Abstract
Bone marrow (BM) fibrosis may occur in myeloproliferative diseases, lymphoma, myelodysplastic syndrome, myeloma, and infectious diseases. In this study, the role of substance P (SP), a peptide with pleiotropic functions, was examined. Some of its functions—angiogenesis, fibroblast proliferation, and stimulation of BM progenitors—are amenable to inducing BM fibrosis. Indeed, a significant increase was found in SP-immunoreactivity (SP-IR) in the sera of patients with BM fibrosis (n = 44) compared with the sera of patients with hematologic disorders and no histologic evidence of fibrosis (n = 46) (140 ±12 vs 18 ±3; P < .01). Immunoprecipitation of sera SP indicated that this peptide exists in the form of a complex with other molecule(s). It was, therefore, hypothesized that SP might be complexed with NK-1, its natural receptor, or with a molecule homologous to NK-1. To address this, 3 cDNA libraries were screened that were constructed from pooled BM stroma or mononuclear cells with an NK-1 cDNA probe. A partial clone (clone 1) was retrieved that was 97% homologous to the ED-A region of fibronectin (FN). Furthermore, sequence analyses indicated that clone 1 shared significant homology with exon 5 of NK-1. Immunoprecipitation and Western blot analysis indicated co-migration of SP and FN in 27 of 31 patients with BM fibrosis. Computer-assisted molecular modeling suggested that similar secondary structural features between FN and NK-1 and the relative electrostatic charge might explain a complex formed between FN (negative) and SP (positive). This study suggests that SP may be implicated in the pathophysiology of myelofibrosis, though its role would have to be substantiated in future research.
Introduction
Bone marrow (BM) fibrosis is characterized by fibrosis, hypercellularity, excessive deposits of extracellular matrix proteins, increased circulating levels of particular cytokines, and neo-angiogenesis in the BM.1-7 BM fibrosis is common to several hematologic disorders, particularly chronic myeloproliferative disorders (MPD). With reference to the onset of BM fibrosis, there are 2 major categories within the various subgroups.1 The first is idiopathic myelofibrosis (IMF) or chronic BM fibrosis, in which the onset correlates with myeloid metaplasia in the BM, spleen, and liver. Although the underlying cause of this subgroup is undefined, fibrosis could develop as a secondary process. The second is BM fibrosis, which occurs as a secondary process at various times after the initial diagnosis of polycythemia vera, essential thrombocythemia, chronic myeloid leukemia,2,8-10 myeloma,11lymphoma, and, infrequently, other nonhematologic disorders.12 13
The mechanism of BM fibrosis remains mostly undefined. Immune-mediated mechanisms in the development of BM fibrosis have been suggested.9,14,15 Monocytes/macrophages and megakaryocytes are included in the types of cells that have roles in the pathophysiology of BM fibrosis.6,16,17 Regardless of the underlying cause, patients with BM fibrosis have excessive proliferation of fibroblastic mesenchymal cells.18 The fibroblasts are heterogeneous, and experimental evidence suggests that their proliferation is secondary to a clonal disorder of the hematopoietic stem cell.19-22 Despite hematopoietic activity in extramedullary tissues of patients with BM fibrosis,23 patients nonetheless have different degrees of cytopenia.2 This dysregulated hematopoiesis is not surprising because in the adult, the population in whom BM fibrosis generally develops, extramedullary hematopoiesis cannot revert to the neonatal state of balanced hematopoiesis.24 Although there have been modest improvements in treatment options and understanding, the complex mechanisms of BM fibrosis are yet to be elucidated, thus deterring satisfactory treatments. A commonly used treatment for myelofibrosis, splenectomy,25,26 could negatively influence and alter a patient's immunocompetence.27Fine-tuned dissection in the development of BM fibrosis could provide therapeutic leads to reverse or prevent BM fibrosis. In this study, we examined a potential role for substance P (SP). Indeed, we found significant increases in SP levels in the sera of patients with BM fibrosis compared to patients with hematologic diseases without BM fibrosis and to healthy controls. SP exerts functions that favor the development of BM fibrosis—for example, induction of angiogenesis,28,29 mitogenic effects on fibroblast, and induction of fibrogenic cytokines from immune and mesenchymal cells—and it stimulates the proliferation of hematopoietic progenitors.28-32 The latter could be relevant to the clonal hematopoietic progenitors that are frequently accompanied by BM fibrosis in patients with MPD.2
SP is an undecapeptide and is the major peptide encoded by the preprotachykinin-I (PPT-I) gene.29 Neural and nonneural cells express PPT-I.29,33-36Neural-derived SP is released in the BM and other lymphoid organs as a neurotransmitter.37,38 Nonneural sources of SP include immune, hematopoietic, and BM stromal cells.28 Other pleiotropic functions of SP and the other related tachykinin–neurokinin family of peptides include immune and hematopoietic regulation.30,31,33 34
PPT-I peptides interact with different affinities to 3 cloned receptors: NK-1, NK-2, and NK-3.39 In BM stromal cells, the expression of NK-1 is induced by cytokines associated with stimulatory hematopoiesis such as stem cell factor. In these same BM cells, the other homologous receptor, NK-2, is constitutively expressed.32,34 Immunoprecipitation has shown that the increased SP levels in patients with BM fibrosis was complexed to another molecule(s). Protection of SP by a larger molecule is important because this peptide could be degraded by endogenous endopeptidases.40 Therefore, we hypothesized that SP may be complexed to its natural high-affinity receptor (NK-1) or to a homologous molecule. To this end, we screened cDNA libraries constructed from BM cells with NK-1 cDNA. Results showed that SP is complexed to fibronectin (FN), suggesting that FN could protect SP and provide chemical stability to the small peptide. This finding could be relevant because FN is increased in patients with myelofibrosis.41 42 The potential for molecular mimicry by FN for NK-1 was examined by computer-assisted molecular modeling. The significance of these findings with previous reports is discussed.
Materials and methods
Study subjects
The Institutional Review Board of UMDNJ-New Jersey Medical School and the VA New Jersey Health Care System approved the use of approximately 10 mL human blood from each study subject. Sera were aliquoted in siliconized tubes and then stored at −70°C until analysis. Participants, summarized in Table1, included patients with different types of hematologic diseases and age- and sex-matched healthy controls (NC; n = 30. Patients were further subdivided into 2 major categories. The first category consisted of MPD with (n = 34) or without (n = 19) BM fibrosis. IMF (n = 15) and other MPD (n = 19) comprised the group with BM fibrosis. The second category consisted of other hematologic diseases (non-MPD) with (n = 10) or without (n = 27) BM fibrosis. Non-MPD with BM fibrosis included patients with T-cell lymphoma (n = 2) and myeloma (n = 8). Non-MPD without BM fibrosis included patients with aplastic anemia (n = 6), B-cell lymphoma (n = 8), pure red cell aplasia (n = 4), myeloma (n = 5), and chronic lymphocytic leukemia (n = 4). Ages of study subjects ranged between 36 and 71 years, and the mean ages for NC and patients were 48 and 54 years, respectively. Patients included in the study had advanced/significant (3-4+) BM fibrosis, based on established clinical and laboratory criteria.2 A clinical hematologist (P.G.) examined the BM biopsy slides of study patients with the hematopathologist to ensure consistency in the grading of BM fibrosis. Patients included in the study had advanced fibrosis graded as 3 to 4+. Patients who underwent multiple transfusions were not included in the study. At the time of blood sampling, hemoglobin levels of patients ranged from 6 to 8 g/dL. Patients received no blood transfusion 3 to 4 months before blood collection. Patients were not included if BM fibrosis developed secondary to myeloproliferative disorder unless the degree of fibrosis was 3 to 4+. One patient became symptomatic, and the sample was taken before transfusion. At the time of the study, patients showed no sign of infection and were not taking medication.
Cytokines, antibodies, and other reagents
Hoffman-La Roche (Nutley, NJ) provided rhIL-1α. Stem cell factor (SCF) was purchased from R&D Systems (Minneapolis, MN). Alkaline phosphatase (Alk Phos)-conjugated goat anti-rabbit IgG was obtained from Kierkegaard & Perry Laboratories (Gaithersburg, MD). Rabbit anti-SP was purchased from Arnel Products (New York, NY). SP and rabbit anti-human FN were purchased from Sigma (St Louis, MO).
Preparation of bone marrow stroma
Bone marrow stroma was prepared as described.43Briefly, BM aspirates were obtained from healthy donors, ages 20 to 35 years, according to guidelines from the institutional review board of UMDNJ-New Jersey Medical School. Unfractionated cells from BM aspirates were cultured at 33°C in α-MEM (Life Technologies, Grand Island, NY) with 12.5% fetal calf serum (Hyclone Laboratories, Logan, UT), 12.5% horse serum (Hyclone Laboratories), 0.1 μM hydrocortisone, 0.1 mM 2-ME, and 1.6 mM glutamine. On day 3 of culture, red blood cells and granulocytes were removed from the nonadherent fraction by Ficoll-Hypaque density gradient (Sigma). Mononuclear cells were replaced in the respective cultures, which were re-incubated with weekly replacement of 50% medium until confluence.
cDNA libraries
Three different cDNA libraries were used to screen for NK-1–related clones. One library, prepared from unstimulated pooled human BM cells, was purchased from Clontech (Palo Alto, CA). Two other cDNA libraries were prepared with poly-A RNA isolated from BM stroma stimulated with 25 ng/mL IL-1α or 10 ng/mL SCF. Each library was prepared with mRNA isolated from at least 9 healthy donors. Donor pool was represented by sex and ethnic diversity. Libraries were constructed with the cDNA synthesis kit and the Zap Express cDNA Gigapack III Gold cloning kit (both purchased from Stratagene, La Jolla, CA). Preparation of the library was according to the manufacturer's instructions. Briefly, second-strand cDNA with XhoI and EcoRI adapters was prepared with 5 μg mRNA and then ligated in pZap. Titration of packaged pZap was approximately 106 to 107 pfu/mL. Each library was screened with 107pfu by plating 5 × 104 pfu/150 mm bacteriologic grade Petri dishes (Fisher Scientific, Springfield, NJ). Plaques were hybridized with human NK-1 cDNA44 using different hybridization and washing parameters.
Quantitation of SP-IR
Competitive enzyme-linked immunosorbent assay (ELISA) quantitated SP-IR as described.43 Briefly, Immulon 96-well plates (Dynatech Laboratories, Chantilly, VA) were coated with 100 μL streptavidin at 5 μg/mL. Each well was incubated with 100 μL biotinylated SP (Chiron Mimotopes, Emeryville, CA) at 750 ng/mL. After this, equal volumes (50 μL) of unknown samples and optimum rabbit anti-SP were added to quadruplicate wells. Each sample was assayed as undiluted and 3-serial dilutions. Bound anti-SP was detected with Alk Phos-conjugated goat anti-rabbit IgG and Sigma 104 phosphatase substrate (Sigma). SP-IR levels were calculated from a standard curve developed with optical density at 405 nm versus 12 serial dilutions of known SP concentrations, ranging from 100 to 0.08 pg/mL. Optimization experiments indicated that the working concentrations of rabbit anti-SP and goat anti-rabbit IgG were 1/15 000 and 150 ng/mL, respectively.
Immunoprecipitation and Western blot analysis
Immunoprecipitation and Western blot analysis were performed as previously described.6 Briefly, SP was immunoprecipitated from sera by incubating with rabbit anti-SP (1/10 000) at 4°C overnight. After this, samples were incubated with protein A Sepharose CL 4B (Sigma) at 4°C for 6 hours and then centrifuged at 4°C, 10 000g for 30 minutes. Pellets were washed (1×) with phosphate-buffered saline, resuspended in sample buffer, and electrophoresed on 12% SDS-PAGE. Proteins were transferred to Immobilon-P membranes (Millipore, Bedford, MA), and SP was detected by overnight incubation at room temperature with rabbit anti-SP (1/15 000). After this, membranes were washed and incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1/5000) for 45 minutes. HRP was developed with ECL Western blot detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ).
Membranes were stripped by washing for 1 hour at room temperature with 62 mM Tris (pH 6.8) containing 100 mM β-ME and 2% SDS and then developed for FN using a detection technique similar to the method used for SP (described above). Rabbit anti-human FN was used at 1/400 dilution. The Mr of developed bands was compared with pre-stained low-, medium-, and high-range protein standards (Diversified Biotech, Newton Center, MA).
Computer-assisted modeling of SP and docking to FN
The structure of SP had to be modeled because it was not solved by crystallography owing to its high flexibility. We modeled the secondary structure of SP and used the partial crystal structure of human FN to explain possible interactions between SP and FN.45 The sequence of SP, an 11-amino acid peptide, gives it probable, yet not fixed, structure. Its 3-dimensional structure was generated using Sybyl 6.6 molecular modeling package (TRIPOS Associates, St Louis, MO), and energy was optimized with Discover.
Statistical analysis
Data were analyzed using the Student t test to determine the significance (P value) between experimental values.
Results
SP-IR levels in the sera of patients with or without BM fibrosis
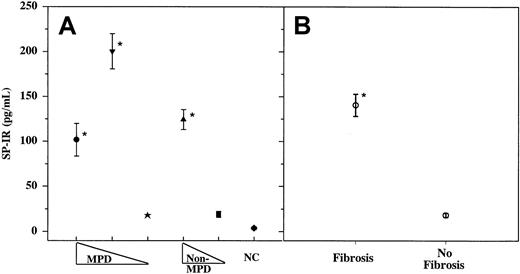
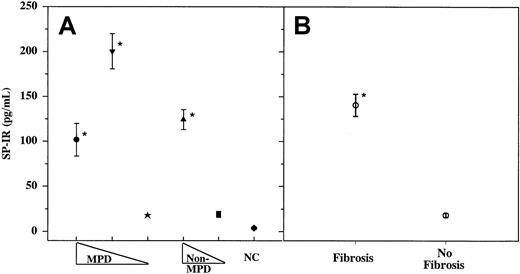
Increased proliferation of fibroblasts in the BM of patients with fibrosis indicates that the BM microenvironment is amenable to the mitogenic effects on fibroblasts. The increased frequency of fibroblast in the BM is heterogeneous rather than clonal.1,2 This suggests that the fibroblasts are responding to factors with mitogenic potential. We, therefore, determined whether SP, a mitogen for fibroblast,28 could be a candidate molecule by quantitating its levels in the sera of patients with fibrosis and compared its levels to those in patients with other hematologic disorders and in age-matched healthy controls. SP-IR levels were quantitated in samples taken at the time of diagnosis (3-4+ reticulin fibers) and at approximately 4-month intervals for up to 2 years. SP-IR levels in the samples collected within this period were not significantly different and ranged from 2 to 5 pg/mL. Results shown for each patient in Figure 1 represent the mean of 4 to 8 samples. Data shown in Figure 1B indicate significant (P < .01) increase in SP-IR in patients with BM fibrosis (162 ± 15.5, ± SD; n = 44) compared to those in patients without fibrosis (26 ± 7, ± SD; n = 46). There was no significant difference (P > .5) in SP-IR levels among the 3 groups of patients with BM fibrosis (MF, IMF, or non-MPD) (Figure1A). The common feature of these 3 groups of patients is the development of BM fibrosis, though the underlying causes are different: MPD and non-MPD, or reactive and nonreactive fibrosis. Data indicate that increased SP-IR level correlates with BM fibrosis rather than with the type of underlying disorder.
Sera were obtained from patients with or without fibrosis and then assayed in triplicate for SP-IR using ELISA.
(A) MPD, with fibrosis (●, other MPD, n = 19; ▾, IMF, n = 15) or without BM fibrosis (★, n = 19). Non-MPD with fibrosis (▴, n = 10) or without fibrosis (▪, n = 27). NC, healthy controls (n = 30). (B) Grouped data from patients, shown in panel A: BM fibrosis (3-4+ reticulin fibers) versus no fibrosis. Results are expressed as mean ± SD. *P < .01 versus no fibrosis.
Sera were obtained from patients with or without fibrosis and then assayed in triplicate for SP-IR using ELISA.
(A) MPD, with fibrosis (●, other MPD, n = 19; ▾, IMF, n = 15) or without BM fibrosis (★, n = 19). Non-MPD with fibrosis (▴, n = 10) or without fibrosis (▪, n = 27). NC, healthy controls (n = 30). (B) Grouped data from patients, shown in panel A: BM fibrosis (3-4+ reticulin fibers) versus no fibrosis. Results are expressed as mean ± SD. *P < .01 versus no fibrosis.
Immunoprecipitation of SP-IR in the sera of patients with BM fibrosis
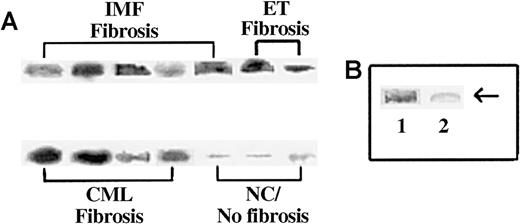
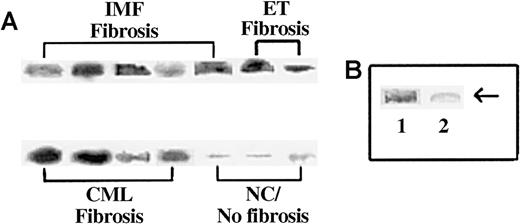
Because SP is a small peptide and is the substrate for several endogenous endopeptidases,40 we next determined whether its presence in the circulation could be due to protection from other complexed proteins. We verified this by determining the approximate molecular weight of the circulating SP-IR by immunoprecipitation. We incubated sera that contained more than 10 pg/mL SP-IR (Figure 1) with anti-SP and then performed Western blot analysis with the immune complexes in nonreducing conditions. Representative results are shown in Figure 2A, and the results of all samples are summarized in Table 2. Strong bands at approximately 225 kd were detected in the samples containing more than 25 pg/mL SP-IR (Figure 2A). We also observed another band at 160 kd, the predicted size for IgG (data not shown). The 160-kd band was confirmed as the precipitating IgG by electrophoresing the samples in the presence or absence of reducing agent (β-ME) and then blotting with the second antibody. In the presence of β-ME, we observed bands at 55 kd (not shown), which is equivalent to the predicted size of a single heavy chain for IgG.
Immunoprecipitation of SP-IR in the sera of subjects with and without BM fibrosis.
Sera (1 mL) were immunoprecipitated with anti-SP and then analyzed by Western blot for SP with the same antibody. (A) Representative samples are shown for patients with fibrosis: IMF, ET/IMF, and CML. Lanes are shown for results obtained for NC or sera with fewer than 5 pg/mL SP and no fibrosis. (B) Immunoprecipitates were analyzed with anti-SP pre-absorbed with SP. Lane 1, regular anti-SP; lane 2, pre-absorbed anti-SP.
Immunoprecipitation of SP-IR in the sera of subjects with and without BM fibrosis.
Sera (1 mL) were immunoprecipitated with anti-SP and then analyzed by Western blot for SP with the same antibody. (A) Representative samples are shown for patients with fibrosis: IMF, ET/IMF, and CML. Lanes are shown for results obtained for NC or sera with fewer than 5 pg/mL SP and no fibrosis. (B) Immunoprecipitates were analyzed with anti-SP pre-absorbed with SP. Lane 1, regular anti-SP; lane 2, pre-absorbed anti-SP.
Because the developed bands shown in Figure 2A were not at the predicted size of SP, we next determined the specificity of the reaction. Western blot analysis was repeated with anti-SP that was pre-absorbed with SP (undiluted antibody incubated with 0.1 mg SP for 2 days at 4°C). Previously developed bands disappeared with the pre-incubated antibody indicating specificity for SP. Representative results are shown in Figure 2B. Results described in this section showed that SP-IR is detected in the sera of patients with BM fibrosis, though at a high molecular weight. These results led to the hypothesis that the increased level of circulating SP-IR in patients with BM fibrosis is complexed to one or more molecules.
Screening of cDNA libraries for structural analogues of NK-1
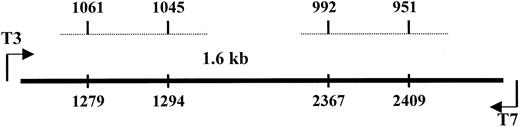
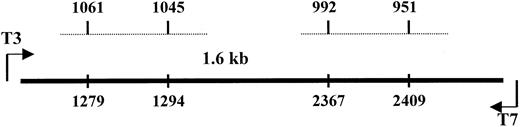
The predicted molecular weight of SP and its natural receptor is approximately 80 kd in nonreducing conditions.46 However, because we consistently observed bands heavier than 200 kd, we investigated whether SP could be bound to a homologue of NK-1 or to a molecule that shares homology with NK-1. To address this, we screened 3 cDNA libraries—IL-1α–stimulated BM stroma, SCF-stimulated BM stroma, and pooled unstimulated BM mononuclear cells. DNA from clone 1 was sequenced in the forward and reverse directions and determined to be 97% homologous with the ED-A region of FN.47 BESTFIT of clone 1 with NK-1 cDNA44 indicated alignment with exon 5 of NK-1. Sequences spanning nt 1045 to 1061 shared 81% homology with clone 1 and 71% with nt 951 to 992 (Figure3). DNA analyses were performed with the Wisconsin Genetics Computer Group (Madison, WI) package of DNA–protein sequence analysis programs (version 10). Results indicated that 2 areas of NK-1 cDNA shared significant homology with the ED-A region of FN.
Alignment of the cDNA of NK-1 and clone 1.
Dashed lines represent the homologous regions of NK-1 with the spanning regions indicated at the top for NK-1 and the bottom for clone 1.
Alignment of the cDNA of NK-1 and clone 1.
Dashed lines represent the homologous regions of NK-1 with the spanning regions indicated at the top for NK-1 and the bottom for clone 1.
Co-precipitation of FN and SP in the sera of patients with BM fibrosis
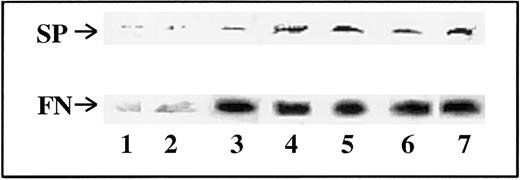
We next determined whether FN could be the carrier protein complexed to SP in the sera of patients with BM fibrosis (Figure 2). Immunoprecipitation with anti-SP was performed, and the complex was determined for the co-migration of SP and FN in Western blots. Indeed, it was shown by consecutive blotting for SP and FN that the developed bands could be superimposed at the same molecular weight. Representative blots are shown in Figure4, and the results of all experiments are summarized in Table 2. Twenty-five patients with myeloproliferative disorders and BM fibrosis showed complexes of SP and FN. Although the total number of patients was low (n = 6) for non-MPD with BM fibrosis, all showed significantly high levels of SP-IR (Figure 1, Table 2). However, only 3 were complexed to FN, and the other 3 migrated to the predicted size (Table 2). Because immunoprecipitation was performed with anti-SP, the zero values in the numerator in Table 2indicate that SP was not complexed to FN. These results indicate that for most of the patients with BM fibrosis, elevated circulating SP-IR is complexed to FN.
Co-migration of SP and FN from the sera of patients with BM fibrosis.
Sera were immunoprecipitated with anti-SP and then developed sequentially for SP and FN in Western blot. Details of the techniques are described in “Materials and methods.” Representative blots of different categories of patients are shown. Lane 1, PV; lanes 2 and 3, ET; lanes 4 and 5, IMF; lanes 6 and 7, CML. No band for SP was observed in patients without BM fibrosis.
Co-migration of SP and FN from the sera of patients with BM fibrosis.
Sera were immunoprecipitated with anti-SP and then developed sequentially for SP and FN in Western blot. Details of the techniques are described in “Materials and methods.” Representative blots of different categories of patients are shown. Lane 1, PV; lanes 2 and 3, ET; lanes 4 and 5, IMF; lanes 6 and 7, CML. No band for SP was observed in patients without BM fibrosis.
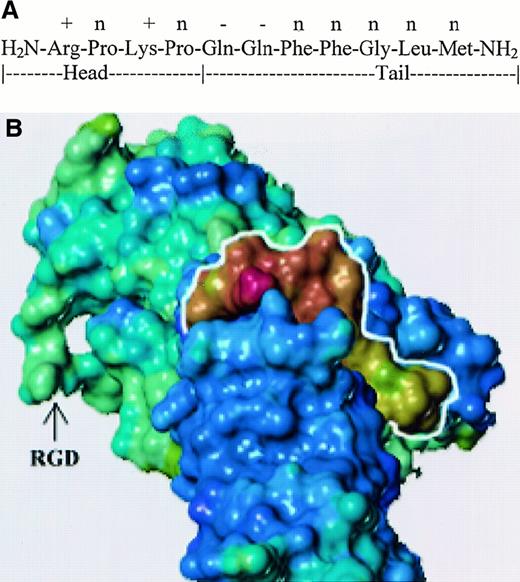
Possible interactions between SP and fibronectin
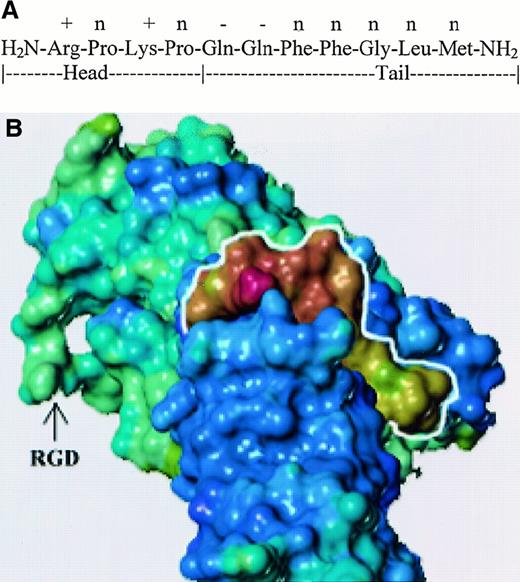
We assigned the N-terminal region of SP structure, SP 1-4, as the head and the C-terminal, SP 5-11, as the tail. Both phenyl rings of the adjacent Phe residues are optimal when they are in trans, and their position lowers the probability of folding between the head and tail ends. Both prolines create a kink in the head, and the electrostatic interaction between the positive and negative residues helps to stabilize the kink. We used SYBYL to dock SP to the crystal structure of repeats 3 to 10 of FN (Protein Data Bank,1FNF).45 This complex was based on the structural, electrostatic, and secondary structural characteristics of both SP and FN. Figure 5B shows the electrostatic potential of SP, outlined in white, to be relatively positive, which is complimentary to the electronegative potential of FN in the binding cleft. The amino terminal head region is shown at the top of SP (marked in white outline). The area of interactions is within repeat 7 of FN, thus leaving the RGD region exposed outside and available for interactions with the family of integrins.45
Docking of SP and fibronectin.
(A) Diagram shows the sequence of SP denoting head and tail regions. Electrostatic potential on the side chains for SP residues are shown: positive (+), negative (−), and neutral (n). (B) Three-dimensional model of SP–FN interaction. Relative electrostatic potential was mapped on the solvent accessible surface of each molecule. Blue-violet represents negatively charged areas, and yellow-red represents positively charged regions. SP is outlined in white.
Docking of SP and fibronectin.
(A) Diagram shows the sequence of SP denoting head and tail regions. Electrostatic potential on the side chains for SP residues are shown: positive (+), negative (−), and neutral (n). (B) Three-dimensional model of SP–FN interaction. Relative electrostatic potential was mapped on the solvent accessible surface of each molecule. Blue-violet represents negatively charged areas, and yellow-red represents positively charged regions. SP is outlined in white.
Discussion
In this study, we describe a potentially novel mechanism for the development of BM fibrosis. Data show a strong correlation between increased levels of SP-IR in the sera and advanced BM fibrosis (Figure1). This correlation is irrespective of the underlying cause of BM fibrosis. However, because the grade of BM fibrosis was greater than 3 in each of the subject groups with fibrosis, a correlate between the degree of fibrosis and SP-IR levels cannot be determined. Results in this study add to the complex mechanism reported for patients with MPD, with or without BM fibrosis.1 The major question that arises from this study is the anatomic and cellular source of SP, which could be produced by neural and nonneural cells.28 One likely source is the macrophage, increased in MPD and activated in BM fibrosis.6,17,48 Because macrophages express NK-1,49-51 our findings might explain a potential mechanism for the activation of circulating monocytes reported for patients with myelofibrosis.6,52 Interactions between circulating SP and NK-1 could induce cytokines.28 Released cytokines could stimulate cells through an autocrine mechanism to activate NF-κB, a transcriptional factor activated in the monocytes of patients with myelofibrosis.50,52 This type of activation signal in myelofibrosis would be in addition to the adhesion–extracellular matrix protein interactions in macrophages or other hematopoietic cells.53 Furthermore, the activation of monocytes could lead to the release of SP and the expression of natural receptor. C-expression of the ligand and receptor in the same cell can result in autocrine stimulation to release other cytokines and adhesion molecules.
The role of SP as an activating signal for monocytes in patients with BM fibrosis is a subject of current investigation in our laboratory. In particular, we are trying to determine whether monocyte activation or cytokine production by monocytes in patients with myelofibrosis6,52 could be partly mediated by the increased levels of circulating SP. We recently cloned the humanPPT-I promoter and found that the activation of BM cells leads to autostimulation, similar to the concept of autoreceptors, described for neurotransmitter release.54,55 We are now studying whether similar autostimulation could occur in the macrophages of patients with myelofibrosis. The findings of this study could help unravel an alternative or synergistic pathway with other identified mechanisms for the activation of monocytes in myelofibrosis.6,52 Despite the angiogenic function of SP,29 this study does not prove that SP is directly involved in the increased angiogenesis and osteosclerosis seen in patients with BM fibrosis. Therefore, further studies are required to show a cause-and-effect relation between the pathophysiology of myelofibrosis and SP levels.
Identification of FN as the carrier molecule for SP provides another mechanism in which the ubiquitous extracellular matrix proteins could be involved in BM fibrosis.14,16 Furthermore, this study also demonstrates a function for the increased FN demonstrated in the BM of patients with myelofibrosis.41 Interestingly, Reilly et al41 showed that enhanced distribution of FN correlated with hypercellularity and vascularity of the BM, both of which could be partly attributed to functions exerted by SP.28-30 As entities, SP and FN are mitogenic to fibroblast. However, a biologic response by the FN–SP complex cannot be eliminated because other authors have shown enhanced chemotactic functions of the FN–TNF-α complex compared to the individual molecules.56 Therefore, a cause-and-effect relation of SP and BM fibrosis appears to be occurring, though this has to be demonstrated in future studies. Of note is the availability of the integrin-interacting site of fibronectin RGD in our model (Figure 5B). The availability of RGD in the complex implies that FN could interact with the immune cells, such as macrophages, while making SP available to the same cell.
TGF-β and its inducible genes have been implicated in the fibrosis of different organs.52,57,58 In BM fibrosis, activated monocytes are one of the cellular sources of TGF-β.6Preliminary evidence indicated that the monocytes could be a source of the increased SP in the BM and circulation (unpublished observations). In inflammatory responses, SP enhances immune responses through the down-regulation of TGF-β in activated normal macrophages.59 Therefore, the simultaneous increase in SP and TGF-β levels in patients with BM fibrosis and their role in maintaining monocyte activation are paradigms that need to be studied further. This would be important not only for understanding BM fibrosis but also for determining whether the clonal disorder could be the initiator of reactive fibrosis in the BM. This is particularly important because SP stimulates the proliferation of BM progenitors.28,30 A source for SP in the clonal cells cannot be eliminated because other transformed cells have been shown to overexpress the PPT-I gene.43 This study presents a major question: why is SP, a relatively small peptide, not degraded or absorbed by specific receptors in immune cells. This question would have to be addressed because all but one patient had normal renal function, and renal clearance might not be altered. One major possibility is that endopeptidases that use SP as their substrate could be dysregulated in these patients.40The function of the reticulo-endothelial system in the study subjects with BM fibrosis is altered. However, it is unlikely that this system is responsible for the non-clearance of SP because there is no evidence for an immune complex. The small size (11 aa) of SP does not make it a likely candidate for opsonization by the reticulo-endothelial system while it is complexed to FN. Furthermore, patients do not show an increased frequency of infection compared to healthy controls, and the reticulo-endothelial system might not be responsible for the inability of SP to be cleared.
If future studies verify a role for PPT-I in BM fibrosis, it would not be unique because dysregulation of this gene is implicated in the pathophysiology of several diseases and infections.43,49,60-62 Current treatments for myelofibrosis have shown modest promise, but there is an obvious need for better treatments.1,25,26 This report, which points to the necessity for further research, might very well add to the understanding of the complex mechanism by which BM fibrosis occurs. If substantiated, such insights could begin to provide a direction toward targets that may be useful in cytoreduction and ultimately restoration of the BM microenvironment. This is particularly important in light of the proliferative effects of SP on the 2 BM cell populations relevant to fibrosis: fibroblasts and hematopoietic progenitors.28
Supported by grants from the National Institutes of Health (HL-54973, HL-57675) and the National Cancer Institute (CA89868).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pranela Rameshwar, Dept of Medicine-Hematology/Oncology, UMDNJ-New Jersey Medical School, MSB, Rm E-579, 185 South Orange Ave, Newark, NJ 07103; e-mail:rameshwa@umdnj.edu.