Abstract
Intensive, myelosuppressive therapy is necessary to maximize outcomes for patients with acute myeloid leukemia (AML). A comparison was made of 3 aggressive postremission approaches for children and adolescents with AML in a randomized trial, CCG-2891. A total of 652 children and adolescents with AML who achieved remission on 2 induction regimens using identical drugs and doses (standard and intensive timing) were eligible for allocation to allogeneic bone marrow transplantation (BMT) based on matched related donor status (n = 181) or randomization to autologous BMT (n = 177) or to aggressive high-dose cytarabine-based chemotherapy (n = 179). Only 115 patients (18%) refused to participate in the postremission phase of this study. Overall compliance with the 3 allocated regimens was 90%. At 8 years actuarial, 54% ± 4% (95% confidence interval) of all remission patients remain alive. Survival by assigned regimen (“intent to treat”) is as follows: allogeneic BMT, 60% ± 9%; autologous BMT, 48% ± 8%; and chemotherapy, 53% ± 8%. Survival in the allogeneic BMT group is significantly superior to autologous BMT (P = .002) and chemotherapy (P = .05); differences between chemotherapy and autologous BMT are not significant (P = .21). No potential confounding factors affected results. Patients receiving intensive-timing induction therapy had superior long-term survival irrespective of postremission regimen received (allogeneic BMT, 70% ± 9%; autologous BMT, 54% ± 9%; chemotherapy, 57% ± 10%). Allogeneic BMT remains the treatment of choice for children and adolescents with AML in remission, when a matched related donor is available. For all others, there is no advantage to autologous BMT; hence, aggressive nonablative chemotherapy should be used.
Introduction
Aggressive induction chemotherapy and postremission treatment are important for optimal treatment of acute myeloid leukemia (AML) in children, adolescents, and young adults.1-3 Prior to 1990, there had been modest improvement in overall outcome in children using standard anthracycline/cytarabine induction followed by intensification with aggressive chemotherapy or, when HLA matching permitted, allogeneic (or “allo”) bone marrow transplantation (BMT) with a sibling donor.4-7 Some, but not all, “biologically randomized” studies have suggested that long-term outcome has been significantly superior with allogeneic BMT.4 7 Unfortunately, these studies have documented an overall long-term survival that has not exceeded 35%.
More recently, several groups have investigated the utility of allogeneic BMT compared with chemotherapy in adults as well as in adolescents and children with AML in first remission.8-11Although not all studies have shown significant differences, overall trends have suggested that younger patients may fare better with allogeneic BMT.9,10,12 Less improvement, if any, has been realized in older individuals.8,11 In addition, the role of autologous (or “auto”) BMT has also been extensively studied, in large part because of the ability to perform this procedure in all patients in remission without regard to availability of a matched sibling donor. Overall results comparing autologous BMT to the other 2 postremission modalities have been inconsistent.8-11,13 Major criticisms have been raised about these studies, including poor compliance with the randomized regimens and relatively short follow-up periods.14
We herein report the results of the largest randomized trial to date in adults or children with AML comparing allogeneic BMT, autologous BMT, and aggressive postremission chemotherapy. Preliminary results were presented in abstract form.15 Overall study results were delayed, however, to allow adequate follow-up of all 3 arms to include late deaths from either leukemia relapse or treatment complications, such as graft-versus-host disease. Participation and compliance in the randomized postremission phase of this study were both high. Allogeneic BMT gave significantly superior results compared with the other 2 treatment modalities. Furthermore, we document that children and adolescents clearly do as well with postremission chemotherapy as with autologous BMT.
Patients and methods
Children's Cancer Group (CCG) Study CCG-2891 opened in October 1989 and closed in April 1995. Children and adolescents younger than 21 years of age with blood and marrow biopsy confirmation of a diagnosis of AML types MO-M7, acute undifferentiated or biphenotypic leukemia with evidence of myeloid differentiation noted on cytologic examination, myelodysplastic syndrome, or granulocytic sarcoma were eligible for participation. French-American-British (FAB) classification was initially recorded by institution pathologists, with central review for most cases, and a consensus diagnosis determined. Patients with known Fanconi anemia or those with Philadelphia chromosome–positive chronic myeloid leukemia in the chronic phase were excluded. Of 1114 children registered, 18 were deemed to be ineligible after central review showed a diagnosis other than those noted above. To define a group of children and adolescents that would parallel AML in young and middle age adults, the following patients were excluded from analysis in this report: (1) Down syndrome as a predisposing factor (n = 104); (2) AML as a second malignant neoplasm (n = 19); (3) granulocytic sarcoma and no evidence of bone marrow involvement (n = 7); and (4) de novo myelodysplastic syndrome (n = 79). The remaining 887 patients form the basis of this report. The other subgroups not analyzed here have been or will be reported separately.
Patients and family members were required to undergo serologic HLA typing at the time of AML diagnosis for study participation. Patients or families signed consent forms after the protocol was approved by each participating CCG member's institutional review board. The study was regularly evaluated by a data monitoring committee.
Therapy
Many of the details of the treatment on CCG-2891 have been previously reported in detail.3 Patients at diagnosis were randomized to 2 induction regimens in which identical drugs and doses were used and administered, with randomization between a “standard-timing” and “intensive-timing” approach. Patients received a 5-drug cycle of induction therapy administered over 4 days: dexamethasone, cytarabine, 6-thioguanine, etoposide, and daunorubicin (DCTER). Daunorubicin, cytarabine, and etoposide were all administered as a continuous infusion for 96 hours. Patients randomized to intensive timing received a second obligatory cycle of DCTER therapy identical to cycle No. 1 after a 6-day rest, irrespective of BM or hematologic status. Delays of 2 to 4 days were permitted for patients who experienced severe ileus or other life-threatening events with cycle No. 1. Patients randomized to standard-timing therapy had a bone marrow examination, including biopsy on day 14. If there was evidence of clearing of circulating blasts and a hypoplastic marrow indicating a large leukemia kill from the first cycle, cycle No. 2—identical to cycle no. 1—was held until the patients' blood counts recovered or there were clear signs of leukemia progression. Patients with residual leukemia documented on day 14, defined as more than 40% blasts in a mildly hypocellular to hypercellular marrow, received cycle No. 2 at that time.
Four total induction cycles were administered to all patients prior to entering the postremission phase, even for patients achieving remission during the first 2 cycles, to guarantee uniform drug dosing. Standard-timing induction therapy was closed in May 1993 after recommendation by the Data Monitoring Committee, with all patients subsequently receiving the intensive-timing arm. Furthermore, filgrastim (granulocyte colony-stimulating factor) was introduced for all patients during the induction phase. This addition has had no overall effect on induction success, postremission outcome, or overall survival; results will be reported separately.
For the 330 patients receiving standard timing and available for analysis, 73% were in remission after 4 cycles. The 271 patients receiving intensive timing without filgrastim had a 77% induction success rate, and 82% of the 251 patients receiving intensive timing plus filgrastim achieved remission. Superiority of intensive-timing induction in both event-free and overall survival has been previously reported.3 15
At the end of induction, patients with 5 or 6 antigen HLA-matched family donors were allocated to allogeneic BMT. All others were eligible for marrow harvesting with 4-hydroperoxycyclophosphamide ex vivo purging.16,17 These patients were randomized between intensification therapy requiring autologous BMT rescue versus non–marrow-ablative therapy consisting of 4 total courses of 3 different chemotherapy regimens, each lasting 4 to 6 weeks. Course 1 consisted of intensively timed high-dose cytarabine used in the previous CCG AML trial for newly diagnosed patients.3 6
Patients allocated to allogeneic BMT or randomized to autologous BMT received a preparative regimen at a CCG-certified BMT center consisting of 4 days of oral busulfan, 16 mg/kg of body weight total; and 4 days of intravenous cyclophosphamide, 200 mg/kg total, with marrow infused after a 1-day rest.3 Graft-versus-host disease prophylaxis for allogeneic patients consisted of intravenous methotrexate over 100 days.3 All other therapeutic considerations and required observations have previously been described.3
Statistical considerations
Analyses of the data obtained in this study through July 1999 were performed with the use of several standard methods. Results were calculated as of the day of last contact, with a cutoff of February 1, 1999. For the postremission randomization, patients were stratified according to induction regimen received.
Differences in survival and disease-free survival (DFS) from the end of induction therapy and in survival and event-free survival from the time on study were tested for significance using the log-rank statistic.18 Patients lost to follow-up were censored at their last known point of study. Survival rates were estimated by the method of Kaplan and Meier, and confidence intervals were calculated using Greenwood's formula.19 The significance of observed differences in proportions was tested using theX2 statistic and Fisher exact test when appropriate for small samples. All reported comparisons were based on regimens to which patients were allocated or randomized at the end of induction (“intent to treat”).
Results
Compliance with the postremission phase
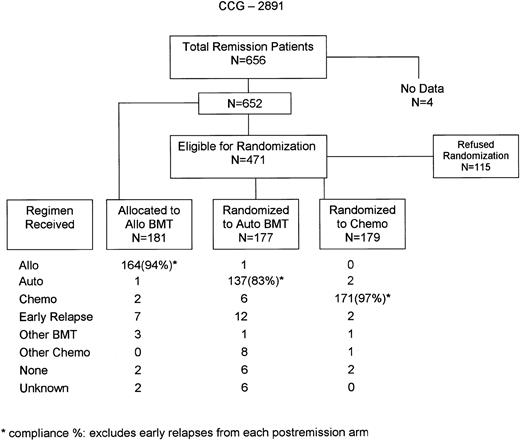
A total of 652 patients with data successfully completed all 4 induction cycles and were eligible for allocation to allogeneic BMT or randomization to autologous BMT or intensive chemotherapy. Figure1 demonstrates the flow of patients in the postremission phase and compliance. Only 115 patients, or 18% of remission patients, refused randomization. Compliance with the 3 postremission arms ranged from 77% to 96%. However, if one excludes patients who had early relapses and hence were not eligible to start the actual postremission chemotherapy, compliance rates were between 83% and 97%. Overall compliance for patients who agreed to participate with the allocations/randomization minus early relapses was 91% and was 88% even if one counts early relapse patients.
Patient characteristics
Table 1 documents presenting characteristics of patients achieving remission on CCG-2891, including many variables that have shown some prognostic significance in previous CCG trials.17 20 In general, various patient characteristics were equally divided among the 3 postremission arms. Of major importance, there were no statistical differences among the ordering of the 3 arms based on white blood count at diagnosis or various cytogenetic abnormalities.
Characteristics of patients achieving remission on CCG-2891 (n = 537)
| . | Allogeneic BMT (n = 181) . | Autologous BMT (n = 177) . | Chemotherapy (n = 179) . | Total . | (%) . | P value for homogeneity . | |||
|---|---|---|---|---|---|---|---|---|---|
| No. . | (%) . | No. . | (%) . | No. . | (%) . | ||||
| Induction regimen | |||||||||
| Intensive | 113 | (64) | 115 | (66) | 108 | (61) | 336 | (64) | |
| Standard | 64 | (36) | 59 | (34) | 69 | (39) | 192 | (36) | .61 |
| Age, y | |||||||||
| 0-1 | 24 | (13) | 43 | (24) | 49 | (27) | 116 | (22) | |
| 2-10 | 78 | (43) | 68 | (38) | 62 | (35) | 208 | (39) | |
| 11-20 | 79 | (44) | 66 | (37) | 68 | (38) | 213 | (40) | .018 |
| White blood count (φL) | |||||||||
| Median | 18.000 | 22.250 | 21.300 | 20.900 | |||||
| Range | .500-351.200 | .500-532.800 | 1.100-345.800 | .500-532.800 | .15 | ||||
| FAB subtype | |||||||||
| M0 | 6 | (4) | 4 | (2) | 5 | (3) | 15 | (3) | |
| M1 | 28 | (17) | 25 | (15) | 31 | (18) | 84 | (16) | |
| M2 | 45 | (28) | 51 | (30) | 44 | (26) | 140 | (27) | |
| M3 | 10 | (6) | 9 | (5) | 11 | (6) | 30 | (6) | |
| M4 | 45 | (28) | 42 | (25) | 39 | (23) | 126 | (25) | |
| M5 | 22 | (14) | 28 | (16) | 31 | (18) | 81 | (16) | |
| M6 | 6 | (4) | 1 | (1) | 4 | (2) | 11 | (2) | |
| M7 | 10 | (6) | 11 | (6) | 5 | (3) | 26 | (5) | |
| Unknown | 9 | — | 6 | — | 9 | — | 24 | — | .77 |
| Cytogenetics | n = 79 | n = 83 | n = 77 | n = 239 | |||||
| Normal | 15 | (19) | 18 | (22) | 17 | (22) | 50 | (21) | .87 |
| 11q23 abnormality | 11 | (14) | 12 | (14) | 19 | (25) | 42 | (18) | .14 |
| t(8;21) | 15 | (19) | 12 | (14) | 5 | (6) | 32 | (13) | .07 |
| t(15;17) | 6 | (8) | 4 | (5) | 6 | (8) | 16 | (7) | .70 |
| 16q abnormality | 9 | (11) | 7 | (8) | 7 | (9) | 23 | (10) | .80 |
| +8 | 2 | (3) | 4 | (5) | 2 | (3) | 8 | (3) | .83 |
| -7/7q- | 3 | (4) | 0 | (0) | 2 | (3) | 5 | (2) | .22 |
| Other | 18 | (23) | 26 | (31) | 19 | (25) | 63 | (26) | |
| . | Allogeneic BMT (n = 181) . | Autologous BMT (n = 177) . | Chemotherapy (n = 179) . | Total . | (%) . | P value for homogeneity . | |||
|---|---|---|---|---|---|---|---|---|---|
| No. . | (%) . | No. . | (%) . | No. . | (%) . | ||||
| Induction regimen | |||||||||
| Intensive | 113 | (64) | 115 | (66) | 108 | (61) | 336 | (64) | |
| Standard | 64 | (36) | 59 | (34) | 69 | (39) | 192 | (36) | .61 |
| Age, y | |||||||||
| 0-1 | 24 | (13) | 43 | (24) | 49 | (27) | 116 | (22) | |
| 2-10 | 78 | (43) | 68 | (38) | 62 | (35) | 208 | (39) | |
| 11-20 | 79 | (44) | 66 | (37) | 68 | (38) | 213 | (40) | .018 |
| White blood count (φL) | |||||||||
| Median | 18.000 | 22.250 | 21.300 | 20.900 | |||||
| Range | .500-351.200 | .500-532.800 | 1.100-345.800 | .500-532.800 | .15 | ||||
| FAB subtype | |||||||||
| M0 | 6 | (4) | 4 | (2) | 5 | (3) | 15 | (3) | |
| M1 | 28 | (17) | 25 | (15) | 31 | (18) | 84 | (16) | |
| M2 | 45 | (28) | 51 | (30) | 44 | (26) | 140 | (27) | |
| M3 | 10 | (6) | 9 | (5) | 11 | (6) | 30 | (6) | |
| M4 | 45 | (28) | 42 | (25) | 39 | (23) | 126 | (25) | |
| M5 | 22 | (14) | 28 | (16) | 31 | (18) | 81 | (16) | |
| M6 | 6 | (4) | 1 | (1) | 4 | (2) | 11 | (2) | |
| M7 | 10 | (6) | 11 | (6) | 5 | (3) | 26 | (5) | |
| Unknown | 9 | — | 6 | — | 9 | — | 24 | — | .77 |
| Cytogenetics | n = 79 | n = 83 | n = 77 | n = 239 | |||||
| Normal | 15 | (19) | 18 | (22) | 17 | (22) | 50 | (21) | .87 |
| 11q23 abnormality | 11 | (14) | 12 | (14) | 19 | (25) | 42 | (18) | .14 |
| t(8;21) | 15 | (19) | 12 | (14) | 5 | (6) | 32 | (13) | .07 |
| t(15;17) | 6 | (8) | 4 | (5) | 6 | (8) | 16 | (7) | .70 |
| 16q abnormality | 9 | (11) | 7 | (8) | 7 | (9) | 23 | (10) | .80 |
| +8 | 2 | (3) | 4 | (5) | 2 | (3) | 8 | (3) | .83 |
| -7/7q- | 3 | (4) | 0 | (0) | 2 | (3) | 5 | (2) | .22 |
| Other | 18 | (23) | 26 | (31) | 19 | (25) | 63 | (26) | |
Postremission outcome
Patients entering the postremission phase have now been followed from a minimum of 4 years to more than 9 years. Overall actuarial survival from AML remission at 8 years is 54% ± 4% (2 SD); DFS for the same period is 48% ± 4%.
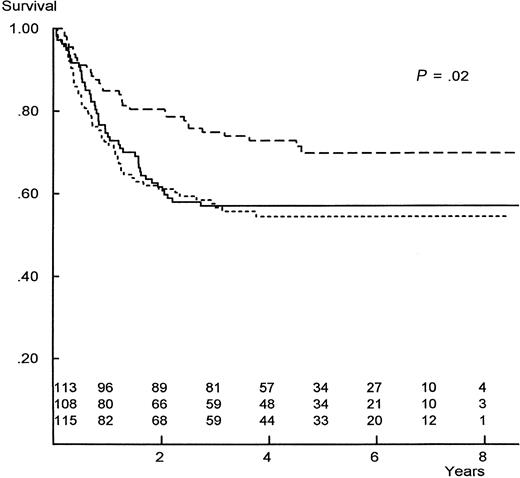
Figure 2 documents the log-rank survival for patients allocated or randomized to the 3 postremission arms, based on an intent-to-treat analysis. Patients allocated to allogeneic BMT show a significantly improved survival over patients randomized to either chemotherapy or autologous BMT, with 8-year actuarial figures of 60% ± 9%, 53% ± 8%, and 48% ± 8%, respectively. No significant differences are noted between the chemotherapy and autologous arms (Figure 2 and Table2). The major improvement in survival for allogeneic BMT is associated with a markedly lower relapse rate compared with the other 2 regimens, as noted in Figure3.
Actuarial survival from AML remission, comparing the 3 postremission regimens from CCG-2891.
Numbers are patients at risk at yearly intervals; rows are in the same order as curves. P value is for homogeneity. Dashed line indicates allogeneic BMT; solid line, intensive non–marrow-ablative chemotherapy; dotted line, autologous BMT.
Actuarial survival from AML remission, comparing the 3 postremission regimens from CCG-2891.
Numbers are patients at risk at yearly intervals; rows are in the same order as curves. P value is for homogeneity. Dashed line indicates allogeneic BMT; solid line, intensive non–marrow-ablative chemotherapy; dotted line, autologous BMT.
Outcomes at 8 years actuarial, comparing the 3 postremission regimens from CCG-2891
| . | Allogeneic BMT . | Autologous BMT . | Chemotherapy . | |||
|---|---|---|---|---|---|---|
| . | P value (allo vs auto) . | . | P value (auto vs chemo) . | . | Pvalue (chemo vs allo) . | |
| All patients (n = 537) | 181 | 177 | 179 | |||
| Survival | 60% ± 9% | .002 | 48% ± 8% | .21 | 53% ± 8% | .05 |
| Disease-free survival | 55% ± 9% | .001 | 42% ± 8% | .31 | 47% ± 8% | .01 |
| Patients receiving intensive-timing induction (n = 336) | 113 | 115 | 108 | |||
| Survival | 70% ± 9% | .006 | 54% ± 9% | .67 | 57% ± 10% | .02 |
| Disease-free survival | 66% ± 9% | .003 | 48% ± 9% | .53 | 53% ± 10% | .02 |
| . | Allogeneic BMT . | Autologous BMT . | Chemotherapy . | |||
|---|---|---|---|---|---|---|
| . | P value (allo vs auto) . | . | P value (auto vs chemo) . | . | Pvalue (chemo vs allo) . | |
| All patients (n = 537) | 181 | 177 | 179 | |||
| Survival | 60% ± 9% | .002 | 48% ± 8% | .21 | 53% ± 8% | .05 |
| Disease-free survival | 55% ± 9% | .001 | 42% ± 8% | .31 | 47% ± 8% | .01 |
| Patients receiving intensive-timing induction (n = 336) | 113 | 115 | 108 | |||
| Survival | 70% ± 9% | .006 | 54% ± 9% | .67 | 57% ± 10% | .02 |
| Disease-free survival | 66% ± 9% | .003 | 48% ± 9% | .53 | 53% ± 10% | .02 |
Actuarial probability of relapse after AML remission, comparing the 3 postremission regimens from CCG-2891.
Numbers are patients at risk at yearly intervals; rows are in the same order as curves. P value is for homogeneity. Dashed line indicates allogeneic BMT; solid line, intensive non–marrow-ablative chemotherapy; dotted line, autologous BMT.
Actuarial probability of relapse after AML remission, comparing the 3 postremission regimens from CCG-2891.
Numbers are patients at risk at yearly intervals; rows are in the same order as curves. P value is for homogeneity. Dashed line indicates allogeneic BMT; solid line, intensive non–marrow-ablative chemotherapy; dotted line, autologous BMT.
Toxicity of the postremission regimens
Table 3 lists the National Cancer Institute (NCI) grade 3-4 (serious, life-threatening) nonhematologic toxicity associated with the 3 postremission regimens. As expected, there was more gastrointestinal and hepatic toxicity among the allogeneic BMT patients. Infections, especially bacteremia/sepsis, were very common in all regimens. Average time to neutrophil recovery (> 0.5 × 109/L [>500/μL]) was 23 days in the allogeneic BMT arm, 47 days in the autologous BMT arm, and 35 days after the high-dose cytarabine chemotherapy course. Overall nonleukemia deaths were 14% in the allogeneic BMT arm, 5% in the autologous BMT arm (7 of 9 deaths from infections), and 4% in the chemotherapy arm (all 8 infections). Only 8 (32%) of the allogeneic deaths occurred in the 100 days post-BMT. There were no apparent differences in toxicity in the postremission arms based on which induction regimen was used. Hence, we found no data to suggest that a more toxic induction regimen leads to more toxicity during aggressive postremission therapy.
Postremission nonhematologic NCI grade 3-4 toxicity from CCG-2891
| Affected organ or system . | Allogeneic BMT . | Autologous BMT . | Chemotherapy . | P value for homogeneity . | |||
|---|---|---|---|---|---|---|---|
| n = 181 No. (%) . | P value (allo vs auto) . | n = 177 No. (%) . | P value (auto vs chemo) . | n = 179 No. (%) . | P value (chemo vs allo) . | ||
| Gastrointestinal tract | 47 (26) | .04 | 30 (17) | < .01 | 15 (8) | < .0001 | < .01 |
| Liver | 59 (33) | < .0001 | 16 (9) | .82 | 15 (8) | < .0001 | < .01 |
| Lungs | 6 (3) | .16 | 2 (1) | .0002 | 19 (14) | .006 | < .01 |
| Heart | 4 (2) | .72 | 3 (2) | .03 | 11 (6) | .06 | .04 |
| Central nervous system | 3 (2) | .98 | 3 (2) | .64 | 2 (1) | .66 | .91 |
| Infection, any | 72 (40) | .54 | 76 (43) | < .0001 | 116 (65) | < .0001 | .001 |
| Infection, bacteremia/sepsis | 47 (26) | .19 | 57 (32) | < .0001 | 97 (54) | < .0001 | .001 |
| Affected organ or system . | Allogeneic BMT . | Autologous BMT . | Chemotherapy . | P value for homogeneity . | |||
|---|---|---|---|---|---|---|---|
| n = 181 No. (%) . | P value (allo vs auto) . | n = 177 No. (%) . | P value (auto vs chemo) . | n = 179 No. (%) . | P value (chemo vs allo) . | ||
| Gastrointestinal tract | 47 (26) | .04 | 30 (17) | < .01 | 15 (8) | < .0001 | < .01 |
| Liver | 59 (33) | < .0001 | 16 (9) | .82 | 15 (8) | < .0001 | < .01 |
| Lungs | 6 (3) | .16 | 2 (1) | .0002 | 19 (14) | .006 | < .01 |
| Heart | 4 (2) | .72 | 3 (2) | .03 | 11 (6) | .06 | .04 |
| Central nervous system | 3 (2) | .98 | 3 (2) | .64 | 2 (1) | .66 | .91 |
| Infection, any | 72 (40) | .54 | 76 (43) | < .0001 | 116 (65) | < .0001 | .001 |
| Infection, bacteremia/sepsis | 47 (26) | .19 | 57 (32) | < .0001 | 97 (54) | < .0001 | .001 |
Potential confounding variables
Several potential prognostic factors, including patient characteristics at diagnosis, were examined for any confounding effect on the results obtained above. Table 4represents an analysis of risk factors, with comparison of the 3 postremission arms given for various subsets. In general, the superiority of allogeneic BMT over both chemotherapy and autologous BMT was documented despite stratification by age, white blood cell count at AML diagnosis, FAB morphologic classification, and cytogenetics. Three particular groups seemed to fare especially poorly with autologous BMT: age 0 to 2 years, FAB M4 histology, and inv(16q) abnormalities. Small numbers of patients in various subsets and many analyses preclude definitive conclusions.
Survival at 8 years actuarial in CCG-2891, comparing the 3 postremission arms for various risk factors
| Risk factor . | Allogeneic BMT . | Pvalue (allo vs auto) . | Autologous BMT . | Pvalue (auto vs chemo) . | Chemotherapy . | Pvalue (chemo vs allo) . | |||
|---|---|---|---|---|---|---|---|---|---|
| No. . | Survival, % . | No. . | Survival, % . | No. . | Survival, % . | ||||
| Age, y | |||||||||
| 0-1 | 24 | 71 | .03 | 43 | 40 | .009 | 49 | 61 | .60 |
| 3-10 | 78 | 56 | .07 | 68 | 49 | .34 | 62 | 55 | .46 |
| 11-20 | 79 | 61 | .17 | 66 | 51 | .43 | 68 | 45 | .03 |
| White blood count at diagnosis (SI units) | |||||||||
| < 20 | 96 | 66 | .08 | 81 | 54 | .59 | 83 | 58 | .22 |
| 20-100 | 67 | 49 | .05 | 66 | 42 | .36 | 63 | 48 | .30 |
| > 100 | 18 | 63 | .10 | 30 | 43 | .38 | 33 | 49 | .28 |
| FAB | |||||||||
| M0 | 6 | 40 | .21 | 4 | 100 | .05 | 5 | 20 | .03 |
| M1 | 28 | 42 | .62 | 25 | 47 | .44 | 31 | 61 | .66 |
| M2 | 45 | 66 | .34 | 51 | 52 | .79 | 44 | 57 | .47 |
| M3 | 10 | 68 | .51 | 9 | 44 | .65 | 11 | 55 | .65 |
| M4 | 45 | 62 | .003 | 42 | 38 | .07 | 39 | 53 | .24 |
| M5 | 22 | 68 | .07 | 28 | 50 | .44 | 31 | 58 | .21 |
| M6 | 6 | 50 | .43 | 1 | 100 | .30 | 4 | 25 | .31 |
| M7 | 10 | 58 | .33 | 11 | 36 | .91 | 5 | 40 | .56 |
| Cytogenetics | |||||||||
| Normal | 15 | 67 | .24 | 18 | 44 | .49 | 17 | 47 | .44 |
| 11q23 abnormality | 11 | 68 | .42 | 12 | 58 | .40 | 19 | 47 | .15 |
| t(8;21) | 15 | 47 | .73 | 12 | 42 | .23 | 5 | 80 | .30 |
| t(15;17) | 6 | 67 | .31 | 4 | 25 | .59 | 6 | 25 | .35 |
| Inv(16q) abnormality | 9 | 78 | .04 | 7 | 29 | .05 | 7 | 71 | .89 |
| Da AB +8 | 2 | 0 | .05 | 4 | 75 | .48 | 2 | 100 | .16 |
| -7/7q- | 3 | 67 | — | 0 | — | — | 2 | 50 | .59 |
| Other | 18 | — | — | 26 | — | — | 19 | — | — |
| Risk factor . | Allogeneic BMT . | Pvalue (allo vs auto) . | Autologous BMT . | Pvalue (auto vs chemo) . | Chemotherapy . | Pvalue (chemo vs allo) . | |||
|---|---|---|---|---|---|---|---|---|---|
| No. . | Survival, % . | No. . | Survival, % . | No. . | Survival, % . | ||||
| Age, y | |||||||||
| 0-1 | 24 | 71 | .03 | 43 | 40 | .009 | 49 | 61 | .60 |
| 3-10 | 78 | 56 | .07 | 68 | 49 | .34 | 62 | 55 | .46 |
| 11-20 | 79 | 61 | .17 | 66 | 51 | .43 | 68 | 45 | .03 |
| White blood count at diagnosis (SI units) | |||||||||
| < 20 | 96 | 66 | .08 | 81 | 54 | .59 | 83 | 58 | .22 |
| 20-100 | 67 | 49 | .05 | 66 | 42 | .36 | 63 | 48 | .30 |
| > 100 | 18 | 63 | .10 | 30 | 43 | .38 | 33 | 49 | .28 |
| FAB | |||||||||
| M0 | 6 | 40 | .21 | 4 | 100 | .05 | 5 | 20 | .03 |
| M1 | 28 | 42 | .62 | 25 | 47 | .44 | 31 | 61 | .66 |
| M2 | 45 | 66 | .34 | 51 | 52 | .79 | 44 | 57 | .47 |
| M3 | 10 | 68 | .51 | 9 | 44 | .65 | 11 | 55 | .65 |
| M4 | 45 | 62 | .003 | 42 | 38 | .07 | 39 | 53 | .24 |
| M5 | 22 | 68 | .07 | 28 | 50 | .44 | 31 | 58 | .21 |
| M6 | 6 | 50 | .43 | 1 | 100 | .30 | 4 | 25 | .31 |
| M7 | 10 | 58 | .33 | 11 | 36 | .91 | 5 | 40 | .56 |
| Cytogenetics | |||||||||
| Normal | 15 | 67 | .24 | 18 | 44 | .49 | 17 | 47 | .44 |
| 11q23 abnormality | 11 | 68 | .42 | 12 | 58 | .40 | 19 | 47 | .15 |
| t(8;21) | 15 | 47 | .73 | 12 | 42 | .23 | 5 | 80 | .30 |
| t(15;17) | 6 | 67 | .31 | 4 | 25 | .59 | 6 | 25 | .35 |
| Inv(16q) abnormality | 9 | 78 | .04 | 7 | 29 | .05 | 7 | 71 | .89 |
| Da AB +8 | 2 | 0 | .05 | 4 | 75 | .48 | 2 | 100 | .16 |
| -7/7q- | 3 | 67 | — | 0 | — | — | 2 | 50 | .59 |
| Other | 18 | — | — | 26 | — | — | 19 | — | — |
Most importantly, when the overall data were analyzed by regimen actually received, the results were the same, including no benefit of autologous BMT over chemotherapy. Furthermore, the superiority of allogeneic BMT over both chemotherapy and autologous BMT was noted when the data were analyzed for patients who received the standard versus intensive-timing regimens separately. Figure4 and Table 2 document the superior survival from AML remission for the 336 patients who received intensive-timing induction, with or without filgrastim, and were subsequently randomized or allocated to 1 of the 3 postremission arms. For the entire study, the overall superiority of intensive-timing induction remains striking, with 49% ± 5% surviving at 8 years from diagnosis versus 34% ± 6% for patients receiving the standard-timing arm, P = .002. These data are similar to those previously reported by us3 but represent an additional 4 years of follow-up.
Actuarial survival from AML remission for the CCG-2891 patients who received intensive-timing induction therapy, comparing the 3 postremission regimens.
Numbers are patients at risk at yearly intervals; rows are in the same order as curves. P value is for homogeneity. Dashed line indicates allogeneic BMT; solid line, intensive non–marrow-ablative chemotherapy; dotted line, autologous BMT.
Actuarial survival from AML remission for the CCG-2891 patients who received intensive-timing induction therapy, comparing the 3 postremission regimens.
Numbers are patients at risk at yearly intervals; rows are in the same order as curves. P value is for homogeneity. Dashed line indicates allogeneic BMT; solid line, intensive non–marrow-ablative chemotherapy; dotted line, autologous BMT.
Discussion
The overall role of aggressive myelosuppressive chemotherapy for children, adolescents, and young adults with AML is now firmly established. Studies have documented improved overall survival using intensified treatment in induction2,3 as well as in the postremission phase.1 21 This aggressive approach, however, is associated with increased morbidity and mortality, primarily related to infection and bleeding from prolonged myelosuppression.
For postremission therapy, there have been 2 major controversies: (1) Is the morbidity and mortality associated with graft-versus-host disease in allogeneic BMT worth the potential benefits of graft-versus-leukemia compared with myeloablative approaches not requiring engraftment across histocompatibility barriers? and (2) Can aggressive chemotherapy not requiring BMT rescue be as effective as a myeloablative approach with autologous rescue? Our study as well as others have attempted to answer these questions.7-11,13,17 One major limitation of previous randomized studies has been the use of less aggressive forms of postremission chemotherapy compared with current standards.4,7 We circumvented this problem by using an intensive high-dose cytarabine-based postremission approach that has been used successfully in previous trials.6 21
Another potential limitation of previous postremission AML trials is inadequate length of follow-up for all patients enrolled, irrespective of the particular approach taken. It has been known for years that survival curves, even for patients undergoing allogeneic BMT, do not plateau until 6 or more years, often with overall rates below 50%.22 Late deaths can be due to both relapse as well as complications of the transplantation, such as chronic graft-versus-host disease. Both were seen in our patients undergoing allogeneic BMT, with less early and late events for patients receiving intensive induction therapy compared with a more standard approach (Figures 2 and 4). For chemotherapy approaches, improvements have interestingly led to plateaus that appear earlier, as noted in our postremission chemotherapy patients who also received intensified induction (Figure4).
Most importantly, previous AML trials involving BMT have had major problems associated with patients refusing randomization or subsequently not complying with the allocated regimen.8,9,11 13 Compliance in this largest-ever postremission AML trial was very high (Figure 1), with only 18% of remission patients refusing randomization and most of those participating in the allocation/randomization complying with the assigned regimen. This high compliance rate greatly increases the validity of our trial, in which results reported by intent to treat were similar to not-reported results for regimen actually received.
Our results clarify some of the controversies noted above. First, for younger patients, including children and adolescents, allogeneic BMT for AML in first remission is the treatment of choice when a matched related donor is available. Every randomized trial in children and adolescents to date has shown a superiority of allogeneic BMT over other approaches,4-7,10 with many of the trials reaching statistical significance.4,7 Longer periods of “quality of life time” are also achieved.23 In older individuals, the role of allogeneic BMT is less clear, possibly due to a higher frequency and severity of graft-versus-host disease.8 11
Second, an aggressive chemotherapy approach not requiring myeloablation appears equally efficacious to autologous BMT when several aggressive chemotherapy courses are used in both the induction and intensification phases. There have been studies, mainly in adults, that have suggested that autologous BMT may be superior to chemotherapy.8,13Zittoun and colleagues from Europe compared autologous BMT with a somewhat less aggressive postremission regimen than ours.8Although DFS was superior in the autologous BMT arm, overall survival was comparable to chemotherapy.8 Burnett and colleagues in their MRC trial (10th United Kingdom Medical Research Council AML trial) added autologous BMT to an aggressive postremission regimen and again showed superior DFS but not significantly different overall survival results.13 One could argue that other preparative regimens may improve autologous BMT outcome,24,25 but none have been tested in large numbers of patients or compared with aggressive chemotherapy in a randomized fashion. Patients who relapse having had chemotherapy appear to fare better than those who relapse after autologous BMT.8,11 13Perhaps the latter modality should be reserved for patients at or after first relapse.
Several large trials involving children and adults confirm that nonmyeloablative therapy can cure a significant number of patients.7 9-11 The major theoretical advantage to allogeneic BMT appears to be its graft-versus-leukemia effect. Future research should be aimed at developing an effective immunotherapy approach to AML not requiring marrow ablation and development of chimerism.
Finally, for the first time in North America we have demonstrated a therapeutic approach to children and adolescents with AML that leads to cure half of the time. This statement is valid irrespective of the presence of a matched family donor; our results confirm those in a similarly aggressive trial of chemotherapy conducted in the United Kingdom.10 With hundreds of children now more than 4 years from AML remission and alive and healthy, cure for this particularly difficult cancer, when it occurs in the child or adolescent, is becoming more reality than not.
Contributing Children's Cancer Group institutions, investigators, and grant numbers follow: Group Operations Center, Arcadia, CA: W. Archie Bleyer, Anita Khayat, Harland Sather, Mark Krailo, Jonathan Buckley, Daniel Stram, and Richard Sposto, CA 13539; University of Michigan Medical Center, Ann Arbor, MI: Raymond Hutchinson, CA 02971; University of California Medical Center, San Francisco, CA: Katherine Matthay, CA 17829; University of Wisconsin Hospital, Madison, WI: Diane Pucccetti, CA 10382; Children's Hospital & Medical Center, Seattle, WA, J. Russell Geyer, CA 10382; Rainbow Babies & Children's Hospital, Cleveland, OH: Eric Kodish, CA 20320; Children's National Medical Center, Washington, DC: Gregory Reaman, CA 03888; Children's Hospital of Los Angeles, Los Angeles, CA: Frederick Ruymann, CA 03750; Columbia Presbyterian College of Physicians & Surgeons, New York, NY: Leonard H. Wexler, CA 03526; Children's Hospital of Pittsburgh, Pittsburgh, PA: A. Kim Ritchey, CA 36015; Vanderbilt University School of Medicine, Nashville, TN: James Whitlock, CA 26270; Doernbecher Memorial Hospital for Children, Portland, OR: H. Stacy Nicholson, CA 26044; University of Minnesota Health Sciences Center, Minneapolis, MN: Joseph Neglia, CA 07306; Children's Hospital Of Philadelphia, Philadelphia, PA: Beverly Lange, CA 11796; Memorial Sloan-Kettering Cancer Center, New York, NY: Peter Steinherz, CA 42764; James Whitcomb Riley Hospital for Children, Indianapolis, IN: Philip Breitfeld, CA 13809; University of Utah Medical Center, Salt Lake City, UT: William L. Carroll, CA 10198; University of British Columbia, Vancouver, Canada: Paul C. Rogers, CA 29013; Children's Hospital Medical Center, Cincinnati, OH: Robert Wells, CA 26126; Harbor/UCLA & Miller Children's Medical Center, Torrance/Long Beach, CA: Jerry Finklestein, CA 14560; University of California Medical Center (UCLA), Los Angeles, CA: Stephen Feig, CA 27678; University of Iowa Hospitals and Clinics, Iowa City, IA: Raymond Tannous, CA 29314; Childrens Hospital of Denver, Denver, CO: Lorrie Odom, CA 28851; Mayo Clinic and Foundation, Rochester, MN: Gerald Gilchrist, CA 28882; Izaak Walton Killam Hospital for Children, Halifax, NS, Canada: Dorothy Barnard; University of North Carolina, Chapel Hill, NC: Stuart Gold; University of Medicine & Dentistry of New Jersey, Camden, NJ: Richard Drachtman; Children's Mercy Hospital, Kansas City, MO: Maxine Hetherington; University of Nebraska Medical Center, Omaha, NE: Peter Coccia; Wyler Children's Hospital, Chicago, IL: James Nachman; MD Anderson Cancer Center, Houston, TX: Beverly Raney; Princess Margaret Hospital, Perth, Australia: David Baker; New York University Medical Center, New York, NY: Aaron Rausen; Childrens Hospital of Orange County, Orange, CA: Violet Shen.
Supported by grants from the Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Bethesda, MD.
Submitted February 7, 2000; accepted August 1, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William G. Woods, Children's Cancer Group, PO Box 60012, Arcadia, CA 91066-6012.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal