Abstract
The cytokine-induced C-C chemokine monocyte chemoattractant protein-1 (MCP-1) is an important regulator of leukocyte recruitment to sites of inflammatory challenge. Here, it is demonstrated that the widely distributed contact hapten NiCl2, like tumor necrosis factor α (TNFα), induces monocyte-chemoattractant activity in primary human endothelial cells via induction of MCP-1. NiCl2 rapidly activated mitogen-activated protein (MAP) kinase p38, and inhibition of p38 partially blocked NiCl2-induced MCP-1 messenger RNA and protein expression. Both NiCl2- and TNFα-induced MCP-1 synthesis was sensitive to D609, an inhibitor of phosphatidylcholine-dependent phospholipase C (PC-PLC). NiCl2-induced MCP-1 synthesis required activation of NF-κB since mutation of NF-κB–binding sites in the promoter resulted in complete loss of inducible promoter activity. Consistent with that finding, stimulation with NiCl2 or TNFα activated IκB kinase-β (IKKβ), and transient transfection of dominant-negative IKKβ strongly inhibited NiCl2- and TNFα-induced MCP-1 expression. However, D609 and the specific p38 inhibitor SB202190 did not affect NiCl2- and TNFα-induced IKKβ activation, NF-κB DNA-binding activity, or transcriptional activity of a Gal4p65 fusion protein. This indicates that p38- and PC-PLC–dependent pathways directly regulate the transcriptional activity of NF-κB factors in the transcriptional complex. Consistent with that, inhibition of p38 blocked enhanced transcriptional activity induced by the transcriptional coactivator p300. Thus, it was concluded that at least 3 independent pathways regulate MCP-1 expression in endothelial cells. Its induction requires activation of the IKKβ/IκBα/NF-κB signaling pathway, resulting in nuclear accumulation of p65 and subsequent recruitment of cofactors. Proper assembly and activity of this transcriptional complex is further modulated by the p38 MAP kinase cascade and a PC-PLC–dependent pathway.
Introduction
Endothelial cells, strategically located between blood and tissue compartments, play an important role for initiation and regulation of inflammatory events. Targeted by cytokines, such as tumor necrosis factor (TNF)-α or interleukin (IL)–1, or by bacterial lipopolysaccharides, they sequentially express adhesion molecules that mediate rolling, adhesion, and transmigration of blood leukocytes from the vessel lumen to the underlying tissue. Moreover, endothelial cells represent a cytokine source that allows them to communicate with other cells and organs. One important cytokine produced by endothelial cells is monocyte chemoattractant protein-1 (MCP-1), a member of the C-C subfamily of chemokines. It exhibits chemoattractive activity on monocytes and T lymphocytes (see Mantovani et al1 and Baggiolini et al2 for a review). MCP-1 expression by endothelial cells contributes to the establishment of a chemokine gradient that facilitates subset-specific recruitment of leukocytes to sites of inflammatory challenge.3 Under flow conditions, MCP-1 triggers firm adhesion of rolling monocytes to E-selectin–expressing vascular endothelium,4 thus contributing to recruitment of monocytes from the vascular compartment. Experimental models with mice lacking MCP-1 or its receptor, CCR2, or employing neutralizing antibodies against MCP-1 have established the prominent role of this chemokine in the pathogenesis of inflammatory disorders.5-8
Induced expression of MCP-1 is strongly dependent on activation of the transcription factor NF-κB, whereas basal transcription is regulated by SP-1.9,10 Exposure of cells to TNFα results in the sequential activation of NF-κB–inducing kinases (NIKs) and IκB kinases α and β (IKKα/β). IKKs promote the critical step in which IκBα phosphorylation finally leads to IκBα degradation and p50/p65 translocation to the nucleus. Data from IKK knock-out mice suggest that the β isoform of IKKs is the main mediator of pro-inflammatory signaling (see Karin11 for a review). The transcriptional activity of nuclear NF-κB factors is further modulated by protein modification and/or cofactor recruitment.12,13 Thus, additional signaling events are required. Indeed several pathways have been shown to interfere with NF-κB activation: Phosphatidylcholine-specific phospholipase C (PC-PLC), a component of the acidic sphingomyelin pathway, may promote the generation of ceramide upon TNFα stimulation and has been implicated in activation of NF-κB.14 Furthermore, members of the mitogen-activated protein (MAP) kinase family such as p38 were shown to be involved in TNFα-mediated gene expression during inflammatory activation of cells. Several groups have demonstrated that pharmacological inhibition of p38 markedly attenuates NF-κB–dependent transcription.15-18 However, the level of p38 interference with the NF-κB–activating pathway is not yet fully understood.19 Some studies favor a direct modulation of the transcriptional activity of NF-κB factors via the p38 MAP kinase pathway; other reports argue for a posttranscriptional regulation,20 eg, by enhancing messenger RNA (mRNA) stability.21 Recent studies demonstrate that activation of p38 even inhibits TNFα-induced NF-κB activation under certain conditions.22 Thus, the cross-talk between the p38 MAP kinase cascade and the NF-κB–activating pathway may occur at different levels, depending on the extracellular stimuli, the individual gene, and the cell type. We have recently demonstrated that p38 significantly contributes to TNFα-inducible expression of MCP-1 at a transcriptional level in vascular endothelium.18 On the basis of these studies, it remained to be elucidated (1) whether the involvement of p38 is specific for TNFα-induced gene expression in endothelial cells; (2) whether additional signaling processes are required; and (3) how these signals converge to mediate a distinct response, such as expression of MCP-1. To further evaluate these mechanisms during endothelial activation, we employed NiCl2, a widely distributed hapten that causes contact hypersensitivity. We previously demonstrated that Ni2+induces expression of endothelial adhesion molecules such as E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in vitro and in an ex vivo skin culture model whereas other bivalent metal ions such as Mn2+, Cu2+, and Zn2+ showed no effect at all.23 Further analyses revealed that NiCl2 induces a strong increase of NF-κB DNA binding in endothelial cells.24 NiCl2 was thus identified as a “hapten with irritant properties” that, beyond its antigen-specific signal, can provide a second defined inflammatory signal that is important for the elicitation of contact hypersensitivity reactions (see Grabbe and Schwarz25 for a review). In the present study, we show that NiCl2 mimics many of the pro-inflammatory effects of TNFα by employing the same signaling mediators. Both agents induce NF-κB–dependent expression of the MCP-1 gene via activation of the p38 MAP kinase cascade and a PC-PLC–dependent mechanism. These pathways appear to interfere directly with the transcriptional activity of NF-κB factors since IKKβ activation, nuclear translocation of p65, and NF-κB DNA-binding activity are not impaired by inhibition of p38 or PC-PLC. We conclude that both the p38 MAP kinase cascade and the PC-PLC–dependent pathway modulate NF-κB–dependent MCP-1 expression, which involves regulation of coactivator function in the transcriptional complex.
Materials and methods
Cytokines and reagents
Human recombinant TNFα was obtained from R&D Systems (Wiesbaden, Germany). Pharmacological inhibitors D609 and SB202190 were purchased from Calbiochem (Bad Soden, Germany) and NiCl2from Merck (Darmstadt, Germany). All other agents were obtained from Sigma-Aldrich (Deisenhofen, Germany) unless otherwise specified.
Cells and cell culture
Primary human umbilical vein endothelial cells (HUVECs) and primary human dermal microvascular endothelial cells (HDMECs) were obtained from Clonetics (via Cell Systems, St Katharinen, Germany). Cells were cultured with endothelial growth medium (EGM) medium (Clonetics) as previously described26 and used between passages 3 and 5.
DNA constructs
MCP-1 promoter luciferase constructs containing the proximal promoter (between −107 and +60) and distal enhancer region (between −2742 and −2513) of MCP-1 (pGLM-ENH) as well as different NF-κB binding–site mutants of pGLM-ENH were kindly provided by T. Yoshimura (Laboratory of Immunobiology, National Cancer Institute, Frederick, MD) and have been previously described.10 The 3xNF-κB–tk or the 5xGal4 promoter constructs contain 3 or 5 copies of a NF-κB– or Gal4-binding motif cloned upstream of a minimal promoter-driven luciferase gene. These plasmids as well as constructs expressing kinase-inactive IKKβ, the transcriptional coactivator p300 (CMVp300), or β-Gal were obtained from T. Wirth, University of Würzburg, Germany. The construct Gal4p65 expressing the DNA-binding domain of the yeast Gal4 protein N-terminally fused to full-length p65 (amino acids 1 through 551) was described previously27 and provided by R. Schreck, University of Würzburg. The GFPS65T expression vector pGreenLantern was purchased from Life Technologies (Karlsruhe, Germany).
In situ hybridization
In situ hybridization experiments were performed as previously described.26 Briefly, stimulated cells were sedimented onto lysine-coated glass slides, fixed in paraformaldehyde, acetylated, dehydrated, and air-dried. Thereafter, cells were overlaid with the hybridization solution containing 35S-labeled antisense and, for controls, sense probes of MCP-1. After hybridization nonhybridized probes were removed by several high-stringency washes. To further minimize nonspecific background, noncomplementary nonhybridized single-stranded probes were digested with RNase A and RNase T1. Slides were then dipped into Kodak NTB-2 solution, exposed for autoradiography, and finally analyzed by means of a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany).
Enzyme-linked immunosorbent assay
Supernatants of HDMECs and HUVECs were collected after exposure to NiCl2 or TNFα, centrifuged at 13 000g to remove cellular debris, and analyzed for MCP-1 synthesis by a sandwich enzyme-linked immunosorbent assay (ELISA) as described.28
Monocyte preparation and chemotaxis assays
Peripheral blood monocytes were obtained from healthy volunteers by gradient density centrifugation and used in chemotaxis assays employing Nucleopore 48-well chemotaxis chamber plates (Costar, Bodenheim, Germany) as described earlier.29 Endothelial cell supernatant obtained after stimulation was studied for monocyte chemoattractive activity in triplicate. In some experiments, chemotaxis was assayed in the presence of neutralizing polyclonal goat immunoglobulin (Ig)–G against recombinant human MCP-1 or macrophage inflammatory protein-1α (MIP-1α) (R&D Systems), which had been added to the endothelial cell supernatant in the lower well of the chemotaxis chamber.
Immunoprecipitation, immune-complex kinase assay, and Western blotting
Endothelial cells were lysed with 20 mM Tris pH 7.4, 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 50 mM sodium-β–glycerophosphate, 20 mM sodium pyrophosphate, 1 mM Pefabloc (Merck), 5 μg/mL aprotinin, 5 μg/mL leupeptin, and 5 mM benzamidine (TLB buffer) at 4°C for 30 minutes. Cell lysates were incubated with protein A agarose (Roche Molecular Biochemicals, Mannheim, Germany) and 1 μg/mL rabbit antiserum against MAP kinase-activated protein (MAPKAP) kinases 2 and 3,30 p38 (C-20), or IKKβ (H-470, Santa Cruz Biotechnology, Heidelberg, Germany) for 2 hours at 4°C. After washing in TLB buffer supplemented with 500 mM NaCl and, thereafter, kinase buffer (25 mM Hepes pH 7.5, 10 mM MgCl2, 25 mM sodium-β–glycerophosphate supplemented with 5 mM benzamidine, 1 mM sodium orthovanadate, and 0.5 mM dithiothreitol), samples were incubated with 3pK K73M (K > M), Hsp27, or GST-IκBα as substrates for p38, MAPKAP kinases 2/3, or IKKβ in the presence of 100 μM unlabeled adenosine triphosphate (ATP), 5 μCi [γ32P]-ATP, and kinase buffer for 15 minutes at 30°C. Samples were subsequently subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, blotted, and visualized by autoradiography or detected by a BioImaging Analyzer BAS 2000 (Fuji; via Raytest, Straubenhardt, Germany). Western blot analysis was performed to confirm equal loading of p38, MAPKAP kinases 2/3, and IKKβ proteins. IκBα and IκBε expression was detected in crude Laemmli lysates with rabbit anti-IκBα (#9242, New England Biolabs, Schwalbach, Germany) or anti-IκBε antisera (M-121, Santa Cruz Biotechnology).
Northern blot
HUVECs were stimulated with NiCl2 or TNFα in the absence or presence of pharmacological inhibitors as indicated. Total cellular RNA was isolated by means of a Qiagen RNeasy kit (Hilden, Germany). Denaturated total RNA (10 μg) was separated on agarose/formaldehyde gels and transferred to Hybond N+ membranes (Amersham Pharmacia Biotech, Freiburg, Germany). Filters were UV–cross-linked and subsequently hybridized with a human MCP-1 complementary DNA probe31 labeled with [α32P]–deoxycytidine triphosphate (dCTP) by means of a random primed DNA-labeling kit (Roche Molecular Biochemicals). Autoradiography was performed at −80°C with the use of Amersham Hyperfilm. For control of RNA loading of lanes, blots were densitometrically analyzed for 28S ribosomal RNA (rRNA) amounts.
Transient transfection and reporter gene assays
Endothelial cells were cultured to 50% to 60% confluence in 6-well plates prior to transient transfection according to a diethylaminoethyl (DEAE)-dextran protocol.32 Cells were incubated with 1 μg reporter construct and 250 μg DEAE-dextran (Amersham Pharmacia Biotech) in 1 mM Hepes/phosphate-buffered saline (PBS) in a final volume of 1 mL for 30 minutes at 37°C. Thereafter, 1.5 mL EGM medium containing 0.15 mM chloroquine was added to each well, and cells were incubated for another 2.5 hours. Medium was then removed and cells were treated with 10% dimethyl sulfoxide in EGM medium for 2.5 minutes. Endothelial cells were subsequently cultured for 36 hours in EGM medium and finally stimulated with NiCl2 or other reagents for the time intervals indicated. Cells of each well were harvested in lysis buffer (50 mM sodium 2-[-morpholino]ethanesulfonic acid, 50 mM Tris-HCl pH 7.8, 10 mM dithiothreitol, and 2% Triton X-100) for 30 minutes at 4°C. We added 50 μL of precleared cell extracts to 50 μL luciferase assay buffer containing 125 mM sodium 2-(N-morpholino)ethanesulfonic acid, 125 mM Tris-HCl pH 7.8, 25 mM magnesium acetate, and 2 mg/mL ATP). After addition of 50 μL of 1 mM D-luciferin (AppliChem, Darmstadt, Germany), luminescence was detected by means of an LB96P luminometer (Berthold, Bad Wildbach, Germany). Induced luciferase activities were normalized on the basis of protein contents and are expressed as “fold” stimulation compared with unstimulated controls.
Flow cytometry
MCP-1 expression by HUVECs cotransfected with a vector expressing dominant-negative IKKβ and GFPS65T was detected as follows. At 36 hours after transfection, cells were exposed to NiCl2 or TNFα for the time intervals indicated. We added 2 μM monensin in order to avoid secretion of the chemokine via the Golgi pathway. Cells were subsequently harvested, washed, fixed with 4% paraformaldehyde in PBS at 4°C for 20 minutes, and then incubated with a monoclonal antibody against MCP-1 (mouse IgG1, clone 5D3-F7) or corresponding isotype control monoclonal antibody (Becton Dickinson, Heidelberg, Germany) that had been diluted in permeabilization buffer containing 1% fetal calf serum, 0.1% saponin, and PBS. Thereafter, cells were successively stained with biotin-SP–conjugated goat–antimouse IgG F(ab′)2 and streptavidin-Cy-chrome (Becton Dickinson). Fluorescence was determined with a FACScalibur (Becton Dickinson). Only cells that expressed GFPS65T (detected in the FL-1 channel) were considered for detection of MCP-1 expression (measured in the FL-3 channel). Nonviable cells were excluded by means of forward scatter and side scatter parameters.
Electrophoretic mobility shift assay
A κB-specific probe33 was labeled in a reaction mixture containing 200 ng double-stranded DNA probe, [α–32P]-dCTP, 1 mM deoxyadenosine triphosphate, 1 mM deoxyguanosine triphosphate, 1 mM thymidine 5′-triphosphate, 500 mM Tris-HCl pH 7.5, 100 mM MgCl2, and 2 U Klenow fragment. After 30 minutes' incubation at 37°C, oligonucleotides were separated on a G-25 Sephadex spin column (Roche Molecular Biochemicals) and finally resuspended in Tris-EDTA (30 000 cpm/μL). For the typical binding reactions, 5 μg of nuclear lysates were incubated on ice for 5 minutes in the absence or presence of competitor DNA in an 18-mL reaction mixture containing 25 mM Tris pH 7.5, 1 mM EDTA, 0.5 mM DDT, 100 mM KCl, 0.1% (vol/vol) NP40, 1 μg (wt/vol) bovine serum albumin, 10% (vol/vol) glycerol, and 0.5 μg (wt/vol) poly(dI-dC); 60 000 cpm of labeled oligonucleotide was added, and the mixture was incubated for 15 minutes at 25°C. The samples were loaded on a 5% nondenaturing polyacrylamide gel equilibrated with 0.5 × TBE (tris borate–EDTA) and electrophoresed for 2.5 hours at 180 V. Gels were dried and DNA-protein complexes were visualized by autoradiography.
Results
NiCl2 induces endothelial MCP-1 mRNA and protein synthesis
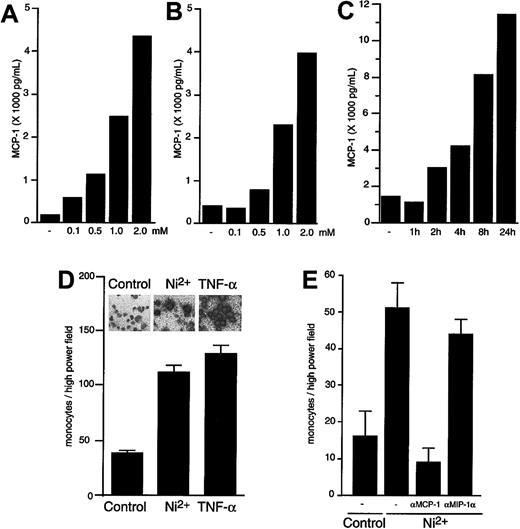
NiCl2, a widely distributed contact sensitizer, is known to activate vascular endothelium (see Grabbe and Schwarz25 for a review). To investigate its effects on endothelial chemokine production, primary HUVECs (Figure1A) or HDMECs (Figure 1B) were exposed to different concentrations of NiCl2, which leads to secretion of MCP-1 in a concentration-dependent manner. Significant amounts of MCP-1 could be already detected 120 minutes after stimulation of HUVECs (Figure 1C) and of HDMECs (data not shown) with NiCl2. Like TNFα, NiCl2 induces a strong up-regulation of MCP-1 message in both cell types (Figure 1D [inset] and data not shown), as evaluated at a single-cell level by in situ hybridization. To exclude the possibility that MCP-1 release into the medium is simply due to toxic effects of NiCl2, we determined cell integrity via detection of lactate dehydrogenase activity in the supernatants. Concentrations of this obligate intracellular enzyme did not significantly differ in supernatants of cells exposed to control medium or NiCl2 (data not shown). To analyze the functionality of NiCl2-induced chemoattractive activity, we performed chemotaxis assays employing peripheral blood monocytes. Conditioned medium of HUVECs (Figure 1D) or HDMECs (Figure 1E) exposed to NiCl2 for 8 hours showed strong chemotactic activity toward monocytes as compared with supernatant obtained from nonstimulated cells. Addition of a neutralizing antiserum against human MCP-1 effectively blocked chemotaxis of monocytes, thus identifying MCP-1 as the principal monocyte-attractive activity produced by endothelial cells upon stimulation with NiCl2 (Figure 1E). A species-matched neutralizing antiserum against the chemokine MIP-1α, which is not inducible in endothelial cells,26 did not show a significant inhibitory effect.
Effect of NiCl2 on expression of functional MCP-1.
NiCl2 induces expression of functional MCP-1 in a time- and concentration-dependent manner. (A-C) HUVECs (A, C) or HDMECs (B) were cultured for 4 hours in the presence of 0, 0.1, 0.5, 1.0, and 2.0 mM NiCl2 (A- B) or for 0, 1, 2, 4, 8, or 24 hours in the presence of 1.5 mM NiCl2 (C). Supernatants were then harvested and studied for MCP-1 content by ELISA. The SEM of each point did not exceed 5% of the mean value. (D-E) HUVECs (D) or HDMECs (E) were exposed to 1.0 mM NiCl2, 1 ng/mL TNFα, or medium as control for 8 hours. Subsequently, supernatants were harvested and studied for monocyte chemotactic activity by means of chemotaxis assays as described in “Materials and methods.” In panel E, chemotaxis was determined in the absence or presence of neutralizing polyclonal goat IgG against human MCP-1 or MIP-1α as indicated. Migrated cells were counted in 4 high power fields per well; each supernatant was evaluated in triplicate. Results are presented as mean ± SEM. The inset in panel D visualizes MCP-1 mRNA expression by HUVECs exposed to medium, 1.0 mM NiCl2, or 1 ng/mL TNFα as indicated. The mRNA expression was detected at a single cell level by in situ hybridization.
Effect of NiCl2 on expression of functional MCP-1.
NiCl2 induces expression of functional MCP-1 in a time- and concentration-dependent manner. (A-C) HUVECs (A, C) or HDMECs (B) were cultured for 4 hours in the presence of 0, 0.1, 0.5, 1.0, and 2.0 mM NiCl2 (A- B) or for 0, 1, 2, 4, 8, or 24 hours in the presence of 1.5 mM NiCl2 (C). Supernatants were then harvested and studied for MCP-1 content by ELISA. The SEM of each point did not exceed 5% of the mean value. (D-E) HUVECs (D) or HDMECs (E) were exposed to 1.0 mM NiCl2, 1 ng/mL TNFα, or medium as control for 8 hours. Subsequently, supernatants were harvested and studied for monocyte chemotactic activity by means of chemotaxis assays as described in “Materials and methods.” In panel E, chemotaxis was determined in the absence or presence of neutralizing polyclonal goat IgG against human MCP-1 or MIP-1α as indicated. Migrated cells were counted in 4 high power fields per well; each supernatant was evaluated in triplicate. Results are presented as mean ± SEM. The inset in panel D visualizes MCP-1 mRNA expression by HUVECs exposed to medium, 1.0 mM NiCl2, or 1 ng/mL TNFα as indicated. The mRNA expression was detected at a single cell level by in situ hybridization.
Induction of p38 MAP kinase by NiCl2
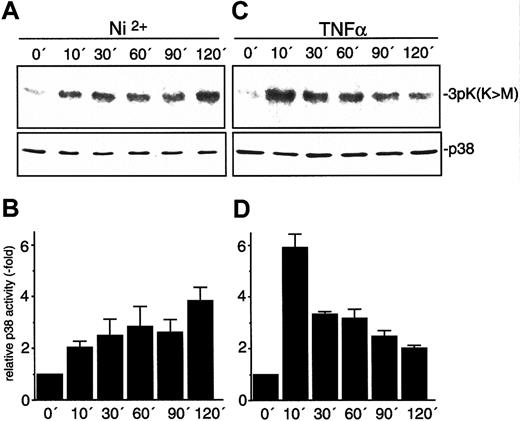
One of the major signaling pathways initiated by environmental stress stimuli results in activation of p38 MAP kinase. We therefore investigated whether NiCl2 mediates its effects via activation of p38. HUVECs were exposed to NiCl2 or TNFα for the time intervals indicated. Subsequently, activity of endogenous p38 was assessed in immune-complex kinase assays (Figure2). We detected a sustained activation of p38 by NiCl2 that steadily increases during the observation period. TNFα also strongly activated p38, albeit with different kinetics: p38 activity was maximal 10 minutes after TNFα stimulation and steadily declined thereafter.
Activation of endothelial p38 by NiCl2 and TNFα.
HUVECs were stimulated with 1.5 mM NiCl2 (A-B) or 1.5 ng/mL TNFα (C-D) for the time intervals indicated. Relative p38 activity was quantified by densitometrical evaluation of autographs and is depicted as mean ± SEM of 3 independent experiments. Immune-complex kinase assays were performed as described in “Materials and methods” with the use of 3pK K73M as substrate. Protein loads were controlled by Western blot with the use of an antiserum against p38.
Activation of endothelial p38 by NiCl2 and TNFα.
HUVECs were stimulated with 1.5 mM NiCl2 (A-B) or 1.5 ng/mL TNFα (C-D) for the time intervals indicated. Relative p38 activity was quantified by densitometrical evaluation of autographs and is depicted as mean ± SEM of 3 independent experiments. Immune-complex kinase assays were performed as described in “Materials and methods” with the use of 3pK K73M as substrate. Protein loads were controlled by Western blot with the use of an antiserum against p38.
p38- and PC-PLC–dependent signal transduction pathways contribute to NiCl2-induced MCP-1 expression
NiCl2 shares some pro-inflammatory properties with TNFα, eg, up-regulation of MCP-1 (see above) or induction of E-selectin and VCAM-123; however, the signaling mechanisms induced by this compound are not well defined. We therefore analyzed whether both stimuli use common pathways of intracellular signaling that finally result in endothelial activation as reflected by, eg, activation of NF-κB.24 At present, it is not fully understood how these different signaling pathways interfere with each other. To address this question, we studied the impact of p38 as well as of PC-PLC on NiCl2-induced activation of endothelium. PC-PLC has earlier been implicated in TNFα-induced activation of NF-κB14 although this role has been challenged after the detection of intact NF-κB signaling in cells derived from acidic sphingomyelinase-deficient mice.34
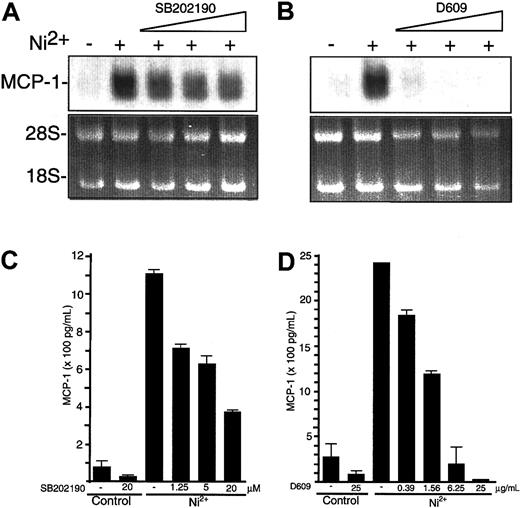
We found that p38 or PC-PLC activities were blocked by the pharmacological inhibitors SB202190 or D609, respectively. Endothelial cells were exposed to 1.5 mM NiCl2; 30 minutes prior to stimulation, SB202190 (Figure 3A,C) or D609 (Figure 3B,D) was added to the culture medium with increasing concentrations. SB202190 partially blocked NiCl2-induced MCP-1 mRNA and protein synthesis (Figure 3A,C). Addition of D609 resulted in a potent dose-dependent inhibition, which was maximal at levels above 6.25 μg/mL D609 (Figure 3B,D).
Inhibition of NiCl2-induced MCP-1 mRNA and protein expression by pharmacological blockade of p38 and PC-PLC.
(A-B) HUVECs were cultured for 8 hours in the absence or presence of 1.25, 5, or 20 μM SB202190 (A) or 1.56, 6.25, or 25 μg/mL D609 (B) and stimulated with 1.5 mM NiCl2 as indicated. Thereafter, HUVECs were processed for detection of MCP-1 mRNA by Northern blot analysis. To control equal RNA loading of Northern blot lanes, ethidium bromide stains of 18S and 28S rRNA are shown. (C-D) Supernatants from HUVECs exposed to 1.25, 5, or 20 μM SB202190 (C) or 0.39, 1.56, 6.25, or 25 μg/mL D609 (D) 30 minutes before and during a 12-hour period of stimulation with 1.5 mM NiCl2 were studied for MCP-1 content by ELISA. Data are expressed as mean ± SE of duplicate wells from 2 independent experiments.
Inhibition of NiCl2-induced MCP-1 mRNA and protein expression by pharmacological blockade of p38 and PC-PLC.
(A-B) HUVECs were cultured for 8 hours in the absence or presence of 1.25, 5, or 20 μM SB202190 (A) or 1.56, 6.25, or 25 μg/mL D609 (B) and stimulated with 1.5 mM NiCl2 as indicated. Thereafter, HUVECs were processed for detection of MCP-1 mRNA by Northern blot analysis. To control equal RNA loading of Northern blot lanes, ethidium bromide stains of 18S and 28S rRNA are shown. (C-D) Supernatants from HUVECs exposed to 1.25, 5, or 20 μM SB202190 (C) or 0.39, 1.56, 6.25, or 25 μg/mL D609 (D) 30 minutes before and during a 12-hour period of stimulation with 1.5 mM NiCl2 were studied for MCP-1 content by ELISA. Data are expressed as mean ± SE of duplicate wells from 2 independent experiments.
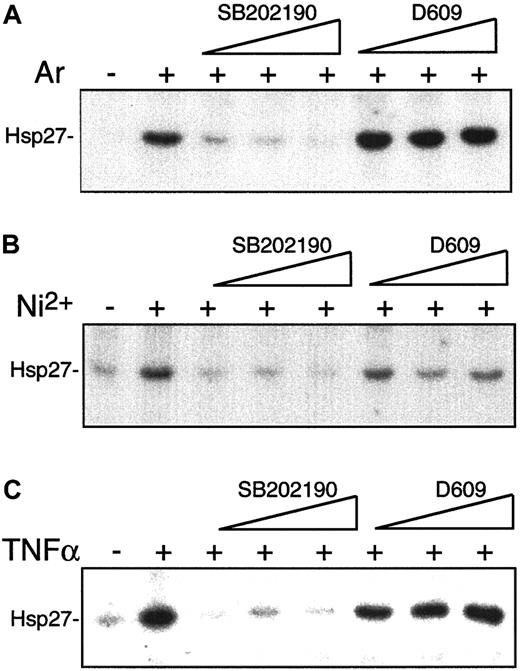
To evaluate whether the SB202190- and D609-sensitive pathways act independently of each other, the activation of the p38 substrates MAPKAP-kinase 2 and 3 in the presence of the inhibitors was analyzed. SB202190 dose-dependently blocked MAPKAP-kinase activation by NiCl2, TNFα, or the classical p38 activator arsenite (Figure 4). D609, however, showed no significant effect on induced MAPKAP-kinase activity, indicating that PC-PLC–dependent signaling processes act parallel to rather than upstream of p38 (Figure 4).
Inhibition of NiCl2-induced endothelial p38 activation in a concentration-dependent manner by SB202190 but not D609.
To study the effects of pharmacological inhibitors SB202190 and D609 on p38 activity, HUVECs were left unstimulated or exposed to arsenite (A) or NiCl2 (B) for 60 minutes or to TNFα (C) for 30 minutes in the absence or presence of 1.25, 5.0, or 20 μM SB202190 or 1.56, 6.25, or 25 μg/mL D609, which was added to the culture medium 30 minutes prior to stimulation. After cell lysis, MAPKAP kinases 2 and 3 were immunoprecipitated and assayed for activity with Hsp27 used as substrate.
Inhibition of NiCl2-induced endothelial p38 activation in a concentration-dependent manner by SB202190 but not D609.
To study the effects of pharmacological inhibitors SB202190 and D609 on p38 activity, HUVECs were left unstimulated or exposed to arsenite (A) or NiCl2 (B) for 60 minutes or to TNFα (C) for 30 minutes in the absence or presence of 1.25, 5.0, or 20 μM SB202190 or 1.56, 6.25, or 25 μg/mL D609, which was added to the culture medium 30 minutes prior to stimulation. After cell lysis, MAPKAP kinases 2 and 3 were immunoprecipitated and assayed for activity with Hsp27 used as substrate.
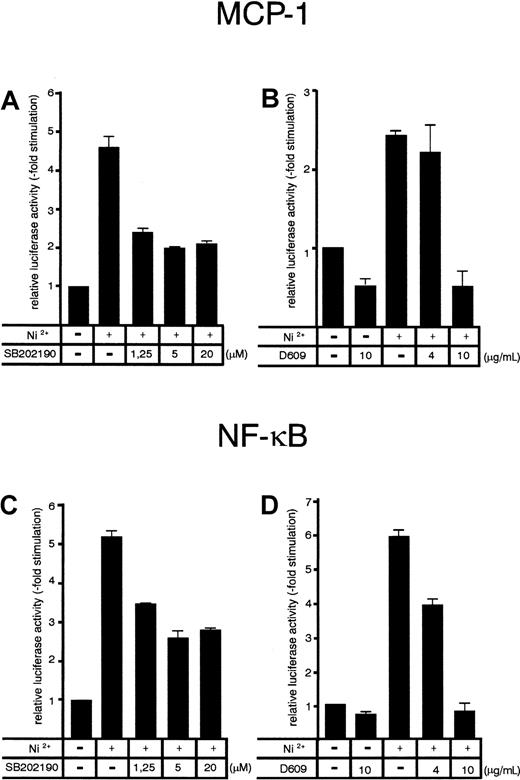
NiCl2-induced transactivation of the MCP-1 promoter and an artificial NF-κB–dependent promoter element are inhibited by SB202190 and D609
Since both SB202190 and D609 inhibited transcription of the MCP-1 gene (Figure 3), we analyzed the effects of the inhibitors on NiCl2-induced MCP-1 promoter–dependent reporter gene activity. Cells transfected with an MCP-1 promoter/enhancer luciferase construct10 were stimulated with NiCl2 at 36 hours after transfection and harvested 12 hours later. NiCl2 induced a strong activation of the MCP-1 luciferase reporter gene (Figure 5A-B). Inhibition of p38 activity by SB202190 was paralleled with a roughly 50% decrease of NiCl2-induced MCP-1 promoter activity (Figure 5A) whereas exposure to D609 dose-dependently resulted in complete inhibition (Figure 5B). The MCP-1 promoter contains 2 NF-κB–binding sites involved in the transcriptional control of the MCP-1 gene.10 To address the question of whether p38- and PC-PLC–dependent pathways might interfere with NF-κB activation, we also studied effects of SB202190 and D609 on an NF-κB–dependent promoter construct. Again, NiCl2-induced NF-κB–dependent luciferase expression was decreased by approximately 50% when p38 was inhibited by SB202190, whereas D609 completely abolished NiCl2-induced NF-κB–dependent reporter gene expression (Figure 5C-D). Thus, NF-κB–mediated transcription appears to be a target for p38- and PC-PLC–dependent signaling pathways. Mutation of one or both of the 2 NF-κB–binding sites of the MCP-1 promoter resulted in complete loss of promoter-inducibility, indicating that NiCl2 induces signals that require intact NF-κB–binding sites (data not shown).
Effect of pharmacological p38 and PC-PLC inhibition on NiCl2-induced MCP-1 promoter and 3xNF-κB–dependent promoter activity in HUVECs.
HUVECs were transiently transfected with an MCP-1 promoter/enhancer construct (A-B) or a 3xNF-κB binding site–containing promoter construct (C-D) according to a DEAE-dextran protocol as described in “Materials and methods.” At 36 hours after transfection, cells were stimulated with 1.5 mM NiCl2 for 8 hours in the absence or presence of SB202190 (A, C) or D609 (B, D) at the concentrations indicated. Inhibitors were added to the culture medium 30 minutes prior to exposure with NiCl2. Relative luciferase activity is expressed as fold stimulation. Mean values ± SE from 4 independent transfections are shown.
Effect of pharmacological p38 and PC-PLC inhibition on NiCl2-induced MCP-1 promoter and 3xNF-κB–dependent promoter activity in HUVECs.
HUVECs were transiently transfected with an MCP-1 promoter/enhancer construct (A-B) or a 3xNF-κB binding site–containing promoter construct (C-D) according to a DEAE-dextran protocol as described in “Materials and methods.” At 36 hours after transfection, cells were stimulated with 1.5 mM NiCl2 for 8 hours in the absence or presence of SB202190 (A, C) or D609 (B, D) at the concentrations indicated. Inhibitors were added to the culture medium 30 minutes prior to exposure with NiCl2. Relative luciferase activity is expressed as fold stimulation. Mean values ± SE from 4 independent transfections are shown.
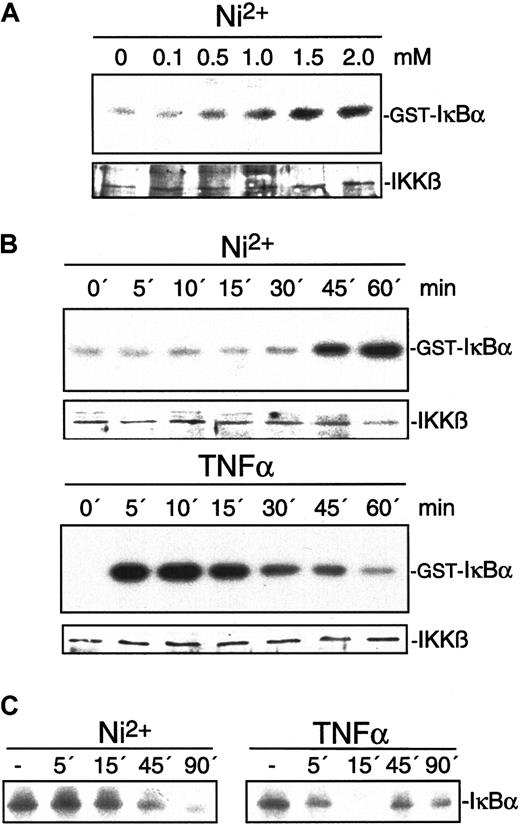
NiCl2 induces activation of IKKβ, degradation of IκBα, and NF-κB DNA-binding activity independent of p38- and PC-PLC–mediated pathways
To further elucidate mechanisms of NiCl2-induced NF-κB–dependent gene expression, we studied its influence on IKKβ, the major IκBα–phosphorylating enzyme during inflammatory activation (see Karin11 for a review). IKKβ activity was determined by immune-complex kinase assay after stimulation of endothelial cells with various concentrations of NiCl2 or TNFα. NiCl2 dose-dependently induced IKKβ activation as visualized by phosphorylation of GST-IκBα (Figure6A). In contrast to the rapid activation of IKKβ by TNFα, NiCl2 induced a delayed but steady increase of activity (Figure 6B). The kinetics of induced IKKβ activity were then compared with the kinetics of IκBα degradation. Under both conditions, IκBα degradation parallels IKKβ activity: TNFα was found to induce IκBα degradation within 5 minutes while NiCl2-induced degradation is retarded (Figure 6C). Similar degradation kinetics were observed for IκBε (data not shown), which has recently been demonstrated to be functional for endothelial cell activation.35 To determine the impact of IKKβ on NiCl2-induced MCP-1 expression, we studied the effect of a dominant-negative IKKβ mutant. MCP-1 expression in cells transiently transfected with vector or dominant-negative IKKβ was determined by flow cytometry as described in “Materials and methods.” Only cells expressing cotransfected GFPS65T to allow identification of cells transfected with the inactive kinase mutant were analyzed. Figure7 demonstrates that dominant-negative IKKβ partially inhibits NiCl2- as well as TNFα-induced MCP-1 synthesis. Similar results could be obtained when IκBα phosphorylation is blocked by the pharmacological inhibitor Bay11-0782 or when degradation of IκBα is blocked by the proteasome inhibitor N-acetyl-leu-leu-norleucinal (ALLN, calpain inhibitor I; data not shown).36
Effect of NiCl2 on IKKβ and IκBα.
Exposure of HUVECs to NiCl2 leads to activation of IKKβ and degradation of IκBα. (A) Endothelial cells were stimulated for 30 minutes with different concentrations of NiCl2 as indicated. Thereafter, cell extracts were prepared and IKKβ was assayed in immune-complex kinase assays using GST-IκBα as a substrate. (B) HUVECs were exposed to 1.5 mM NiCl2 for 0, 5, 10, 15, 30, 45, or 60 minutes. IKKβ activity was determined by immune-complex kinase assays as described above. Protein loads were controlled by Western blot with the use of an antiserum to IKKβ. (C) HUVECs stimulated with 1.5 mM NiCl2 or 2 ng/mL TNFα for 0, 5, 15, 45, or 90 minutes were processed for Western blot analysis.IκBα immunoreactivity is visualized as a 40-kd protein.
Effect of NiCl2 on IKKβ and IκBα.
Exposure of HUVECs to NiCl2 leads to activation of IKKβ and degradation of IκBα. (A) Endothelial cells were stimulated for 30 minutes with different concentrations of NiCl2 as indicated. Thereafter, cell extracts were prepared and IKKβ was assayed in immune-complex kinase assays using GST-IκBα as a substrate. (B) HUVECs were exposed to 1.5 mM NiCl2 for 0, 5, 10, 15, 30, 45, or 60 minutes. IKKβ activity was determined by immune-complex kinase assays as described above. Protein loads were controlled by Western blot with the use of an antiserum to IKKβ. (C) HUVECs stimulated with 1.5 mM NiCl2 or 2 ng/mL TNFα for 0, 5, 15, 45, or 90 minutes were processed for Western blot analysis.IκBα immunoreactivity is visualized as a 40-kd protein.
Inhibition of NiCl2-induced endothelial MCP-1 expression by dominant-negative IKKβ.
HUVECs were transfected in a 1:3 ratio with pGreenLantern expressing GFPS65T and a plasmid expressing either empty expression vector or dominant-negative IKKβ (dn IKKβ). Cells were left unstimulated or exposed to 1.5 mM NiCl2 or 2 ng/mL TNFα for 12 hours. Successfully transfected cells, ie, cells labeled by green fluorescent protein, were analyzed for MCP-1 expression by flow cytometry as described in “Materials and methods.” Flow cytometry profiles of one representative experiment are shown. Open profiles represent isotype controls; shaded profiles indicate cells intracellularly labeled for MCP-1 expression.
Inhibition of NiCl2-induced endothelial MCP-1 expression by dominant-negative IKKβ.
HUVECs were transfected in a 1:3 ratio with pGreenLantern expressing GFPS65T and a plasmid expressing either empty expression vector or dominant-negative IKKβ (dn IKKβ). Cells were left unstimulated or exposed to 1.5 mM NiCl2 or 2 ng/mL TNFα for 12 hours. Successfully transfected cells, ie, cells labeled by green fluorescent protein, were analyzed for MCP-1 expression by flow cytometry as described in “Materials and methods.” Flow cytometry profiles of one representative experiment are shown. Open profiles represent isotype controls; shaded profiles indicate cells intracellularly labeled for MCP-1 expression.
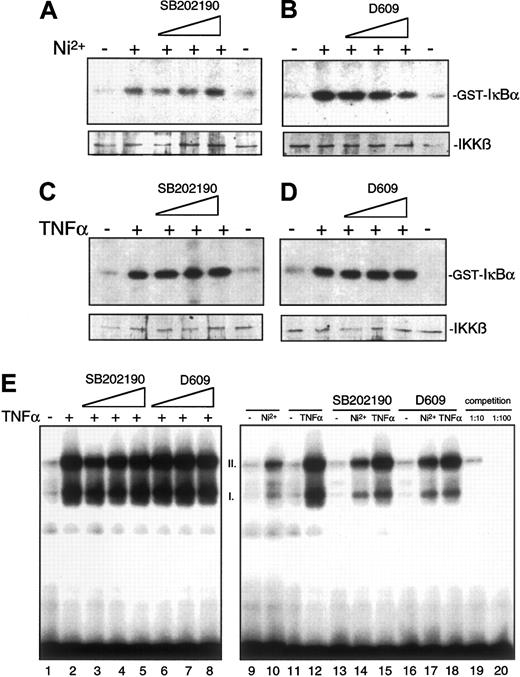
When these findings are taken together, induction of MCP-1 synthesis by NiCl2 requires activation of IKKβ and degradation of IκBα as well as functional NF-κB–binding sites in the promoter. Further, MCP-1 synthesis is dependent on p38 and PC-PLC activity; however, it is unclear where the different pathways converge. To analyze whether IKKβ is a target of p38 or PC-PLC–dependent signaling pathways (or both), IKKβ immune-complex kinase assays were performed from lysates of HUVECs pretreated with increasing concentrations of inhibitors SB202190 or D609 prior to stimulation. SB202190 did not reveal any effect on NiCl2- or TNFα-induced activation of IKKβ (Figure8A,C). Exposure to higher concentrations of D609 resulted in a marginal inhibition of NiCl2-mediated IKKβ activation whereas TNFα-induced activation was virtually not affected (Figure 8B,D). Similarly, nuclear translocation of p65, which is a component of TNFα- and NiCl2-induced activated NF-κB complexes in HUVECs,24 is not significantly blocked by SB202190 or D609 (data not shown). TNFα- or NiCl2-induced NF-κB DNA-binding activity is not blocked by either p38 or PC-PLC inhibition (Figure 8E and data not shown). Furthermore, addition of inhibitors SB202190 or D609 directly to nuclear extracts during the EMSA reaction procedure (Figure 8E, lanes 13-18) did not affect binding of NF-κB to its cognate binding site. Collectively, these data indicate that p38- and PC-PLC–dependent signaling pathways do not interfere with NF-κB activation at the level of IKKβ, nuclear translocation of p65, or NF-κB DNA binding.
Pharmacological inhibition of p38 or PC-PLC and its effect on induced activation of IKKβ and NF-κβ DNA-binding affinity.
(A-D) HUVECs were preincubated for 30 minutes with increasing concentrations of SB202190 (1.25, 5, or 20 μM) (A, C) or D609 (1.56, 6.25, or 25 μg/mL) (B, D) and subsequently either left untreated or stimulated for 1 hour with 1.5 mM NiCl2 or 2 ng/mL TNFα as indicated. Cell lysates were subsequently assayed for IKKβ activity. (E) HUVECs were pre-incubated with 1.25 (lane 3), 5 (lane 4), or 20 μM SB202190 (lane 5) or with 1.56 (lane 6), 6.25 (lane 7), or 25 μg/mL D609 (lane 8) and subsequently stimulated with NiCl2 or TNFα as indicated. Thereafter, nuclear extracts were obtained and an electrophoretic mobility shift assay (EMSA) was performed as described in “Materials and methods.” For lanes 13 through 18, 20 μM SB202190 or 25 μg/mL D609 was added to nuclear extracts only during the EMSA reaction procedure in order to study potential interaction of inhibitors with the binding of NF-κB to its cognate binding site. I and II indicate the major NF-κB complexes formed as determined by competition assays with unlabeled probes (lanes 19 and 20).
Pharmacological inhibition of p38 or PC-PLC and its effect on induced activation of IKKβ and NF-κβ DNA-binding affinity.
(A-D) HUVECs were preincubated for 30 minutes with increasing concentrations of SB202190 (1.25, 5, or 20 μM) (A, C) or D609 (1.56, 6.25, or 25 μg/mL) (B, D) and subsequently either left untreated or stimulated for 1 hour with 1.5 mM NiCl2 or 2 ng/mL TNFα as indicated. Cell lysates were subsequently assayed for IKKβ activity. (E) HUVECs were pre-incubated with 1.25 (lane 3), 5 (lane 4), or 20 μM SB202190 (lane 5) or with 1.56 (lane 6), 6.25 (lane 7), or 25 μg/mL D609 (lane 8) and subsequently stimulated with NiCl2 or TNFα as indicated. Thereafter, nuclear extracts were obtained and an electrophoretic mobility shift assay (EMSA) was performed as described in “Materials and methods.” For lanes 13 through 18, 20 μM SB202190 or 25 μg/mL D609 was added to nuclear extracts only during the EMSA reaction procedure in order to study potential interaction of inhibitors with the binding of NF-κB to its cognate binding site. I and II indicate the major NF-κB complexes formed as determined by competition assays with unlabeled probes (lanes 19 and 20).
Inhibition of p38 or PC-PLC signaling does not block either basal or induced transcriptional activity of nuclear Gal4p65
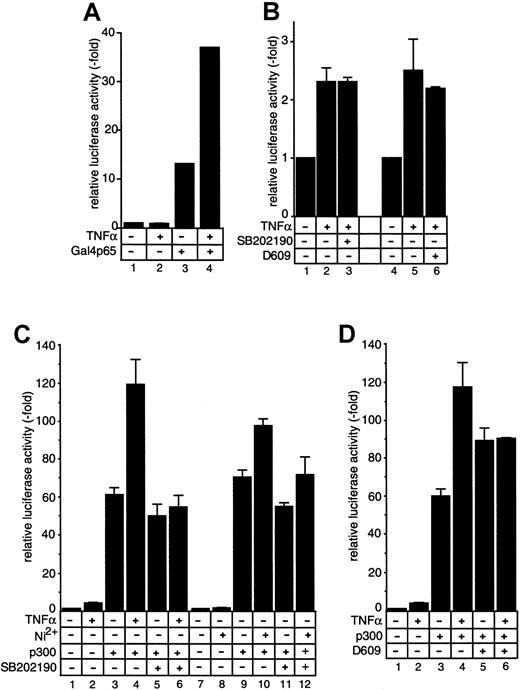
To analyze the transactivating capacity of nuclear p65, we overexpressed the factor that results in a nuclear accumulation. The p65 was expressed as a Gal4–DNA-binding domain (Gal4BD) fusion protein, which allows a selective assay for the transactivating features of transfected p65 by recruiting the factor to an artificial 5xGal4–binding site promoter luciferase construct.27 In the absence of the Gal4BD fusion protein, the 5xGal4 promoter is completely silent, no matter whether cells were stimulated or not (Figure 9A, lanes 1 and 2). Cotransfection of Gal4p65 results in a strong induction of promoter activity (Figure 9A, lane 3), which was further enhanced after stimulation of cells with TNFα (Figure 9A, lane 4). This indicates that TNFα-induced signaling events result in an enhanced transactivating capacity of p65, presumably by inducing a modification of the transcription factor. This modification is most likely a phosphorylation event, since it was reported earlier that TNFα induces phosphorylation of p65 resulting in an enhanced transcriptional activity.37 However, stimulus-induced promoter activity indicative of p65 modification was not blocked by either SB202190 (Figure 9B, lane 3) or D609 (Figure 9B, lane 6), suggesting that the pathways leading to an enhanced p65 transactivating capacity are not dependent on p38 or PC-PLC.
Effect of SB202190 and D609 on p65 activity and of SB202190 on TNFα- and NiCl2-induced function of p300.
SB202190 and D609 do not inhibit modulation of p65 activity, but SB202190 blocks TNFα- and NiCl2-induced function of p300. (A-B) HUVECs were transiently transfected with plasmids expressing luciferase under control of a 5xGal4 binding site–containing promoter (A, lanes 1 and 2) or were cotransfected with a vector expressing a Gal4-p65 fusion protein (A, lanes 3 and 4, and B). (C) HUVECs were transiently transfected with 1 μg MCP-1 ENH promoter or were cotransfected with 0.5 μg CMVp300 (lanes 3 through 6 and 9 through 12). In all experiments, 36 hours after transfection, cells were either left untreated or stimulated with 2 ng/mL TNFα (lanes 2, 4, 6) or 1.5 mM NiCl2 (lanes 8, 10, 12) in the presence or absence of 20 μM SB202190. Equal transfection and expression efficiencies were controlled by coexpression of a CMV-driven β-Gal reporter gene construct (data not shown). (D) Experiments were performed as in panel C, but SB202190 was replaced by 10 μg/mL D609. Relative luciferase activity is expressed as fold stimulation. In panel B, Gal4-promoter transactivation by expression of Gal4p65 in the absence of TNFα is arbitrarily set as 1, and fold activation of TNFα-induced activity is calculated. Mean values ± SEM from up to 8 independent transfections are shown.
Effect of SB202190 and D609 on p65 activity and of SB202190 on TNFα- and NiCl2-induced function of p300.
SB202190 and D609 do not inhibit modulation of p65 activity, but SB202190 blocks TNFα- and NiCl2-induced function of p300. (A-B) HUVECs were transiently transfected with plasmids expressing luciferase under control of a 5xGal4 binding site–containing promoter (A, lanes 1 and 2) or were cotransfected with a vector expressing a Gal4-p65 fusion protein (A, lanes 3 and 4, and B). (C) HUVECs were transiently transfected with 1 μg MCP-1 ENH promoter or were cotransfected with 0.5 μg CMVp300 (lanes 3 through 6 and 9 through 12). In all experiments, 36 hours after transfection, cells were either left untreated or stimulated with 2 ng/mL TNFα (lanes 2, 4, 6) or 1.5 mM NiCl2 (lanes 8, 10, 12) in the presence or absence of 20 μM SB202190. Equal transfection and expression efficiencies were controlled by coexpression of a CMV-driven β-Gal reporter gene construct (data not shown). (D) Experiments were performed as in panel C, but SB202190 was replaced by 10 μg/mL D609. Relative luciferase activity is expressed as fold stimulation. In panel B, Gal4-promoter transactivation by expression of Gal4p65 in the absence of TNFα is arbitrarily set as 1, and fold activation of TNFα-induced activity is calculated. Mean values ± SEM from up to 8 independent transfections are shown.
SB202190 blocks stimulus-induced coactivator function of p300
It was recently reported that phosphorylation of p65 results in an enhanced transactivating capacity by regulating association of transcriptional coactivators of the CBP/p300 family. We therefore cotransfected a plasmid expressing p300 to analyze its effects on the MCP-1 promoter. The p300 cotransfection resulted in a strong transcriptional activation of the MCP-1 promoter, which was further enhanced after treatment of cells with TNFα (Figure 9C-D). Interestingly, D609 treatment of nonstimulated cells slightly enhanced p300-induced promoter activity (Figure 9D, lane 5) whereas in stimulated cells D609 minimally reduced p300 coactivation (Figure 9D, lane 6). However, this inhibition does not fully explain the inhibitory effect of D609, which at the same concentration resulted in a complete loss of promoter activity in the absence of p300 (Figure 5B,D). This was different in the presence of SB202190. Although the basal coactivating capacity of p300 was only marginally affected (Figure 9C, lane 5), the inhibitor completely blocked TNFα-induced, p300-dependent transcriptional coactivation (Figure 9C, lane 6). Essentially similar results were seen in the case of NiCl2(Figure 9C, lanes 7-12). This observation is compatible with the model in which the p38 MAP kinase pathway regulates stimulus-induced coactivator functions of such factors as CBP/p300, presumably by facilitating the proper formation of the transcriptional complex.
Discussion
The molecular mechanisms of NF-κB–dependent gene expression are complex and involve a variety of different signaling inputs and coactivators. Here we analyzed NF-κB–mediated transcription in the context of MCP-1 expression in primary human endothelial cells. We demonstrate that the contact hapten NiCl2, which is the most common inductor of allergic contact dermatitis in industrialized countries, induces MCP-1 expression in a time- and dose-dependent manner, sharing many of the pro-inflammatory properties of TNFα by employing the same signaling mediators. Both agents activate IKKβ, p38 MAP kinase, and a PC-PLC–dependent mechanism. These 3 signals appear to be independently transduced to converge at the level of formation of the transcriptional complex, presumably by facilitating activation of cofactor function.
Although several important components of NiCl2-induced signaling were identified here, the initial signaling events generated by this agent are not yet clear. One clue might be that the transition metals Ni2+ and Co2+ induce formation of reactive oxygen intermediates according to the Fenton reaction.38-40 These intermediates are used as second-messenger molecules, which integrate a diverse variety of stimuli into the NF-κB pathway.41 Accordingly, NiCl2-induced NF-κB–binding activity and adhesion molecule expression by endothelial cells were blocked by an antioxidant, such as pyrrolidine dithiocarbamate.24
Another mechanism of signal transmission induced by environmental stimuli involves activation of the p38 MAP kinase module. As outlined in this study, NiCl2 can be added to the list of environmental factors, such as heat shock, osmotic stress, and UV light, that activate p38. The role of reactive oxygen intermediates for p38 activation is not clear. They may weakly activate p38; however, antioxidants have been shown not to influence42 or even induce p38 activation16 but also not to inhibit it.43 It is striking that NiCl2-induced activation of both p38 (shown in this study) and NF-κB24is delayed in comparison with that evoked by TNFα. This might indicate different modes of signal transduction at early stages after acquisition of the initial signal. Considering the still relatively rapid activation kinetics, it appears to be unlikely that de novo synthesis of intermediate proteins is necessary. The finding that NiCl2-induced activation of NF-κB does not depend on novel synthesis or release of cytokines such as IL-124further argues against an autocrine indirect mechanism.
Another pathway activated by NiCl2 and TNFα is susceptible to inhibition by the metabolic inhibitor D609, which almost completely blocked induction of MCP-1 expression. This pathway involved neither p38 activation nor induction of NF-κB DNA-binding activity via the IKKβ/IκBα pathway; however, NF-κB–dependent transactivation was completely blocked. D609 is thought to inhibit generation of diacylgylycerol from phosphatidylcholine, which activates acidic sphingomyelinase.14 Subsequent generation of ceramide directly or indirectly interferes with MAP kinase (Raf/MEK/ERK and JNK/SAPK) and PKCζ-dependent pathways (see Kolesnick and Krönke44 and Levade and Jaffrézou45for a review). Since D609 did not inhibit agonist-induced activation of ERK or JNK/SAPK (K.K., unpublished data, September 1997), we suppose that D609 may additionally target other pathways than the pathway described by Schütze et al,14 which has so far not been characterized in detail. Accordingly, exposure of endothelial cells to synthetic ceramide analogues or to sphingomyelinase induced only a very moderate expression of MCP-1, and ceramide treatment did not overcome the effect of D609 (M.G., unpublished observations, October 1999). Conflicting data exist regarding the interference of D609 with NF-κB activation. Whereas in some cells inhibition of NF-κB DNA-binding activity was observed,14,46 in others such as in HUVECs no effect on agonist-induced NF-κB DNA binding could be detected.47 The mode of D609 inhibition on NiCl2- and TNFα-induced NF-κB–dependent transcription is so far not fully understood; however, it does not involve either ceramide or p38.
The p38 MAP kinase does not modulate activity of IKKβ or influence translocation of NF-κB to the nucleus. The latter observation is quite compatible with other studies reporting no effect of pharmacological p38 inhibitors on stimulus-induced NF-κB DNA binding.15-17 However, blockade of p38 function partially inhibited transcription of a MCP-1 promoter/luciferase reporter gene construct, indicating that p38 activation is required.18Moreover, mutation of both or even one of the NF-κB–binding sites of the MCP-1 promoter completely abrogated its inducibility by NiCl2 or TNFα, indicating that p38 acts at the level of transactivation. One of the potential modes of interaction with the NF-κB pathway might be interaction with NF-κB subunits. In endothelial cells, transcriptional activity of nuclear Gal4p65 could indeed further be enhanced after stimulation (Figure 9A-B), suggesting that p65 is modified by a TNFα-activated pathway. This modification is most likely a phosphorylation event, which would be quite compatible with earlier observations showing that endothelial p65 is phosphorylated upon activation,48 resulting in enhanced transcriptional activity.12 In our experiments, TNFα-induced transcriptional activity of Gal4p65 is not blocked by either SB202190 or D609, indicating that modification/phosphorylation of p65 is not modulated by p38- or PC-PLC–dependent pathways. Regarding p38, our finding contrasts with observations reported by Vanden Berghe and colleagues,49 who found a direct inhibitory effect of SB203580 on the transactivation potential of the p65 NF-κB subunit when analyzing murine L929sA cells. This discrepancy may be due to the use of a different cell type or a different Gal4–binding site promoter construct that still contained 50 IL-6 promoter-specific nucleotides.49 Our data are consistent with the recent observation by Wang and Baldwin37, who could not confirm inhibition of TNFα- induced p65 phosphorylation by SB203580. In addition, Carter et al50 showed that lipopolysaccharide-induced p65 phosphorylation in THP-1 cells is not blocked by SB203580. In the latter study, inhibition of p38 signaling resulted in hypophosphorylation and decreased DNA binding of the TATA-binding protein (TBP). The authors therefore concluded that p38 MAP kinase regulates NF-κB–dependent transcription in part by modulation of TBP function. Taking into account our observation that TNFα-induced enhanced transactivating potency of p300 is sensitive to SB202190, we propose the following model of signal transduction: Upon activation of IKK and migration of the active NF-κB factors to the nucleus, a transcriptional complex is formed at the DNA by recruitment of multiple cofactors.13 This recruitment is facilitated by phosphorylation events, such as protein kinase A–mediated p65 phosphorylation, which has been shown to result in a bivalent interaction with the transcriptional coactivator CBP/p300.12 CBP/p300 also interacts with TBP,51 which binds to DNA in a p38-dependent fashion.50 Thus, the proper formation of the transcriptional complex, in particular, the coactivator functions of CBP/p300 family members, might be regulated by a p38-dependent pathway either directly or in cooperation with TBP. A downstream target of PC-PLC may target other essential factors during transcriptional complex formation. Although D609 only marginally affects coactivation by p300, MCP-1 promoter activity and protein synthesis are completely abrogated in the presence of the drug. This indicates involvement of other, so far not identified targets.
The impact of accessory pathways supporting NF-κB–dependent transcription may be dependent on the specific requirement of different cofactors regulating an individual gene in a certain cell type. In conclusion, our data indicate that multiple signaling pathways converge to regulate NF-κB–dependent expression of MCP-1 in primary endothelial cells. IKK-mediated IκB phosphorylation is essential for nuclear accumulation of the transcription factors, and p38- and PC-PLC–dependent signaling events are required to optimize the function of the transcriptional complex.
Acknowledgments
We thank Martina Gropengiesser, Heide Häfner, Sybille Schmid, and Atiye Toksoy for excellent technical assistance; Peifeng Chen for her help in performing EMSA assays; Teizo Yoshimura for providing MCP-1 promoter luciferase constructs; and Bernd Baumann, Stephan Feller, Alex McLellan, Conny Seitz, and Thomas Wirth for critically reading the manuscript and helpful discussions.
Supported by grants GO 811/1-1 and LU 477/2-4 from the Deutsche Forschungsgemeinschaft and from the Fonds der Chemischen Industrie (M.G. and S.L.) and by grant 95.064.2 from the W.-Sander-Stiftung (R.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Matthias Goebeler, Department of Dermatology, University of Würzburg, Josef-Schneider-Str. 2, 97080 Würzburg, Germany; e-mail:goebeler-m.derma@mail.uni-wuerzburg.de; and Dr. Stephan Ludwig, Institut für Medizinische Strahlenkunde und Zellforschung, University of Würzburg, Versbacher Str. 5, 97078 Würzburg, Germany; e-mail: s.ludwig@mail.uni-wuerzburg.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal