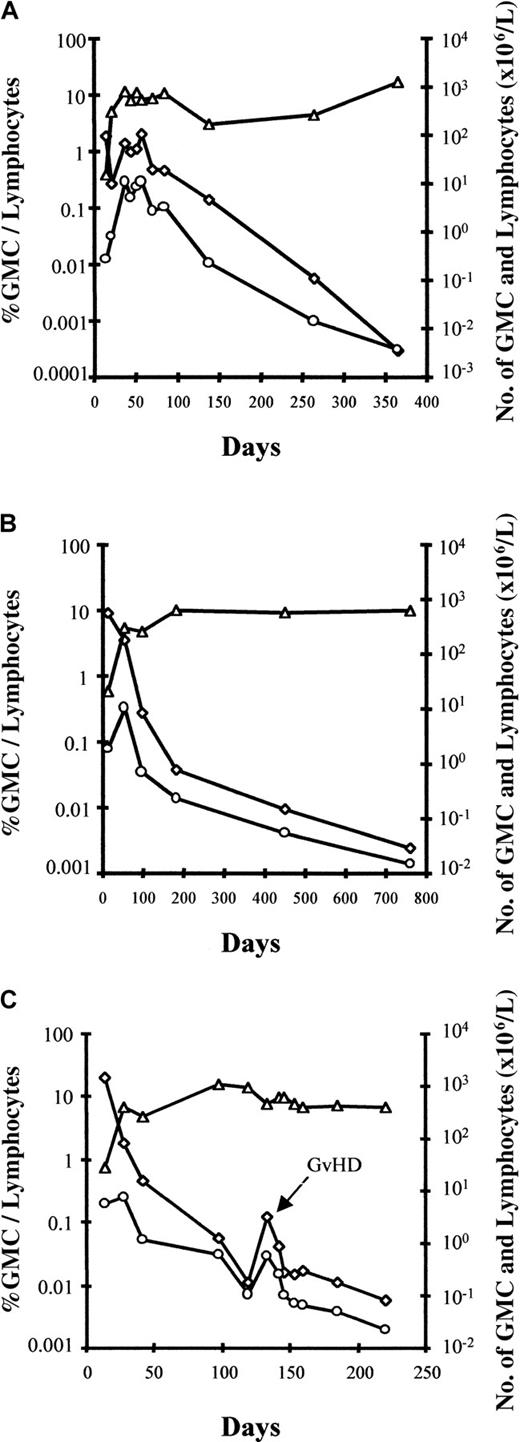
Interestingly, an early increase in the number of circulating GMCs was observed, at which time GMCs comprised a substantial fraction of circulating lymphocytes, before a subsequent progressive decrease in the fraction and absolute number of circulating GMCs over time (Figure2). An estimate of the total number of circulating GMCs early after BMT suggests that these cells expanded in vivo after BMT, with a maximum number of circulating GMCs between day 20 and 35 before progressively decreasing, at least within the circulating compartment. Using the assumption that 2% of the total pool of lymphocytes are circulating in the blood,28 maximum in vivo circulating GMC expansion varied significantly from one patient to another, with a 2-fold to more than 1200-fold cell expansion (median, 62-fold). The patient with the highest in vivo GMC expansion was diagnosed with EBV-LPD 2 weeks later. In all evaluable patients, the number of GMCs decreased progressively in the blood. However, circulating GMCs continued to be detectable in all patients who were evaluable for more than one year after adoptive transfer. Importantly, as evidenced in Figure 2C for patient No. 9, GVHD occurrence was associated with an increased fraction of GMCs among circulating lymphocytes as well as an increased absolute number of circulating GMCs. Such an increase in circulating GMCs at the time of GVHD occurrence was observed in all evaluable patients.
Early increase in the number of circulating GMCs observed before a subsequent progressive decrease in the fraction and absolute number of circulating GMCs.
Kinetics of circulating cells in patient no. 7 (A), patient no. 8 (B), and patient no. 9 (C) with a follow-up of more than 1 year. ◊ indicates the percentage of GMCs among circulating lymphocytes; ○, the absolute number of circulating GMCs; and ▵, lymphocytes. For patient no. 9 (panel C), circulating GMCs after day 220 were still detected, but only by nested PCR (less than 10−5 cells); the latest date analyzed is day 500. The fourth patient, no. 6, with a follow-up of more than 1 year had very low (nonquantifiable) levels of circulating GMCs early after BMT before receiving GCV for acute GVHD (D20).
Early increase in the number of circulating GMCs observed before a subsequent progressive decrease in the fraction and absolute number of circulating GMCs.
Kinetics of circulating cells in patient no. 7 (A), patient no. 8 (B), and patient no. 9 (C) with a follow-up of more than 1 year. ◊ indicates the percentage of GMCs among circulating lymphocytes; ○, the absolute number of circulating GMCs; and ▵, lymphocytes. For patient no. 9 (panel C), circulating GMCs after day 220 were still detected, but only by nested PCR (less than 10−5 cells); the latest date analyzed is day 500. The fourth patient, no. 6, with a follow-up of more than 1 year had very low (nonquantifiable) levels of circulating GMCs early after BMT before receiving GCV for acute GVHD (D20).
Effect of GCV treatment on the number of circulating GMCs
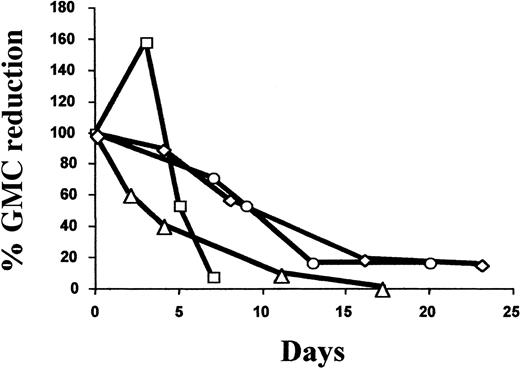
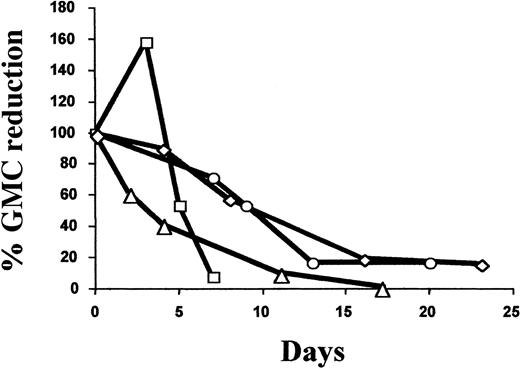
Six patients (50%) received GCV treatment. Five patients (Nos. 1, 3, 6, 9, and 11) received GCV treatment for greater than grade I acute (n = 4) or chronic (n = 1) GVHD. Patient No. 11 received GCV for refractory GVHD after infusion of unmanipulated donor lymphocytes at day 205 because of tumor relapse (B-cell lymphoma). Patient No. 12 was treated with GCV for CMV lung infection associated with grade I acute GVHD. As detailed in Table4, the fraction of GMCs among circulating lymphocytes at the time of GVHD diagnosis and initiation of GCV varied widely. In all evaluable cases, GCV treatment significantly reduced the percentage and absolute number of GMCs among circulating lymphocytes, with a mean decrease of 92.7% (range, 85% to 98.1%; n = 4) and 85.3% (range, 75.8% to 99.7%; n = 3), respectively (Table 3). As detailed in Figure 3, the decrease in the percentage of circulating GMCs occurred progressively over a 2-week period. Importantly, GCV treatment resulted in a similar decrease in circulating GMCs soon after transplantation as well as later (3-8 months after transplantation). This constant susceptibility suggests stable HS-tk gene expression. With the exception of patient No. 6, who had low nonquantifiable levels of circulating GMCs, GCV treatment was associated with the subsequent persistence of circulating GMCs, albeit at a low level.
Kinetics of the GCV-induced reduction of circulating GMCs.
Arbitrarily, the 100% value corresponds to the percentage of GMCs among PBMCs at day 0, the date when GCV treatment was started. Values for subsequent dates are calculated as the percent reduction of GMCs among PBMCs compared with the day 0 value. ▵ indicates patient no. 1; ○, patient no. 9; ■, patient no. 11; and ◊, patient no. 12.
Kinetics of the GCV-induced reduction of circulating GMCs.
Arbitrarily, the 100% value corresponds to the percentage of GMCs among PBMCs at day 0, the date when GCV treatment was started. Values for subsequent dates are calculated as the percent reduction of GMCs among PBMCs compared with the day 0 value. ▵ indicates patient no. 1; ○, patient no. 9; ■, patient no. 11; and ◊, patient no. 12.
GVHD occurrence and outcome
Following BMT and GMC infusion, 3 patients developed greater than grade I acute GVHD, and one patient developed extensive chronic GVHD (Table 1). Two patients (Nos. 1 and 6) developed grade II acute GVHD involving the skin at days 31 and 20, respectively; patient No. 3 developed grade III GVHD involving both skin and liver. Skin biopsy confirmed the diagnosis of acute GVHD in all cases. All patients were receiving cyclosporine for GVHD prophylaxis. GCV treatment was initiated as the only treatment in all cases and was associated with a CR in 3 of the 4 patients. Improvement in the acute GVHD skin lesions of responding patients was observed 24-48 hours after GCV initiation, with a CR occurring within one week. Bilirubin levels in the patient with grade III GVHD returned to normal levels with slower kinetics within 3 weeks. In the nonresponding patient (No. 6), skin lesions remained unmodified after 3 days of GCV treatment. The subsequent addition of a glucocorticoid (2 mg/kg methylprednisolone) resulted in rapid CR. Unfortunately, lack of biological material prevented the quantification of circulating GMCs in this last patient at the time of GVHD. However, semiquantitative PCR suggested a very low fraction of GMCs before GCV treatment and a disappearance of identifiable GMCs after GCV. Patient No. 9 developed extensive chronic GVHD with biopsy-proven skin (lichenoid GVHD) and salivary gland involvement at day 134. The patient was not taking cyclosporine. GVHD was treated by GCV alone for 5 days. Six days after GCV initiation, a significant reduction in the cutaneous lesions, as well as the disappearance of the sicca syndrome, were observed. Concomitant treatment included a one-time dose of 400 mg/kg IV immunoglobulin for hypogammaglobulinemia. Three weeks later, at a time when chronic GVHD lesions had disappeared, the patient developed extensive depigmentation. Vitiligo was confirmed by skin biopsy. There were no signs of chronic lichenoid GVHD observed. PCR for the presence of GMCs in the skin was negative.29
EBV-induced lymphoproliferative disease
Three patients developed EBV-LPD. Patient No. 1 developed diffuse EBV-lymphoma at day 45 (2 weeks after GCV-sensitive acute GVHD). Interruption of CSA and reinfusion of GMC (2 × 106CD3+ cells per kg) resulted in achieving a CR. Unfortunately, the patient subsequently died of a cerebral toxoplasmosis. Patient No. 5 was diagnosed with polyclonal EBV-LDP at day 60 and died at day 72. Autopsy revealed the concomitant presence of invasive lung aspergillosis. Lastly, a third patient (No. 10) developed a lethal EBV-lymphoma 4 weeks after a second transplantation from the same donor. The second transplantation (granulocyte colony-stimulating factor [G-CSF] mobilized unmanipulated peripheral blood stem cells), performed on day 152 because of secondary pancytopenia, was conditioned by cyclophosphamide and antithymocyte globulin. In all 3 cases, there was no evidence for the presence of vector in the tumor cells or circulating RCRs.
Overall survival
Four patients are alive in CR with a follow-up of 29-38 months. Among the patients (Nos. 2 and 5-10) who received transplantations in early-stage disease, the overall survival is 4 of 7 patients. The causes of death are listed in Table 1.
Discussion
The primary objectives of this study are to evaluate the safety of administrating HS-tk–expressing donor T cells in conjunction with a T-cell–depleted marrow graft, to study the in vivo survival and circulation of the GMCs, and to evaluate the effects of GCV on the survival of such cells. With such objectives in mind, the study combines both gene-marking aspects, namely the in vivo fate of mature donor T cells infused with a marrow graft, and gene-therapy aspects, ie, the feasibility of selectively destroying donor T cells in vivo in the presence of deleterious alloreactivity. Results from the first 12 patients with an HLA-identical sibling donor included in the study provide valuable information with respect to feasibility of the approach, toxicity associated with GMC administration, in vivo survival and GCV sensitivity of such cells, and GVHD response to GCV.
Gene-modified HS-tk–expressing donor T cells, meeting all quality control criteria, can be reproducibly prepared. Administration of GMCs at the first 3 dose levels (2 × 105 to 2 × 106 CD3+ GMCs/kg) was associated with no acute toxicity. Early after BMT, GMCs comprise a significant fraction of the circulating PBMCs before progressively decreasing both as a percentage of the total lymphocytes and in absolute numbers. In addition, the increase in the number of circulating GMCs early after BMT clearly demonstrates initial in vivo expansion of such cells. Exact interpretation of these findings is hampered by variables such as the phenotype of circulating GMCs; their circulation pattern between blood, lymphoid organs, and other host tissues; and the number of unmodified T or NK cells provided by the marrow graft or stemming from post-engraftment lymphopoiesis. A majority of the infused GMCs are CD3+ T cells. However, CD56+ NK cells are also present among the GMCs, and NK can constitute a high fraction of circulating PBMCs early after BMT.30 Unfortunately, the limited number of GMCs available for analysis has prevented us from identifying the T- or NK-cell phenotype of the circulating GMCs. The development of techniques combining flow-PCR and cell surface phenotype analysis26 might, in the future, be helpful to address this question.
The progressive reduction in the number of circulating GMCs could be due to factors related to the use of GMCs, or the reduction could just reflect the fate of the mature T cells infused with an HSC graft. A significant fraction of the GMCs probably circulates out of the blood stream and into the tissues in response to various factors including allogeneic or microbial antigens. Such cells clearly circulate within the body, as demonstrated by the presence of GMCs in tissues such as skin, liver, lymph nodes, and brain (data not shown). In addition, the increase in circulating GMCs observed both early after BMT and at the time of GVHD further substantiates in vivo function of such cells. A limited lifespan and/or altered function of such cells could also contribute to the observed progressive reduction in the number of circulating GMCs. GMC preparation required ex vivo culture for 12 days in the presence of IL-2. Such a process might impair functional properties of the T cells. In vitro, GMCs are capable of proliferating in response to HLA-mismatched irradiated PBMCs, albeit less strongly than unmanipulated cells from the same donor.14Polyclonality of GMCs, as measured by V-β T-cell repertoire analysis (CDR3 size analysis by immunoscope), reveals the presence, within a mostly preserved repertoire, of limited donor-specific “skewing” within various V-β subfamilies.31 Importantly, identical repertoire alterations are found in control-cultured untransduced donor T cells, thus strongly suggesting that such findings result from the ex vivo culture per se and not from the transduction and/or selection process.
In vivo, we have demonstrated that similarly cultured murine T cells are capable of strong alloreactivity, as evidenced by the occurrence of lethal acute GVHD in an allogeneic BMT model.32 However, GVHD-related mortality after administration of the cultured T cells was observed later than after administration of the same number of fresh T cells, indicating reduced in vivo alloreactivity of the cultured T cells. A number of factors, including prior ex vivo activation, impaired migration, and increased sensitivity to apoptosis (as suggested by increased Fas expression), could contribute to these findings. Nevertheless, the demonstration of long-term GMC circulation confirms the prolonged life span of at least a fraction of such cells, as initially suggested by studies involving ADA- and NeoR-expressing autologous T cells in ADA-deficient patients.25,33 In addition, our study demonstrates for the first time that allogeneic mature T cells infused with an HSC graft can persist for more than 2 years after transplantation. Such a long-term persistence of GMCs also argues against the presence of cytotoxic immune responses againstHS-tk and/or NeoR transgenes, as described by Riddell et al34 and Verzeletti et al.35Indeed, such an immune response was associated with a disappearance of the GMCs. In the latter case, BMT recipients had received repeated administration of HS-tk–expressing donor T cells late after BMT (ie, after immune restoration). In our study, GMCs were administered only once, together with a TCD HSC graft, following an intensified conditioning regimen and in the presence of cyclosporine.
GVC treatment resulted in a significant rapid reduction in the number of circulating GMCs in evaluable patients. These results were obtained in the absence of any chemotherapeutic agent. The reduction in the number of circulating GMCs occurred during a 10- to 15-day period, with a percentage reduction in circulating GMCs comparable to the 80% to 93% in vitro GCV-induced growth inhibition of GMCs before their infusion. Importantly, GCV treatment resulted in a similar decrease in circulating GMCs both early as well as late after transplantation, thus suggesting stable HS-tk gene expression. GVC treatment did not prevent the long-term persistence of very low levels (often below the threshold for quantification) of circulating GMCs. Our findings differ significantly from the findings reported by Bonini et al12 in 2 aspects. Indeed, they observed a very rapid GCV-induced reduction in the number of GMCs with, in less than 8 days, a disappearance (less than 10−4) of circulating GMCs. Our findings could be explained by a number of factors. GCV inhibition of HS-tk–expressing T cells requires cell division and should therefore leave unaffected noncycling cells.36 In addition, recent studies have shown that viral LTR-driven expression of a transgene (HS-tk in the present case) is dependent on the state of activation of target T cells.37 One could therefore postulate that nonactivated HS-tk–expressing donor T cells could be preserved from GCV toxicity. Although unproved at the moment, such an in vivo selectivity of GCV toxicity could be of considerable clinical relevance by preserving nonactivated donor T cells that could subsequently contribute to immune competence. Defective expression ofHS-tk and/or expression of a nonfunctional HS-tktransgene could also contribute to persisting circulating GMCs. Indeed, we have established that packaging cell lines, such as G1Tk1SvNa, can produce a low level of virions containing a spliced HS-tkgene that can result, after transduction, in GCV-resistant T cells.38 However, such a finding should not significantly contribute to the differences between our data and the data from Bonini et al,12 as a similar HS-tk splicing also occurs in the packaging cell line used in their study.38Other factors, such as the level of HS-tk expression or the sensitivity of the PCR analysis used for detection, might also account for the observed differences.
Nevertheless, GCV treatment was associated with a complete remission in 2 of 3 patients experiencing acute GVHD in the absence of any other form of immunosuppressive therapy. Such a result further confirms the potential of this approach to efficiently control acute GVHD. An additional patient with chronic GVHD also responded to GCV with a complete disappearance of both skin lesions and sicca syndrome. This latter patient received concomitant IV immunoglobulin because of hypogammaglobulinemia. Although this is not standard treatment for chronic GVHD and although it was only administered once, one cannot totally rule out the possibility that the immunoglobulin infusion may have contributed to the disappearance of the chronic GVHD lesions.39 One patient with grade II acute GVHD skin lesions did not respond to GCV and had persisting lesions after 3 days of treatment. Interestingly, this patient had very low nonquantifiable levels of circulating GMCs before GVHD occurrence, so that untransduced donor T cells infused with the marrow might have significantly contributed to the occurrence of GVHD. Other possible reasons for GCV resistance included defective HS-tk expression, as discussed above, as well the possibility that specific ablation of the donor T cell infused with the graft might not always be sufficient to adequately control ongoing acute GVHD. Such a possibility is further substantiated by our findings in a murine BMT model involving the use of HS-tk–expressing donor T cells.40 GCV treatment, initiated at the time of acute GVHD, significantly prevented GVHD-induced death. However, a number of recipients still died of GVHD, thus suggesting that combining GCV with other treatment modalities might sometimes be necessary to control ongoing GVHD.
Acute GVHD occurred in 3 of 12 patients despite the low number of gene-modified T cells infused and the use of cyclosporine after BMT. We recently reported a pilot study involving the infusion of 2-2.5 × 105 nonmodified donor T lymphocytes per kg (same amount as for the first 5 patients in our protocol) with a similar conditioning regimen as well as a T-cell–depleted allogeneic graft in patients over 40 years old.41 Cyclosporine and steroids were administered following BMT. Of 21 patients, 5 patients developed greater than grade I acute GVHD, and 6 of 17 evaluable patients developed chronic GVHD. In addition, cyclosporine might affect differently the alloreactivity of ex vivo cultured versus unmanipulated T cells. We have indeed demonstrated, in a murine GVHD model, that prophylactic cyclosporine had no effect on GVHD induced by ex vivo–expanded T cells, while as expected, delayed GVHD-related mortality was observed in mice receiving fresh splenocytes.42 These findings could be highly relevant when considering allogeneic T-cell therapy approaches.
The occurrence of EBV-related LPD in 3 of 12 patients is a disturbing finding. EBV-LPD is a well-established complication following allogeneic BMT.43 Patients in our protocol accumulated risk factors: recipient age; intensive conditioning regimen; low T-cell dose; use of cyclosporine; and in one patient, a second transplantation following ATG treatment. Direct involvement of the GMCs in the occurrence of the EBV-LPD is highly unlikely. We did not find a vector in the tumor cells, nor did we find circulating RCRs. Lastly, the LPD was unequivocally linked to EBV in all cases. In our previous study involving nonmodified T cells, EBV-LPD occurred in 2 of 21 patients.41 The occurrence of these 3 cases of EBV-LPD clearly highlights defective immune surveillance despite the GMC infusion. Our protocol called for starting with the infusion of low numbers (2 × 105 cells per kg for the first patients) of gene-modified T cells and a slow dose escalation. Low numbers of T cells, which were also cultured ex vivo, as discussed above, almost certainly contributed to defective immunosurveillance and thus to the occurrence of EBV-LPD. Preliminary data from our laboratory suggest that frequency of pre-CTL precursors against EBV is indeed reduced in GMCs compared to fresh T cells.
In an effort to reduce such a risk, a B-cell depletion was added to the T-cell graft depletion. Indeed, graft B-cell depletion has been associated with a reduced EBV-LPD incidence.44 In addition, in view of the absence of uncontrolled GVHD in the first 12 patients, we have also significantly increased the number of infused gene-modified T cells (2 × 107 cells per kg) as well as abandoned GVHD prophylaxis by cyclosporine for subsequent patients. Lastly, introducing a CD3/CD28 costimulation instead of CD3/IL-2 might reduce GMC T-cell repertoire skewing31 and enhance T-cell immune function (E. Robinet, unpublished data, May 2000).
Overall, we demonstrate that the use of HS-tk–expressing donor T cells at the time of allogeneic BMT is feasible and is associated with the absence of acute toxicity, persistent circulation of the GMCs, and GCV-sensitive GVHD. Further studies exploring the administration of increasing numbers of suicide-expressing donor T cells after transplantation are warranted to further evaluate and confirm these early findings and to improve post-transplant immune competence. Such studies will hopefully demonstrate that “squaring the alloreactive circle” (adequate engraftment, tightly controlled GVHD, enhanced GVL effect, and immune competence) is indeed a reasonable goal. If so, the use of such gene-modified donor T cells could significantly contribute to expanding the use of alloreactivity as a treatment modality in settings such as unrelated and/or HLA-mismatched transplantation,1 nonmyeloablative conditioning regimens,45,46 older recipients, treatment of solid tumors,47,48 autoimmune diseases,49 or induction of tolerance after organ transplantation.50
Acknowledgments
We would like to thank Francis Ruscetti and Jonathan Keller for their important contribution to the preclinical studies as well as Will Jacob, Gerard McGarrity, Ed Otto, Zhifeng Long, Chris Lockey, Sheila Weir, and Kerry Atkinson for their contribution to the clinical trial.
Supported by the Programme Hospitalier de Recherche Clinique (PHRC No 950898), la Ligue Nationale contre le Cancer (Centre Réseau de Développement des Thérapies Géniques [CRTG]), l'Association Française contre la Myopathie (CRTG), le Ministère de l'Enseignement Supérieur et de la Recherche (CRTG), Genetic Therapy Inc, Novartis, and the European Community (Biomed contract No CT97-2074).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pierre Tiberghien, Etablissement Français du Sang, Bourgogne/ Franche-Comté, 1 bvd Fleming, 25000 Besançon, France; e-mail: pierre.tiberghien@univ-fcomte.fr.