Abstract
The 2-phenylaminopyrimidine derivative STI571 is a selective inhibitor of c-Abl, c-kit, and platelet-derived growth factor–receptor tyrosine kinases and is presently in phase II-III clinical studies. Here, this study reports on a novel pharmacologic activity of the compound, ie, enhancement of the cyto-differentiating, growth-inhibitory, and apoptogenic actions of all-trans-retinoic acid (ATRA). Whereas STI571 is not a cytodifferentiating agent by itself, the compound interacts with ATRA and enhances the myeloid maturation program set in motion by the retinoid in the PML-RARα+ acute promyelocytic leukemia NB4 and the PML-RARα− myeloblastic HL60 and U937 cell lines. In addition, STI571 relieves the cyto-differentiation block observed in the ATRA-resistant cell lines, NB4.R1, NB4.306, and NB4.007. In NB4 promyelocytes, a RARα agonist, but not an RXR agonist, can substitute for ATRA and interact with STI571. By contrast, STI571 is unique among c-Abl–specific tyrosine kinase inhibitors in modulating the pharmacologic activity of ATRA. In NB4 cells, enhanced cyto-differentiation results in increased up-regulation of the expression of a number of genes coding for myeloid differentiation markers, including CD11b, CD11c, and some of the components of the nicotinamide adenine dinucleotide phosphate–oxidase enzymatic complex. All this is accompanied by inhibition of c-Abl tyrosine phosphorylation and retardation of the retinoid-dependent degradation of PML-RARα and RARα. Stabilization of the 2 retinoic acid receptors is likely to be the result of augmented and accelerated inhibition of the proteasome-dependent proteolytic activity observed on ATRA treatment.
Introduction
All-trans-retinoic acid (ATRA) represents the sole example of clinically useful cytodifferentiating agent.1 Treatment of patients with acute promyelocytic leukemia (APL) with ATRA alone or in combination with chemotherapy results in high rates of complete clinical remission.1,2However, the therapeutic use of this compound is limited by a number of problems, which include serious systemic toxicity3 and induced ATRA resistance.4,5 In addition, the use of ATRA is limited to the APL subset of acute myelogenous leukemia, as all the other French-American-British subtypes are generally refractory to the cytodifferentiating action of the retinoid. One way to circumvent some or all of the aforementioned problems is to identify agents capable of enhancing the pharmacologic activity of ATRA. In previous reports, we and others demonstrated that agents such as granulocyte colony-stimulating factor, cell-permeable analogs of cyclic adenosine 3′,5′-monophosphate (cAMP), and interferons enhance the cytodifferentiating action that ATRA exerts on APL and other myeloid cell lines.6-11
The 2-phenylaminopyrimidine derivative STI571 is a tyrosine kinase inhibitor, acting on a restricted number of target proteins,12,13 ie, c-Abl and its pathologic derivative BCR-Abl,12,14,15 c-kit16,17, and the platelet-derived growth factor (PDGF) receptor.17,18 In vitro, the compound selectively inhibits the growth and induces apoptosis of chronic myelogenous leukemia (CML) blasts synthesizing p210 BCR-Abl19,20 and lymphoblastic leukemia cells expressing p185 BCR-Abl.21 In vivo, the tyrosine kinase inhibitor causes regression of human CML xenografts in nude mice.22 The compound is currently undergoing phase II-III clinical trials for the treatment of patients with CML with very promising results.23
In this study, we report on an unexpected pharmacologic effect of STI571 in acute myelogenous leukemia cells, which do not express the p210 or p185 BCR-Abl oncogenic protein. We observe that STI571 enhances the cyto-differentiating, antiproliferative, and apoptogenic activity of ATRA. In addition, in certain APL cell lines made resistant to the retinoid, the tyrosine kinase inhibitor is capable of relieving resistance. These effects are associated with a decrease in ATRA-induced degradation of RARα and the APL-specific PML-RARα fusion protein, which may explain, albeit partially, the observed interaction.
Materials and methods
Chemicals
ATRA and 8-(4-chlorophenylthio) adenosine 3′:5′-cyclic monophosphate (8-CPT-cAMP) were obtained from Sigma (St. Louis, MO), the RARα agonist AM58024 and the RXR agonist CD291525 were synthesized by CIRD-Galderma Research and Development (Sophia Antipolis, France). The 2-phenylaminopyrimidine derivative STI571 was a kind gift of Dr Elisabeth Buchdunger (Novartis, Basel, Switzerland). AG957,26-28 NSC68410, and NSC676448 were synthesized by the Chemical Branch of the National Cancer Institute (Bethesda, MD) and kindly provided by Dr Edward Sausville.
Cell culture and treatment
The NB4 cell line29 and the NB4.R130ATRA-resistant subline were obtained from Dr Michel Lanotte (Hopital Saint Louis, Paris, France). The 2 ATRA-resistant NB4.30631and NB4.00732 sublines were a kind gift of Dr Carlo Passerini-Gambacorti (Istituto Nazionale dei Tumori di Milano, Italy). HL-60 and U937 myeloid leukemia cells were obtained from the American Type Culture Collection (Rockville, MD). Cell lines were cultured in the presence of RPMI-1640 containing 10% fetal calf serum and were free from Mycoplasma contamination, as assessed by staining with Hoechst 33258.
Cellular proliferation, apoptosis, and cytodifferentiation
The total number of cells and cell viability were determined by manual counting in Burker chambers following staining with erythrosin (Sigma). To prevent apoptosis, control cultures were kept in the logarithmic phase of growth (3-6 × 105 cells/mL) by dilution in fresh medium every 2 days. Cultures treated in parallel were similarly diluted in drug-containing medium to keep the cell density between 3-6 × 105 cells/mL. For the determination of the apoptotic index (percentage of cells with fragmented nuclei), cells were stained with 4′-6-diamidine-2-phenylindole (DAPI), as described.33 The surface markers CD11b, CD11c, and CD33 were measured by flow cytometry as previously described.34 The nitrobluetetrazolium (NBT) reduction assay was performed on extracts of PMA-stimulated cells as detailed in a previous study.35 In some experiments, we determined the percentage of NBT positivity by counting (3 microscopic fields/experimental point, in double blind) the number of cells reducing NBT to formazans (blue cells) and that of cells devoid of NBT-reducing activity (white cells). We expressed the data as the percentage of the following ratio: blue cells/blue + white cells.
Western blot analysis immunoprecipitation and determination of the proteasome activity
Total extracts from NB4, HL60, or U937 cells (approximately 3 × 106 cells) were subjected to Western blot analysis as reported.33 The experiments were performed with antibodies against the F-domain of RARα (RPαF′36), RXRα, 4RX1F6,37 CEBPε (C-22; Santacruz Biotechnology, Santa Cruz, CA), STAT1 p91 (C-24; Santacruz), c-kit (C-14; Santacruz), c-Abl (24-11; Santacruz), phosphotyrosine (PY20; Signal Transduction Laboratories, Lexington, KY), or β-actin (C-11; Santacruz). Bands were visualized with the enhanced chemiluminescence detection kit (Amersham, Little Chalfont, United Kingdom). Immunoprecipitations with antibodies recognizing c-kit (C-19; Santacruz) or c-Abl (K-12; Santacruz) were performed as described by the manufacturer, using 2 × 107 cells. The in vitro activity of the proteasome was determined according to the fluorimetric method of Grune et al38 on cell lysates (2.5 × 106cells).
RNA extraction, Northern blot analysis, and microarray screening
Northern blot analysis was performed on total RNA,6using complementary DNA (cDNA) probes for cEBPε, IRF-1, STAT1, CD11b, p22/phox, p47/phox, p67/phox, gp91/phox, rac1, rac2, and β-actin.35 Except for β-actin, all the cDNA probes were obtained by reverse transcriptase-polymerase chain reaction (RT-PCR) with the use of specific amplimers: CD11b (sense primer, nucleotide 2471-2490; antisense primer, nucleotide 3271-329039); cEBPε (sense primer, nucleotide 196-215; antisense primer, nucleotide 940-970); p22/phox (sense primer, nucleotide 131-150; antisense primer, nucleotide 651-67040); p47/phox (sense primer, nucleotide 191-210; antisense primer, nucleotide 931-95041); p67/phox (sense primer, nucleotide 821-840; antisense primer, nucleotide 1631-165042); gp91/phox (sense primer, nucleotide 911-930; antisense primer, nucleotide 1641-166043); rac1 (sense primer, nucleotide 141-160; antisense primer, nucleotide 911-93044); rac2 (sense primer, nucleotide 11-30; antisense primer, nucleotide 491-51045). For the microarray experiment, we used the ATLAS Human Cytokine/Receptor Array (cat. no. 7744-1; Clontech, Palo Alto, CA). Imaging and quantitation of Northern blots were performed with the STORM 460 phosphoimager (Amersham-Pharmacia Instrumentation, Little Chalfont, United Kingdom).
Results
STI571 enhances the growth-inhibitory, apoptogenic, and cyto-differentiating effects of ATRA on APL cells
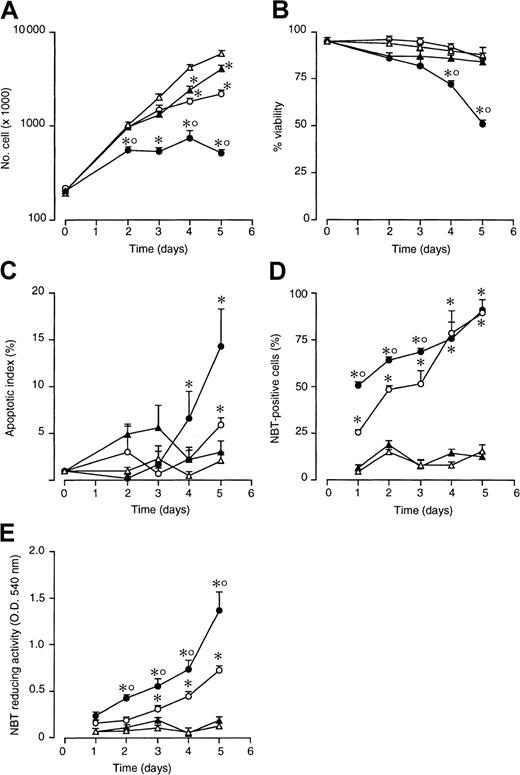
As shown in Figure 1A, treatment of NB4 cells with STI571 (5 × 10−6 M) slows down the logarithmic growth of NB4 cells. The effect is of the same order of magnitude as that observed following treatment with a suboptimal concentration of ATRA (10−7 M). When cells are simultaneously treated with STI571 and ATRA, a more than additive effect is observed. As illustrated in Figure 1B, from the fourth day on, the STI571 + ATRA combination causes a significant decrease in cell viability relative to what is observed in cultures treated with vehicle alone, ATRA, or STI571. The loss of viability is accompanied by a significant increase in the number of apoptotic cells (Figure 1C). More importantly, STI571 accelerates the process of NB4 blasts differentiation, as assessed by determining the percentage of cells expressing NBT-reducing activity, a phenotypic marker of granulocytic maturation (Figure 1D). This is associated with a stronger induction of the marker enzyme in STI571 + ATRA than in ATRA-differentiated cells, as indicated by the data obtained following measurement of NBT-reducing activity on whole cell extracts (Figure1E). With reference to this last point, when the majority of cells is NBT+ following treatment with ATRA + STI571 and ATRA, cell-associated NBT-reducing activity is significantly higher in the former than in the latter experimental group (compare the results at 4 and 5 days in Figure 1D,E).
Effect of ATRA and STI571 alone or in combination on the growth, granulocytic maturation, and apoptosis in NB4 cells.
NB4 cells were seeded at an initial concentration of 2 × 105/mL and treated for the indicated amount of time with medium alone (▵) or medium containing ATRA (10−7 M) (○), STI571 (5 × 10−6 M) (▴), and STI571 + ATRA (●). Aliquots of the cultures were withdrawn at each time point and subjected to counting, following erythrosin staining for the determination of the total number of viable cells (A), the percentage of viable cells (B), the degree of apoptosis as assessed by DAPI staining (C), and the level of granulocytic maturation, as assessed by measuring the percentage of NBT-positive cells (D) or cell-associated NBT-reducing activity (E). Results are the mean ± SD of 3 separate culture dishes and are representative of at least 3 independent experiments. *, significantly higher or lower than the untreated control (P < .01 according to the Studentt test). °, significantly higher or lower than the sum of the effects observed in the corresponding STI571- and ATRA-treated groups, following 2-way analysis of variance and measurement of the F of interaction (P < .01 according to Tukey test).
Effect of ATRA and STI571 alone or in combination on the growth, granulocytic maturation, and apoptosis in NB4 cells.
NB4 cells were seeded at an initial concentration of 2 × 105/mL and treated for the indicated amount of time with medium alone (▵) or medium containing ATRA (10−7 M) (○), STI571 (5 × 10−6 M) (▴), and STI571 + ATRA (●). Aliquots of the cultures were withdrawn at each time point and subjected to counting, following erythrosin staining for the determination of the total number of viable cells (A), the percentage of viable cells (B), the degree of apoptosis as assessed by DAPI staining (C), and the level of granulocytic maturation, as assessed by measuring the percentage of NBT-positive cells (D) or cell-associated NBT-reducing activity (E). Results are the mean ± SD of 3 separate culture dishes and are representative of at least 3 independent experiments. *, significantly higher or lower than the untreated control (P < .01 according to the Studentt test). °, significantly higher or lower than the sum of the effects observed in the corresponding STI571- and ATRA-treated groups, following 2-way analysis of variance and measurement of the F of interaction (P < .01 according to Tukey test).
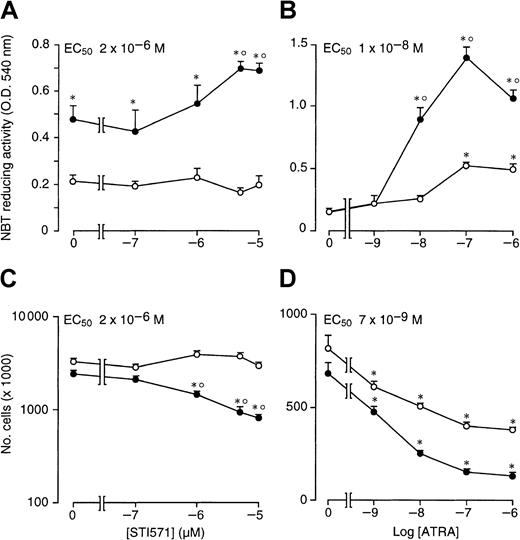
As documented by Figure 2A, when STI571 is incubated for 4 days in the presence of ATRA (10−7 M), the enhancing effect of the compound on retinoid-induced granulocytic maturation of NB4 cells is already evident at 1 × 10−6M and plateaus at 5 × 10−6 M, with a median effective concentration value of approximately 2 × 10−6 M. This concentration is the same as that necessary to cause half maximal inhibition of NB4 cell growth by STI571 in combination with the retinoid (Figure 2C). The results indicate that there is a tight relationship between the growth-inhibitory and the cyto-differentiating effects of the combination of ATRA + STI571. The minimal concentration of ATRA causing significant interaction with STI571 (5 × 10−6) both in terms of cyto-differentiation (Figure 2B) and growth inhibition (Figure 2D) is approximately 10−8 M. However, the maximal effect of the retinoid is observed at 10−7 M.
Dose-dependent effects of STI571 and ATRA on the growth and granulocytic maturation of NB4 cells.
NB4 cells were seeded at an initial concentration of 2 × 105/mL and treated for 4 days with vehicle alone, STI571, ATRA, or the combination of the 2 compounds. In (A) and (C), cells were treated with the indicated concentrations of STI571 in the absence (○) or presence of ATRA (10−7 M) (●). In (B) and (D), cells were treated with the indicated concentrations of ATRA in the absence (○) or presence of STI571 (5 × 10−6 M) (●). The level of NBT-reducing activity is presented in (A) and (B), whereas the number of viable cells is shown in (C) and (D). Results are the mean ± SD of 3 separate culture dishes and are representative of 2 independent experiments. *, significantly higher or lower than the untreated control (P < .01 according to the Studentt test). °, significantly higher or lower than the sum of the effects observed in the corresponding STI571- and ATRA-treated groups, following 2-way analysis of variance and measurement of the F of interaction (P < .01 according to Tukey test).
Dose-dependent effects of STI571 and ATRA on the growth and granulocytic maturation of NB4 cells.
NB4 cells were seeded at an initial concentration of 2 × 105/mL and treated for 4 days with vehicle alone, STI571, ATRA, or the combination of the 2 compounds. In (A) and (C), cells were treated with the indicated concentrations of STI571 in the absence (○) or presence of ATRA (10−7 M) (●). In (B) and (D), cells were treated with the indicated concentrations of ATRA in the absence (○) or presence of STI571 (5 × 10−6 M) (●). The level of NBT-reducing activity is presented in (A) and (B), whereas the number of viable cells is shown in (C) and (D). Results are the mean ± SD of 3 separate culture dishes and are representative of 2 independent experiments. *, significantly higher or lower than the untreated control (P < .01 according to the Studentt test). °, significantly higher or lower than the sum of the effects observed in the corresponding STI571- and ATRA-treated groups, following 2-way analysis of variance and measurement of the F of interaction (P < .01 according to Tukey test).
Interaction between STI571 and ATRA is not limited to NB4 blasts but is also observed in retinoid-responsive HL-60 and U937 myeloblastic cells as well as in retinoid-resistant NB4 clones
HL-60 and U937 are PML-RARα− myeloblastic cell lines that respond to the cyto-differentiating action of ATRA.46 47 As illustrated by Figure3A, following 4 days of treatment, STI571 has an inducing effect on NBT-reducing activity similar to that of ATRA in both HL-60 and U937 blasts. When both cell lines are incubated with STI571 and ATRA simultaneously, NBT-reducing activity is significantly increased relative to treatment with the tyrosine kinase inhibitor or the retinoid alone. As expected, NB4.306 and NB4.007 cells are refractory to the cyto-differentiating action of ATRA. When NB4.306 blasts are treated with STI571 alone, a slight but reproducible increase in NBT-reducing activity is observed, whereas this differentiation marker is left substantially unaffected by the tyrosine kinase inhibitor in NB4.007 cells. In both cell lines, the STI571 + ATRA combination has a stronger inducing effect than STI571 or ATRA alone. However, the most interesting results are observed in ATRA-resistant NB4.R1 blasts (Figure 3B). In this cell line, coadministration of STI571 and ATRA results in a significant induction of NBT-reducing activity relative to what is observed following treatment with vehicle, STI571, or ATRA alone. The cyto-differentiating effect of combinations between STI571 and ATRA is further confirmed by increased expression of the 2 membrane markers CD11b and especially CD11c. These phenomena are accompanied by cell growth inhibition, which is largely the result of the STI571 activity, although the effect is slightly augmented by addition of ATRA to the growth medium.
Effect of STI571 and ATRA alone or in combination on the differentiation and growth of ATRA-sensitive and ATRA-resistant myeloid cell lines.
The ATRA-sensitive NB4, HL-60, and U937 cells as well as the ATRA-resistant NB4-derived sublines NB4.306, NB4.007, and NB4.R1 cells were seeded at an initial concentration of 2 × 105/mL and treated for 4 days with vehicle alone (control, ■), STI571 (▨, 5 × 10−6 M; 1 × 10−5 M in the case of HL-60), ATRA (░, 10−7 M), or the combination of the 2 compounds (▪). (A) The level of NBT-reducing activity was measured in the indicated cell line. (B) The level of NBT-reducing activity (left panel); the percentage of CD11b, CD11c, or CD33 (middle panel); and the total number of viable cells (right panel) were determined in NB4.R1 cells (the percentage of cell viability is shown above columns). Results are the mean ± SD of 3 separate culture dishes and are representative of at least 2 independent experiments. *, significantly lower than the corresponding group treated with medium alone (P < .01 according to the Student t test). °°, significantly higher than the sum of the effects observed in the corresponding STI571- and ATRA-treated groups, following 2-way analysis of variance and measurement of the F of interaction (P < .01 according to Tukey test).
Effect of STI571 and ATRA alone or in combination on the differentiation and growth of ATRA-sensitive and ATRA-resistant myeloid cell lines.
The ATRA-sensitive NB4, HL-60, and U937 cells as well as the ATRA-resistant NB4-derived sublines NB4.306, NB4.007, and NB4.R1 cells were seeded at an initial concentration of 2 × 105/mL and treated for 4 days with vehicle alone (control, ■), STI571 (▨, 5 × 10−6 M; 1 × 10−5 M in the case of HL-60), ATRA (░, 10−7 M), or the combination of the 2 compounds (▪). (A) The level of NBT-reducing activity was measured in the indicated cell line. (B) The level of NBT-reducing activity (left panel); the percentage of CD11b, CD11c, or CD33 (middle panel); and the total number of viable cells (right panel) were determined in NB4.R1 cells (the percentage of cell viability is shown above columns). Results are the mean ± SD of 3 separate culture dishes and are representative of at least 2 independent experiments. *, significantly lower than the corresponding group treated with medium alone (P < .01 according to the Student t test). °°, significantly higher than the sum of the effects observed in the corresponding STI571- and ATRA-treated groups, following 2-way analysis of variance and measurement of the F of interaction (P < .01 according to Tukey test).
STI571 interacts with RARα or PML-RARα agonists but not with RXR agonists
AM580 is a selective RARα and PML-RARα agonist with strong cyto-differentiating activity in APL cells.24 Figure4A demonstrates that peak induction of NBT-reducing activity in NB4 cells is already observed following 4 days of treatment with AM580 at concentrations as low as 10−8M. STI571 enhances AM580-dependent stimulation of the marker enzyme, indicating that activation of RARα and/or PML-RARα is necessary and sufficient for the STI571-dependent enhancement of the granulocytic maturation program triggered by retinoids.
Effect of synthetic retinoids and tyrosine kinase inhibitors on the granulocytic differentiation and growth of NB4 cells.
NB4 cells were seeded at an initial concentration of 2 × 105/mL and treated for 4 days (A and B) or 3 days (C and D) with the indicated compounds. (A) NBT-reducing activity in NB4 cells treated with the indicated concentrations of the RARα agonist AM580 in the absence (○) or presence of STI571 (●, 5 × 10−6 M). (B) NBT-reducing activity in cells treated with vehicle (control), STI571 (5 × 10−6 M), the RXR agonist CD2915 (10−6 M), 8-CPT-cAMP (1 × 10−4 M; cAMP), ATRA (10−7 M), and the indicated combinations. (C) and (D) NBT-reducing activity and growth of cells treated with vehicle (control), STI571 (5 × 10−6M), ATRA (10−7 M), the tyrosine kinase inhibitors AG957 (1 × 10−6 M), NSC68 410 (1 × 10−6 M), and NSC676448 (1 × 10−6 M) or the indicated combinations. Results are the mean ± SD of 3 separate culture dishes and are representative of at least 2 independent experiments. *, significantly higher or lower than the corresponding group treated with medium alone (P < .01 according to the Studentt test). °, significantly higher than the sum of the effects observed in the corresponding STI571- and ATRA-treated groups, following 2-way analysis of variance and measurement of the F of interaction (P < .01 according to Tukey test).
Effect of synthetic retinoids and tyrosine kinase inhibitors on the granulocytic differentiation and growth of NB4 cells.
NB4 cells were seeded at an initial concentration of 2 × 105/mL and treated for 4 days (A and B) or 3 days (C and D) with the indicated compounds. (A) NBT-reducing activity in NB4 cells treated with the indicated concentrations of the RARα agonist AM580 in the absence (○) or presence of STI571 (●, 5 × 10−6 M). (B) NBT-reducing activity in cells treated with vehicle (control), STI571 (5 × 10−6 M), the RXR agonist CD2915 (10−6 M), 8-CPT-cAMP (1 × 10−4 M; cAMP), ATRA (10−7 M), and the indicated combinations. (C) and (D) NBT-reducing activity and growth of cells treated with vehicle (control), STI571 (5 × 10−6M), ATRA (10−7 M), the tyrosine kinase inhibitors AG957 (1 × 10−6 M), NSC68 410 (1 × 10−6 M), and NSC676448 (1 × 10−6 M) or the indicated combinations. Results are the mean ± SD of 3 separate culture dishes and are representative of at least 2 independent experiments. *, significantly higher or lower than the corresponding group treated with medium alone (P < .01 according to the Studentt test). °, significantly higher than the sum of the effects observed in the corresponding STI571- and ATRA-treated groups, following 2-way analysis of variance and measurement of the F of interaction (P < .01 according to Tukey test).
Combinations of cell-permeable analogs of cAMP and RXR agonists have been recently shown to cause granulocytic maturation of NB4 cells through an intracellular pathway that is entirely different from that activated by ATRA and RAR agonists.48 As shown in Figure4B, we confirm this with the use of the cell-permeable analog of cAMP, 8-CPT-cAMP, and CD2915, a powerful and selective RXR ligand.25 The level of NBT-reducing activity observed following treatment with cAMP + CD2915 is higher than that observed in NB4 cells treated with cAMP + ATRA or STI571 + ATRA. Thus, we determined whether STI571 could substitute for or complement cAMP and activate the RXR-dependent pathway leading to granulocytic maturation of NB4 cells. Combinations between STI571 and CD2915 are as ineffective as the single agents in inducing cyto-differentiation in NB4 cells. In addition, the tyrosine kinase inhibitor has no significant effect on the induction of NBT-reducing activity triggered by cAMP + ATRA. These data indicate that RXRs do not mediate the interaction between STI571 and retinoids.
Other structurally unrelated c-Abl inhibitors, such as AG957, NSC68410, and NSC676448,26-28 have no significant effects on the induction of NBT-reducing activity caused by ATRA (Figure 4C), although, in the presence or absence of the retinoid, they inhibit cell growth to variable extents (Figure 4D). Thus, STI571 is unique among c-Abl tyrosine kinase inhibitors in enhancing the cyto-differentiating effect of ATRA. This is likely to be the result of the fact that AG957, NSC68410, and NSC676448 are cytotoxic to NB4 cells and can be used only at concentrations below the 50% inhibitory concentration, presumably affording only limited intracellular inhibition of the target tyrosine kinase(s).
STI571 enhances the expression of other ATRA-induced granulocytic differentiation markers acting at the messenger RNA level
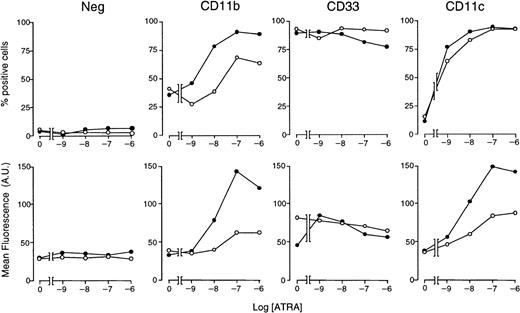
With the use of optimal concentrations of STI571 (5 × 10−6 M) and variable amounts of ATRA, we determined the effects of these combinations on markers of myeloid differentiation other than NBT staining (Figure5). In control conditions, approximately 35% of NB4 cells are CD11b+ and less than 10% are CD11c+, whereas the whole cell population is positive for the early myeloid marker CD33. As expected,34 ATRA increases the number of CD11b+ and CD11c+ cells in a dose-dependent manner, whereas it does not alter the proportion of CD33-synthesizing cells to a significant extent. ATRA-dependent increases in the number of CD11b+ and CD11c+cells are accompanied by a corresponding elevation in the amount of cell-associated fluorescence. When given in combination with the retinoid, STI571 augments the ATRA-induced surface expression of both CD11b and CD11c, leaving unaffected CD33. Notice that the enhancing effect on CD11c is limited to an increase in cell-associated fluorescence. Taken together, these results indicate that the tyrosine kinase inhibitor has positive effects on various phenotypic markers of the granulocytic differentiation program set in motion by ATRA in NB4 cells.
Effect of STI571 and ATRA alone or in combination on the expression of CD11b, CD11c, and CD33 myeloid markers in NB4 cells.
NB4 cells were seeded at an initial concentration of 2 × 105/mL and treated for 3 days with vehicle alone, STI571 (5 × 10−6 M), the indicated concentrations of ATRA (○), or the combination of the 2 compounds (●). The level of expression of the indicated phenotypic markers was determined by flow cytometry, using fluorescein-labeled monoclonal antibodies against CD11b, CD33, and CD11c or irrelevant isotype-matched antibodies (Neg). The percentage of marker-positive cells is shown in the upper panels, whereas the amount of cell-associated fluorescence in arbitrary units is illustrated in the lower panels. Results are the mean ± SD of 3 separate culture dishes and are representative of at least 2 independent experiments.
Effect of STI571 and ATRA alone or in combination on the expression of CD11b, CD11c, and CD33 myeloid markers in NB4 cells.
NB4 cells were seeded at an initial concentration of 2 × 105/mL and treated for 3 days with vehicle alone, STI571 (5 × 10−6 M), the indicated concentrations of ATRA (○), or the combination of the 2 compounds (●). The level of expression of the indicated phenotypic markers was determined by flow cytometry, using fluorescein-labeled monoclonal antibodies against CD11b, CD33, and CD11c or irrelevant isotype-matched antibodies (Neg). The percentage of marker-positive cells is shown in the upper panels, whereas the amount of cell-associated fluorescence in arbitrary units is illustrated in the lower panels. Results are the mean ± SD of 3 separate culture dishes and are representative of at least 2 independent experiments.
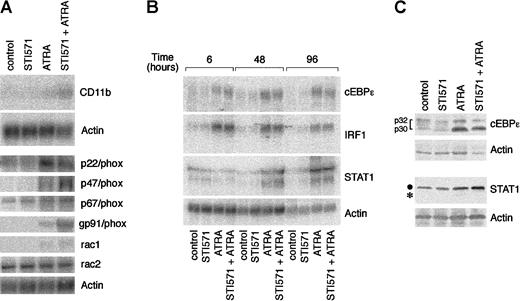
To gain insight into the molecular mechanisms underlying the interaction between STI571 and ATRA, we determined the steady-state levels of the transcripts coding for CD11b and the multiple components of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase responsible for NBT-reducing activity. Following treatment of NB4 cells with medium alone or STI571 for 4 days, very low levels of CD11b messenger RNA (mRNA) are evident. ATRA up-regulates the transcript, and this effect is enhanced a further 3-fold if STI571 is added to the retinoid (Figure 6A). This is similar to what is observed in the case of the mRNAs encoding p47/phox and gp91/phox, 2 of the NADPH oxidase components. By contrast, the tyrosine kinase inhibitor has no significant effects on the induction of rac1 and p22/phox mRNAs by ATRA. As to the remaining constituents of the NADPH oxidase enzymatic complex, the basal levels of expression of p67/phox and rac2 mRNAs are left unaltered by all the treatments considered.
Effect of STI571 and ATRA on the steady-state levels of the transcripts coding for CD11b; the components of the NADPH-oxidase complex; the transcription factors cEBPε, IRF-1, and STAT1; as well as cEBPε and STAT1 proteins.
NB4 cells were seeded at an initial concentration of 2 × 105/mL and treated for 4 days (A and C) or the indicated amount of time (B) with vehicle alone, STI571 (5 × 10−6 M), ATRA (10−7 M), and the combination of the 2 compounds. In (A) and (B), the steady-state levels of the indicated transcripts were measured by Northern blot analysis, using total RNA (20 μg/lane) and 32P-labeled specific cDNA probes. In (C), cEBPε and STAT1 protein levels were determined by Western blot analysis (●, intact form of STAT1; *, degradation product of the protein). Determination of NBT-reducing activity in parallel cultures at the time of RNA harvesting gave the following results in ΔO.D. at 540 nm: control = 0.21; STI571 = 0.21; ATRA = 0.41; STI571 + ATRA = 0.92.
Effect of STI571 and ATRA on the steady-state levels of the transcripts coding for CD11b; the components of the NADPH-oxidase complex; the transcription factors cEBPε, IRF-1, and STAT1; as well as cEBPε and STAT1 proteins.
NB4 cells were seeded at an initial concentration of 2 × 105/mL and treated for 4 days (A and C) or the indicated amount of time (B) with vehicle alone, STI571 (5 × 10−6 M), ATRA (10−7 M), and the combination of the 2 compounds. In (A) and (B), the steady-state levels of the indicated transcripts were measured by Northern blot analysis, using total RNA (20 μg/lane) and 32P-labeled specific cDNA probes. In (C), cEBPε and STAT1 protein levels were determined by Western blot analysis (●, intact form of STAT1; *, degradation product of the protein). Determination of NBT-reducing activity in parallel cultures at the time of RNA harvesting gave the following results in ΔO.D. at 540 nm: control = 0.21; STI571 = 0.21; ATRA = 0.41; STI571 + ATRA = 0.92.
To investigate whether enhanced cyto-differentiation by STI571 + ATRA is the result of a modulation in the expression of genes coding for transcription factors that are involved in the process of myeloid maturation, we measured the levels of the mRNAs for cEBPε,49 STAT1,50 and IRF-1.51However, as shown in Figure 6B, in no instance did we measure increases in the 3 mRNAs by the combination STI571 + ATRA over what is observed following treatment of NB4 cells with the retinoid alone. At the protein level, ATRA elevates STAT1, although it also induces degradation of the transcription factor, as indicated by the appearance of a lower molecular weight band (Figure 6C). Addition of STI571 to the medium protects STAT1 from ATRA-induced proteolysis, ultimately resulting in a further increase in the levels of the protein. A similar effect is not observed in the case of cEBPε, whose ATRA-induced up-regulation is not significantly affected by STI571.
STI571 alone or in combination with ATRA alters the expression profile of many other genes
With the use of a cDNA microarray containing clones corresponding to cytokine and cytokine receptors, we studied the differential expression of almost 400 genes in NB4 cells. As indicated in Table1, on treatment with STI571, ATRA, or STI571 + ATRA for 2 days, approximately 6% of the cDNAs represented in the array show reproducible alterations in their basal levels of expression. These cDNAs can be divided into 4 groups according to their expression profile. The first group contains genes (the myeloid chemokine monocyte chemotactic protein 1 precursor [MCP-1], migration-inhibitory factor-related protein 8 [MRP-8], MRP-14, and corticotropin-releasing factor receptor type 1), which are expressed at low levels in control conditions, are induced by ATRA and further augmented by the combination of ATRA + STI571. The second one consists of genes, such as those for the transcription factor interferon regulatory factor 1 (IRF-1) and the ligand/receptor couple brain-derived neurotrophic factor/neuromodulin, whose expression is induced by ATRA but not further enhanced by ATRA + STI571. The genes for thymosine β3, the neurotrophic growth factor receptor, and heparin-binding neurite outgrowth-promoting factor 1, whose expression is inhibited by STI571 regardless of the presence or absence of ATRA, are gathered in a third group. A fourth subset combines genes, including predominantly those coding for cytokines (such as granulocyte macrophage colony-stimulating factor, interferon γ, IL-4, and IL-13), which are up-regulated by STI571 and whose induction is inhibited by the addition of ATRA. At present, tumor necrosis factor α (TNFα) is the only example of a gene that is down-regulated by ATRA and further inhibited in its expression by STI571 + ATRA. The profile of expression of a number of genes modulated by STI571 was confirmed by RT-PCR and Northern blot experiments (data not shown except for IRF-1 in Figure 6B) or by immunoassay, as in the case of the MCP-1 protein (data not shown).
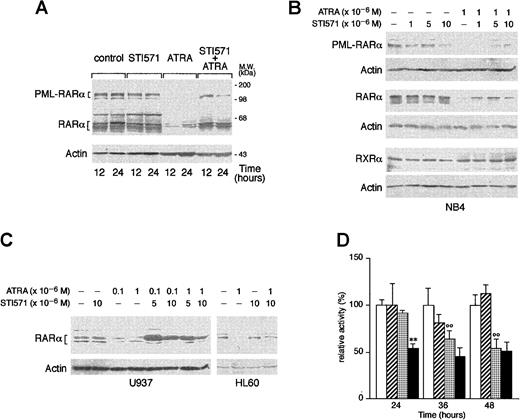
STI571 delays the ATRA-dependent degradation of RARα and PML-RARα
ATRA is known to induce the degradation of PML-RARα and RARα, and this phenomenon has been related to the ability of the retinoid to induce granulocytic differentiation of APL cells.52-55 As shown in Figure 7A, treatment of NB4 cells with ATRA for 12 and 24 hours causes the disappearance of the bands corresponding to PML-RARα and a remarkable decrease in the levels of those corresponding to RARα. These effects are the result of specific degradation of the 2 proteins, as the amounts of the relative mRNAs are left unaltered by the tyrosine kinase inhibitor or the retinoid (data not shown). Whereas STI571 alone does not modify the quantities of RARα or PML-RARα observed in control conditions, combinations between the tyrosine kinase inhibitor and ATRA lead to a decrease in the degradation of the 2 receptors that is evident at least until 24 hours of treatment. As shown in Figure 7B, inhibition of RARα and PML-RARα degradation is observed at the same concentrations of STI571 that induce growth inhibition and granulocytic maturation. The protective effect on ATRA-dependent RARα proteolysis afforded by STI571 is not limited to NB4 but is also observed in HL-60 and U937 cells (Figure 7C). Because the retinoid-dependent catabolism of nuclear retinoic acid receptors is largely, although not exclusively, mediated by the proteasome complex,55 56 we evaluated the effect of STI571 and ATRA on the proteolytic activity of this subcellular organelle, using a fluorogenic peptide substrate. As shown in Figure 7D, treatment of NB4 cells with the combination STI571 + ATRA results in a significant inhibition of the proteolytic activity of the proteasome. The effect is already observed at 24 hours, a time at which neither STI571 nor ATRA affects proteasome activity. This interaction is progressively lost at 36 and 48 hours, since ATRA alone starts to exert an inhibitory action that is not further modified by the tyrosine kinase inhibitor. The proteolytic activity measured in our experimental conditions is largely due to the activity of the proteasome as it is more than 80% inhibited by the specific proteasome inhibitor z-Ile-Glu(Obut)-Ala-Leu-H(aldehyde) (data not shown).
Effect of STI571 and ATRA on the degradation of PML-RARα, RARα, and RXR as well as proteasome activity in NB4 cells.
NB4 (A and B), or HL-60 and U937 (C) cells were seeded at an initial concentration of 2 × 105/mL and treated for 3 days (B and C) with the indicated compounds and for the indicated amount of time. (A and D) Western blot analysis (50 μg protein/lane) was performed with specific antibodies against PML-RARα, RARα, RXRα, and β-actin. On the right side of panel A, the position of the molecular weight standard is indicated. (D) Proteasome-dependent proteolytic activity was measured with a specific fluorogenic peptide substrate. Results are the mean ± SD of 3 separate culture dishes. The results are representative of at least 3 independent experiments. ■, control; ▨, STI571; ░, ATRA; ▪, STI571 plus ATRA; **, significantly lower than the corresponding group treated with ATRA alone (P < .01 according to the Student ttest); °°, significantly lower than the corresponding control group (P < .01 according to the Student t test).
Effect of STI571 and ATRA on the degradation of PML-RARα, RARα, and RXR as well as proteasome activity in NB4 cells.
NB4 (A and B), or HL-60 and U937 (C) cells were seeded at an initial concentration of 2 × 105/mL and treated for 3 days (B and C) with the indicated compounds and for the indicated amount of time. (A and D) Western blot analysis (50 μg protein/lane) was performed with specific antibodies against PML-RARα, RARα, RXRα, and β-actin. On the right side of panel A, the position of the molecular weight standard is indicated. (D) Proteasome-dependent proteolytic activity was measured with a specific fluorogenic peptide substrate. Results are the mean ± SD of 3 separate culture dishes. The results are representative of at least 3 independent experiments. ■, control; ▨, STI571; ░, ATRA; ▪, STI571 plus ATRA; **, significantly lower than the corresponding group treated with ATRA alone (P < .01 according to the Student ttest); °°, significantly lower than the corresponding control group (P < .01 according to the Student t test).
STI571 inhibits c-Abl but not c-kit tyrosine phosphorylation
As shown in Figure 8A, in basal conditions, NB4 cells express significant amounts of the STI571 targets, c-Abl and c-kit. Concentrations of the tyrosine kinase inhibitor (5 × 10−6 M) capable of enhancing the maturation effect of ATRA do not affect c-Abl or c-kit protein levels significantly. Similarly, either alone or in combination with STI571, ATRA does not alter the levels of the 2 proteins. As shown in Figure8B, both c-Abl and c-kit are constitutively tyrosine-phosphorylated, as indicated by Western blot experiments conducted with antiphosphotyrosine antibodies on immunoprecipitates of the 2 proteins. Following 18 hours of continuous treatment, STI571 decreases tyrosine-phosphorylation of c-Abl (54% in the experiment shown), whereas it has no significant effect on c-kit. ATRA is ineffective in modulating the level of c-Abl (50% inhibition) or c-kit tyrosine-phosphorylation observed in the absence or presence of STI571.
Effects of STI571 and ATRA on the levels tyrosine phosphorylation of c-Abl and c-kit.
NB4 cells seeded at an initial concentration of 2 × 105/mL were treated with STI571 (5 × 10−6 M), ATRA (10−7 M), or the combination of the 2 compounds for the indicated amount of time (A) or for 16 hours (B). In (A), cell extracts (50 μg protein/lane) were subjected to Western blot analysis with c-Abl, c-kit, or β-actin antibodies. In (B), cell extracts were immunoprecipitated (ip) with c-Abl or c-kit antibodies. The immunoprecipitates were run on a 6% polyacrylamide gel and subjected to Western blot analysis (WB) with antiphosphotyrosine antibodies (p-Tyr Ab), c-Abl, or c-kit antibodies. The results are representative of 2 independent experiments.
Effects of STI571 and ATRA on the levels tyrosine phosphorylation of c-Abl and c-kit.
NB4 cells seeded at an initial concentration of 2 × 105/mL were treated with STI571 (5 × 10−6 M), ATRA (10−7 M), or the combination of the 2 compounds for the indicated amount of time (A) or for 16 hours (B). In (A), cell extracts (50 μg protein/lane) were subjected to Western blot analysis with c-Abl, c-kit, or β-actin antibodies. In (B), cell extracts were immunoprecipitated (ip) with c-Abl or c-kit antibodies. The immunoprecipitates were run on a 6% polyacrylamide gel and subjected to Western blot analysis (WB) with antiphosphotyrosine antibodies (p-Tyr Ab), c-Abl, or c-kit antibodies. The results are representative of 2 independent experiments.
Discussion
In this article, we demonstrate that STI571 enhances and accelerates the cyto-differentiating activity of ATRA in retinoid-sensitive cell lines derived from APL and other types of myeloblastic leukemia. In addition, the compound relieves ATRA resistance in NB4 sublines selected for their insensitivity to the retinoid. The interaction between STI571 and ATRA is mediated by PML-RARα or RARα but not by RXRs, as indicated by the results obtained with specific agonists of the 2 types of retinoic acid nuclear receptors. STI571 is unique among selective tyrosine kinase inhibitors in its ability to interact with ATRA and to stimulate granulocytic maturation of APL cells. This is likely to be the result of its low cytotoxic activity relative to the other structurally unrelated compounds used in this study. Enhanced cyto-differentiation by STI571 + ATRA is accompanied by accelerated growth inhibition in both ATRA-sensitive and ATRA-resistant NB4 sublines and followed by increased spontaneous apoptosis.
Stimulation of granulocytic maturation by STI571 + ATRA is observed at the level of many myeloid markers, whose expression is up-regulated by the retinoid alone. These include CD11b, CD11c, MRP-8, MRP1-4, MCP-1, and some of the components of the enzymatic complex, NADPH oxidase, which is responsible for NBT-reducing activity. Elevation of these markers is primarily the result of transcriptional or post-transcriptional events, as the phenomenon is associated with an increase of the steady-state levels of the corresponding transcripts. Treatment of NB4 cells with STI571, ATRA, or the combination of the 2 compounds has also significant effects on the expression profile of a substantial number of genes coding for cytokine and cytokine receptors. Among these, down-regulation of the TNFα gene by ATRA + STI571 could be of clinical relevance, since overproduction of this cytokine may contribute to the tissue damage effects observed in ATRA syndrome. In this context, it is reassuring that STI571-dependent up-regulation of the potentially harmful proinflammatory cytokines IL-6, IL-8, and migration-inhibitory factor is blunted or inhibited by the addition of ATRA. Finally, of potential interest for the growth-inhibitory effect of STI571 + ATRA is also the down-regulation of thymosin β3 afforded by the tyrosine kinase inhibitor, as thymosins have been implicated in the control of the cell cycle.57
In APL cells, the process of granulocytic maturation triggered by ATRA is likely to depend on the activation of a complex network of genes controlled directly or indirectly by retinoic acid receptors and other transcription factors. Thus, it is conceivable that STI571 enhances the pharmacologic action of ATRA by modulating the levels or the state of activation of this type of nuclear proteins.
As to retinoic acid receptors, the APL blast expresses significant amounts of PML-RARα, RARα, and the 3 RXR isoforms.6,35 PML-RARα is believed to interfere with the normal physiologic function of RARα and/or PML.5 These phenomena are thought to underlie the maturation block and the abnormal expansion of the leukemic clone. Degradation of PML-RARα afforded by pharmacologic concentrations of ATRA is thought to induce release of the dominant-negative effect on RAR5 and to cause PML relocalization.5 These 2 effects are deemed necessary and/or sufficient to overcome the maturation arrest and allow blasts to proceed along the granulocytic differentiation pathway. Interestingly, enhanced granulocytic maturation observed in NB4 cells treated with STI571 + ATRA is associated with inhibition of ligand-dependent PML-RARα proteolysis. This indicates that granulocytic maturation of the APL blast proceeds independently of PML-RARα degradation. Indeed, the finding suggests that delayed disappearance of the oncogenic fusion protein may be instrumental in accelerating and enhancing granulocytic maturation. This is compatible with the observations made in PML-RARα–expressing U937 cells47 and with very recent reports demonstrating that PML-RARα in its homodimeric form58,59 is the crucial determinant of the ATRA-mediated cyto-differentiation effect observed in APL cells.58STI571 inhibits not only the ATRA-dependent degradation of PML-RARα but also that of RARα, whereas it has no apparent effect on RXRα. Stabilization of RARα is observed in NB4, HL-60, and U937 cells, ie, in all the retinoid-sensitive myeloid cell lines in which the tyrosine kinase inhibitor enhances the cyto-differentiating effect of ATRA. The correlation between stabilization of nuclear retinoic acid receptors and enhanced granulocytic maturation suggests that the 2 phenomena are causally related and explain, albeit partially, the pharmacologic action of the STI571 + ATRA combination in ATRA-sensitive cellular contexts. This mechanism may be active also in ATRA-resistant APL cells, since constitutive degradation of PML-RARα has been demonstrated in the NB4.007 subline and suggested to be important for the resistant phenotype.56
In retinoid-sensitive APL cells, ATRA and synthetic retinoids degrade the nuclear retinoic acid receptors through activation of a caspase- and/or a proteasome-dependent pathway.52-55 Within the first 4 days of culture, caspase activity is very low in NB4 cells and is left unaffected by treatment with ATRA,25 STI571, or the combination of the 2 compounds (M.G. and E.G., unpublished results, January 2000). This suggests that inhibition of caspases is not involved in the stabilization of nuclear retinoic acid receptors observed following treatment with STI571 + ATRA. By contrast, when given in association with ATRA, STI571 has a significant inhibitory effect on the activity of the proteasome complex. The phenomenon is likely to explain the stabilization effect on nuclear retinoic acid receptors and is of a complex nature, since it requires the contemporaneous presence of the tyrosine kinase inhibitor and the retinoid.
As to the transcription factors other than retinoic acid receptors, potentially involved in ATRA-induced myeloid maturation of the APL blast, we took into consideration STAT1, cEBPε, and IRF-1. Whereas STI571 does not enhance ATRA-dependent increases in the expression of the STAT1 mRNA, it inhibits degradation of the relative protein, eventually resulting in super induction of the transcription factor. Given the number of genes implicated in the processes of differentiation, growth arrest, and apoptosis regulated by STAT1, it is possible that the factor plays a role in the observed interaction between STI571 and ATRA. By contrast, cEBPε and IRF-1 are unlikely to be involved in the enhancement of ATRA-dependent cell differentiation triggered by STI571. In fact, the levels of the 2 transcription factors are not further increased by STI571 over what is observed in the presence of ATRA alone.
A key question in the observed interaction between the tyrosine kinase inhibitor and ATRA is whether enhanced cyto-differentiation, growth inhibition, apoptosis, as well as retarded PML-RARα and RARα degradation are ultimately the result of STI571 action on its recognized molecular targets, ie, c-Abl, c-kit, and PDGF-R. NB4 cells contain detectable amounts of c-Abl and c-Kit, and the steady-state levels of the 2 proteins are not affected by STI571, ATRA, or the combination of the 2 compounds. By contrast, these leukemic blasts do not express significant amounts of the receptor for PDGF (at least as indicated by analysis of the cDNA microarray used in this study). Both c-Abl and c-kit show constitutive tyrosine phosphorylation in NB4 promyelocytes. Treatment with STI571 alone inhibits phosphorylation of c-Abl, whereas, surprisingly, it has no effect on c-kit. Thus, although we cannot rule out the involvement of other as yet unrecognized molecular targets, our results are compatible with the idea that c-Abl inhibition is the first step in the chain of events leading to STI571-dependent potentiation of the granulocytic maturation process triggered by ATRA in NB4 cells. Interestingly, as inhibition of c-Abl is not significantly affected by the presence of ATRA, it is likely that the site(s) of interaction between the tyrosine kinase inhibitor and the retinoid lays downstream of c-Abl itself.
In conclusion the observed interaction between STI571 and ATRA could be exploited at the clinical level in the first- or second-line treatment of APL and perhaps other types of PML-RARα− acute myelogenous leukemia. We are currently exploring the potential of this promising combination using in vivo animal models of APL.34
We are thankful to Prof Silvio Garattini and Dr Mario Salmona for critical reading of the manuscript. We would like to thank Dr Julia Szeberenyi and Mr Massimiliano Marini for skillful experimental work performed. We would also like to thank Prof P. Chambon and Dr Cecile Rochette-Egly (IGBMC, Strasbourg, France) for the kind gift of anti-RAR and anti-RXR antibodies.
Supported by grants to E.G. from the Associazione Italiana per la Ricerca contro il Cancro (AIRC), from the “Istituto Superiore di Sanità,” and from the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST). I.P. is the recipient of a fellowship from “La Via di Natale.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Enrico Garattini, Laboratorio di Biologia Molecolare, Centro Catullo e Daniela Borgomainerio, Istituto di Ricerche Farmacologiche “Mario Negri,” via Eritrea 62, 20157 Milano, Italy; e-mail: egarattini@irfmn.mnegri.it.