Abstract
The G protein–coupled 7 transmembrane (STM) chemoattractant receptors can be inactivated by heterologous desensitization. Earlier work showed that formly peptide receptor-like 1 (FPRL1), an STM receptor with low affinity for the bacterial chemotactic peptide formyl-methionyl-leucyl-phenylalamine (fMLF), is activated by peptide domains derived from the human immunodeficiency virus (HIV)-1 envelope glycoprotein gp120 and its activation results in desensitization and down-regulation of the chemokine receptors CCR5 and CXCR4 from monocyte surfaces. This study investigated the possibility of interfering with the function of CCR5 or CXCR4 as HIV-1 coreceptors by activating FPRL1. Cell lines were established expressing FPRL1 in combination with CD4/CXCR4 or CD4/CCR5 and the effect of a synthetic peptide, WKYMVm, a potent activator of formyl peptide receptors with preference for FPRL1 was determined. Both CXCR4 and CCR5 were desensitized by activation of the cells with WKYMVm via a staurosporine-sensitive pathway. This desensitization of CXCR4 and CCR5 also attenuated their capacity as the fusion cofactors for HIV-1 envelope glycoprotein and resulted in a significant inhibition of p24 production by cell lines infected with HIV-1 that use CCR5 or CXCR4 as coreceptors. Furthermore, WKYMVm inhibited the infection of human peripheral monocyte–derived macrophages and CD4+ T lymphocytes by R5 or X4 strains of HIV-1, respectively. These results indicate that heterologous desensitization of CCR5 and CXCR4 by an FPRL1 agonist attenuates their major biologic functions and suggest an approach to the development of additional anti-HIV-1 agents.
Introduction
Chemokine receptors CCR5 and CXCR4 are 2 major coreceptors for human immunodeficiency virus (HIV)-1 infection and belong to the 7 transmembrane (STM), G protein–coupled receptor superfamily.1 Such receptors are composed of 7 hydrophobic transmembrane domains, an N-terminus outside the cell surface, 3 extracellular and 3 intracellular loops, and a C-terminus in the cytoplasmic compartment.2 The C-termini of these receptors contain serine and threonine residues which, on phosphorylation, are involved in signaling and receptor desensitization.2,3Agonist-induced phosphorylation of chemokine receptors, such as CCR5, can result in homologous desensitization and internalization of the receptors.4,5 Chemokine receptors may also be subjected to “heterologous desensitization” when the cells are activated by agonists selective for unrelated STM receptors.3 For example, activation of formyl peptide receptor (FPR), the receptor for the bacterial chemotactic peptide fMLF, effectively desensitizes a number of chemoattractant receptors, including the receptors for the chemokine interleukin (IL) 8.3 Both homologous and heterologous desensitization results in impairment of various biologic functions of chemokine receptors in response to further stimulation by their cognate ligands.3 Because human leukocytes express a variety of chemoattractant receptors, the “cross-talk” among those receptors has been proposed to be important in fine-tuning of cell responses in the presence of multiple stimulants.
Two STM receptors that can be activated by the bacterial chemotactic peptide fMLF have been identified and cloned.6,7 FPR interacts with low concentrations of fMLF and thus acts as a high-affinity fMLF receptor. On the other hand, the low-affinity variant receptor formyl peptide receptor-like 1 (FPRL1) exhibits Ca++ flux only in response to high concentrations of fMLF.6,7 Despite the fact that both FPR and FPRL1 are among the earliest chemoattractant receptors discovered on human phagocytic leukocytes, their biologic significance in host defense and immune responses is not clear. Although the nature of the host-derived ligands for FPR remains obscure, at least 2 endogenous molecules in humans, the lipid metabolite lipoxin A4 (LXA4)8 and a chemotactic acute-phase protein serum amyloid A (SAA),9have been found to activate FPRL1. HIV-1 envelope glycoprotein gp120 and gp41 also contain domains that preferentially activate either FPR or FPRL1.10-12 A 20 amino acid peptide domain derived from gp120 of the HIV-1LAI strain specifically stimulates phagocyte chemotaxis and Ca++ flux through FPRL1.12 By activating a protein kinase C (PKC)-mediated signaling pathway, this peptide also down-regulates the expression and chemotactic function of the chemokine receptors CCR5 and CXCR4 in monocytes.12 Furthermore, we have observed that activation of FPRL1 by its agonists induced serine phosphorylation of CCR5 in monocytes.13 These observations motivated us to investigate whether the desensitization of CCR5 and CXCR4 by activation of FPRL1 could compromise their capacity as HIV-1 coreceptors. In this study, we show that a synthetic peptide WKYMVm, originally derived from a random peptide library and a highly potent agonist for FPRs with preference for FPRL1,14 desensitizes chemokine receptors CCR5 and CXCR4 and attenuates their capacity as HIV-1 coreceptors. Our results suggest a novel approach to the development of anti–HIV-1 reagents.
Materials and methods
Chemicals
WKYMVm (Trp-Lys-Tyr-Met-Val-D-Met, designated W peptide) was synthesized and purified by the Department of Biochemistry, Colorado State University (Fort Collins, CO), based on the published sequence.14 15 The purity of the peptide was more than 90%, and the amino acid composition was verified by mass spectrometry. The endotoxin levels in the dissolved peptide were undetectable. A scrambled peptide with the sequence of KWMNVY, which does not activate FPRs, was used as control. The PKC inhibitor staurosporine was purchased from Sigma (St Louis, MO). All chemokines were purchased from Pepro Tech (Rocky Hill, NJ).
Cells
Human osteosarcoma cells coexpressing CD4/CCR5 or CD4/CXCR4 (Hos/CD4/CCR5 or Hos/CD4/CXCR4) were obtained through the NIH AIDS Research and Reference Reagents Program. These cells were further transfected with plasmid containing a full-length complementary DNA (cDNA) coding for FPRL1 (a kind gift of Drs P. M. Murphy and J. Gao, National Institute of Allergy and Infectious Diseases, Bethesda, MD), and were designated Hos/CD4/CCR5/FPRL1 or Hos/CD4/CXCR4/FPRL1 cells. Control cells were cotransfected with pcDNA3 plasmid vector alone and were designated Hos/CD4/CCR5/Mock or Hos/CD4/CXCR4/Mock cells. The transfected cell lines were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 500 μg/mL puromycine, and 2000 μg/mL geneticin. HeLa cells were purchased from American Tissue Culture Collection (Rockville, MD). TF228 cells, which stably express HIV-1 T-tropic envelope protein gp120 + 41LAI, were kindly supplied by Dr D. Dimitrov (National Cancer Institute, Frederick, MD). Human peripheral blood mononuclear cells (PBMC) were isolated from leukopacks obtained from the Transfusion Medicine Department, NIH Clinical Center, Bethesda, MD. Monocytes were further purified by elutriation to yield more than 90% pure preparations. CD4+ T lymphocytes were further isolated by negative selection through anti-CD8 columns (Miltenyi Biotech, Bergisch Gladbach, Germany) to yield more than 95% pure preparations.
Chemotaxisassays
Chemotaxis assays were performed using a 48-well chemotaxis chamber technique (Neuro Probe, Cabin John, MD) as described previously.9 11 For migration of receptor transfected Hos cells, the incubation period was 3 hours and polycarbonate filters (10 μm pore size) precoated with collagen type 1 were used. For human monocyte–derived macrophages, the incubation period was 90 minutes with 5 μm pore-sized polycarbonate filters. The results were expressed as chemotaxis index (CI), which represents the fold increase in the number of migrated cells in 3 high-powered fields (HPF) in response to chemoattractants over spontaneous cell migration in response to control medium.
Calciummobilization
Calcium mobilization was measured by incubating cells (2 × 107/mL) in loading medium (DMEM, 10% FCS) with 5 μM Fura-2 am (Molecular Probes, Eugene, OR) for 1 hour at room temperature in the dark. The dye-loaded cells were washed 3 times and resuspended in saline buffer (138 mM NaCl, 6 mM KCl, 1 mM CaCl2, 10 mM Hepes, pH 7.4, 5 mM glucose, 0.1% bovine serum albumin [BSA]). The cells were then transferred into quartz cuvettes (1 × 106 cells in 2 mL), which were placed in a luminescence spectrometer (LS-50B, Perkin Elmer, Beaconsfield, United Kingdom). Stimulants at different concentrations were added in a volume of 20 μL to cuvettes at the indicated time points. The ratio of fluorescence at 340 and 380 nm was calculated using a FL-WinLab program (Perkin Elmer). To examine the effect of W peptide, both FPRL1 and mock transfected Hos cells were preincubated with 1 μM W peptide or with medium alone for 40 to 60 minutes at 37°C. The cells were thoroughly washed and then were examined for calcium mobilization. For PKC-dependent heterologous desensitization, FPRL1-transfected Hos cells were pretreated with the PKC inhibitor staurosporine at 1.4 ng/mL for 1 hour at 37°C, washed thoroughly with RPMI 1640, and then treated with W peptide.
Phosphorylation of CCR5 and CXCR4
The phosphorylation of CCR5 was studied using an approach as described previouly.13 Briefly, Hos/CD4/CCR5/FPRL1 cells treated with RANTES or W peptide were lysed and immunoprecipitated with a polyclonal antiphophoserine antibody (Zymed Laboratory, South San Francisco, CA) followed by immunoblotting with a polyclonal anti-CCR5 antibody (a kind gift of Dr R. Sargeant, Millenium Biotechnology, Ramona, CA). The image was visualized by using a horseradish peroxidase–conjugated goat antirabbit IgG and SuperSignal Chemiluminescent Substrate Stable Peroxide Solution (Pierce, Rockford, IL). CXCR4 phosphorylation was measured as described previously.16 Briefly, Hos/CD4/CXCR4/FPRL1 cells were labeled with [32P] orthophosphate (Amersham, Piscataway, NJ) and then were stimulated with stromal cell–derived factor (SDF)-1α or W peptide. The cell lysates were prepared and imunoprecipitated with a rabbit polyclonal anti-CXCR4 antibody (a kind gift of Dr R. Sargeant, Millennium Biotechnology). The immune complexes were captured with protein A-Sepharose (Novex, Carlsbad, CA) and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
Quantitative assays for HIV-1 envelope glycoprotein-mediated fusion
The assays were performed as described previously.17 Briefly, Hos/CD4/CCR5 or Hos/CD4/CXCR4 cells in the presence or absence of FPRL1 were infected with recombinant vaccinia virus vTF7-3 encoding the bacteriophage T7 RNA polymerase under the control of the natural P7.5 early-late vaccinia promoter. HeLa cells were coinfected with a recombinant vaccinia virus vBC43 expressing monocyte tropic HIV-1Env (Bal 31) and vBC21R containing a T7 promoter linked to the LacZ reporter gene. TF 228 cells, which express T-tropic HIV-1 envelope protein gp120 + 41LAI, were infected with recombinant vaccinia virus vBC21R alone. After infection with vaccinia viruses for 2 hours at 37°C, the cells were washed thoroughly with culture medium and kept in fresh medium at 31°C overnight. Hos cells expressing or not expressing FPRL1 were pretreated with W peptide at the designated concentrations for 1 hour at 37°C, then were mixed with the Env-expressing cells. The 2 populations of cells (1 × 105 each) were seeded in triplicate in the wells of 96-well tissue culture plates. After 12 hours of culture at 37°C, the cells were lysed with 0.05% Nonidet P-40 and spun at 2500 rpm for 5 minutes. The 50-μL cell lysate was mixed with 50 μL of 16 mM chlorophenol red-β-galactopyranoside (CPRG) dissolved in phosphate buffer (0.12 M Na2HPO4, 0.08 M NaH2PO4, 0.02 M KCI, 0.002 M MgSO4, 0.01 M β-mercaptoethanol). The reactions were kept at room temperature for 30 minutes before the color was measured with an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm.
HIV-1 infection
The Hos cells expressing combinations of receptors were pretreated with W peptide at the designated concentrations for 1 hour and infected with M-tropic (R5) HIV-1Bal (MOI 0.1, for Hos/CD4/CCR5/FPRL1) or T-tropic (X4) HIV-1PNL4-3 (MOI 0.1, for Hos/CD4/CXCR4/FPRL1) at 37°C for 2 hours. For infection of primary cells, CD4+ T lymphocytes were first stimulated with phytohemagglutin (PHA; Sigma) at 2 μg/mL and cultured with IL-2 at 50 U/mL for 4 days. Monocytes were cultured with 5% human AB serum for 4 to 6 days. The cells were then pretreated with W peptide at the designated concentrations at 37°C for 1 hour before infection with X4 or R5 HIV-1 for 2 hours. At the end of infection, the cells were washed 3 times and cultured in fresh medium containing W peptide. The culture supernatants were collected at 72 hours for measurement of HIV-1gag gene product P24 by ELISA (Zeptometrix, Buffalo, NY, and AIDS Vaccine Program, NCI-FCRDC, Frederick, MD). In each experiment, CXC chemokine SDF-1α (or CC chemokines RANTES, MIP-1α, and MIP-1β) in combination were used as control inhibitors.
Statisticalanalysis
All experiments were performed at least 3 times and results from representative experiments are shown. The significance of the difference between test and control groups was analyzed with Student t test.
Results
Establishment of cell lines expressing FPRL1 and HIV-1 coreceptors
The Hos cells genetically engineered to express CD4 and CCR5 or CXCR4 were additionally transfected with the full-length cDNA of FPRL1. The transfected cells were cloned by limited dilution and selected with both puromycin and geneticin. The coexpression of FPRL1 was examined by their responsiveness to a synthetic peptide WKYMVm (W peptide) that is a highly efficacious chemotactic agonist for FPRL1.14 As shown in Figure 1, Hos cells expressing only CD4/CCR5 or CD4/CXCR4 migrated in response to the chemokine ligands but not to W peptide. In contrast, cells cotransfected with FPRL1 exhibited a response to both chemokines and W peptide. The cell response to W peptide occurred when low nanomolar concentrations of the peptide were used, indicating FPRL1 was effectively expressed by these cells (Figure 1A,B).
Chemotactic response of cells to chemokines or W peptide.
Hos/CD4/CCR5 or Hos/CD4/CXCR4 cells were stably transfected with FPRL1 cDNA or pcDNA3 vector alone. Cell migration in response to chemokines was examined. (A) Migration of Hos/CD4/CCR5 cells in the presence (FPRL1+) or absence (Mock) of FPRL1 in response to MIP-1α or W peptide compared to spontaneous migration (medium). ▨ indicates FPRL1+; ▪, Mock. (B) Migration of Hos/CD4/CXCR4 cells in the presence (FPRL1+) or absence (Mock) of FPRL1 in response to SDF-1α or W peptide compared to migration in the absence of chemoattractants (medium). ■ indicates FPRL1+; ▪, Mock. *P < .05 and **P < .01.
Chemotactic response of cells to chemokines or W peptide.
Hos/CD4/CCR5 or Hos/CD4/CXCR4 cells were stably transfected with FPRL1 cDNA or pcDNA3 vector alone. Cell migration in response to chemokines was examined. (A) Migration of Hos/CD4/CCR5 cells in the presence (FPRL1+) or absence (Mock) of FPRL1 in response to MIP-1α or W peptide compared to spontaneous migration (medium). ▨ indicates FPRL1+; ▪, Mock. (B) Migration of Hos/CD4/CXCR4 cells in the presence (FPRL1+) or absence (Mock) of FPRL1 in response to SDF-1α or W peptide compared to migration in the absence of chemoattractants (medium). ■ indicates FPRL1+; ▪, Mock. *P < .05 and **P < .01.
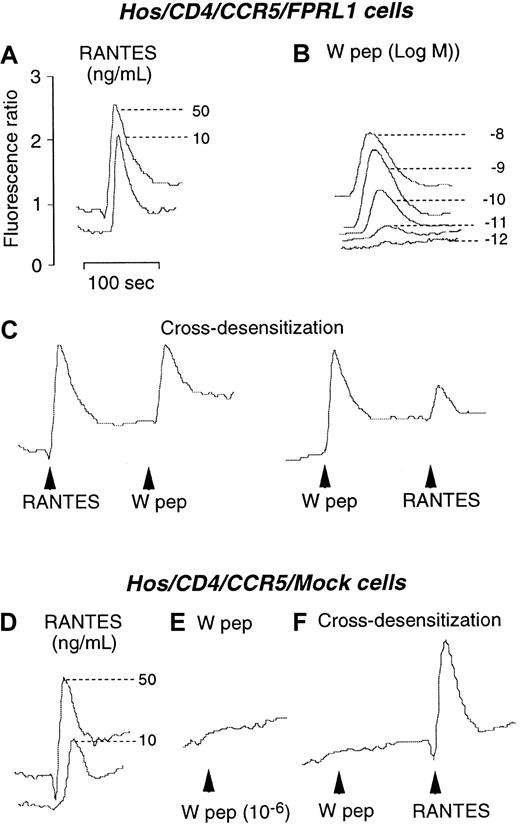
Desensitization of CCR5 and CXCR4 in Hos cells by activation of FPRL1
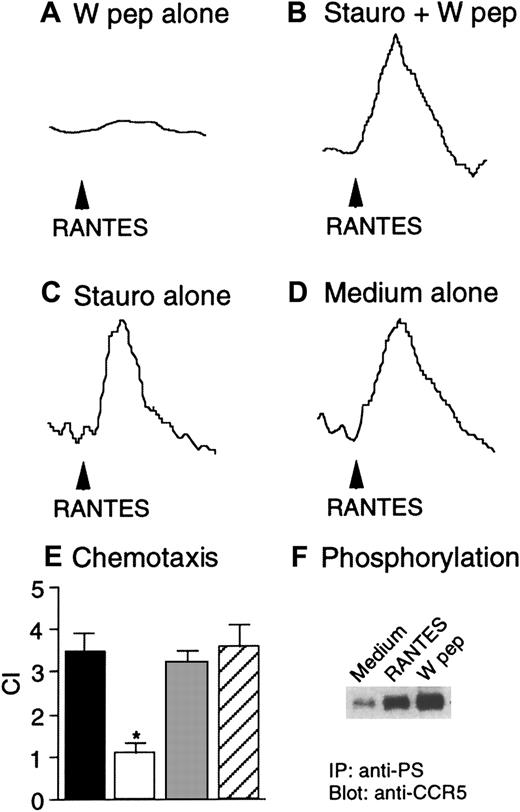
We next investigated the capacity of W peptide to desensitize CCR5 and CXCR4 in Hos cells following activation of FPRL1. As shown in Figure 2, both W peptide and CCR5 ligand RANTES induced dose-dependent Ca++ flux in CD4/CCR5/FPRL1 cells (Figure 2A,B). In cross-desensitization experiments, prior stimulation of the cells with RANTES did not affect the subsequent response to W peptide. However, stimulation of the cells with W peptide significantly reduced the cell response to RANTES, suggesting a desensitizing capacity of FPRL1 activation on cell response to CCR5 ligand (Figure 2C). In contrast, RANTES, but not W peptide, could induce similar levels of Ca++ mobilization in cells in the absence of FPRL1 (Figure 2D,E). Prior exposure of CD4/CCR5/Mock cells to W peptide did not affect the cell response to RANTES (Figure 2F), suggesting the activity of W peptide is dependent on the presence of FPRL1. Prolonged incubation of CD4/CCR5/FPRL1 Hos cells with W peptide at 37°C for 60 minutes completely abolished the response to RANTES (Figure 3A). However, cells preincubated with the PKC inhibitor staurosporine maintained a response to RANTES even after prolonged incubation of cells with W peptide at 37°C for 60 minutes (Figure 3B). As a control, treatment of the cells with staurosporine alone also did not affect the response to RANTES (Figure3C,D). These results suggest that the cross-desensitization of CCR5 following FPRL1 activation is mediated by PKC. We also investigated the capacity of W peptide to desensitize CCR5-mediated cell migration, another crucial function of the chemokine receptor. Figure 3, panel E, shows that preincubation of Hos cells expressing CD4/CCR5/FPRL1 with W peptide inhibited cell migration induced by CCR5 ligand RANTES, and the effect of W peptide was significantly reduced when the cells were pretreated by staurosporine, in support of the involvement of PKC activation in FPRL1-mediated CCR5 desensitization. Furthermore, W peptide induced serine phosphorylation of CCR5 in receptor transfected Hos cells (Figure 3F). As predicted, the cognate CCR5 ligand RANTES also induced the phosphorylation of CCR5. These results are in agreement with our observations in monocyte/macrophages,13in which several specific FPRL1 agonists heterologously desensitize the function of CCR5 in association with CCR5 phosphorylation.
Calcium mobilization in Hos/CD4/CCR5 cells coexpressing FPRL1 or pcDNA3 vector (Mock).
(A) and (B) Ca++ flux by Hos/CD4/CCR5/FPRL1 cells in response to RANTES and W peptide. (C) Sequential stimulation of Hos/CD4/CCR5/FPRL1 cells with RANTES (50 ng/mL) and W peptide (10 nM) or vice versa. (D) and (E) Ca++ flux in Hos/CD4/CCR5/Mock cells induced by RANTES and W peptide. (F) Sequential stimulation of Hos/CD4/CCR5/Mock cells with W peptide (1 μM) and RANTES (50 ng/mL).
Calcium mobilization in Hos/CD4/CCR5 cells coexpressing FPRL1 or pcDNA3 vector (Mock).
(A) and (B) Ca++ flux by Hos/CD4/CCR5/FPRL1 cells in response to RANTES and W peptide. (C) Sequential stimulation of Hos/CD4/CCR5/FPRL1 cells with RANTES (50 ng/mL) and W peptide (10 nM) or vice versa. (D) and (E) Ca++ flux in Hos/CD4/CCR5/Mock cells induced by RANTES and W peptide. (F) Sequential stimulation of Hos/CD4/CCR5/Mock cells with W peptide (1 μM) and RANTES (50 ng/mL).
Heterologous desensitization of CCR5 and the effect of staurosporine.
Hos/CD4/CCR5/FPRL1 cells were preincubated in the absence (A) or presence (B) of staurosporine (1.4 ng/mL) at 37°C for 1 hour, followed by treatment with W peptide (1 μM) at 37°C for an additional 60 minutes. Ca++ flux in response to RANTES (50 ng/mL) was measured. Hos/CD4/CCR5/FPRL1 cells were preincubated with (C) or without (D) staurosporine (1.4 ng/mL) at 37°C for 1 hour, and RANTES-induced Ca++ flux was then measured. (E) Hos/CD4/CCR5/FPRL1 cells were preincubated at 37°C with medium, W peptide (1 μM, 1 hour; W pep), staurosporine (1.4 ng/mL, 1 hour) followed by W peptide (1 μM, 1 hour; Stauro+Wpep), or staurosporine alone (1.4 ng/mL, 1 hour; Stauro alone). After washing, cell migration in response to RANTES (50 ng/mL) was measured. The asterisk indicates a significantly reduced cell migration after W peptide treatment, compared with cells treated with medium only (medium). ▪ indicates medium; ■, W pep; ░, stauro + W pep; ▨, stauro alone. (F) Hos cells expressing CD4/CCR5/FPRL1 were treated at 37°C with RANTES (1 μg/mL, 1 minute) or W peptide (1 μM, 60 minutes). The cell lysates were detected for CCR5 phophorylation by using an antiphosphoserine antibody (anti-PS) for immunoprecipitation (IP) followed by immunoblot with anti-CCR5 antibody.
Heterologous desensitization of CCR5 and the effect of staurosporine.
Hos/CD4/CCR5/FPRL1 cells were preincubated in the absence (A) or presence (B) of staurosporine (1.4 ng/mL) at 37°C for 1 hour, followed by treatment with W peptide (1 μM) at 37°C for an additional 60 minutes. Ca++ flux in response to RANTES (50 ng/mL) was measured. Hos/CD4/CCR5/FPRL1 cells were preincubated with (C) or without (D) staurosporine (1.4 ng/mL) at 37°C for 1 hour, and RANTES-induced Ca++ flux was then measured. (E) Hos/CD4/CCR5/FPRL1 cells were preincubated at 37°C with medium, W peptide (1 μM, 1 hour; W pep), staurosporine (1.4 ng/mL, 1 hour) followed by W peptide (1 μM, 1 hour; Stauro+Wpep), or staurosporine alone (1.4 ng/mL, 1 hour; Stauro alone). After washing, cell migration in response to RANTES (50 ng/mL) was measured. The asterisk indicates a significantly reduced cell migration after W peptide treatment, compared with cells treated with medium only (medium). ▪ indicates medium; ■, W pep; ░, stauro + W pep; ▨, stauro alone. (F) Hos cells expressing CD4/CCR5/FPRL1 were treated at 37°C with RANTES (1 μg/mL, 1 minute) or W peptide (1 μM, 60 minutes). The cell lysates were detected for CCR5 phophorylation by using an antiphosphoserine antibody (anti-PS) for immunoprecipitation (IP) followed by immunoblot with anti-CCR5 antibody.
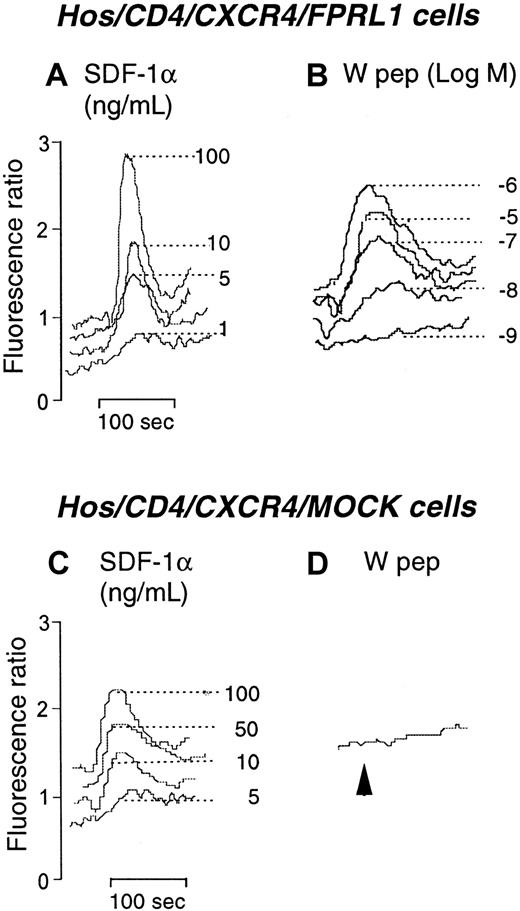
Additional experiments showed that W peptide had a similar desensitizing effect on CXCR4 resulting in reduction of the response to the chemokine ligand SDF-1α. As shown in Figure4, Hos/CD4/CXCR4 cells cotransfected with FPRL1 mobilized Ca++ in response to both SDF-1α (Figure4A) and W peptide (Figure 4B). In contrast, cells in the absence of FPRL1 responded only to SDF-1α (Figure 4C) but not to W peptide (Figure 4D). Preincubation of Hos/CD4/CXCR4/FPRL1 cells (at 37°C for 1 hour) with W peptide (10−6 M) markedly reduced the response to SDF-1α (Figure 5A). However, the effect of W peptide was reduced when the cells were pretreated with the PKC inhibitor staurosporine (Figure 5B). Treatment of the cells with staurosporine alone did not affect the cell response to SDF-1α (Figure 5C) compared to medium alone (Figure 5D). Similar to its desensitizing effect on CXCR4-mediated Ca++ flux induced by SDF-1α in receptor transfected Hos cells, W peptide also attenuated cell migration in response to SDF-1α in a PKC-activation–dependent manner, because cells pretreated with staurosporine were resistant to the desensitizing activity of W peptide (Figure 5E). As observed for CCR5, the functional attenuation of CXCR4 by FPRL1 activation was also associated with phosphorylation of CXCR4 in Hos cells transfected with both CXCR4 and FPRL1 (Figure 5F).
Calcium mobilization in receptor-transfected Hos cells.
(A) and (B) Ca++ flux by Hos/CD4/CXCR4/FPRL1 cells in response to SDF-1α and W peptide. (C) and (D) Ca++ flux in Hos/CD4/CXCR4/Mock cells in response to SDF-1α and W peptide (1 μM).
Calcium mobilization in receptor-transfected Hos cells.
(A) and (B) Ca++ flux by Hos/CD4/CXCR4/FPRL1 cells in response to SDF-1α and W peptide. (C) and (D) Ca++ flux in Hos/CD4/CXCR4/Mock cells in response to SDF-1α and W peptide (1 μM).
Desensitization of CXCR4 by W peptide pretreatment and the effect of staurosporine.
Hos/CD4/CXCR4/FPRL1 cells were preincubated in the absence (A) or presence (B) of staurosporine (1.4 ng/mL) at 37°C for 1 hour, followed by treatment with W peptide (1 μM) at 37°C for an additional 1 hour. Ca++ flux in response to SDF-1α (100 ng/mL) was measured. Hos/CD4/CXCR4/FPRL1 cells were preincubated with (C) or without (D) staurosporine (1.4 ng/mL) at 37°C for 1 hour, and SDF-1α–induced Ca++ flux was then measured. (E) Hos/CD4/CXCR4/FPRL1 cells were preincubated at 37°C with medium, W peptide (1 μM, 1 hour; W pep), staurosporine (1.4 ng/mL, 1 hour) followed by W peptide (1 μM, 1 hour; Stauro+Wpep), or staurosporine alone (1.4 ng/mL, 1 hour; Stauro alone). After washing, cell migration in response to SDF-1α (50 ng/mL) was measured. The asterisk indicates a significantly reduced cell migration after W peptide treatment, compared with cells treated with medium only. ▪ indicates medium; ■, W pep; ░, stauro + W pep; ▨, stauro alone. (F) [32P] orthophosphate-labeled Hos/CD4/CXCR4/FPRL1 cells were treated at 37°C with SDF-1α (1 μg/mL, 1 minute) or W peptide (1 μM, 60 minutes). The cell lysates were immunoprecipitated (IP) by using an anti-CXCR4 polyclonal antibody to detect increased phosphorylation of CXCR4.
Desensitization of CXCR4 by W peptide pretreatment and the effect of staurosporine.
Hos/CD4/CXCR4/FPRL1 cells were preincubated in the absence (A) or presence (B) of staurosporine (1.4 ng/mL) at 37°C for 1 hour, followed by treatment with W peptide (1 μM) at 37°C for an additional 1 hour. Ca++ flux in response to SDF-1α (100 ng/mL) was measured. Hos/CD4/CXCR4/FPRL1 cells were preincubated with (C) or without (D) staurosporine (1.4 ng/mL) at 37°C for 1 hour, and SDF-1α–induced Ca++ flux was then measured. (E) Hos/CD4/CXCR4/FPRL1 cells were preincubated at 37°C with medium, W peptide (1 μM, 1 hour; W pep), staurosporine (1.4 ng/mL, 1 hour) followed by W peptide (1 μM, 1 hour; Stauro+Wpep), or staurosporine alone (1.4 ng/mL, 1 hour; Stauro alone). After washing, cell migration in response to SDF-1α (50 ng/mL) was measured. The asterisk indicates a significantly reduced cell migration after W peptide treatment, compared with cells treated with medium only. ▪ indicates medium; ■, W pep; ░, stauro + W pep; ▨, stauro alone. (F) [32P] orthophosphate-labeled Hos/CD4/CXCR4/FPRL1 cells were treated at 37°C with SDF-1α (1 μg/mL, 1 minute) or W peptide (1 μM, 60 minutes). The cell lysates were immunoprecipitated (IP) by using an anti-CXCR4 polyclonal antibody to detect increased phosphorylation of CXCR4.
We extended our observations of W peptide to primary monocyte-derived macrophages, which express CCR5 and CXCR4 as well as FPRL1. Cells preincubated with W peptide showed a markedly reduced Ca++flux (Figure 6A) and chemotaxis (Figure6B) in response to either RANTES or SDF-1α. In contrast, macrophages that were pretreated with staurosporine retained their responsiveness to chemokines in both Ca++ flux and chemotaxis experiments, confirming the requirement for PKC activation in mediating the desensitizing effect of the W peptide. It should be noted that our previous study has shown that W peptide is a potent agonist for both FPRL1 and its prototype FPR,14 despite its higher efficacy on FPRL1. Because both FPRL1 and FPR are expressed in mononuclear phagocytes and are capable of transducing signals that culminate in desensitization of chemokine receptors, it would be difficult to distinguish which of the FPRs plays a major role in mediating the effect of the W peptide in macrophages. Our results nevertheless suggest that W peptide is a potent desensitizer of CCR5 and CXCR4 in primary macrophages.
Effect of W peptide on macrophage response to chemokines RANTES and SDF-1α.
Human macrophages generated by incubating monocytes with 5% human AB serum for 4 days were preincubated at 37°C with W peptide (1 μM, 1 hour; W pep) of staurosporine (1.4 ng/mL, 1 hour) followed by W peptide (1 μM, 1 hour; Stauro+W pep). The cells were washed and examined for Ca++ flux (A) and chemotaxis (B) in response to RANTES or SDF-1α (50 ng/mL each). The asterisk indicates significantly reduced cell response in cells treated with W peptide in comparison with cells treated with medium (Medium).
Effect of W peptide on macrophage response to chemokines RANTES and SDF-1α.
Human macrophages generated by incubating monocytes with 5% human AB serum for 4 days were preincubated at 37°C with W peptide (1 μM, 1 hour; W pep) of staurosporine (1.4 ng/mL, 1 hour) followed by W peptide (1 μM, 1 hour; Stauro+W pep). The cells were washed and examined for Ca++ flux (A) and chemotaxis (B) in response to RANTES or SDF-1α (50 ng/mL each). The asterisk indicates significantly reduced cell response in cells treated with W peptide in comparison with cells treated with medium (Medium).
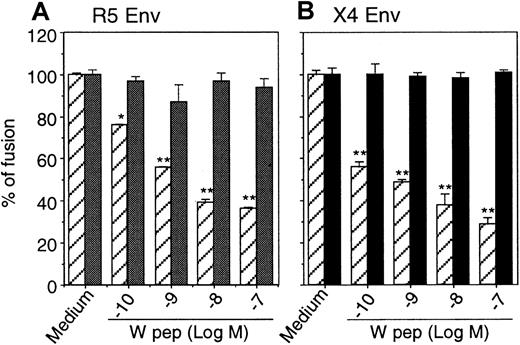
Inhibition of HIV-1 Env-mediated fusion
We then investigated the effect of desensitization of CCR5 and CXCR4 by W peptide on HIV-1 Env-mediated fusion. Quantitative colorimetric fusion assays were performed, and our results showed that the fusion between HIV-1 Env-expressing cells and cells bearing CD4/CCR5/FPRL1 (Figure 7A) or CD4/CXCR4/FPRL1 (Figure 7B) was significantly inhibited by pretreatment of the cells with W peptide. In contrast, W peptide did not inhibit HIV-1 Env-mediated fusion with cells that expressed CD4 and chemokine receptors without FPRL1. Taken together, these results indicate that the activation of FPRL1 by W peptide not only attenuated the Ca++-mobilizing or chemotactic activity of CCR5 and CXCR4, but also impaired their capacity to act as coreceptors for HIV-1 Env-mediated fusion.
Inhibition of HIV-1 Env-mediated fusion by W peptide.
Hos/CD4/CCR5 (▨ indicates FPRL1+; ░, Mock) (A) or Hos/CD4/CXCR4 (B) cells in the presence (FPRL1+) or absence (Mock) of FPRL1 were pretreated with different concentrations of W peptide at 37°C for 1 hour, then were mixed with HeLa cells expressing R5 HIV-1 Env or TF-228 cells expressing X4 HIV-1 Env. After 12 hours of incubation at 37°C, the cells were lysed, and the lysate was allowed to react with chlorophenol red-β-mercaptopyranoside (CPRG) followed by measurement of OD at 570 nm. ▨ indicates FPRL1+; ▪, Mock.
Inhibition of HIV-1 Env-mediated fusion by W peptide.
Hos/CD4/CCR5 (▨ indicates FPRL1+; ░, Mock) (A) or Hos/CD4/CXCR4 (B) cells in the presence (FPRL1+) or absence (Mock) of FPRL1 were pretreated with different concentrations of W peptide at 37°C for 1 hour, then were mixed with HeLa cells expressing R5 HIV-1 Env or TF-228 cells expressing X4 HIV-1 Env. After 12 hours of incubation at 37°C, the cells were lysed, and the lysate was allowed to react with chlorophenol red-β-mercaptopyranoside (CPRG) followed by measurement of OD at 570 nm. ▨ indicates FPRL1+; ▪, Mock.
Inhibition of HIV-1 infection by W peptide
The inhibition of HIV-1 Env-mediated fusion by W peptide in cell lines coexpressing FPRL1 prompted us to investigate further whether W peptide could suppress viral infection by activating FPRL1. As shown in Figure 8, pretreatment of Hos cells expressing HIV-1 coreceptors and FPRL1 with W peptide significantly reduced P24 production when the cells were infected with M-tropic (R5) HIV-1 (Figure 8A) or T-tropic (X4) HIV-1 (Figure 8B). In contrast, W peptide did not reduce P24 production in HIV-1 infected cell lines that were not cotransfected with FPRL1 (data not shown). These results suggest that the anti-HIV-1 activity of W peptide is dependent on the presence of FPRL1 in these cell lines.
Inhibition of HIV-1 replication by pretreatment with W peptide.
Hos/CD4/CCR5/FPRL1 (A) or Hos/CD4/CXCR4FPRL1 (B) cells were preincubated with W peptide at 37°C for 1 hour. The cells were then infected with R5 (Bal) or X4 (PNL4-3) HIV-1 at 37°C for 2 hours, and P24 production was measured after 72 hours. (C) Human monocyte–derived macrophages (R5, [Bal] ▪) or activated CD4+ T lymphocytes (X4, [Pnl4-3] ■) were preincubated with W peptide or chemokines (MIP-1α, MIP-1β, and RANTES at 500 ng/mL each with macrophages, or SDF-1α at 1 μg/mL for CD4+ T cells) at 37°C for 1 hour, followed by infection with R5 (macrophages) or X4 (CD4+ T cells) HIV-1 at 37°C for 2 hours. The cell culture supernatants were then measured for P24 production after 72 hours. *P < .01 compared with cells not treated with W peptide.
Inhibition of HIV-1 replication by pretreatment with W peptide.
Hos/CD4/CCR5/FPRL1 (A) or Hos/CD4/CXCR4FPRL1 (B) cells were preincubated with W peptide at 37°C for 1 hour. The cells were then infected with R5 (Bal) or X4 (PNL4-3) HIV-1 at 37°C for 2 hours, and P24 production was measured after 72 hours. (C) Human monocyte–derived macrophages (R5, [Bal] ▪) or activated CD4+ T lymphocytes (X4, [Pnl4-3] ■) were preincubated with W peptide or chemokines (MIP-1α, MIP-1β, and RANTES at 500 ng/mL each with macrophages, or SDF-1α at 1 μg/mL for CD4+ T cells) at 37°C for 1 hour, followed by infection with R5 (macrophages) or X4 (CD4+ T cells) HIV-1 at 37°C for 2 hours. The cell culture supernatants were then measured for P24 production after 72 hours. *P < .01 compared with cells not treated with W peptide.
Because FPRL1 is expressed in monocytes6,11 14 and CD4+ T lymphocytes (Le, unpublished observation), we then asked whether W peptide could also inhibit HIV-1 infection of primary mononuclear cells. Our results (Figure 8C) show that W peptide inhibited the infection by R5 and X4 HIV-1 strains in peripheral blood monocyte-derived macrophages and CD4+ T lymphocytes cultured with IL-2. The inhibition of HIV-1 P24 production by W peptide in PBMC was dose-dependent with more than 50% of the inhibition achieved at a 10 nM concentration of the peptide. At 100 nM, W peptide showed an effect equal to or greater than that of a combination of CCR5 chemokines (MIP-1α, MIP-1β, and RANTES) at 500 ng/mL each or 1 μg/mL CXC chemokine SDF-1α.
Discussion
In this study, we showed that W peptide potently inhibits HIV-1 Env-mediated fusion and viral infection through heterologous desensitization of the chemokine receptors CCR5 and CXCR4. The effect of W peptide on CCR5 and CXCR4 desensitization was dependent on its capacity to activate FPRL1 in receptor-transfected Hos cells. This was supported by the observation that a scrambled peptide with similar amino acid composition to W peptide, KWMNVY, which was not an agonist for FPRs, did not desensitize CCR5 and CXCR4, and did not inhibit HIV-1 Env-mediated fusion or viral infection of the cells (data not shown). W peptide was initially identified as an activator of human leukocytes by inducing chemotaxis and mediator release.14,15 Our previous efforts to characterize its cellular receptors on leukocytes revealed that W peptide activates 2 STM receptors used by the bacterial chemotactic peptide fMLF, FPR and FPRL1, with much higher potency for FPRL1.14 FPRL1 possesses 69% identity at the amino acid level to the prototype receptor FPR. Unlike FPR, FPRL1 is activated to mobilize Ca++ only by high concentrations of fMLF and does not mediate cell migration in response to fMLF.9,11,14 We recently identified several other chemotactic agonists for FPRL1, including synthetic peptide domains of the HIV-1 envelope proteins11-13 and the acute-phase protein SAA.9 In addition, LXA4 was reported to bind FPRL1-transfected cells with high affinity and to activate guanosine triphosphatase through this receptor. LXA4 is generated in a number of inflammatory and immunologic diseases and appears to have opposing effects on the proinflammatory responses of neutrophils when compared with monocytes.18-20 The expression of FPRL1 is not restricted to phagocytic leukocytes as originally postulated. In fact, B and T lymphocytes, as well as a number of nonhematopoietic cell types, also express FPRL1 (Le, unpublished data). When viewed together with the great diversity of agonists, these characteristics suggest that FPRL1 may play an important role in host defense and immunologic responses.
The signal transduction pathway mediated by FPRL1 has not been extensively studied. However, its high homology to the prototype FPR and sensitivity to pertussis toxin suggest that FPRL1 might share many common features of secondary messenger activation with FPR. The binding of FPR by its agonists results in a G-protein signaling cascade that leads to cell adhesion, migration, release of oxygen intermediates, and activation of mitogen-activated protein kinase.6,7,10Activation of FPR by fMLF also attenuates the expression and function of several chemokine receptors such as CXCR23 and CCR521 through heterologous desensitization. Our previous study showed that preincubation of human leukocytes with the FPRL1 agonist SAA inhibited the response to subsequent stimulation with other chemoattractants,22 suggesting that activation of FPRL1 may, similar to the activation of FPR, result in desensitization of other chemotactic receptors. The effect of F peptide, a synthetic peptide domain of HIV-1 gp120 that activates FPRL1 in monocytes and down-regulates the expression/function of chemokine receptor CCR5 and CXCR4, supported this hypothesis.12 The heterologous desensitization of CCR5 by activated FPRL1 was associated with a PKC-mediated serine phosphorylation of CCR5.13 However, in contrast to the effect on monocytes, desensitization of CCR5 or CXCR4 in receptor-transfected Hos cells by W peptide did not significantly reduce the cell surface expression of these chemokine receptors as assessed by FACS analyses using specific antibodies (Li, unpublished observation). These results suggest that in Hos cells, heterologous desensitization of CCR5 and CXCR4 by activated FPRL1 may not result in receptor internalization. Nevertheless, the signaling of CCR5 and CXCR4 in response to their cognate chemokine ligands is similarly impaired in both monocytes and HOS cells. Whether activation of FPRs could also affect the expression and signaling of other cell surface molecules such as CD4, the primary HIV-1 receptor, requires further investigation. It has been reported that activation of CCR5 by its chemokine ligand could attenuate CD4-mediated T-cell chemotaxis induced by the CD4 ligand IL-16.23 24 This CD4 desensitizing activity appears to be a specific property of CCR5, because stimulation of other chemokine receptors such as CCR1, CCR2, CCR3, and CXCR4 did not cause similar CD4 desensitization. We also explored the possible cross-talk between FPRL1 and CD4. Our preliminary results showed that activation of FPRL1 in receptor transfected Hos cells or in primary mononuclear cells did not reduce the binding of radiolabeled HIV-1 gp120 (MN and CM stains) (Li, unpublished observation). In addition, treatment for up to 1 hour with W peptide of CD4+ T lymphocytes, which express FPRL1, did not enhance tyrosine phosphorylation of the src-like kinase p56lck(Gong, unpublished observation). We therefore postulated that activation of FPRL1 might not transduce signals that significantly affected CD4 expression and function. However, further research is required to fully elucidate this issue; in particular, the effect of long-term exposure of cells to FPR agonists on phenotypic and functional alterations is a subject of our ongoing investigation.
Our present study demonstrated that W peptide, by activating FPRL1, potently desensitized CCR5 and CXCR4 in cell lines transfected with FPRL1. In addition, W peptide inhibited HIV-1 Env-mediated cell fusion and viral infection of these cell lines. Furthermore, the productive infection of human PBMC by HIV-1 of both the M-tropic and T-tropic strains was inhibited by W peptide. These results are in support of a recent observation in which activation of human PBMC with the B oligomer subunit of the pertussis toxin, desensitizes CCR5 and inhibits HIV-1 fusion and infection.25 26 However, B oligomer did not affect the cell surface expression of CCR5, suggesting that disturbance of the signaling cascade at the level of CCR5 may be sufficient to compromise its capacity to act as an HIV-1 coreceptor. At maximal concentrations, the W peptide inhibited HIV-1 Env-mediated fusion by approximately 60%, while inhibiting viral infectivity by more than 80%. Although these levels of inhibition are within an order of magnitude, the results may suggest that the desensitization of the HIV-1 coreceptors leads to a high level of interference with infectivity without completely blocking viral fusion. In any case, desensitization of chemokine receptors may represent a novel approach to the development of anti–HIV-1 agents. In this context, W peptide has several unique characteristics. First, it is a small peptide with only 6 amino acids and, therefore, may not be antigenic in vivo; second, it contains a D amino acid at the C-terminus and may be more resistant to peptidase degradation; and third, it recruits leukocytes through at least 2 FPRs and enhances phagocytosis and release of reactive intermediates that support host defense. Therefore, W peptide and its analogues may have potential as a basis for developing novel anti–HIV-1 agents and immune adjuvants.
The authors thank P. M. Murphy and J. Gao for providing FPRL1 cDNA; J. J. Oppenhiem for reviewing the manuscript; N. M. Dunlop, C. Sadowski, Y. Feng, and A. Brinnan for technical support; and C. Fogle and T. Covell for secretarial assistance.
Supported in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health (NIH), under contract no. NO1-CO-56000, and by NIH grants DA06650 and DA11130 (to T.J.R.), DA05894 and T32DA07237 (to M.A.W.), and DA12113 (to E.E.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ji Ming Wang, LMI, DBS, NCI-FCRDC, Bldg 560, Rm 31-40, Frederick, MD 21702; e-mail: wangji@mail.ncifcrf.gov.


![Fig. 5. Desensitization of CXCR4 by W peptide pretreatment and the effect of staurosporine. / Hos/CD4/CXCR4/FPRL1 cells were preincubated in the absence (A) or presence (B) of staurosporine (1.4 ng/mL) at 37°C for 1 hour, followed by treatment with W peptide (1 μM) at 37°C for an additional 1 hour. Ca++ flux in response to SDF-1α (100 ng/mL) was measured. Hos/CD4/CXCR4/FPRL1 cells were preincubated with (C) or without (D) staurosporine (1.4 ng/mL) at 37°C for 1 hour, and SDF-1α–induced Ca++ flux was then measured. (E) Hos/CD4/CXCR4/FPRL1 cells were preincubated at 37°C with medium, W peptide (1 μM, 1 hour; W pep), staurosporine (1.4 ng/mL, 1 hour) followed by W peptide (1 μM, 1 hour; Stauro+Wpep), or staurosporine alone (1.4 ng/mL, 1 hour; Stauro alone). After washing, cell migration in response to SDF-1α (50 ng/mL) was measured. The asterisk indicates a significantly reduced cell migration after W peptide treatment, compared with cells treated with medium only. ▪ indicates medium; ■, W pep; ░, stauro + W pep; ▨, stauro alone. (F) [32P] orthophosphate-labeled Hos/CD4/CXCR4/FPRL1 cells were treated at 37°C with SDF-1α (1 μg/mL, 1 minute) or W peptide (1 μM, 60 minutes). The cell lysates were immunoprecipitated (IP) by using an anti-CXCR4 polyclonal antibody to detect increased phosphorylation of CXCR4.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/10/10.1182_blood.v97.10.2941/6/m_h81011073005.jpeg?Expires=1767858932&Signature=T3pmylFZVToa2RwNUmxLYR4D55Zy8CPrZM59UWeqJ1m2R7HI-ZmUjK4~Q4C8uhF~Pt8O5rrTJ4al4jzvVkypMiwXlrynXHLfSx1FT25GEh5thtDB7DqLYLfsHUePCHJp4E-G3FbG4w9Kv6KUjM~S8MbB9IAZqBkXn7okT4Pj69iMn42HrUj2Mcr~TlNzvOB-L4F6bJrCeKwc0kJXTHqH1HU7ut4tRYddJksAI5mXG-QLqV6XEXEf2BRSR7FF3D~k9clVufwlSoIAvb5fLndsg9OVV~Ac~Hg3KlQ5~i7AzBP69uCxjQrvewFFXvbnGs81kXIY2xqbL24jkkA~nOZSDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 8. Inhibition of HIV-1 replication by pretreatment with W peptide. / Hos/CD4/CCR5/FPRL1 (A) or Hos/CD4/CXCR4FPRL1 (B) cells were preincubated with W peptide at 37°C for 1 hour. The cells were then infected with R5 (Bal) or X4 (PNL4-3) HIV-1 at 37°C for 2 hours, and P24 production was measured after 72 hours. (C) Human monocyte–derived macrophages (R5, [Bal] ▪) or activated CD4+ T lymphocytes (X4, [Pnl4-3] ■) were preincubated with W peptide or chemokines (MIP-1α, MIP-1β, and RANTES at 500 ng/mL each with macrophages, or SDF-1α at 1 μg/mL for CD4+ T cells) at 37°C for 1 hour, followed by infection with R5 (macrophages) or X4 (CD4+ T cells) HIV-1 at 37°C for 2 hours. The cell culture supernatants were then measured for P24 production after 72 hours. *P < .01 compared with cells not treated with W peptide.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/10/10.1182_blood.v97.10.2941/6/m_h81011073008.jpeg?Expires=1767858932&Signature=RBP1On4o248N32R8o-MG9uccrTS-ZTXQ-d72t0Hi5gJYWuabRPAJQAh8pxDq3btX4GOtVruh~gtJubAvxBUUYsOHk-xqLA-OcSlprkKDd58TcMQkhrMBqvulD-ONBlafVVCklNOb2l1V-zsdn9erUtQur96mrh96QFcG1e-grBZpg-5rjcpuoDrhfkRj4ieVxI7znx6Uo7QdfbU2tIjqXEwZwVoNlEnY1sv3R6ylD-A3B48~sw1b74LOoACitExNIvO4prN1LKCmOi8YCPOf8KI3umCZx6d8jqja47nLSMFMcQ5fHDcvQ2-6QVJWppRxFoAyfmX8xBPhNcAPV3gE0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
